Abstract
Functional capacity as assessed by 6-minute walk test distance (6MWTD) has been shown to predict outcomes in selected cohorts with cardiovascular disease. To evaluate the association between 6MWTD and outcomes after transcatheter aortic valve implantation (TAVI) among participants in the Placement of AoRTic TraNscathetER valve (PARTNER) trial, TAVI recipients (n = 484) were stratified into 3 groups according to baseline 6MWTD: unable to walk (n = 218), slow walkers (n = 133), in whom 6MWTD was below the median (128.5 meters), and fast walkers (n = 133) with 6MWTD >128.5 meters. After TAVI, among fast walkers, follow-up 6MWTD decreased by 44 ± 148 meters at 12 months (p <0.02 compared with baseline). In contrast, among slow walkers, 6MWTD improved after TAVI by 58 ± 126 meters (p <0.001 compared with baseline). Similarly, among those unable to walk, 6MWTD distance increased by 66 ± 109 meters (p <0.001 compared with baseline). There were no differences in 30-day outcomes among 6MWTD groups. At 2 years, the rate of death from any cause was 42.5% in those unable to walk, 31.2% in slow walkers, and 28.8% in fast walkers (p = 0.02), driven primarily by differences in noncardiac death. In conclusion, among high-risk older adults undergoing TAVI, baseline 6MWTD does not predict procedural outcomes but does predict long-term mortality. Nonetheless, patients with poor baseline functional status exhibit the greatest improvement in 6MWTD. Additional work is required to identify those with poor functional status who stand to benefit the most from TAVI.
Transcatheter aortic valve implantation (TAVI) is an established treatment for severe symptomatic aortic stenosis (AS) in older adults considered to be inoperable or at high risk for traditional surgery. However, intermediate to long-term mortality rates among patients undergoing TAVI have been high,1,2 largely reflecting underlying comorbidities of the treated population. Accordingly, identifying those who stand to benefit the most from TAVI is a priority. The 6-minute walk test distance (6MWTD) is a widely accepted measure of exercise capacity and functional status3; it correlates with peak oxygen consumption4,5 and predicts mortality after aortic valve replacement,6–8 after coronary revascularization,9 and in chronic heart failure.4 Accordingly, we sought to evaluate the prognostic value of 6MWTD among older adults considered to be inoperable or at high surgical risk who received TAVI in the Placement of AoRTic TraNscathetER Valve (PARTNER) Trial. Our primary objectives were (1) to evaluate the association between baseline 6MWTD and functional improvement after TAVI and (2) to evaluate the association between baseline 6MWTD and mortality after TAVI. We hypothesized that those with poor 6MWTD performance at baseline would experience less improvement in functional capacity and would have higher mortality after TAVI.
Methods
The design and initial results of the PARTNER trial have been published previously.10,11 The PARTNER trial enrolled patients with severe symptomatic AS. Patients were divided into 2 cohorts: those who were considered to be candidates for surgery despite being at high surgical risk (cohort A) and those who were not considered to be suitable candidates for surgery because of severe coexisting conditions (cohort B). Patients in cohort B with a suitable iliofemoral vessel were randomized to transfemoral TAVI with the Edwards-Sapien heart valve system (Edwards Lifesciences, Irvine, California) or to standard medical care. Patients in cohort A were randomized to TAVI (transfemoral if iliofemoral vessels were suitable or transapical if not) or to conventional surgical aortic valve replacement. The current analyses pooled patients from cohorts A and B who underwent TAVI via a transfemoral or transapical approach. The study was approved by the institutional review board at each participating site, and all patients provided written informed consent.
The 6-minute walk test was attempted at baseline and then at 1, 6, and 12 months after TAVI. It was conducted according to a standardized protocol, using an internal hallway with the 50-foot distance marked.12 Participants were told that “the purpose of this test is to see how far you can walk in 6 minutes.” They were then instructed to “walk from end to end of the hallway at your own pace, in order to cover as much ground as possible.” Participants were allowed to stop and rest during the test but were instructed to resume walking as soon as they were able to do so. The technician counted the number of laps completed and used a timer to stop the participant 6 minutes after the walk started.
The primary functional outcome was follow-up 6MWTD at 1, 6, and 12 months after TAVI. All available follow-up 6MWTD data were included in this analysis without imputation for those with missing data due to death or failure to return for follow-up visits. The primary clinical outcome measure was the time to death from any cause over 2-years of follow-up. Other clinical outcomes of interest included the 30-day frequency of death from cardiac cause, repeat hospitalization due to AS or complications of the valve procedure, stroke, major bleeding, major vascular complications, permanent pacemaker, and renal failure requiring dialysis. Cardiac death, stroke, and major vascular complications were defined according to a modified version of the Valve Academic Research Consortium criteria13 as described in the PARTNER trial protocol.10,11 The 2-year rates of cardiovascular death and noncardiac death were also analyzed. All events were adjudicated by an independent clinical events committee.
All statistical analyses were based on the population of patients who actually received TAVI. Continuous variables are summarized as median (interquartile range) and were compared using the Mann-Whitney rank-sum test. Categorical variables are presented as proportions and were compared by the chi-square test. The 6MWTD was analyzed as a continuous and categorical variable. Those subjects for whom there was documentation that they were unable to perform the 6-minute walk test were categorized as “unable to walk” and assigned a distance of 0 meters. Otherwise, subjects without 6MWTD at baseline were considered missing and excluded from this analysis. For categorical analyses, baseline 6MWTD was categorized into a 3-level variable: those unable to walk, those with 6MWTD less than or equal to the median value among patients with 6MWTD >0 (128.5 meters, “slow”), and those with 6MWTD greater than the median value (“fast”). To evaluate the change in 6MWTD over time according to baseline 6MWTD group, follow-up 6MWTD at 1, 6, and 12 months were compared with the patient’s baseline distance using paired t test. Between-group comparisons of 6MWTD distances according to baseline 6MWTD groups (unable/slow/fast) were performed using analysis of variance followed by comparisons of individual groups using t tests with Tukey correction.
