Abstract
Objective
To determine if low to moderate doses of anthracycline-based chemotherapy (Anth-bC) are associated with subclinical cardiovascular (CV) injury.
Background
Cancer survivors that receive Anth-bC experience premature CV events. It is unknown whether low to moderate doses of anthracyclines a) promote early subclinical CV disease manifested by deteriorations in left ventricular ejection fraction (LVEF) or increases in aortic stiffness, or b) are associated with change in quality of life (QOL).
Methods
In 53 men and women with breast cancer, leukemia, or lymphoma, we assessed left ventricular volumes, LVEF, circumferential strain, aortic pulse wave velocity (PWV), late gadolinium enhancement, serum B-type natriuretic peptide (BNP), troponin I (TnI), and the impact of treatment on QOL before, and 1, 3, and 6 months after receipt of Anth-bC.
Results
Participants averaged 50±2 (range 19–80) years in age, 58% were women, 17% were black, and they each received a range of 50 to 375 mg/m2 of doxorubicin equivalent chemotherapy. Left ventricular end systolic volume (LVESV; 48±3 to 54±3 ml; p=0.02), left ventricular strain (−17.7±0.4 to −15.1±0.4; p=0.0003), PWV (6.7±0.5 to 10.1±1 m/sec; p=0.0006), and QOL deterioration (15.4±3.3 to 28.5±3.9; p=0.008) increased, while LVEF (58±1 to 53±1%; p=0.0002) decreased within 6 months after low to moderate doses of Anth-bC. All findings persisted after accounting for age, gender, race (white/black), doxorubicin equivalent dose, doxorubicin administration technique, comorbidities associated with CV events, and cancer diagnosis (p=0.02 to 0.0001 for all). There were no new late gadolinium enhancement findings after 6 months.
Conclusions
Low to moderate doses of Anth-bC are associated with the early development of subclinical abnormalities of cardiac and vascular function that in other populations are associated with the future occurrence of CV events.
Keywords: Cardio-oncology, chemotherapy, cardiotoxicity
Introduction
Although anthracycline-based chemotherapeutic regimens for breast and hematologic malignancies have improved cancer-related survival, studies have recognized an unanticipated increase in premature cardiovascular (CV) events among individuals receiving these regimens (1–4). Today, CV events including systolic or diastolic left ventricular dysfunction, congestive heart failure, stroke, and myocardial ischemia and infarction are the second leading cause of premature morbidity and mortality among those surviving >5 years after their initial diagnosis and treatment for breast cancer (2), Hodgkin’s disease or non-Hodgkin’s lymphoma (3,4). Currently, anthracycline- based chemotherapeutic agents are typically limited to cumulative doses of ≤450 mg/m2 to reduce the incidence of clinical CV events (5), but to date, studies have not addressed whether lower doses of anthracyclines administered in the course of cancer treatment could promote early subclinical CV injury that in other populations (e.g., those with hypertension or atherosclerosis) are associated with future CV events.
Accordingly, we hypothesized that the administration of low to moderate doses of anthracycline- based chemotherapy (Anth-bC) would be associated with the early onset of subclinical CV disease that in other populations is associated with future CV events. To address this hypothesis, we measured imaging, biochemical, and quality of life markers that have been used in other larger population-based studies to identify subclinical CV disease predictive of adverse CV outcomes. We performed these measurements in a cohort of subjects recently diagnosed with either breast cancer or a hematologic malignancy who were scheduled to receive low to moderate doses of Anth-bC for the first time.
Methods
Study population and design
This study was approved by the Institutional Review Board of the Wake Forest University School of Medicine, and funded by the National Cancer Institute (R33CA12196). All participants provided witnessed, written informed consent. The study population consisted of 53 participants who were scheduled to receive Anth-bC for the first time. They were consecutively recruited over a 1.5 year period from the hematology/oncology outpatient clinics and inpatient hospital facilities within the Comprehensive Cancer Center at Wake Forest School of Medicine. Participants were asked to identify their race/ethnicity when enrolled in the study and this data was utilized as a variable in reporting participant demographic characteristics.
Cardiovascular magnetic resonance (CMR), serum biomarkers, and questionnaires assessing quality of life (QOL) were performed according to specifications used in other large National Institutes of Health sponsored population based studies of CV disease such as the Multi-Ethnic Study of Atherosclerosis or MESA (6). These markers were acquired before cancer treatment, and at 1, 3, and 6 months after initiation of low to moderately dosed Anth-bC. The 1, 3, and 6 month CMR imaging and serum biomarker acquisitions were obtained early each morning prior to the scheduled administration of chemotherapy. Analyses of all images were performed by individuals blinded to all study participant identifiers including prior imaging and biomarker results: a blinded, unpaired read. Participants were ineligible for enrollment if they had a contraindication to CMR (implanted metal or electronic devices). No participants received radiation therapy during the study.
