Abstract
Objective
Studies of the association between transportation barriers and HIV-related health outcomes have shown both positive and negative effects, possibly because a reliable, validated measure of transportation barriers has not been identified.
Design
Prospective cohort study of HIV-infected patients in rural Uganda.
Methods
Participants were enrolled from the HIV clinic at the regional referral hospital in Mbarara, Uganda as part of the Uganda AIDS Rural Treatment Outcomes (UARTO) Study. We collected the following measures of transportation barriers to HIV clinic: global positioning systems (GPS)-tracked distance measured by driving participants to their homes along their typical route; straight-line GPS distance from clinic to home, calculated with the Great Circle Formula; self-reported travel time; and self-reported travel cost. We assessed inter-measure agreement using linear regression, correlation coefficients and κ statistics (by measure quartile) and validated measures by fitting linear regression models to estimate associations with days late for clinic visits.
Results
One hundred and eighty-eight participants were tracked with GPS. Seventy-six percent were women, with a median age of 40 years and median CD4 cell count of 193 cells/μl. We found a high correlation between GPS-based distance measures (β = 0.74, P < 0.001, R2 = 0.92, k = 0.73), but little correlation between GPS-based and self-reported measures (all R2 ≤ 0.4). GPS-based measures were associated with days late to clinic (P < 0.001); but neither self-reported measure was associated (P > 0.85).
Conclusion
GPS-measured distance to clinic is associated with HIV clinic absenteeism and should be prioritized over self-reported measures to optimally risk-stratify patients accessing care in rural, resource-limited settings.
Keywords: distance to clinic, global positioning systems, HIV/AIDS, linkage to care, sub-Saharan Africa, transportation, Uganda
Background
Structural barriers continue to thwart HIV treatment programs in resource-limited settings (RLS) with large catchment areas [1]. HIV-infected patients commonly cite transportation costs as a challenge [2–6]. Multiple studies have shown an association between transportation barriers and health-related outcomes including decreased ART adherence, decreased patient retention, and increased mortality [7–12]. However, several other studies have failed to show an association between transport barriers and outcomes [13–17]. One possibility for this discrepancy is inconsistent measurement of transportation barriers across studies, which have including self-reported travel distance [8,13,14,18], self-reported travel time [9,19–21], calculated travel distance [19], linear travel distance [19], and travel cost [19].
Global positioning systems (GPS) tracking enables highly accurate and reproducible measurement of distance. We collected both objective and self-reported measures of transportation barriers to clinic, using GPS tracking and patient interviews among patients at a publicly funded HIV clinic in southwestern Uganda. We examined the correlation between the measures as well as their association with missed clinic appointments. We hypothesized that GPS-based measures of transportation would correlate with each other and be more reliable predictors of missed clinic time.
Methods
Study participants and procedures
Participants were enrolled in the Ugandan AIDS Rural Treatment Outcomes Study (UARTO), a cohort study of HIV-infected adults taking antiretroviral therapy (ART) in southwestern Uganda, described in detail previously [22]. Adult patients receiving care at the Mbarara Regional Referral Hospital are eligible for enrollment at the time of ART initiation. Participants completed questionnaires at baseline and every 3–4 months, which included information about socioeconomic status, transportation time and transportation costs. Participants receive stipends for research visit transportation costs, but not for clinic or medication refill visits. In August 2011, study drivers began transporting individual participants to or from their residence with a GPS device, which recorded route coordinates in 20-m intervals (Garmin GPSMAP 60CSx, Chicago, Illinois, USA). Drivers recorded the route and type of transportation used for each leg (walking, motorbike, shared taxi, or bicycle) on the GPS device. Data on scheduled and attended clinic visits was obtained from the clinic database. All participants gave informed consent to participate in the study. The study procedures and the clinic database data-sharing plan were approved by the ethics committees of Partners Healthcare, the University of California at San Francisco, and the Mbarara University of Science and Technology.