Thirty-day event rates were compared between groups; only unadjusted analyses were performed to evaluate the association between baseline 6MWTD category and 30-day clinical outcomes. Time to event variables, including death from any cause, noncardiac death, and cardiac death were summarized by means of Kaplan-Meier estimates and compared with the log-rank test. Cox proportional hazards models were used to evaluate the independent association between baseline 6MWTD and all-cause mortality. Multivariable models were built to avoid overfitting using a ratio of 1 covariate for every 10 events. Variables of clinical interest or that satisfied an entry criterion of p <0.1 in the univariate analysis were selected as candidate variables for multivariable models. A 2-sided alpha level of 0.05 was used for all significance testing. All statistical analyses were performed with the use of SAS software, version 9.2 (SAS Institute, Cary, North Carolina).
Results
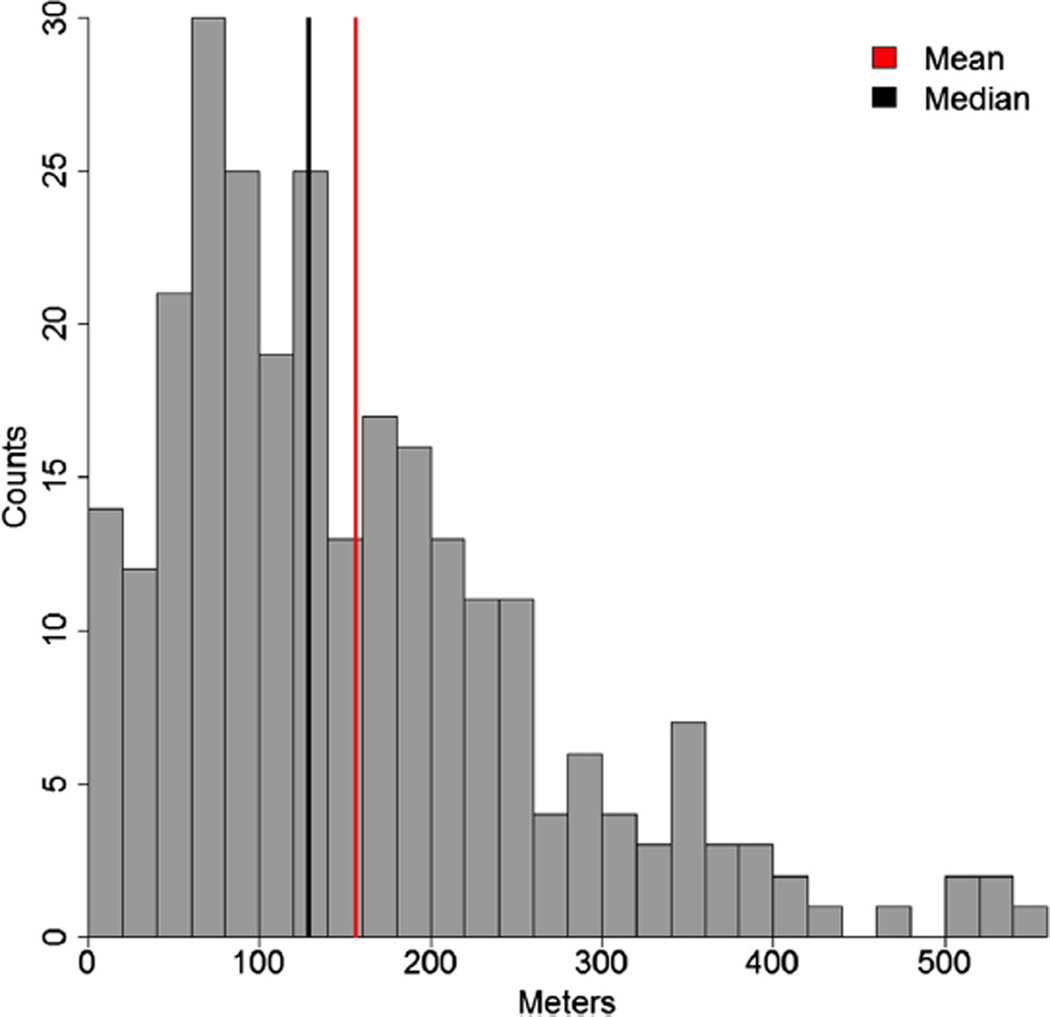
Among the 699 participants enrolled in the PARTNER trial cohort A and the 358 participants enrolled in the PARTNER trial cohort B, 322 participants from cohort A and 162 from cohort B received TAVI and attempted the 6-minute walk test at baseline and therefore were included in this analysis (n = 484). Among the 484 participants, 218 (124 from cohort A and 94 from cohort B) were unable to perform the 6-minute walk test at baseline and were categorized as “unable” to walk. Among the patients who were able to perform the 6-minute walk test at baseline, the median 6MWTD was 128.5 meters, and the mean 6MWTD was 155.7 ± 110.8 (Figure 1). Of these patients, 133 (94 from cohort A and 39 from cohort B) were categorized as “slow” walkers based on a baseline 6MWTD of ≤128.5 meters, and 133 (104 from cohort A and 29 from cohort B) were categorized as “fast” walkers based on a 6MWTD of >128.5 meters.
Figure 1.
Distribution of baseline 6-minute walk distance (meters) among 266 participants who were able to perform the baseline 6-minute walk test.