CMR technique and measurements
Determination of left ventricular volumes and ejection fraction
All participants were scanned using a 1.5 Tesla Magnetom Avanto Scanner (Siemens, Erlangen, Germany). Left ventricular volumes and left ventricular ejection fraction (LVEF) were measured according to previously published methods (7) using cine white blood steady state free precession techniques with a 256×128 matrix, a 40 cm field of view (FOV), a 10-ms repetition time (TR), a 4-ms echo time (TE), a 20-degree flip angle (FA), an 8 mm thick slice, and a 40-ms temporal resolution. Ventricular volumes were also indexed to body surface area.
Left ventricular circumferential strain
Strain imaging was performed according to previously published methods using the spatial modulation of magnetization (SPAMM) technique at the middle papillary muscle level of the left ventricular short axis as identified on the 4 chamber view (8). Imaging parameters included a 34 cm FOV, a 10 ms TR, a 4 ms TE, a 20-degree FA, an 8 mm thick slice, a 256×160 matrix, and a 32 kHz bandwidth. Mean mid-wall left ventricular circumferential strain was measured according to previously published methods using harmonic phase (HARP) analyses (Diagnosoft, Cary, NC) (9).
Aortic Stiffness
Thoracic aortic pulse wave velocity (PWV) was determined according to previously published methods (6,10). Phase-contrast CMR imaging parameters included a 34 to 36 cm FOV, a 256×912 matrix, a 6 ms TR, a 3 to 5 ms TE, a 15–20-degree FA, an 8 mm thick slice, through-plane velocity encoding of 150 cm/sec, temporal resolution 25–35ms, GRAPPA acceleration factor of 2, 4–8 lines of K-space segmentation, and 30 frames reconstructed. The distance between ascending and descending thoracic aorta was obtained by tracing the centerline of the aortic lumen on a sagittal localizing image. The transit time of the flow wave was determined as the time delay between the ascending and descending aortic waveforms at 50% of their respective peaks (10).
Late Gadolinium Enhancement
Late gadolinium enhancement imaging was performed according to previously published methods (11). Inversion time sequences were performed 10 minutes following gadolinium injection, with an inversion time to provide a uniform dark non-infarcted myocardium. Multi-slice short-axis inversion recovery late gadolinium enhancement images were obtained scanning the heart base to the left ventricular apex. Scan parameters included a 784 ms TR, a 3.3 ms TE, a 130 Hz bandwidth, and 317 * 390 field of view.
Serum BNP and Troponin-I
At each study visit, patients underwent venipuncture for blood sampling which was accomplished prior to receipt of chemotherapy. Serum troponin-I or TnI (Beckman Coulter) and B-type natriuretic peptide or BNP (Phoenix Pharmaceuticals Inc.) levels were assessed according to previously published methods (12,13). Serum TnI values ≤ 0.06 ng/ml were considered negative for myocardial injury; values of 0.06≤x<1.0 ng/ml were considered as subclinical and values ≥1.0 ng/ml were considered as a clinical injury (14).
Impact on Quality of Life
At each study visit, patients completed the Minnesota Living with Heart Failure Questionnaire (MLHFQ) to assess the impact of cancer treatment on their QOL (15). This well-established questionnaire contains 21 items that assess key areas of physical, emotional, social, and mental dimensions of QOL. Respondents rate each item on a 6-point Likert scale ranging from 0=no impact to 5=very much impact.
Statistical analysis
Descriptive statistics, including means and standard errors for continuous measures, and counts and percentages for categorical measures were calculated for participants in the study. Next, a series of repeated measures mixed models were fit to examine the impact of time on the outcomes of interest; participants in these models were treated as random effects. Time was entered into the models as a random effect and considered as a continuous, time varying measure. A series of pre-defined covariates were assessed to determine whether the time effect on the outcome of interest was impacted by the covariate. To do this, time by covariate interactions was fit separately for each covariate. These covariates included age, race, gender, doxorubicin equivalent chemotherapy dose (16), type of cancer, the number of CV risk factors present at baseline (e.g., hypertension, diabetes, hyperlipidemia, tobacco use, and history of myocardial infarction or revascularization), and associated chemotherapy (defined as cyclophosphamide, trastuzumab, or all other agents). If the time by risk factor interaction was not significant, then mixed models were fit only including the main effects for time and the risk factor. In particular, for left ventricular volumes, PWV and strain, where differences between men and women were expected, we examined whether the time effects were consistent for men and women. All analyses were performed using SAS Software (Version 9.2) by personnel within the Department of Biostatistics in the Wake Forest School of Medicine with a p-value of ≤0.05 considered significant.
Results
Demographic data from the 53 participants are displayed in Table 1. The age averaged 50±2 (range 19 to 80) years. The majority (58%) of participants were women and of white race (83%). Four participants had known pre-existing coronary artery disease with previous revascularization. Forty-two percent (42%), 32%, 24%, and 2% of the participants carried the diagnosis of breast cancer, lymphoma, leukemia, or myelodysplastic syndrome, respectively.
