Abstract
Although organophosphate pesticides are not usually characterized as “endocrine disruptors,” recent work points to potential, long-term reductions of circulating thyroid hormones after developmental exposures to chlorpyrifos that are devoid of observable toxicity. We administered chlorpyrifos to developing rats on gestational days 17–20 or postnatal days 1–4, regimens that produce distinctly different, sex-selective effects on neurobehavioral performance. The prenatal regimen produced a small, but statistically significant reduction in brain thyroxine levels from juvenile stages through adulthood; in contrast, postnatal exposure produced a transient elevation in young adulthood. However, in neither case did we observe the sex-selectivity noted earlier for neurobehavioral outcomes of these specific treatment regimens, or as reported earlier for effects on serum T4 in developing mice. Thus, although chlorpyrifos has the potential to disrupt thyroid status sufficiently to alter brain thyroid hormone levels, the effect is small, and any potential contribution to neurobehavioral abnormalities remains to be proven.
Keywords: Chlorpyrifos, Organophosphate pesticides, Thyroid hormone
1. INTRODUCTION
The chief health concern about organophosphate pesticides is their propensity to elicit developmental neurotoxicity at exposures below the threshold for inhibition of cholinesterase, the biomarker used for safety assessment (Colborn, 2006; Landrigan, 2001; Slotkin, 2004, 2005). Recent studies in three different cohorts of children have verified the correspondence of prenatal organophosphate exposures to subsequent neurobehavioral anomalies (Bouchard et al., 2011; Engel et al., 2011; Rauh et al., 2011), including specific abnormalities of brain structure (Rauh et al., 2012) as predicted from animal studies (Slotkin, 2004, 2005). Chlorpyrifos (CPF), one of the most widely-used and best-studied organophosphates, disrupts neuronal cell replication and differentiation through a variety of cellular mechanisms, culminating in loss of neurons, “mis-wiring” of brain circuits and deficiencies in synaptic function (Slotkin, 2004, 2005).
However, little attention has been paid to potential endocrine disruption as a contributor to adverse neurobehavioral outcomes. CPF is only weakly estrogenic (Andersen et al., 2002) and doses above the threshold for systemic toxicity are required for effects on testosterone catabolism (Usmani et al., 2003) or to elicit secondary endocrine alterations (Guven et al., 1999), unlike the exquisite sensitivity for effects on neurodevelopment. Nevertheless, a recent study points to potential effects on thyroid function at otherwise nontoxic CPF levels (De Angelis et al., 2009). Mice exposed to CPF postnatally, at doses that did not cause cholinesterase inhibition, showed a small, but significant reduction in serum concentrations of triiodothyronine and thyroxine (T4). The effect was selective for males and was associated with cellular abnormalities in the thyroid gland itself. In adults, CPF is directly thyrotoxic only at higher doses (Jeong et al., 2006) and acute poisoning reduces circulating T4 (Rawlings et al., 1998) and/or T3 (Guven et al., 1999), so the results in developing mice suggest that a heightened sensitivity to thyroid toxicity in the immature organism could produce endocrine deficiencies. However, it is not known whether the reductions in serum T4 are sufficient to produce functional hypothyroidism. In the current study, we examined the effects of developmental exposure to CPF in rats, focusing on whether brain T4 levels are affected, a precondition for a functional impact on brain development. We used treatment regimens known to produce neurobehavioral abnormalities and that are just at or below the threshold for barely-detectable cholinesterase inhibition (Slotkin, 2004, 2005). Specifically, we compared the effects of exposure in late gestation to those seen with early postnatal treatment because the neurobehavioral effects of the two exposure paradigms show key differences (Levin et al., 2001, 2002). For the same reason, we also looked for sex-selective effects that could correspond to those seen for neurobehavioral outcomes.