Baseline demographic, clinical, and echocardiographic characteristics stratified by baseline 6MWTD are summarized in Table 1. Notably, those unable to walk were more likely to be women, have higher Society of Thoracic Surgery scores, were less likely to have previous coronary bypass surgery and carotid artery disease, and were more likely to have undergone previous balloon aortic valvuloplasty. The proportion of those with oxygen-dependent chronic obstructive pulmonary disease was highest among those unable to walk and lowest among those who were categorized as fast walkers.
Table 1.
Demographic, clinical, and echocardiographic characteristics by baseline 6-minute walk test performance
| Variable | Unable to Walk | Slow Walkers | Fast Walkers | p Value |
|---|---|---|---|---|
| Age (yrs) | 84.6 (79.1–88.9) | 85.9 (81.7–88.5) | 83.6 (78.3–87.6) | 0.01 |
| Male gender | 98 (45%) | 73 (55%) | 89 (67%) | 0.0003 |
| Body mass index (kg/m2) | 26.2 (22.7–30.4) | 26.0 (22.1–30.2) | 25.5 (22.8–29.0) | 0.60 |
| Transfemoral TAVI | 180 (83%) | 104 (78%) | 103 (77%) | 0.43 |
| STS Score | 11.4 (10.0–14.0) | 11.1 (9.4–14.0) | 10.5 (8.8–12.0) | 0.001 |
| Diabetes mellitus | 90 (41%) | 46 (35%) | 56 (42%) | 0.37 |
| Hypertension | 193 (89%) | 116 (87%) | 119 (90%) | 0.83 |
| Angina pectoris | 45 (21%) | 37 (28%) | 37 (28%) | 0.19 |
| Heart failure | 216 (99%) | 130 (98%) | 128 (96%) | 0.19 |
| NYHA Class IV | 118 (54%) | 64 (48%) | 60 (45%) | 0.23 |
| CAD | 148 (68%) | 96 (72%) | 103 (77%) | 0.15 |
| Previous PCI | 61 (28%) | 45 (34%) | 39 (29%) | 0.53 |
| Previous coronary bypass | 78 (36%) | 46 (35%) | 64 (48%) | 0.04 |
| Cerebrovascular disease | 59 (29%) | 32 (26%) | 43 (34%) | 0.37 |
| Peripheral vascular disease | 91 (42%) | 49 (37%) | 47 (36%) | 0.47 |
| Previous BAV | 32 (15%) | 23 (17%) | 8 (6%) | 0.01 |
| Permanent pacemaker | 37 (17%) | 37 (29%) | 25 (19%) | 0.04 |
| Renal disease | 41 (19%) | 24 (18%) | 23 (17%) | 0.94 |
| Liver disease | 5 (2%) | 1 (1%) | 8 (6%) | 0.03 |
| Oxygen-dependent COPD | 41 (19%) | 15 (11%) | 10 (8%) | 0.007 |
| AV mean gradient (mm Hg) | 41.9 (32.5–53.3) | 40.4 (33.0–51.2) | 41.3 (33.3–49.2) | 0.67 |
| AV area (EOA) (cm2) | 0.63 (0.51–0.76) | 0.63 (0.53–0.73) | 0.66 (0.56–0.79) | 0.26 |