Table 1.
Demographic Data (n=53)
| Variables | Characteristics |
|---|---|
| Age (yrs) | 50 ± 2 |
| Gender, % female | 58% |
| Height (cm) | 170 ± 2 |
| Weight (kg) | 84 ± 3 |
| Body Mass Index (kg/m2) | 29.6 ± 1.6 |
| Race (% white/black) | 83/17 |
| Cardiovascular risk factors | |
| Coronary artery disease | 8% |
| Diabetes | 13% |
| Hypertension | 40% |
| Hyperlipidemia | 25% |
| Smoking | 45% |
| Medications | |
| Beta Blocker | 17% |
| Calcium antagonist | 8% |
| Angiotensin-converting enzyme inhibitor | 26% |
| Angiotensin receptor blocker | 6% |
| Vasodilator | 4% |
| Statin | 21% |
| Diuretic | 26% |
Data are means +/− standard error of the estimate
Doxorubicin was administered to 37 participants (240 mg/m2 median cumulative dose; range 50 to 375 mg/m2) and daunorubicin to 16 participants (180 mg/m2 median cumulative dose; range 36 to 500 mg/m2). The anthracycline doxorubicin equivalents for all participants ranged from 50 to 375 mg/m2. The majority of participants (92%) received the anthracycline as a 15 minute intravenous infusion during consecutive days. The remaining 4 participants received their anthracycline as a 24 hour intravenous infusion as part of their chemotherapy regimen.
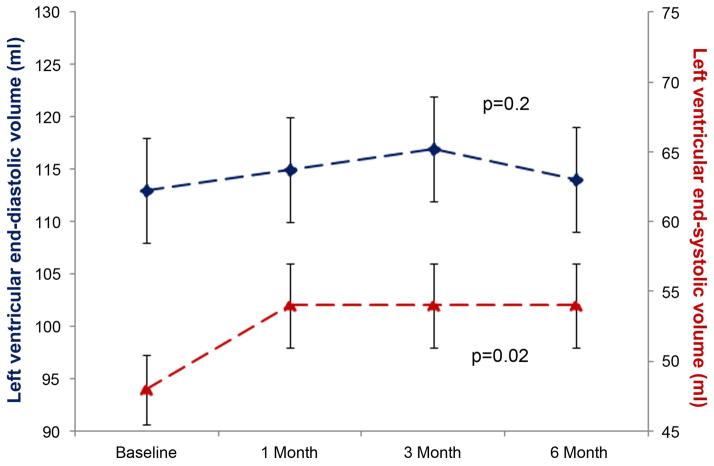
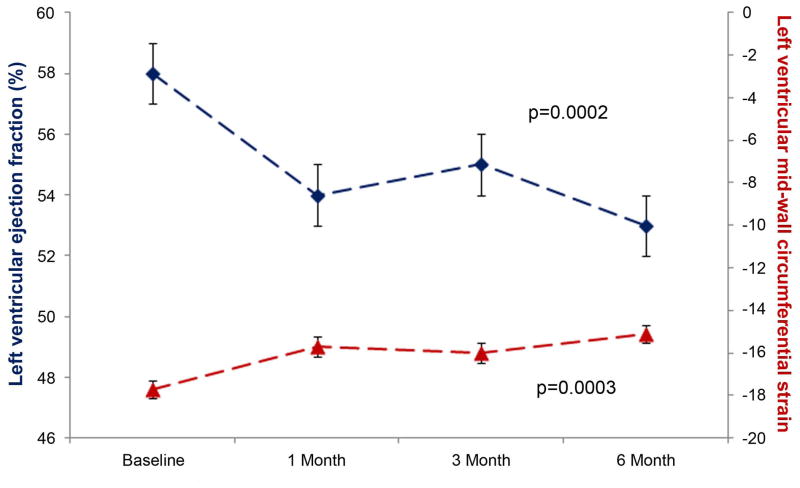
Left ventricular end diastolic volume (LVEDV) remained similar (113±5 ml at baseline and 114±5 ml at study end) throughout the study (p=0.2; Figure 1A). Left ventricular end systolic volume (LVESV) increased from 48±3 ml to 54±3 ml (p=0.02) during the study. As a result of these changes in left ventricular volumes, LVEF diminished (from 58±1 to 53±1% [p=0.0002]; Figure 1B) over 6 months. At baseline, 11% of the cohort (n=6) exhibited a LVEF < 50%, but this increased to 34% after completion of Anth-bC (p=0.002). Of the 47 participants that had a LVEF > 50% at baseline, 26% experienced a LVEF of <50% (a metric used clinically to distinguish mildly impaired left ventricular systolic dysfunction) 6 months after receipt of Anth-bC (p<0.01). The decline in LVEF also paralleled a 6 month increase in left ventricular mean mid-wall circumferential strain (less negative reflecting worsening regional systolic function [−17.7±0.4 to −15.1±0.4; p=0.0003; Figure 1B]).