Statistical methods
We evaluated four measures of transportation-related barriers: self-reported travel time; self-reported travel cost; GPS-tracked distance measured by driving participants to their homes along their typical route with a GPS device; and GPS-measured straight-line distance from clinic to home, calculated with the Great Circle Distance Formula, which measures point-to-point distance along the surface of a spherical object. We summarized intra-measure distribution and assessed inter-measure agreement using linear regression, correlation coefficients, and κ statistics by dividing each measure into quartiles. We conducted sensitivity analyses by excluding outliers with values greater than three SD from the mean of each transportation-related measure. We validated each measure by estimating its association with clinic absenteeism, that is days late for clinic appointments. For our outcome of interest, we used the number of days late for clinic appointments per each 360 days of study time, log-transformed to satisfy model assumptions of normality. We fit linear regression models for each measure of transportation adjusted for predicted correlates of missed clinic time including age, sex, educational attainment, CD4 cell count, and socio-economic status using the Filmer–Pritchett asset-index score [23]. The asset index is the first principal component derived from a principal components analysis applied to 25 binary indicators for household-owned assets (e.g. bicycle, radio) and housing characteristics (e.g. type of toilet facilities, water source). Demographic and CD4 cell count results were used on the survey date closest in time to the home-based tracking date. We graphically depicted the relationships between the measures and number of missed clinic days by computing the predicted number of days late at multiple percentiles of each transportation measure, holding other covariates constant at their median value. All statistical analyses were performed using Stata 11 (Stata Corp, College Station, Texas, USA).
Results
As of September 2012, 188 participants completed GPS tracking, of whom 76% were women, with a median age of 40 years and median CD4 cell count of 193 cells/μl. Median self-reported cost to clinic was $4 [interquartile range (IQR) 2–6]; self-reported travel time was 60 min (IQR 30–90); GPS-measured straight-line distance was 9.6 km (km) (IQR 3.2–15.3); and GPS-tracked actual travel distance was 12.7 km (IQR 4.9–19.3). Participants reported requiring one (17%), two (48%) or three (35%) methods of transportation to arrive at clinic, with the most commons being paid motorbike (72%) followed by shared taxi (41%), walking (38%) and bicycle (2%).
Correlation between measures of transportation
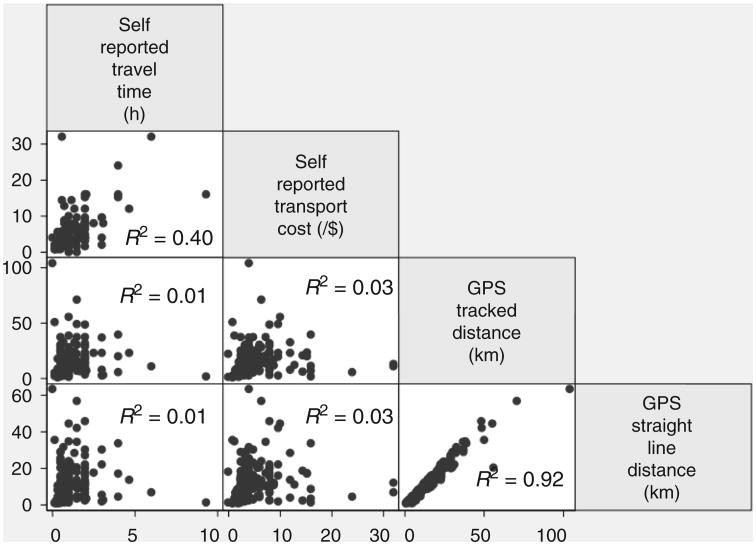
There was a high correlation between GPS-tracked and straight-line distance measures (β = 0.74, P < 0.001, R2 = 0.92, k = 0.73) (Fig. 1). However, we found low correlations between GPS measured straight-line distance and self-reported transport time (β = 0.75, P = 0.31, R2 = 0.14, k = 0.14) or self-reported cost to clinic (β = 0.36, P = 0.03, R2 = 0.03, k = 0.10) and low correlations between GPS-tracked distance to clinic and self-reported transport time (β = 0.91, P = 0.33, R2 = 0.01, k = 0.21) or self-reported cost to clinic (β = 0.45, P = 0.03, R2 = 0.03, k = 0.10). There was moderate correlation between the two self-reported measures (β = 2.66, P < 0.001, R2 = 0.40, k = 0.35). In sensitivity analyses excluding seven participants with a measure of distance greater than three SDs from the mean, the R2 value for correlation between objective measures remained 0.92, and all other correlates remained within 0.1 of their initial value, with none exceeding an R2 > 0.35.
Fig. 1.
Scatter plots comparing measures of transportation for participants in care at an HIV clinic in southwestern Uganda.