| Ejection fraction (%) | 55.4 (44.4–61.7) | 53.0 (37.6–61.3) | 57.5 (48.5–64.4) | 0.06 |
| Severe mitral regurgitation | 3 (1%) | 6 (5%) | 6 (5%) | 0.14 |
AV = aortic valve; BAV = balloon aortic valvuloplasty; BMI = body mass index; CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; EOA = effective orifice area; NYHA = New York Heart Association; PCI = percutaneous coronary intervention; STS = Society of Thoracic Surgery; TAVI = transcatheter aortic valve implantation.
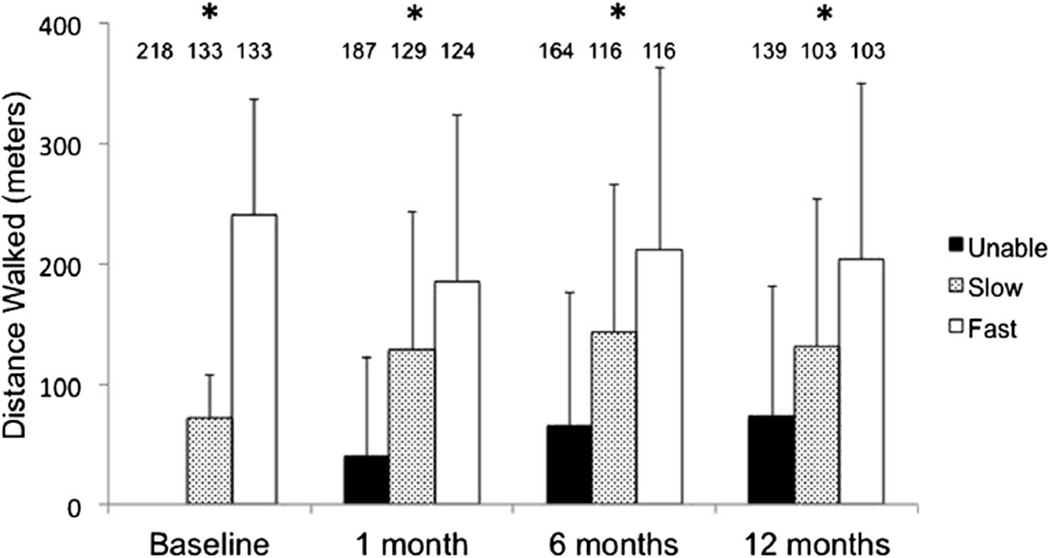
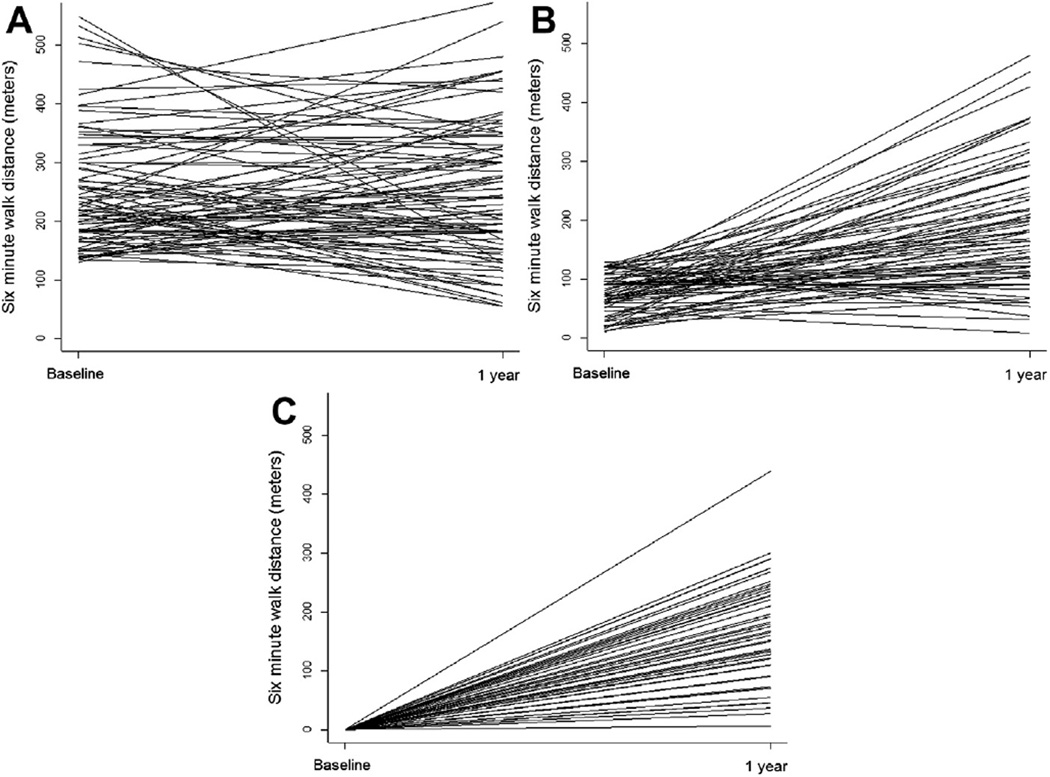
Mean 6MWTD at baseline and follow-up stratified by baseline category is summarized in Figure 2. Baseline mean 6MWTD was 240 ± 96 meters among the fast walkers and 72 ± 34 meters among the slow walkers. After TAVI, among fast walkers, follow-up 6MWTD decreased by 53 ± 148 meters (n = 124), 31 ± 136 meters (n = 116), and 44 ± 148 meters (n = 103) at 1, 6, and 12 months, respectively (p <0.02 for all comparisons to baseline). In contrast, among slow walkers, 6MWTD improved after TAVI by 53 ± 118 meters (n = 129), 69 ± 121 meters (n = 116), and 58 ± 126 meters (n = 103) at 1, 6, and 12 months, respectively (all p <0.001 compared with baseline). Similarly, among those unable to walk, 6MWTD increased by 38 ± 80 meters (n = 187), 56 ± 101 meters (n = 164), and 66 ± 109 (n = 139) meters at 1, 6, and 12 months, respectively (all p <0.001 compared with baseline). Figure 3 depicts the pairwise comparison of 6MWTD at baseline and 12 months for all available pairs. The trajectory of 6MWTD over time was heterogeneous in all 3 groups.
Figure 2.
Six-minute walk test distance at baseline, 1 month, 6 months, and 1 year after TAVI. Asterisk (*) indicates overall comparison significant at a level of p <0.001 (analysis of variance) and all pairwise comparisons significant at a level of p <0.05 (Tukey corrected t tests). The number of participants at each time point is indicated over each bar.
Figure 3.
(A) Pairwise comparison of 6MWTD (meters) at baseline and 12 months after transcatheter aortic valve implantation among fast walkers. (B) Pairwise comparison of 6MWTD (meters) at baseline and 12 months after TAVI among slow walkers. (C) Pairwise comparison of 6MWTD (meters) at baseline and 12 months after TAVI among those unable to walk.