Figure 1.
Time dependent changes in left ventricular end diastolic volume (LVEDV) (left y-axis; panel A) and left ventricular end systolic volume (LVESV) (right y-axis; panel A); in left ventricular ejection fraction (LVEF) (left y-axis; panel B) and mean mid wall circumferential strain (right y-axis; panel B). The mean ± the standard error are shown. There was a significant increase in LVESV and mean mid-wall circumferential strain while at the same time a decrease in LVEF from baseline to 6 months after administration of low to moderate doses of anthracycline-based chemotherapy.
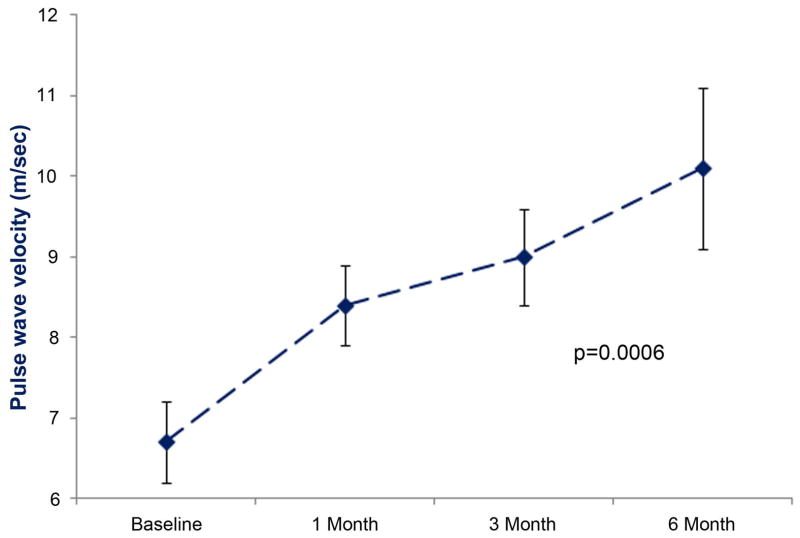
PWV increased steadily from 6.7±0.5 to 10.1±1 m/sec (p=0.0006) after Anth-bC administration (Figure 2). Adjusting baseline heart rate had a minimal impact on the change in PWV over time. However, after adjusting for baseline systolic blood pressure in a fitted model, participants with a higher systolic blood pressure had a higher PWV at baseline in addition to a faster increase in PWV over time compared to participants with lower systolic blood pressures (p=0.036).
Figure 2.
Time dependent changes in pulse wave velocity (y-axis). The mean ± the standard error are shown. There was a significant increase in pulse wave velocity from baseline to 6 months after administration of low to moderate doses of anthracycline-based chemotherapy.
Three participants with prior myocardial infarction and revascularization had late gadolinium enhancement imaging consistent with myocardial scar. All other baseline and 6 month late gadolinium enhancement studies were unremarkable. Serum BNP did not increase and trended (p=0.2) toward a decrease throughout the study (63±14 pg/ml at baseline and 46±4 pg/ml at study end). Serum TnI averaged 0.02±0.003 ng/ml at baseline and 0.04±0.01 ng/ml at the 6 month visit (p=0.12). More importantly, 26% of the cohort exhibited evidence of subclinical cardiac injury (0.06<TnI≤1.0) on the morning before their final dose of anthracycline.
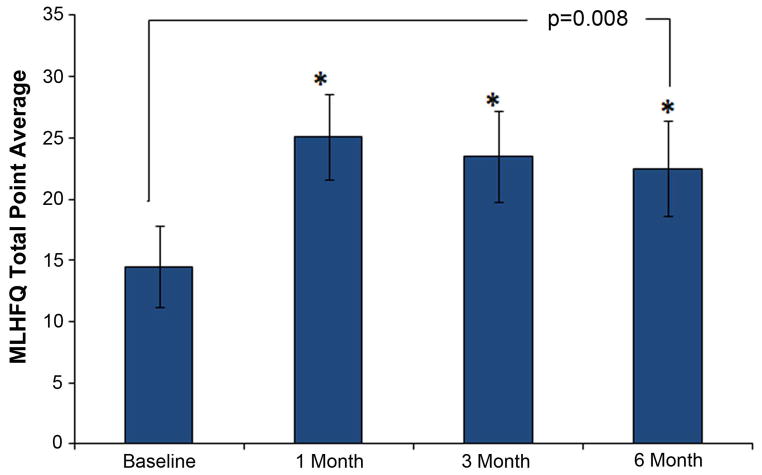
The negative impact of low to moderate Anth-bC on QOL significantly increased over time (p=0.008). Mean score on the MLHFQ was 15.4±3.3 at baseline, 27.8±3.5 after treatment, and 28.5±3.9 at study end (Figure 3). The change observed in QOL trended toward a positive association with the change in LVEF (r=0.085) and myocardial strain (r=0.24), but neither was found to be statistically significant (p=0.64 and 0.17 respectively).