2. MATERIALS AND METHODS
All procedures utilized tissues that were archived from earlier studies and maintained frozen at −45° C, so that no additional animals were actually used for this study. Details of animal husbandry, institutional approvals, maternal and litter characteristics, and growth curves, have all been presented in earlier work from the original animal cohorts (Aldridge et al., 2004; Meyer et al., 2004; Slotkin et al., 2013). Timed-pregnant Sprague-Dawley rats (Charles River, Raleigh, NC) were housed individually and given free access to food and water. For prenatal exposure, dams received daily subcutaneous injections of 1 or 5 mg/kg of CPF (Chem Service, West Chester, PA) on gestational days (GD) 17–20; CPF was dissolved in dimethylsulfoxide (1 ml/kg; Sigma Chemical Co., St. Louis, MO) so as to provide consistent absorption (Whitney et al., 1995) and control animals received vehicle injections on the same schedule. For postnatal (PN) exposure, pups were given 1 mg/kg of CPF daily on PN1-4 at a dose of 1 mg/kg. These doses span the threshold for barely-detectable inhibition of brain cholinesterase activity and are devoid of systemic toxicity (Garcia et al., 2003; Qiao et al., 2002; Song et al., 1997).
On PN1 and every few days thereafter, pups were weighed, litters were randomized within treatment groups and dams were rotated among litters so as to distribute differential effects of maternal caretaking equally among all litters, making sure that all the pups in a given litter were from the same treatment group to avoid the possibility that the dams might distinguish among pups with different treatments. Animals were weaned on PN21. Brain samples were obtained at ages ranging from PN4 (24 hr after the preceding day’s CPF injection) through adulthood, with no more than one male and one female used from any single litter (defined from the final litter assignment in the randomization procedure), with group sizes of 8–12 for each treatment group at each age. For prenatal exposures we used the brainstem at all age points; for postnatal exposures, we sampled whole brain on PN4, brainstem on PN10 and cerebellum on PN50 and PN150. For each treatment and time point, the CPF and control groups were always from the same animal cohorts. Samples were flash-frozen in liquid nitrogen and maintained frozen at −45° C until assayed.
Tissue preparation, T4 separation and analysis were all conducted by established procedures utilizing isotope-dilution liquid chromatography tandem mass spectrometry (Kunisue et al., 2011). T4 and triiodothyronine labeled with 13C were used as internal standards and values were corrected for recovery of the standards, which averaged 75% and 85%, respectively. Brain triiodothyronine levels were too close to the assay detection limit (! 0.3 ng/g) to permit accurate assessment, so these are not reported. Data were compiled as means and standard errors and treatment effects were evaluated with multivariate ANOVA (factors of treatment, sex and age); data were log-transformed for the ANOVA because of heterogeneous variance across ages Because the results showed no main effect of sex or interaction of treatment × sex, results for males and females were combined for presentation. Where there was a treatment × age interaction, Fisher’s Protected Least Significant Difference Test was applied to identify individual ages at which treatment effects were significant; in the absence of an interaction, only main treatment effects were reported.