Association between transportation measures and missed clinic visits
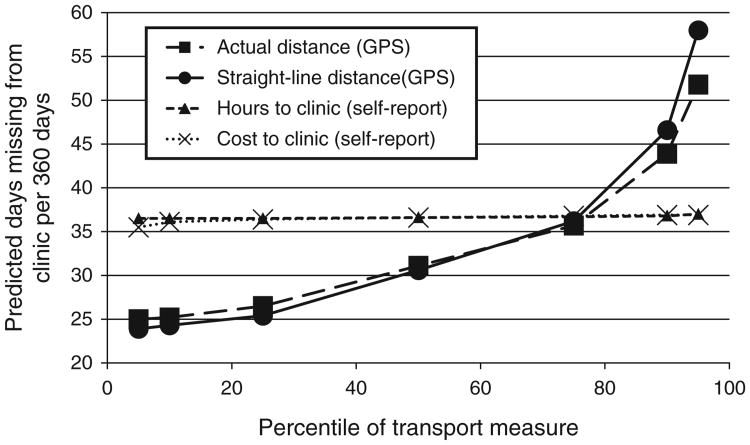
Participants contributed a median of 748 days (IQR 678–783) of study time to the missed visits analysis. In multivariable regression models adjusted for age, sex, asset index score, educational attainment and baseline CD4 cell count, increasing GPS-tracked distance (β-coefficient = 0.029, 95% CI 0.018–0.041, P < 0.001) and GPS straight-line measures (β-coefficient = 0.021, 95% CI 0.012–0.030, P < 0.001) of transportation were significantly associated with increasing log-transformed days late for clinic visits (Fig. 2). The magnitudes of these associations between GPS-measured distance and clinic absenteeism were large. For example, evaluated at the median of the covariates, the predicted number of days late to clinic for participants at the 10th percentile of distance to clinic (2.6 km) was 25 days (95% CI, 18–33), whereas the predicted number of days late to clinic for participants at the 90th percentile (29.0 km) was 44 days (95% CI, 36–52). There was no association between self-reported time to clinic (β-coefficient = –0.006, 95% CI –0.136 to 0.147, P = 0.94) or self-reported cost of transportation (β-coefficient = 0.003, 95% CI =0.034– 0.029, P = 0.86) and clinic days missed per 360-day period.
Fig. 2.
Estimated days late to clinic at 5th, 10th, 25th percentile, median, 75th, 90th and 95th percentile of measures of transportation assuming median of model covariates (age, sex, as set index, educational attainment, baseline CD4 cell count).
Discussion
In a cohort of HIV-infected patients taking ART in southwestern Uganda, GPS-based measures of transportation distance were highly correlated with each other and associated with late presentation to HIV clinic appointments. In contrast, self-reported measures were poorly correlated with objective measures and were not associated with missed clinic days. Our results suggest distance to clinic is an important barrier to sustained treatment and that objective measures of transportation are necessary to optimally risk-stratify patients accessing HIV care in RLS.
A large body of public health literature has described deleterious effects of transportation barriers on health outcomes [24–27]. In the non-HIV literature, many of these studies support the use of objective measures of distance from home to clinic. A study in Bangladesh found that increasing straight-line distance to a health center was associated with decreased clinic attendance and increased attributable mortality for cases of diarrhea [26]. In a population-based study of childhood mortality in Burkina Faso, both time and distance to clinic were calculated using maps to estimate routes of travel [25]. The authors found a significant association for both distance and time to clinic with child mortality, and a high correlation between the two measures. In Kenya, GPS measured distance to clinic has been associated with decreased healthcare utilization [27].