Figure 3.
Time dependent changes in quality of life (QOL) assessed by the Minnesota Living with Heart Failure Questionnaire (MLHFQ). The total point mean ± the standard error are shown. There was a significant increase in the total score, indicating a negative impact on QOL, from baseline to 1 month after administration of low to moderate doses of anthracycline-based chemotherapy that was maintained through 6 months.
Measures of LVESV, LVEF, left ventricular strain, PWV, and QOL remained different at the 6 month visit relative to baseline values after accounting for CV risk factors regardless of the number of co-morbid conditions experienced by a participant (p=0.02 to 0.0001 for all) and after accounting for the presence of baseline late gadolinium enhancement imaging (p=0.02 to 0.0001). Abnormal trends in these parameters (LVESV, LVEF, left ventricular strain, PWV, and QOL) also persisted at the end of the study relative to the baseline after adjusting for race only (p=0.02 to 0.0002 for all), gender only (p=0.015 to 0.0001 for all), cancer diagnosis only (p=0.018 to 0.0002 for all), anthracycline administration protocol only (p=0.02 to 0.0002 for all), and in a model that adjusted for gender, age, race, and doxorubicin equivalent cumulative dose (p=0.016 to 0.0001 for all).
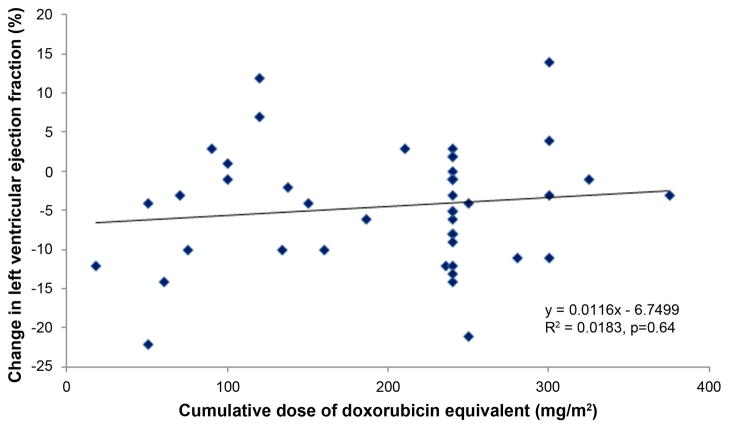
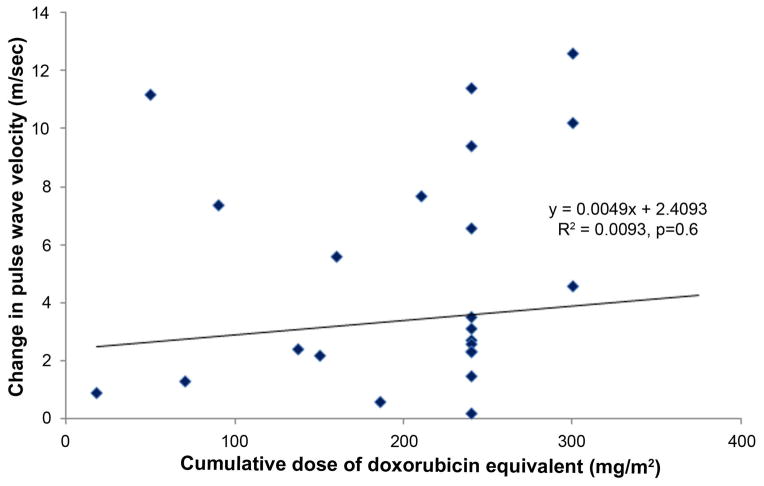
The cumulative doses of doxorubicin equivalents administered in this study did not correlate with greater reductions in LVEF or increases in PWV (Figure 4). This finding was similar for myocardial strain (p=0.12). Thirty-nine participants (74%) received cyclophosphamide. The addition of cyclophosphamide, trastuzumab, or all other adjuvant chemotherapy agents in combination with anthracyclines was not associated with larger decrements in LVEF or increases in PWV or strain.
Figure 4.
Changes in left ventricular ejection fraction (LVEF; y-axis; panel A) and pulse wave velocity (PWV; y-axis; panel B) versus the cumulative doses of doxorubicin equivalent (x-axes; panels A and B). Each symbol represents data from a participant, and the regression line and equations are shown for each panel. No association between changes in LVEF or PWV and the doses of doxorubicin equivalent chemotherapy were observed.
