The relative importance of bone marrow-derived cell populations to adult neovascularization is not clear. There are increasing evidences that myeloid cell lineage may play a role in neovascularization1–4. In a previous report, we demonstrated that Gr-1+CD11b+ myeloid cells improve both angiogenesis and vasculogenesis in tumor1. A recent report by Kim et al showed that Muscle-derived Gr1(dim)CD11b(+) myeloid cells enhance neovascularization in an ischemic hind limb model3. Similarly, a study with the same ischemic hindlimb model also verified the enhanced neovascularization properties uniquely associated with proangiogenic cells derived from common myeloid progenitors4. In the present study, we further investigated whether intravenous administration of Gr1+CD11b+ myeloid cells increases neovascularization and improves cardiac function after heart infarction.
C57BL/6 mice were purchased from Jakson Lab. All animals received humane care and the study protocols were approved by Vanderbilt Medical University Animal Care and Use Committee.
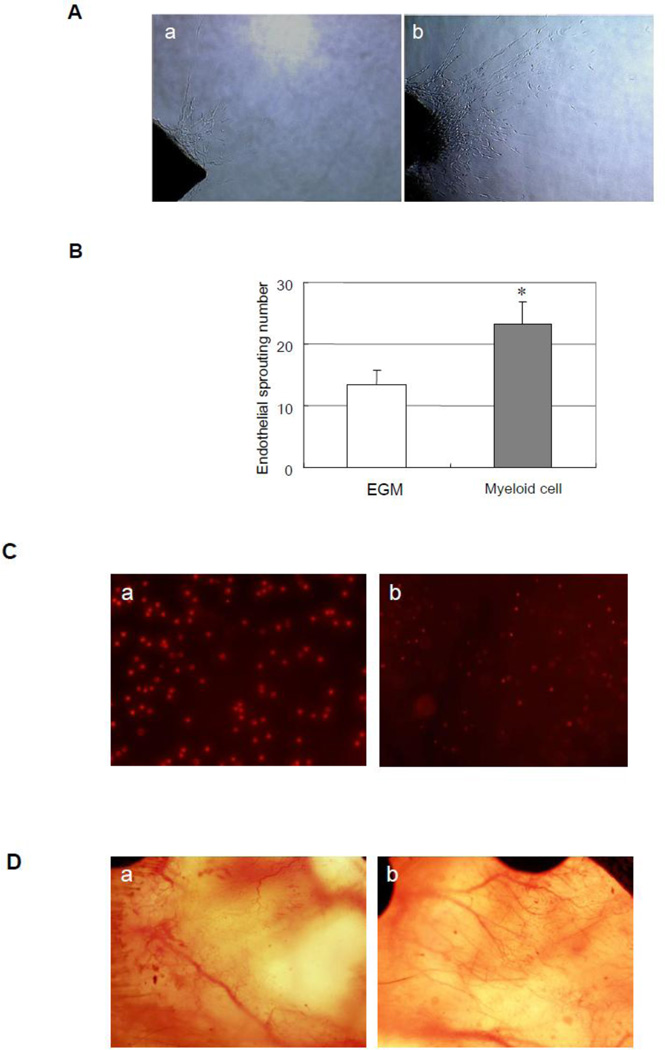
We sorted Gr-1+CD11b+ myeloid cells from the spleens of mice as previously described5. To investigate whether Gr-1+CD11b+ myeloid cells increase angiogenesis in vitro, we cultured Gr-1+CD11b+ myeloid cells with aortic ring 6. At the seventh day, we found that endothelial sprouts in Gr-1+CD11b+ myeloid cell treated group are significantly more than that in no cell treated group (23.2 ± 3.7 versus 13.4 ± 2.4 control, p < 0.01, n = 5 in each group, fig 1A and B), which indicated that Gr-1+CD11b+ myeloid cells increase angiogenesis in vitro. In order to see whether Gr-1+CD11b+ myeloid cells increase angiogenesis in vivo, we performed dorsal window model in mice7. We injected Gr-1+CD11b+ myeloid cells into tail vain of mice to see whether injected Gr-1+CD11b+ myeloid cells home to the site of window chamber. By tracing Gr-1+CD11b+ myeloid cells marked with PKH-26 (Fig 1C-a), we found that the Gr-1+CD11b+ myeloid cells home to the site of the window chamber after intravenous injection (Fig 1C-b). We further found the intravenous injection of Gr-1+CD11b+ myeloid cells obviously increase blood vessel branches at the site of window chamber, compared with saline treated groups, which suggested that the Gr-1+CD11b+ myeloid cells also increase angiogenesis in vivo (Fig 1D).