Access to and sustained retention in HIV care remains a major challenge in RLS, in which HIV-associated mortality rates remain high [28–30]. Linkage failures occur at multiple points along the care continuum due to a complex array of structural barriers including difficulty accessing and affording transport [1,3,14]. Though dozens of prior studies in the HIV literature have reported measures of transportation to clinic and its association with health-related outcomes in RLS [4–6,11,12,14,16,17,20,21,31–33], only one to our knowledge utilized GPS measurements [19]. In that study of HIV-infected patients in Zambia, GPS-measured distance to clinic was not associated with self-reported ART adherence, but the authors excluded adherence measurement in patients with missed visits. Because the association between adherence and distance is likely mediated through missed visits, the lack of an association in their study is not unexpected. Among the numerous other studies measuring the effects of transportation on HIV outcomes, there are reports of negative [7,10–12], null [9,16,17], and positive [13–15] effects of self-reported transportation barriers on HIV associated outcomes. Given our results, the variation in findings from these studies might be explained by the low correlation between self-reported measures of distance and both actual distance and patient outcomes. Though we cannot discern from our data the reason for low correlation between self-reported measures and clinic visitation, two explanations might include imprecise estimation of distance by self-report and over-reporting of clinical distance and/or cost to increase travel compensation. Though the former explanation is supported by the random association between self-reported and objective measures that we found (Fig. 1), the latter would instead have been suggested by a systematic over-reporting of distance.
Our study was limited to participants taking ART in rural, southwestern Uganda and might not pertain to urban settings or patients who have not begun ART. Variations in geography, patient population and means of transportation likely modify the inter-measure correlation and health-related effects of transportation barriers [34,35]. Further study of the varying impact of wealth, road development, topography and population density will help clarify how these differences affect reliability of transportation measures across settings.
In summary, GPS-based objective measures of distance to clinic, but not self-reported assessments, are highly correlated and predict missed clinic days at an HIV clinic in rural, southwestern Uganda. Our findings have implications for both optimizing measurement of transportation barriers and improving access to care in rural, RLS. Although self-reported measures of travel cost and time are less costly and simpler to perform, our results should call into question their value. Research groups that have the requisite resources should prioritize objective measures of distance. Because GPS-measured straight line and GPS-tracked distance are highly correlated and have similar associations with missed clinic time, a single measurement of home coordinates is likely sufficient to calculate distance to clinic. In our experience, the single-waypoint method is less laborious and costly than tracked measurement because it can be performed by one staff member for multiple clients simultaneously. For programs unable to perform objective measurements, estimating straight-line distance from maps based on location of residence might be an accurate alternative to GPS measurement, though this method requires further investigation. Finally, study of the relationships between measures of transportation and health outcomes across varying climates, living standards, road and infrastructure conditions, and topographies will be useful to corroborate our findings and inform development of site-specific interventions to mitigate the effect of transportation-related barriers on clinic absenteeism.
Acknowledgments
We thank the UARTO participants and staff who made this study possible; and the study drivers for their tireless efforts to perform GPS tracking of participants, rain or shine: Richard Jumba, Ibrahim Kiviiri, Silver Mugisha, Ambrose Mulumba, Medard Tushemereirwe, and Ssenyondo Wahab.
Sources of funding: the Uganda AIDS Rural Treatment Outcomes Study is funded by U.S. National Institutes of HealthR01 MH54907and P30AI27763. The authors also acknowledge the following additional sources of salary support: NIH K23 MH087228 (Haberer); NIH K23 MH096620 (Tsai); NIH K24 MH87227 (Bangsberg); the Harvard Institute for Global Health, the Fogarty International Clinical Research Scholars and Fellows Program at Vanderbilt University (R24 TW007988), and NIH T32 AI007433 (M.J.S.); and the Doris Duke Charitable Foundation through Harvard Medical School (A.L.). Additional study funding was provided by the Mark and Lisa Schwartz Family Foundation, the Sullivan Family Foundation, and the Bacca Foundation.
Footnotes
Author contributions: M.J.S. took the lead on conception and design, performed data analysis, wrote the first draft of the article and performed editing of subsequent drafts.
A.L. assisted with study design, performed data analysis and interpretation, and made substantial edits and critical revision of the article.
A.C.T. assisted with study design, performed data analysis and interpretation, and made substantial edits and critical revision of the article.
C.M. assisted with study design, performed data collection, and performed critical revision of the article.
P.H. participated in study conception and design, performed data acquisition and performed article editing and revision.
J.M. participated in study conception and design, performed data acquisition and performed article editing and revision.
J.H. participated in study conception and design, performed data acquisition and performed article editing and revision.
D.B. was the principal investigator and developer of the UARTO study. He participated in study conception, design and article editing and revision
Conflicts of interest: The authors have no conflicts of interest to report.