Discussion
Our results demonstrate 5 important findings. First, measures of left ventricular systolic performance (LVESV, LVEF, and mean mid-wall circumferential strain) deteriorate early and remain abnormal 6 months after initiation of low to moderate doses of Anth-bC (Figure 1). Second, participants exhibit an increase in PWV (a measure of aortic stiffening) which occurs early during the first 6 months after initiation of low to moderate doses of Anth-bC (Figure 2). Third, we did not appreciate new infarcts or fibrosis by late gadolinium enhancement that were commensurate with the other subclinical abnormalities that occurred during the 6 months of receipt of Anth-bC. Fourth, these changes in subclinical abnormalities of cardiac and vascular function occurred in parallel with worsening effects on physical, emotional, social, and mental dimensions of QOL (Figure 3), but due to our small sample size, we did not appreciate a significant association between CV function and QOL. Finally, the magnitude of subclinical deterioration in cardiac and vascular function is not correlated with the total amount of anthracycline received after treatment for cancer (Figure 4).
In the 1970s, investigators recognized that relatively high doses (>500 mg/m2) of anthracyclines were associated with increases in morbidity and mortality related to overt clinical heart failure (4). Over the past 10 years, premature CV events have emerged in long-term survivors of adult and pediatric lymphoma and leukemia, and women with breast cancer (2–4). Individuals in our study were not exposed to high but rather low to moderate doses of Anth-bC. Our results suggest that early during the exposure to these low doses individuals develop subclinical abnormalities of both left ventricular performance and aortic stiffness.
Three metrics of left ventricular systolic function (LVESV, LVEF, and strain) deteriorated over the course of the study. Changes similar to these (2–6 ml increase in LVESV or a 5% drop from a normal resting LVEF) were associated with an adverse CV prognosis in a recent study of patients with heart failure and a preserved resting LVEF (17). Importantly, within 6 months of the receipt of chemotherapy, 26% of individuals that exhibited a LVEF >50% at baseline experienced a deterioration in LVEF <50%, a threshold which identifies abnormal left ventricular performance that often warrants initiation of medical therapy (18,19). Of note, LVEDV did not decrease during the study; thus the changes we observed in LVEF and mid-wall myocardial strain were not due to decreases in left ventricular preload that occasionally occur with decreased oral intake or vomiting during receipt of anthracyclines.
Echocardiographically, changes in mid-wall myocardial strain have been shown to deteriorate prior to changes in left ventricular fractional shortening or LVEF in individuals receiving moderate to high doses of Anth-bC (20,21). In this study, myocardial strain diminished early, commensurate with a decrement in LVEF. The difference in our results could be explained by the heightened accuracy of CMR to measure left ventricular volumes and LVEF (22). After multivariable analyses, the subclinical abnormalities we observed in LVESV, LVEF, and mid-wall circumferential strain occurred regardless of gender, race, associated CV comorbidities, or the total amount of Anth-bC received. Of note, our mid-wall, mean circumferential strain short axis image acquisitions were similar to those performed in MESA (7), and has prognostic value for both CV events and left ventricular remodeling after myocardial infarction (23). Future studies could consider the addition of longitudinal strain measures acquired from left ventricular apical views that have been found useful clinically during transthoracic echocardiography (23).
Anth-bC was associated with an early and persistent increase in PWV, a measure of vascular stiffening that is associated with increased left ventricular afterload, left ventricular dysfunction and hypertrophy, and adverse CV events including mortality (24,25). The average change in PWV of >3m/sec to a mean value of 10.1±1 m/sec that we observed in this study has been found clinically relevant in other patient populations. In 483 hypertensive patients aged 50±13 years, aortic PWV measures of 11.5±3.4 m/sec were associated with an odds ratio of 2.14 for cardiac events (p<0.0001) (25). The rapid (1 month) increase in PWV suggests an acute (endothelial dysfunction, increase in smooth muscle tone) rather than chronic (atherosclerosis, increased collagen synthesis) process influenced aortic stiffening in our participants (10); however at this time, we are uncertain of the mechanism of this stiffening.
Although late gadolinium enhancement imaging exhibits diagnostic and prognostic utility in multiple CV disorders (26), its use in assessment of the effects of Anth-bC is limited to one case series of 2 patients with Ewing’s sarcoma who developed subendocardial enhancement >10 years after receipt of an anthracycline (27). Our results demonstrated no new late gadolinium enhancement during receipt of low doses of Anth-bC at a time when other subclinical functional CV abnormalities occurred.
Serum TnI levels demonstrated an upward trend 6 months after initiation of Anth-bC, and 26% of the participants met the criteria (0.06≤x<1.0 mg/ml) for subclinical CV injury 3 months into treatment with Anth-bC. Previously, investigators demonstrated an association between similar subclinical elevations of serum TnI and future cardiac events in individuals treated with chemotherapy (28). It is also important to note that serum BNP did not increase during the duration of this study, which reflects that participants were not in clinical decompensated heart failure. This suggests that BNP does not contribute to the screening of subclinical CV disease in this patient population.