3. RESULTS & DISCUSSION
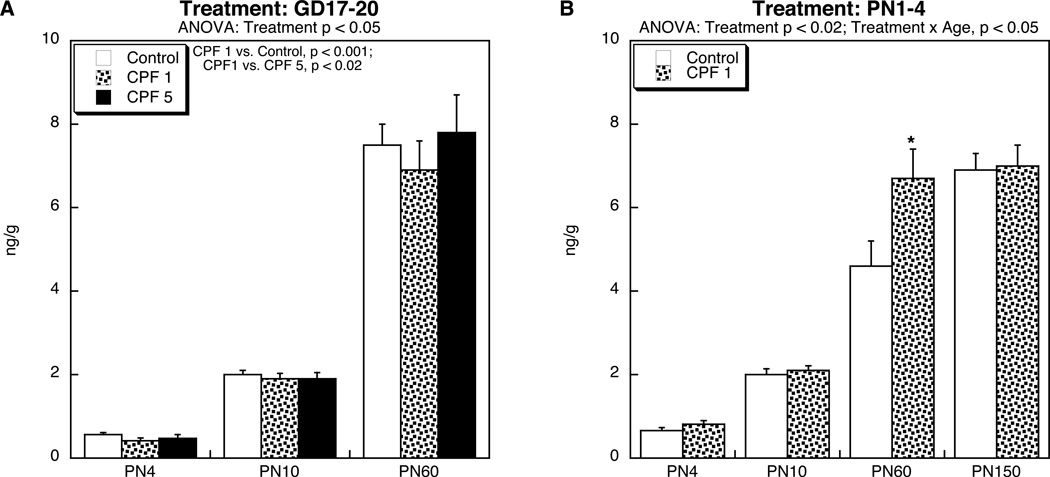
Prenatal exposure to 1 mg/kg of CPF produced a small, but statistically significant reduction in brain T4 levels from PN4 through young adulthood (Figure 1A), without sex-selectivity (no treatment × sex interaction). Notably, the higher CPF dose did not produce a significant overall decrement, and the lack of effect was statistically distinguishable from the reduction caused by 1 mg/kg. The magnitude of the reduction seen at the lower dose was comparable to the effect on serum T4 concentrations as reported earlier for developmental CPF exposure in mice (De Angelis et al., 2009) but those effects were restricted to mice receiving postnatal CPF rather than prenatal treatment. In contrast, in the present study, postnatal CPF exposure (Figure 1B) produced the opposite effect on brain T4 levels, namely a significant overall increase (main treatment effect), that was most pronounced in young adulthood (PN60; significant age × treatment interaction). Again, we did not observe any sex-selectivity, in contrast to the effects of postnatal CPF on serum T4 in the mouse study (De Angelis et al., 2009).
Figure 1.
Effects of CPF exposure (A) during gestation (GD17-20), or (B) postnatally (PN1-4) on T4 levels in the brain. Data represent means and standard errors obtained from 8–12 animals in each treatment group at each age. In (A), we used brainstem samples at all ages, whereas in (B) we used whole brain on PN4, brainstem on PN10 and cerebellum on PN60 and PN150. Results of multivariate ANOVA are shown at the top of each panel. Values for males and females were combined because of the absence of either a main effect of sex or a treatment × sex interaction. In (A), there was a main treatment effect without a treatment × age interaction, so post-hoc analysis was limited to main effects only, showing a significant reduction caused by CPF 1 mg/kg as compared to either the controls or the CPF 5 mg/kg group. In (B), there was a main treatment effect (CPF 1 > Control) but also a treatment × age interaction; the asterisk denotes the individual age at which CPF evoked a significant increase.
Our results in rats thus point to a critical prenatal period in which otherwise nontoxic CPF exposure leads to persistent, but small decrements in brain T4, whereas postnatal exposure produces a small increase. Although our findings differ in detail from the prior results for serum T4 in mice (different stage, lack of sex selectivity for brain T4 in the rat), both studies support a general principle of persistent alterations in thyroid status from developmental CPF exposure in a critical developmental period; the differences may reside either in developmental timetables of the two species or in disparities between effects on serum T4 as compared to brain T4. In either case, the important question is the extent to which this small degree of endocrine disruption contributes to the neurobehavioral consequences of CPF exposure. In support of such a relationship, the nonmonotonic dose-effect curve seen here for the gestational exposure group (significant reduction at 1 mg/kg but not 5 mg/kg) does correspond to some of the behavioral anomalies caused by these treatments, which likewise peak at the lower dose (Levin et al., 2002). Similarly, our earlier work distinguished important differences in the neurochemical and behavioral consequences of prenatal vs. postnatal CPF exposure (Aldridge et al., 2003; Levin et al., 2001; Slotkin, 2004, 2005), consistent with the current finding of reduced brain T4 levels from prenatal CPF, but increased T4 levels from postnatal CPF. Nonetheless, for neurobehavioral effects, we identified major, sex-selective differences, whereas we did not detect such differences for effects on T4.