Fig 1.
Gr-1+CD11b+ myeloid cells increase angiogenesis in vitro and in vivo (A) Representive endothelial sprouts after 7 days of aortic ring sprouts assay. a, aorta ring culture with no cells; b, aorta ring culture with Gr-1+CD11b+ myeloid cells. Magnification ×10. (B) Gr-1+CD11b+ myeloid cells significantly increase endothelial sprouts compared with no cell treatment, *p < 0.01 versus EGM. (C) Gr-1+CD11b+ myeloid cells home to the site of window chamber after injection from tail vain. a, Gr-1+CD11b+ myeloid cells marked with PKH-26. Magnification ×100. b, Gr-1+CD11b+ myeloid cells homed to the site of window chamber. Magnification ×40. (D) Gr-1+CD11b+ myeloid cells increase angiogenesis in window chamber. a, saline treatment; b, Gr-1+CD11b+ myeloid cells treatment. Magnification ×10.
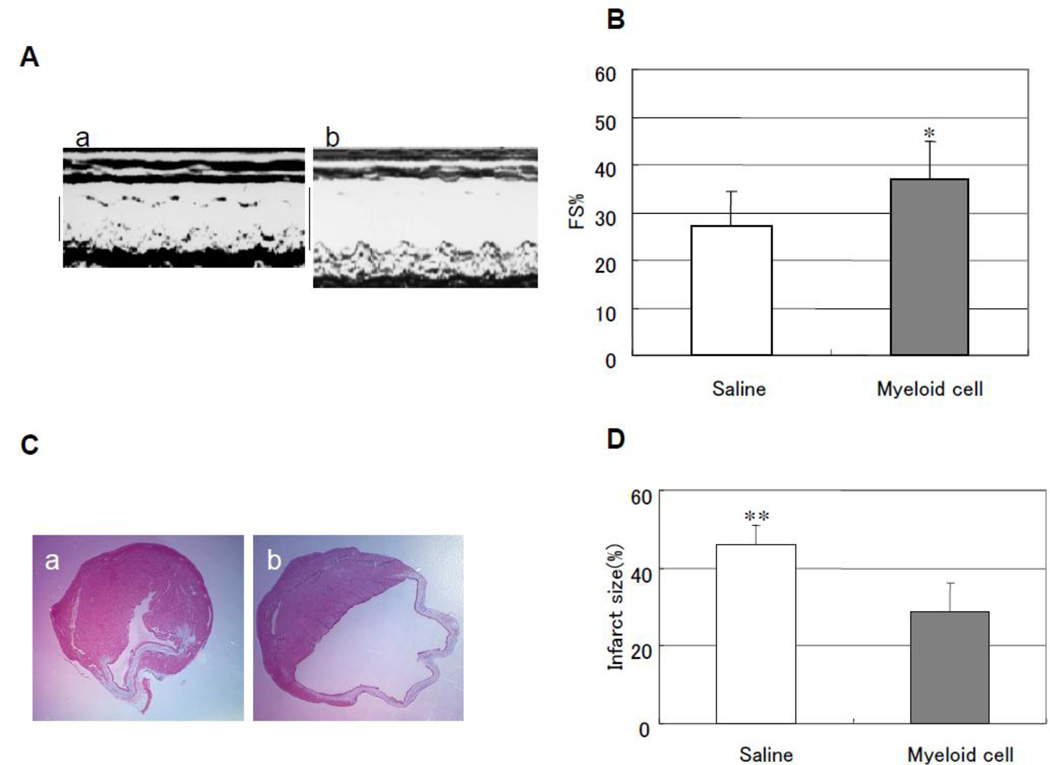
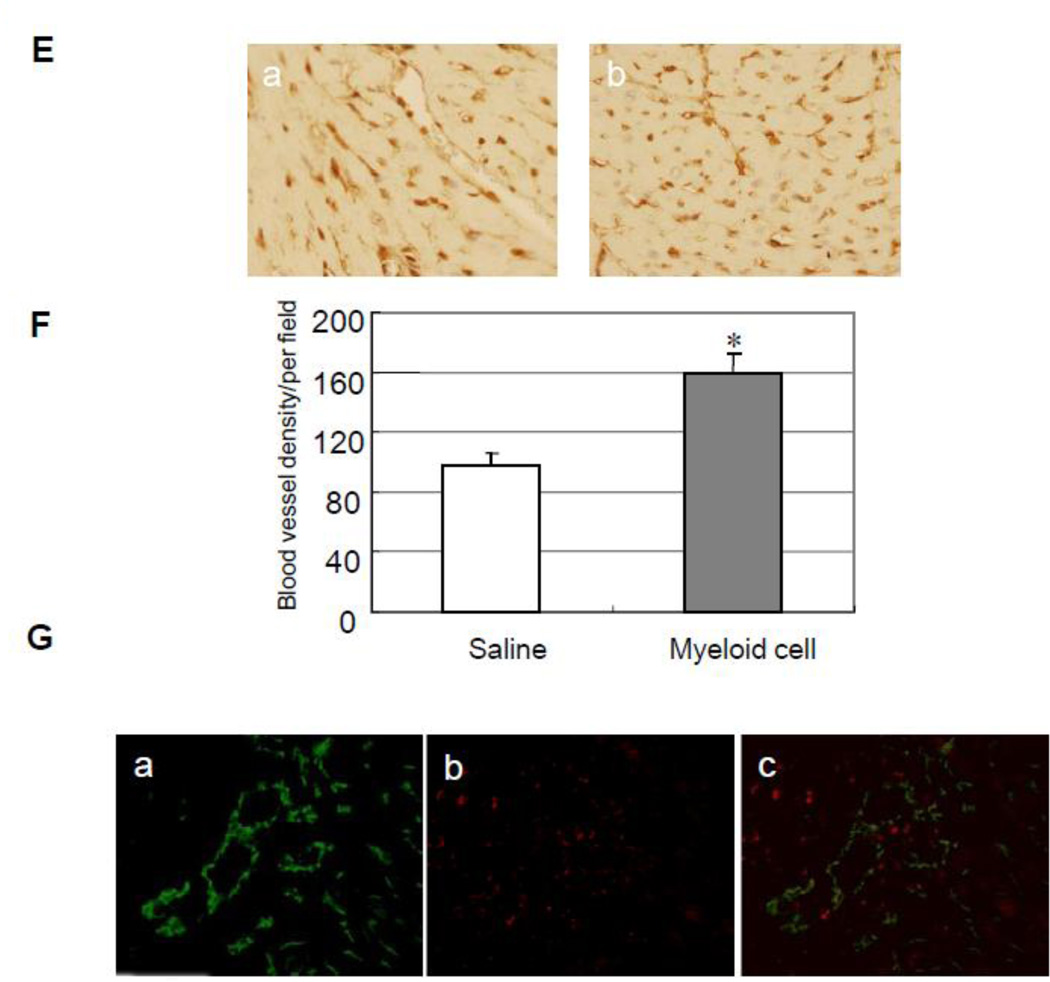
Next, we injected Gr-1+CD11b+ myeloid cells via tail vain to see whether Gr-1+CD11b+ myeloid cells improve heart function after heart infarction with a model of LAD ligation in mice8. After 4 weeks of LAD ligation and myeloid cells injection, the cardiac function was evaluated by echocardiography. The measured index indicated that myeloid cells significantly improve heart FS (37.2 ± 7.8% versus 27.3 ± 7.2% control, p < 0.05, n = 5 in each group, Fig 2A and B). The hearts were harvested after 4 weeks, and Masson's trichrome staining was performed9. The results indicated that myeloid cells significantly decrease infarct size after LAD ligation (28.9 ± 7.4% versus 45.8 ± 5.0% control, p < 0.01, n = 5 in each group, Fig 2C and D). We further performed capillary density measurement and tracing myeloid cells marked with PKH-26 in the infarct hearts. The results showed that myeloid cells significantly increase capillary density in the board area of infarction (159.2 ± 13.3 versus 96.8 ± 9.0 control, p < 0.01, n = 5 in each group Fig 2E and F). By tracing the myeloid cells and immunostaining of endothelial cells with anti-CD31 antibody, we found a few Gr-1+CD11b+ myeloid cells incorporate into vasculature (Fig 2G). These indicated Gr-1+CD11b+ myeloid cells improve cardiac function after heart infarction via both angiogenesis and vasculogenesis.