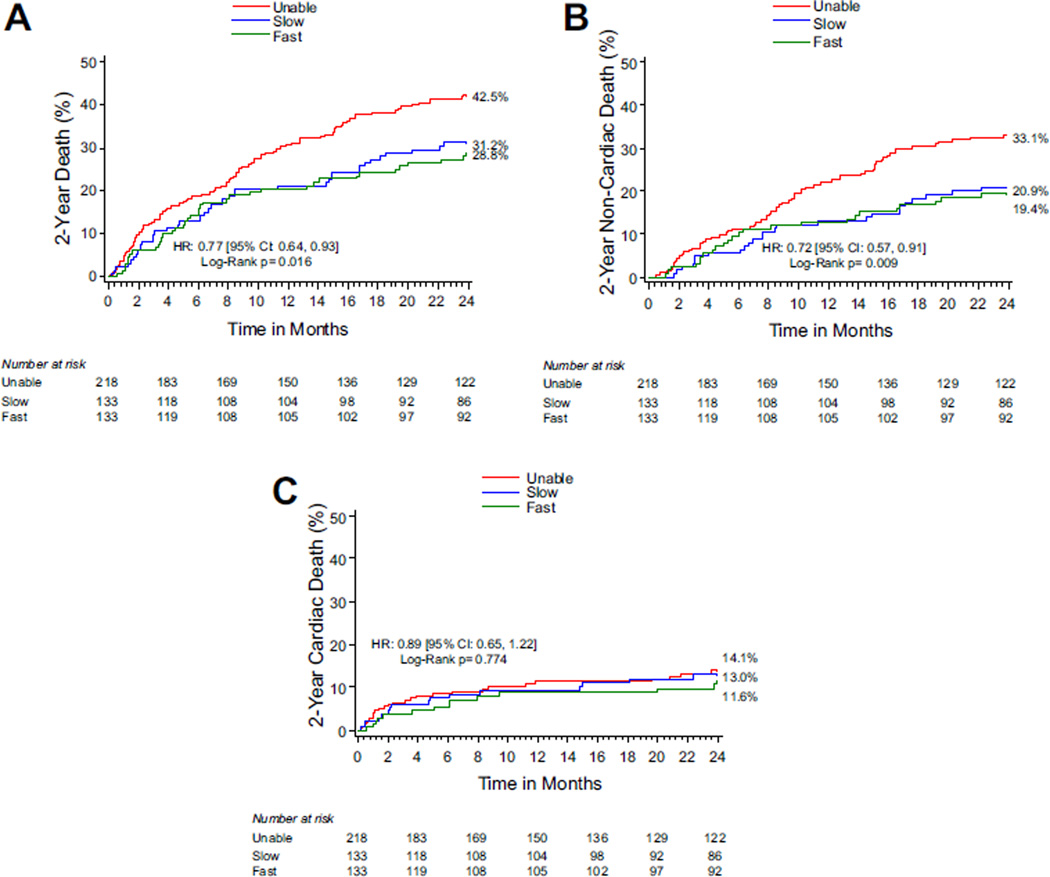
At 30 days, there were no differences in rates of major adverse clinical events including death, cardiac death, stroke, or repeat hospitalization according to baseline 6MWTD categories (Table 2). At 2 years, the rate of death from any cause was 42.5% among those unable to walk at baseline, 31.2% among slow walkers at baseline, and 28.8% among fast walkers at baseline (p = 0.016; Figure 4). This difference in all-cause mortality was driven primarily by differences in noncardiac death. At 2 years, the rates of noncardiac death were 33.1%, 20.9%, and 19.4% among unable, slow, and fast walkers at baseline (p = 0.009, Figure 4), whereas rates of cardiac death were 14.1%, 13.0%, and 11.6% for the same 6MWTD categories (p = 0.77, Figure 4).
Table 2.
Unadjusted 30-day clinical outcomes stratified by baseline 6-minute walk test distance
| 30 Day | ||||
|---|---|---|---|---|
| Unable to Walk |
Slow Walkers |
Fast Walkers |
p Value |
|
| Death | ||||
| Any cause | 15 (7%) | 4 (3%) | 6 (5%) | 0.26 |
| Cardiovascular cause | 11 (5%) | 4 (3%) | 4 (3%) | 0.51 |
| Repeat hospitalization* | 12 (6%) | 10 (8%) | 7 (5%) | 0.68 |
| Major stroke | 5 (2%) | 1 (1%) | 1 (1%) | 0.57 |
| Major bleeding | 30 (14%) | 18 (14%) | 15 (11%) | 0.76 |
| Major vascular complications | 31 (14%) | 12 (9%) | 13 (10%) | 0.25 |
| Permanent pacemaker | 5 (2%) | 5 (4%) | 9 (7%) | 0.11 |
| Renal failure (dialysis required) | 8 (4%) | 3 (2%) | 3 (2%) | 0.64 |
Due to AS or complications of the valve procedure.
Figure 4.
(A) Kaplan Meier estimates of death stratified by baseline 6MWTD category. (B) Kaplan-Meier estimates of noncardiac death stratified by baseline 6MWTD category. (C) Kaplan-Meier estimates of cardiac death stratified by baseline 6MWTD category.
Table 3 summarizes the results of multivariable analysis to assess the prognostic significance of baseline 6MWTD in the study population. After adjustment for age, gender, body mass index, history of carotid artery disease, previous balloon aortic valvuloplasty, chronic liver disease, oxygen-dependent chronic obstructive pulmonary disease, Society of Thoracic Surgery risk score, and access route (transfemoral vs transapical), inability to perform the 6-minute walk test at baseline was associated with an increased risk of 2-year death compared with fast walkers (adjusted hazard ratio [HR] 1.80, 95% confidence interval [CI] 1.20 to 2.69; p = 0.004). In contrast, slow walking at baseline was not associated with an increased risk of 2-year mortality compared with fast walking (adjusted HR 1.24, 95% CI 0.78 to 1.95; p = 0.36). A similar relationship was seen when baseline 6MWTD was modeled as a continuous variable (adjusted HR 1.14 per 50 meter decrease in baseline 6MWTD, 95% CI 1.01 to 1.28, p = 0.04). There was no statistically significant interaction between access route (transfemoral vs transapical) and baseline 6MWTD.
Table 3.