In addition to CV structural and functional changes seen with low to moderate Anth-bC, there were also negative changes in QOL that involved physical, emotional, social, and mental aspects. These changes were significant early after receipt of chemotherapy and remained significant at 6 months. A recent study has validated the MLHFQ and reported that an increase of 5 total points is clinically significant (29).
Those treated for breast cancer or a hematologic malignancy, or those receiving doxorubicin versus daunorubicin experienced similar increases in PWV and decreases in left ventricular performance. It is important to note that there was no correlation between the decrease in LVEF, or the increase in PWV, and the cumulative dose of doxorubicin equivalent administered to participants in the study. In addition, our resting measures of LVEF (57.9±1%) and PWV (6.7±0.5 m/sec) are similar to measures observed in other populations without clinical CV disease (25,30,31); thus we did not appreciate a resting abnormality of CV function that would necessarily contribute to our results.
Limitations of this study include the following: first, while the high accuracy and reproducibility of CMR allowed us to identify subclinical abnormalities of CV function in patients receiving treatment for cancer with a small sample size, studies with larger numbers of participants could identify whether specific cardiovascular risk factors predispose individuals to develop early subclinical CV injury upon receipt of Anth-bC. Second, there were no Hispanic or Asian participants in the study. For this reason, we are unable to derive data regarding the relationship between anthracycline administration and subclinical CV disease in Hispanics or Asians. Third, our serum TnI and BNP samples were acquired the morning before anthracycline administration; therefore, the results and conclusions related to chronic TnI elevation observed in this study may differ from those that are recognized within hours after anthracycline administration. Larger elevations in serum TnI have been observed in children immediately after receipt of an anthracycline (32).
In conclusion, independent of gender, age, black or white race, and the number of CV risk factors, cardiac systolic performance declines and aortic stiffness increases early after exposure to low to moderate doses of anthracycline-based chemotherapeutic regimens for breast cancer or a hematologic malignancy. These data indicate that low dose Anth-bC is associated with subclinical abnormalities of CV function that in other high risk populations are associated with CV events. Further investigations are warranted to determine if these markers of subclinical CV disease that develop early after anthracycline administration are associated with future CV events in cancer survivors.
Acknowledgments
Research supported in part by National Institute of Health grants R33CA12196, R01HL076438, M01RR07122, and P30CA012197, and a grant (BCTR0707769) from the Susan B. Komen Foundation.
The authors appreciate the expert assistance of Amanda Keith in the preparation of the manuscript.
Abbreviations list
- Anth-bC
anthracycline-based chemotherapy
- BNP
B-type natriuretic peptide
- CMR
cardiovascular magnetic resonance
- CV
cardiovascular
- LVEF
left ventricular ejection fraction
- LVEDV
left ventricular end diastolic volume
- LVESV
left ventricular end systolic volume
- QOL
quality of life
- PWV
pulse wave velocity
- TnI
troponin I
Footnotes
Disclosures: All authors confirm that no conflicts of interest exist for this manuscript and that the manuscript represents valid work and that neither this manuscript nor one with substantially similar content under their authorship has been published or is being considered for publication elsewhere.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Du XL, Fox EE, Lai D. Competing causes of death for women with breast cancer and change over time from 1975 to 2003. Am J Clin Oncol. 2008;31:105–16. doi: 10.1097/COC.0b013e318142c865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst. 2007;99:365–75. doi: 10.1093/jnci/djk064. [DOI] [PubMed] [Google Scholar]
- 3.Swerdlow AJ, Higgins CD, Smith P, et al. Myocardial infarction mortality risk after treatment for Hodgkin’s disease: A collaborative British cohort study. J Natl Cancer Inst. 2007;99:206–14. doi: 10.1093/jnci/djk029. [DOI] [PubMed] [Google Scholar]
- 4.Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Eng J Med. 1998;339:900–5. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 5.Eschenhagen T, Force T, Ewer MS, et al. Cardiovascular side effects of cancer therapies: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2011;13:1–10. doi: 10.1093/eurjhf/hfq213. [DOI] [PubMed] [Google Scholar]