Conclusion
Thus, our main conclusion is that CPF has the potential to disrupt thyroid status sufficiently to alter brain T4 levels, but only to a small extent, and in light of the disparity between sex-selective neurobehavioral effects and the lack of sex-selectivity for brain T4, any contribution of thyroid disruption to functional abnormalities remains to be proven.
Highlights.
Chlorpyrifos given to fetal rats led to small, but significant reductions in brain thyroxine
The same treatment given postnatally evoked a small, but significant increase
Unlike chlorpyrifos’ neurobehavioral actions, effects on thyroxine were not sex-selective
Thyroid disruption is not likely to account for the neuroteratogenicity of chlorpyrifos
Acknowledgment
Research was supported by NIH ES010356.
Abbreviations
- ANOVA
analysis of variance
- CPF
chlorpyrifos
- GD
gestational day
- PN
postnatal day
- T4
thyroxine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: TAS has received consultant income in the past three years from the following firms: The Calwell Practice (Charleston WV), Finnegan Henderson Farabow Garrett & Dunner (Washington DC), Carter Law (Peoria IL), Gutglass Erickson Bonville & Larson (Madison WI), The Killino Firm (Philadelphia PA), Alexander Hawes (San Jose, CA), Pardieck Law (Seymour, IN), Tummel & Casso (Edinburg, TX), Shanahan Law Group (Raleigh NC), and Chaperone Therapeutics (Research Triangle Park, NC).
REFERENCES
- Aldridge JE, Seidler FJ, Meyer A, Thillai I, Slotkin TA. Serotonergic systems targeted by developmental exposure to chlorpyrifos: effects during different critical periods. Environ. Health Perspect. 2003;111:1736–1743. doi: 10.1289/ehp.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Seidler FJ, Slotkin TA. Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environ. Health Perspect. 2004;112:148–155. doi: 10.1289/ehp.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen HR, Vinggaard AM, Rasmussen TH, Gjermandsen IM, Bonefeld-Jorgensen EC. Effects of currently used pesticides in assays for estrogenicity, androgenicity, and aromatase activity in vitro. Toxicol. Appl. Pharmacol. 2002;179:1–12. doi: 10.1006/taap.2001.9347. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, Trujillo C, Johnson C, Bradman A, Barr DB, Eskenazi B. Prenatal exposure to organophosphate pesticides and IQ in 7-year old children. Environ. Health Perspect. 2011 doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colborn T. A case for revisiting the safety of pesticides: a closer look at neurodevelopment. Environ. Health Perspect. 2006;114:10–17. doi: 10.1289/ehp.7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis S, Tassinari R, Maranghi F, Eusepi A, Di Virgilio A, Chiarotti F, Ricceri L, Venerosi Pesciolini A, Gilardi E, Moracci G, Calamandrei G, Olivieri A, Mantovani A. Developmental exposure to chlorpyrifos induces alterations in thyroid and thyroid hormone levels without other toxicity signs in CD-1 mice. Toxicol. Sci. 2009;108:311–319. doi: 10.1093/toxsci/kfp017. [DOI] [PubMed] [Google Scholar]
- Engel SM, Wetmur J, Chen J, Zhu C, Barr DB, Canfield RL, Wolff MS. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ. Health Perspect. 2011 doi: 10.1289/ehp.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia SJ, Seidler FJ, Slotkin TA. Developmental neurotoxicity elicited by prenatal or postnatal chlorpyrifos exposure: effects on neurospecific proteins indicate changing vulnerabilities. Environ. Health Perspect. 2003;111:297–303. doi: 10.1289/ehp.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guven M, Bayram F, Unluhizarci K, Kelestimur F. Endocrine changes in patients with acute organophosphate poisoning. Human Exp. Toxicol. 1999;18:598–601. doi: 10.1191/096032799678839419. [DOI] [PubMed] [Google Scholar]