Fig 2.
Intravenous injection of Gr-1+CD11b+ myeloid cells improves cardiac function and decreases cardiac infarct size after heart infarction via increasing neovascularization. (A) Representive echocardography of heart after 4 weeks of LAD ligation. a, Gr-1+CD11b+ myeloid cells injected from tail vain; b, saline injected from tail vain. (B) Gr-1+CD11b+ myeloid cells significantly improve FS after heart infarction compared with saline, *p < 0.05 versus saline, n=5 in each group. (C) Representive Masson's trichrome staining of the heart after 4 weeks of heart infarction, a, Gr-1+CD11b+ myeloid cells treatment; b, saline treatment. (D) Gr-1+CD11b+ myeloid cells significantly decrease cardiac infarct size after heart infarction compared with saline, **p < 0.01 versus saline, n=5 in each group. (E) Representive capillary density measurement in peri-infarct area of the heart. a, saline treatment; b, Gr-1+CD11b+ myeloid cells treatment. Magnification ×200. (F) Gr-1+CD11b+ myeloid cells significantly increase angiogenesis in the peri-infarct area after heart infarction, *p < 0.01 versus saline, n=5 in each group. (G) Florescent imaging of the heart after intravenous injection of Gr-1+CD11b+ myeloid cells. a, endothelial cells stained with anti-CD31 (green); b, Gr-1+CD11b+ myeloid cells marked with PKH-26 (red); c, some of the Gr-1+CD11b+ myeloid cells incorporate into vasculature (yellow). Magnification ×200.
In conclusion, the intravenous administration of Gr-1+CD11b+ myeloid cells increases neovascularization and improves cardiac function after heart infarction. The Gr-1+CD11b+ myeloid cells could be used as a potential cell source in the field of cell therapy for ischemic cardiovascular diseases.
Acknowledgements
This work was supported by the National Natural Foundation of China [30960379 to J.H], the Scientific and Technological Agency of NingXia, China [KGX-13-09-25 to J.H], and the National Institutes of Health [CA108856 to P.C.L ].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yang L, DeBusk LM, Fukuda K, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 2.Grunewald M, Avraham I, Dor Y, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 3.Kim JA, March K, Chae HD, et al. Muscle-derived Gr1(dim)CD11b(+) cells enhance neovascularization in an ischemic hind limb mouse model. Blood. 2010;116(9):1623–1626. doi: 10.1182/blood-2009-08-237040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wara AK, Croce K, Foo S, et al. Bone marrow-derived CMPs and GMPs represent highly functional proangiogenic cells: implications for ischemic cardiovascular disease. Blood. 2011;118(24):6461–6464. doi: 10.1182/blood-2011-06-363457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ljung BM, Mayall B, Lottich C, et al. Cell dissociation techniques in human breast cancer--variations in tumor cell viability and DNA ploidy. Breast Cancer Res Treat. 1989;13:153–159. doi: 10.1007/BF01806527. [DOI] [PubMed] [Google Scholar]
- 6.Huang L, Sankar S, Lin C, et al. HCPTPA, a protein tyrosine phosphatase that regulates vascular endothelial growth factor receptor-mediated signal transduction and biological activity. J Biol Chem. 1999;274:38183–38188. doi: 10.1074/jbc.274.53.38183. [DOI] [PubMed] [Google Scholar]
- 7.Lin P, Polverini P, Dewhirst M, Shan S, Rao PS, Peters K. Inhibition of tumor angiogenesis using a soluble receptor establishes a role for Tie2 in pathologic vascular growth. J Clin Invest. 1997;100:2072–2078. doi: 10.1172/JCI119740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang R, Khoo MS, Wu Y, et al. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi K, Ito Y, Morikawa M, et al. Adenoviral-delivered angiopoietin-1 reduces the infarction and attenuates the progression of cardiac dysfunction in the rat model of acute myocardial infarction. Mol Ther. 2003;8:584–592. doi: 10.1016/s1525-0016(03)00230-2. [DOI] [PubMed] [Google Scholar]