Multivariable association of 6-minute walk test performance with 2-year mortality
| HR (95% CI) | p Value | |
|---|---|---|
| Model 1 | ||
| Fast walkers | (Reference) | |
| Slow walkers | 1.06 (0.68–1.64) | 0.80 |
| Unable to walk at baseline | 1.64 (1.12–2.38) | 0.01 |
| Model 2 | ||
| Fast walkers | (Reference) | |
| Slow walkers | 1.12 (0.72–1.75) | 0.61 |
| Unable to walk at baseline | 1.85 (1.26–2.72) | 0.002 |
| Model 3 | ||
| Fast walkers | (Reference) | |
| Slow walkers | 1.24 (0.78–1.95) | 0.36 |
| Unable to walk at baseline | 1.80 (1.20–2.69) | 0.004 |
Model 1: unadjusted association between baseline walking test category and 2-year all-cause mortality. Model 2: model 1 + the following candidate variables: age, gender, and body mass index. Model 3: model 1 + the following candidate variables: age, gender, body mass index, history of carotid artery disease, previous balloon aortic valvuloplasty, chronic liver disease, oxygen-dependent chronic obstructive pulmonary disease, Society of Thoracic Surgery risk score, and access route (transfemoral vs transapical).
Discussion
The current report, drawn from a cohort of 484 patients with severe symptomatic AS who underwent TAVI, evaluated the association between physical performance as estimated by the 6MWTD and long-term prognosis after TAVI. We found that compared with those with 6MWTD above the median value, those who were unable to walk experienced a higher rate of death after TAVI. In contrast, patients who were unable to walk and those were slow walkers at baseline experienced an improvement in functional status after TAVI, whereas the fast walkers did not improve and actually experienced a modest decrease in 6MWTD.
It is noteworthy that the 6MWTD reported in this study are substantially lower than predicted for healthy older adults, both at baseline and during follow-up. Casanova found that the 10th percentile of 70- to 80-year-old healthy male and female subjects walked >400 meters in 6 minutes.14 Enright derived a reference equation for predicting 6MWTD among healthy older adults.15 According to this equation, the predicted lower limit of normal for the women and men in this study would be approximately 230 and 250 meters, respectively, values that are considerably greater than the observed average distances at all time points in our TAVI population. Moreover, among 2,054 participants in the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) registry (median age 59 years, 36% with New York Heart Association class III or IV heart failure symptoms) median 6MWTD was 372 meters (~3 times the median value in this study), and 75% of patients had distances of ≥300 meters.16 The lower 6MWTD in the PARTNER trial population most likely reflect a combination of factors including the severity of the exercise impairment seen among those with severe AS, advanced age, and multiple comorbidities.
The 6-minute walk test has been shown to be responsive to clinically important changes in symptoms and health status in cohorts of patients with chronic pulmonary disease or cardiac disease.17–22 For example, in a small study of patients with chronic heart failure, changes in 6MWTD of 25 to 50 meters were associated with clinically meaningful changes in health status.22 If this magnitude of change is applicable to patients with severe AS many of whom have impaired mobility, our results suggest that TAVI was associated with clinically important improvements.
Ours is not the first study to evaluate changes in functional status after TAVI. Gotzmann performed 6-minute walk tests on 44 participants at baseline and 1 month after TAVI and found that median 6MWTD improved after TAVI.23 Similarly, Bagur performed 6-minute walk tests on 64 participants at baseline and 6 months after TAVI and demonstrated that although overall 6MWTD increased, 25% of subjects did not improve their 6MWTD at follow-up compared with baseline.24 In a similar cohort (n = 76), using the Duke Activity Status Index questionnaire to evaluate changes in functional status after TAVI, Bagur reported that 30% of patients did not demonstrate functional improvement 6 months after TAVI.25 In our study, derived from an analysis of data from a large multicenter randomized controlled trial, approximately 37% of patients with paired data available did not improve (or even worsened) at 6-month follow-up—results that are similar to previous studies.
Physical function as estimated by 6MWTD has also been shown in previous studies to predict outcomes among adults with severe AS. In the True Or Pseudo severe Aortic Stenosis (TOPAS) study, among those with low-flow low-gradient AS, a 6MWTD of >320 meters was associated with improved survival after surgical aortic valve replacement or medical therapy.7 In the Aortic Stentless versus Stented valve assessed by Echocardiography Randomised Trial (ASSERT) study, baseline 6MWTD was the only independent predictor of the composite endpoint of death, myocardial infarction, and stroke among 208 patients who underwent surgical aortic valve replacement.6 Mok demonstrated an association between baseline 6MWTD and all-cause mortality among 212 TAVI recipients at a single center who were able to perform the test (mean Society of Thoracic Surgery score 7.0%, mean 6MWTD 182 meters, HR 1.08, 95% CI 1.04 to 1.13 for each 10-meter decrease in 6MWTD).8 Therefore, the findings of our study are consistent with previous studies but extend the association to a higher-risk and lower-functioning population of patients undergoing TAVI from multiple centers.
Although the 6-minute walk test has been used for decades as an estimate of cardiopulmonary reserve,4,5 walking speed is actually a summary indicator of overall physical vitality because walking requires integration of circulatory, respiratory, nervous, and musculoskeletal systems.26 As such, walking speed, or the time it takes to walk a short hallway (i.e., 5 meters), is an established marker of frailty in the general population and in cohorts with cardiovascular disease.26–28 Furthermore, frailty is highly prevalent and emerging as an important predictor of outcomes in the TAVI population.29,30 In this population, the prognostic value of 6MWTD may be derived from the association of baseline 6MWTD with impaired cardiopulmonary reserve or as a marker of multisystem impairment and frailty or both. Future studies are needed to attempt to distinguish between these 2 overlapping syndromes in older adults with AS.
Finally, the high mortality rates seen after TAVI among inoperable patients enrolled in the PARTNER trial (43.3% at 2 years) have motivated clinicians to attempt to identify patients in whom TAVI would be considered “futile.”2 Our study does not suggest that TAVI is futile in those who are unable to walk. Rather, despite the high mortality rate seen among those unable to walk, those who do survive after TAVI actually experience the greatest improvement in walking distance. In contrast, the overall decrease in walking performance after TAVI seen among the fastest walkers at baseline may reflect either regression to the mean or a “ceiling effect” in which factors other than AS per se limit walking speed for this cohort. Among such patients, it is therefore important to ensure that AS is truly severe to ensure that TAVI would be expected to provide a meaningful survival benefit.