- Jeong SH, Kim BY, Kang HG, Ku HO, Cho JH. Effect of chlorpyrifos-methyl on steroid and thyroid hormones in rat F0- and F1-generations. Toxicology. 2006;220:189–202. doi: 10.1016/j.tox.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Kunisue T, Fisher JW, Kannan K. Determination of six thyroid hormones in the brain and thyroid gland using isotope-dilution liquid chromatography/tandem mass spectrometry. Anal. Chem. 2011;83:417–424. doi: 10.1021/ac1026995. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ. Pesticides and polychlorinated biphenyls (PCBs): an analysis of the evidence that they impair children's neurobehavioral development. Mol. Genet. Metab. 2001;73:11–17. doi: 10.1006/mgme.2001.3177. [DOI] [PubMed] [Google Scholar]
- Levin ED, Addy N, Baruah A, Elias A, Christopher NC, Seidler FJ, Slotkin TA. Prenatal chlorpyrifos exposure in rats causes persistent behavioral alterations. Neurotoxicol. Teratol. 2002;24:733–741. doi: 10.1016/s0892-0362(02)00272-6. [DOI] [PubMed] [Google Scholar]
- Levin ED, Addy N, Christopher NC, Seidler FJ, Slotkin TA. Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Dev. Brain Res. 2001;130:83–89. doi: 10.1016/s0165-3806(01)00215-2. [DOI] [PubMed] [Google Scholar]
- Meyer A, Seidler FJ, Slotkin TA. Developmental effects of chlorpyrifos extend beyond neurotoxicity: critical periods for immediate and delayed-onset effects on cardiac and hepatic cell signaling. Environ. Health Perspect. 2004;112:170–178. doi: 10.1289/ehp.6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Padilla S, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: What is the vulnerable period? Environ. Health Perspect. 2002;110:1097–1103. doi: 10.1289/ehp.021101097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, Whyatt R. 7-Year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ. Health Perspect. 2011 doi: 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Perera FP, Horton MK, Whyatt RM, Bansal R, Hao X, Liu J, Barr DB, Slotkin TA, Peterson BS. Brain anomalies in children exposed to a common organophosphate pesticide. Proc. Natl. Acad. Sci. 2012;109:7871–7876. doi: 10.1073/pnas.1203396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings NC, Cook SJ, Waldbillig D. Effects of the pesticides carbofuran, chlorpyrifos, dimethoate, lindane, triallate, trifluralin, 2,4-D, and pentachlorophenol on the metabolic endocrine and reproductive endocrine system in ewes. J. Toxicol. Environ. Health. 1998;54:21–36. doi: 10.1080/009841098159006. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol. Appl. Pharmacol. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Developmental neurotoxicity of organophosphates: a case study of chlorpyrifos. In: Gupta RC, editor. Toxicity of Organophosphate and Carbamate Pesticides. San Diego: Elsevier Academic Press; 2005. pp. 293–314. [Google Scholar]
- Slotkin TA, Card J, Infante A, Seidler FJ. Prenatal dexamethasone augments the sex-selective developmental neurotoxicity of chlorpyrifos: implications for vulnerability after pharmacotherapy for preterm labor. Neurotoxicol. Teratol. 2013 doi: 10.1016/j.ntt.2013.02.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Seidler FJ, Saleh JL, Zhang J, Padilla S, Slotkin TA. Cellular mechanisms for developmental toxicity of chlorpyrifos: targeting the adenylyl cyclase signaling cascade. Toxicol. Appl. Pharmacol. 1997;145:158–174. doi: 10.1006/taap.1997.8171. [DOI] [PubMed] [Google Scholar]
- Usmani KA, Rose RL, Hodgson E. Inhibition and activation of the human liver microsomal and human cytochrome P450 3A4 metabolism of testosterone by deployment-related chemicals. Drug Metab. Dispos. 2003;31:384–391. doi: 10.1124/dmd.31.4.384. [DOI] [PubMed] [Google Scholar]
- Whitney KD, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: cellular mechanisms. Toxicol. Appl. Pharmacol. 1995;134:53–62. doi: 10.1006/taap.1995.1168. [DOI] [PubMed] [Google Scholar]