- 6.Rerkpattanapipat P, D’Agostino RB, Jr, Link KM, et al. Location of arterial stiffening differs in those with impaired fasting glucose versus diabetes: Implications for left ventricular hypertrophy from the Multi-Ethnic Study of Atherosclerosis. Diabetes. 2009;58:946–53. doi: 10.2337/db08-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heckbert SR, Post W, Pearson GD, et al. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48:2285–92. doi: 10.1016/j.jacc.2006.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shehata ML, Cheng S, Osman NF, Bluemke DA, Lima JA. Myocardial tissue tagging with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2009;11:55. doi: 10.1186/1532-429X-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sampath S, Derbyshire JA, Atalar E, Osman NF, Prince JL. Real-time imaging of two-dimensional cardiac strain using a harmonic phase magnetic resonance imaging (HARP-MRI) pulse sequence. Magn Reson Med. 2003;50:154–63. doi: 10.1002/mrm.10509. [DOI] [PubMed] [Google Scholar]
- 10.Chaosuwannakit N, D’Agostino R, Jr, Hamilton CA, et al. Aortic stiffness increases upon receipt of anthracycline chemotherapy. J Clin Oncol. 2010;28:166–72. doi: 10.1200/JCO.2009.23.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–53. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 12.John J, Woodward DB, Wang Y, et al. Troponin-I as a prognosticator of mortality in severe sepsis patients. J Crit Care. 2010;25:270–5. doi: 10.1016/j.jcrc.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Daugaard G, Lassen U, Bie P, et al. Natriuretic peptides in the monitoring of anthracycline induced reduction in left ventricular ejection fraction. Eur J Heart Fail. 2005;7:87–93. doi: 10.1016/j.ejheart.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. New Engl J of Med. 2009;361:868–77. doi: 10.1056/NEJMoa0903515. [DOI] [PubMed] [Google Scholar]
- 15.Rector TS. A conceptual model of the quality of life in relation to heart failure. J Cardiac Failure. 2005;11:173–6. doi: 10.1016/j.cardfail.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Keefe DL. Anthracycline-induced cardiomyopathy. Semin Oncol. 2001;28:2–7. [PubMed] [Google Scholar]
- 17.Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk of vascular events. N Engl J Med. 2008;358:1547–59. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 18.Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 19.Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–9. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 20.Cheung Y, Hong WJ, Chan GC, Wong SJ, Ha SY. Left ventricular myocardial deformation and mechanical dyssynchrony in children with normal ventricular shortening fraction after anthracycline therapy. Heart. 2010;96:1137–41. doi: 10.1136/hrt.2010.194118. [DOI] [PubMed] [Google Scholar]
- 21.Stanton T, Leano R, Marwick TH. Prediction of all cause mortality from global longitudinal speckle strain: Comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2:356–64. doi: 10.1161/CIRCIMAGING.109.862334. [DOI] [PubMed] [Google Scholar]
- 22.Bellenger NG, Davies LC, Francis JM, Coats AJ, Pennell DJ. Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2000;2:271–8. doi: 10.3109/10976640009148691. [DOI] [PubMed] [Google Scholar]
- 23.Hung CL, Verma A, Uno H, et al. Longitudinal and circumferential strain rate, left ventricular remodeling, and prognosis after myocardial infarction. J Am Coll Cardiol. 2010;56:1812–22. doi: 10.1016/j.jacc.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 24.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–41. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 25.Boutouyrie P, Tropeano Al, Asmar R, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: A longitudinal study. Hypertension. 2002;39:10–5. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 26.Green JJ, Berger JS, Kramer CM, Salerno M. Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging. 2012;5:370–7. doi: 10.1016/j.jcmg.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 27.Perel RD, Slaughter RE, Strugnell WE. Subendocardial late gadolinium enhancement in two patients with anthracycline cardiotoxicity following treatment for Ewing’s sarcoma. J Cardiovasc Magn Reson. 2006;8:789–91. doi: 10.1080/10976640600737664. [DOI] [PubMed] [Google Scholar]
- 28.Cardinale D, Sandri MT, Colombo A, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004;109:2749–54. doi: 10.1161/01.CIR.0000130926.51766.CC. [DOI] [PubMed] [Google Scholar]
- 29.Rector TS, Carson PE, Anand IS, et al. Assessment of long-term effects of irbesartan on heart failure with preserved ejection fraction as measured by the Minnesota Living with Heart Failure Questionnaire in the Irbesartan in Heart Failure with Perserved Systolic Function (I-PRESERVE) Trial. Circ Heart Fail. 2012;5:217–225. doi: 10.1161/CIRCHEARTFAILURE.111.964221. [DOI] [PubMed] [Google Scholar]
- 30.Quinones MA, Greenberg BH, Kopelen HA, et al. Echocardiographic predictors of clinical outcome in patients with left ventricular dysfunction enrolled in the SOLVD registry and trials: significance of left ventricular hypertrophy. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 2000;35:1237–44. doi: 10.1016/s0735-1097(00)00511-8. [DOI] [PubMed] [Google Scholar]
- 31.Dahlof B. Cardiovascular disease risk factors: Epidemiology and risk assessment. Am J Cardiol. 2010;105(1 Supp):3A–9A. doi: 10.1016/j.amjcard.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Germanakis I, Anagnostatou N, Kalmanti M. Troponins and natriuretic peptides in the monitoring of anthracycline cardiotoxicity. Pediatr Blood Cancer. 2008;51:327–33. doi: 10.1002/pbc.21633. [DOI] [PubMed] [Google Scholar]