There are several important limitations to this study. First, this is a retrospective analysis of prospectively collected data within the PARTNER Trial. Consequently, our findings should be considered hypothesis generating. Second, because detailed information concerning the reasons for inability to perform the 6-minute walk test were not collected, we were unable to evaluate whether differences in the reasons for not performing the test (i.e., overall immobility vs severe shortness of breath at rest) have different prognostic implications. Finally, other markers of frailty were not collected systematically among this cohort and, as such, the incremental prognostic value of 6MWTD over that of the frailty phenotype cannot be determined.
Acknowledgments
The PARTNER trial was funded by Edwards Lifesciences and designed collaboratively by the Steering Committee and the sponsor. The present analysis was carried out by academic investigators with no additional funding.
Footnotes
Disclosures
Dr. Cohen has received research grant support from Medtronic and Edwards Lifesciences and consulting income from Medtronic. Dr. Mack is a nonpaid member of the Scientific Advisory Board of Edwards Lifesciences and has received travel reimbursement from Edwards for activities related to his participation on the Executive Committee of the PARTNER Trial. Dr. Williams is a consultant to Edwards Lifesciences. Dr. Kodali is a consultant to Medtronic and Edwards Lifesciences and a member of the advisory boards of Thubrikar Aortic Valve, Inc., Paieon Medical, and St. Jude Medical. Dr. Leon is a nonpaid member of the Scientific Advisory Board of Edwards Lifesciences and has received travel reimbursement from Edwards for activities related to his participation on the Executive Committee of the PARTNER Trial. The other authors have nothing to disclose.
References
- 1.Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, Fontana GP, Dewey TM, Thourani VH, Pichard AD, Fischbein M, Szeto WY, Lim S, Greason KL, Teirstein PS, Malaisrie SC, Douglas PS, Hahn RT, Whisenant B, Zajarias A, Wang D, Akin JJ, Anderson WN, Leon MB. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366:1686–1695. doi: 10.1056/NEJMoa1200384. [DOI] [PubMed] [Google Scholar]
- 2.Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, Douglas PS, Thourani VH, Babaliaros VC, Webb JG, Herrmann HC, Bavaria JE, Kodali S, Brown DL, Bowers B, Dewey TM, Svensson LG, Tuzcu M, Moses JW, Williams MR, Siegel RJ, Akin JJ, Anderson WN, Pocock S, Smith CR, Leon MB. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J of Med. 2012;366:1696–1704. doi: 10.1056/NEJMoa1202277. [DOI] [PubMed] [Google Scholar]
- 3.Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, Berman LB. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–923. [PMC free article] [PubMed] [Google Scholar]
- 4.Cahalin LP, Mathier MA, Semigran MJ, Dec GW, DiSalvo TG. The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest. 1996;110:325–332. doi: 10.1378/chest.110.2.325. [DOI] [PubMed] [Google Scholar]
- 5.Ross R, Murthy J, Wollak I, Jackson A. The six minute walk test accurately estimates mean peak oxygen uptake. BMC Pulm Med. 2010;10:31–40. doi: 10.1186/1471-2466-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Arenaza DP, Pepper J, Lees B, Rubinstein F, Nugara F, Roughton M, Jasinski M, Bazzino O, Flather M. Preoperative 6-minute walk test adds prognostic information to Euroscore in patients undergoing aortic valve replacement. Heart. 2010;96:113–117. doi: 10.1136/hrt.2008.161174. [DOI] [PubMed] [Google Scholar]
- 7.Clavel MA, Fuchs C, Burwash IG, Mundigler G, Dumesnil JG, Baumgartner H, Bergler-Klein J, Beanlands RS, Mathieu P, Magne J, Pibarot P. Predictors of outcomes in low-flow, low-gradient aortic stenosis: results of the multicenter TOPAS study. Circulation. 2008;118:S234–S242. doi: 10.1161/CIRCULATIONAHA.107.757427. [DOI] [PubMed] [Google Scholar]
- 8.Mok M, Nombela—Franco L, Urena M, Dumont E, DeLarochelliere R, Doyle D, Ribeiro HB, Cote M, Pibarot P, DeLarochelliere H, Laflamme L, Poirier P, Rodes—Cabau J. Pre-procedural six-minute walk test as a predictor of mortality in patients undergoing transcatheter aortic valve implantation. J Am Coll Cardiol. 2013;61:897–898. doi: 10.1016/j.jacc.2012.10.050. [DOI] [PubMed] [Google Scholar]
- 9.Cacciatore F, Abete P, Mazzella F, Furgi G, Nicolino A, Longobardi G, Testa G, Langellotto A, Infante T, Napoli C, Ferrara N, Rengo F. Six-minute walking test but not ejection fraction predicts mortality in elderly patients undergoing cardiac rehabilitation following coronary artery bypass grafting. Eur J Cardiovasc Prev Cardiol. 2012;19:1401–1409. doi: 10.1177/1741826711422991. [DOI] [PubMed] [Google Scholar]
- 10.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 11.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 12.ATS Statement: guidelines for the six-minute walk test. Am J Resp Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 13.Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, Krucoff MW, Mack M, Mehran R, Miller C, Morel M-a, Petersen J, Popma JJ, Takkenberg JJM, Vahanian A, van Es G-A, Vranckx P, Webb JG, Windecker S, Serruys PW. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. J Am Coll Cardiol. 2011;57:253–269. doi: 10.1016/j.jacc.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Casanova C, Celli BR, Barria P, Casas A, Cote C, de Torres JP, Jardim J, Lopez MV, Marin JM, Montes de Oca M, Pinto-Plata V, Aguirre-Jaime A. The 6-min walk distance in healthy subjects: reference standards from seven countries. Euro Resp J. 2011;37:150–156. doi: 10.1183/09031936.00194909. [DOI] [PubMed] [Google Scholar]
- 15.Enright PL, McBurnie MA, Bittner V, Tracy RP, McNamara R, Arnold A, Newman AB. The 6-min walk test: a quick measure of functional status in elderly adults. Chest. 2003;123:387–398. doi: 10.1378/chest.123.2.387. [DOI] [PubMed] [Google Scholar]
- 16.Forman DE, Fleg JL, Kitzman DW, Brawner CA, Swank AM, McKelvie RS, Clare RM, Ellis SJ, Dunlap ME, Bittner V. 6-min walk test provides prognostic utility comparable to cardiopulmonary exercise testing in ambulatory outpatients with systolic heart failure. J Am Coll Cardiol. 2012;60:2653–2661. doi: 10.1016/j.jacc.2012.08.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puhan MA, Chandra D, Mosenifar Z, Ries A, Make B, Hansel NN, Wise RA, Sciurba F National Emphysema Treatment Trial Research Group. The minimal important difference of exercise tests in severe COPD. Eur Respir J. 2011;37:784–790. doi: 10.1183/09031936.00063810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wise RA, Brown CD. Minimal clinically important differences in the six-minute walk test and the incremental shuttle walking test. COPD. 2005;2:125–129. doi: 10.1081/copd-200050527. [DOI] [PubMed] [Google Scholar]
- 19.Redelmeier DA, Bayoumi AM, Goldstein RS, Guyatt GH. Interpreting small differences in functional status: the six minute walk test in chronic lung disease patients. Am J Respir Crit Care Med. 1997;155:1278–1282. doi: 10.1164/ajrccm.155.4.9105067. [DOI] [PubMed] [Google Scholar]
- 20.Gremeaux V, Troisgros O, Benaim S, Hannequin A, Laurent Y, Casillas JM, Benaim C. Determining the minimal clinically important difference for the six-minute walk test and the 200-meter fast-walk test during cardiac rehabilitation program in coronary artery disease patients after acute coronary syndrome. Arch Phys Med Rehab. 2011;92:611–619. doi: 10.1016/j.apmr.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 21.Shoemaker MJ, Curtis AB, Vangsnes E, Dickinson MG. Triangulating clinically meaningful change in the six-minute walk test in individuals with chronic heart failure: a systematic review. Cardiopulm Phys Ther J. 2012;23:5–15. [PMC free article] [PubMed] [Google Scholar]
- 22.O’Keeffe ST, Lye M, Donnellan C, Carmichael DN. Reproducibility and responsiveness of quality of life assessment and six minute walk test in elderly heart failure patients. Heart. 1998;80:377–382. doi: 10.1136/hrt.80.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gotzmann M, Hehen T, Germing A, Lindstaedt M, Yazar A, Laczkovics A, Mumme A, Mugge A, Bojara W. Short-term effects of transcatheter aortic valve implantation on neurohormonal activation, quality of life and 6-minute walk test in severe and symptomatic aortic stenosis. Heart. 2010;96:1102–1106. doi: 10.1136/hrt.2009.180661. [DOI] [PubMed] [Google Scholar]
- 24.Bagur R, Rodes-Cabau J, Dumont E, Larochelliere RD, Doyle D, Bertrand OF, Cote M, Poirier P, Pibarot P. Exercise capacity in patients with severe symptomatic aortic stenosis before and six months after transcatheter aortic valve implantation. Am J Cardiol. 2011;108:258–264. doi: 10.1016/j.amjcard.2011.03.031. [DOI] [PubMed] [Google Scholar]
- 25.Bagur R, Rodes-Cabau J, Dumont E, De Larochelliere R, Doyle D, Pibarot P, Cote M, Clavel MA, Villeneuve J, Gutierrez M, Poirier P, Bertrand OF. Performance-based functional assessment of patients undergoing transcatheter aortic valve implantation. Am Heart J. 2011;161:726–734. doi: 10.1016/j.ahj.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 26.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Purser JL, Kuchibhatla MN, Fillenbaum GG, Harding T, Peterson ED, Alexander KP. Identifying frailty in hospitalized older adults with significant coronary artery disease. J Am Geriatr Soc. 2006;54:1674–1681. doi: 10.1111/j.1532-5415.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- 28.Afilalo J, Eisenberg MJ, Morin JF, Bergman H, Monette J, Noiseux N, Perrault LP, Alexander KP, Langlois Y, Dendukuri N, Chamoun P, Kasparian G, Robichaud S, Gharacholou SM, Boivin JF. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56:1668–1676. doi: 10.1016/j.jacc.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 29.Green P, Woglom AE, Genereux P, Maurer MS, Kirtane AJ, Hawkey M, Schnell S, Sohn J, Moses JW, Leon MB, Smith CR, Williams M, Kodali S. Gait speed and dependence in activities of daily living in older adults with severe aortic stenosis. Clin Cardiol. 2012;35:307–314. doi: 10.1002/clc.21974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green P, Woglom AE, Genereux P, Daneault B, Paradis JM, Schnell S, Hawkey M, Maurer MS, Kirtane AJ, Kodali S, Moses JW, Leon MB, Smith CR, Williams M. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience. JACC Cardiovasc Interv. 2012;5:974–981. doi: 10.1016/j.jcin.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]