References
- 1.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8:e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardon AP, Akurut D, Comoro C, Ekezie C, Irunde HF, Gerrits T, et al. Hunger, waiting time and transport costs: time to confront challenges to ART adherence in Africa. AIDS Care. 2007;19:658–665. doi: 10.1080/09540120701244943. [DOI] [PubMed] [Google Scholar]
- 3.Tuller DM, Bangsberg DR, Senkungu J, Ware NC, Emenyonu N, Weiser SD. Transportation costs impede sustained adherence and access to HAART in a clinic population in southwestern Uganda: a qualitative study. AIDS Behav. 2010;14:778–784. doi: 10.1007/s10461-009-9533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amuron B, Namara G, Birungi J, Nabiryo C, Levin J, Grosskurth H, et al. Mortality and loss-to-follow-up during the pre-treatment period in an antiretroviral therapy programme under normal health service conditions in Uganda. BMC Public Health. 2009;9:290. doi: 10.1186/1471-2458-9-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krebs DW, Chi BH, Mulenga Y, Morris M, Cantrell RA, Mulenga L, et al. Community-based follow-up for late patients enrolled in a district-wide programme for antiretroviral therapy in Lusaka, Zambia. AIDS Care. 2008;20:311–317. doi: 10.1080/09540120701594776. [DOI] [PubMed] [Google Scholar]
- 6.McGuire M, Munyenyembe T, Szumilin E, Heinzelmann A, Le Paih M, Bouithy N, et al. Vital status of pre-ART and ART patients defaulting from care in rural Malawi. Trop Med Int Health. 2010;15(Suppl 1):55–62. doi: 10.1111/j.1365-3156.2010.02504.x. [DOI] [PubMed] [Google Scholar]
- 7.Ingle SM, May M, Uebel K, Timmerman V, Kotze E, Bachmann M, et al. Outcomes in patients waiting for antiretroviral treatment in the Free State Province. South Africa: prospective linkage study AIDS. 2010;24:2717–2725. doi: 10.1097/QAD.0b013e32833fb71f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taiwo BO, Idoko JA, Welty LJ, Otoh I, Job G, Iyaji PG, et al. Assessing the viorologic and adherence benefits of patient-selected HIV treatment partners in a resource-limited setting. J Acquir Immune Defic Syndr. 2010;54:85–92. doi: 10.1097/01.qai.0000371678.25873.1c. [DOI] [PubMed] [Google Scholar]
- 9.Charurat M, Oyegunle M, Benjamin R, Habib A, Eze E, Ele P, et al. Patient retention and adherence to antiretrovirals in a large antiretroviral therapy program in Nigeria: a longitudinal analysis for risk factors. PLoS One. 2010;5:e10584. doi: 10.1371/journal.pone.0010584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elbireer S, Guwatudde D, Mudiope P, Nabbuye-Sekandi J, Manabe YC. Tuberculosis treatment default among HIV-TB co-infected patients in urban Uganda. Trop Med Intern Health. 2011;16:981–987. doi: 10.1111/j.1365-3156.2011.02800.x. [DOI] [PubMed] [Google Scholar]
- 11.Kempf MC, Allen S, Zulu I, Kancheya N, Stephenson R, Brill I, et al. Enrollment and retention of HIV discordant couples in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2008;47:116–125. doi: 10.1097/QAI.0b013e31815d2f3f. [DOI] [PubMed] [Google Scholar]
- 12.Sutcliffe CG, Bolton-Moore C, van Dijk JH, Cotham M, Tambatamba B, Moss WJ. Secular trends in pediatric antiretroviral treatment programs in rural and urban Zambia: a retrospective cohort study. BMC Pediatrics. 2010;10 doi: 10.1186/1471-2431-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook RE, Ciampa PJ, Sidat M, Blevins M, Burlison J, Davidson MA, et al. Predictors of successful early infant diagnosis of HIV in a rural district hospital in Zambezia, Mozambique. J Acquir Immune Defic Syndr. 2011;56:e104–e109. doi: 10.1097/QAI.0b013e318207a535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geng EH, Glidden DV, Bwana MB, Musinguzi N, Emenyonu N, Muyindike W, et al. Retention in care and connection to care among HIV-infected patients on antiretroviral therapy in Africa: estimation via a sampling-based approach. PLoS One. 2011;6:e21797. doi: 10.1371/journal.pone.0021797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haberer JE, Kiwanuka J, Nansera D, Ragland K, Mellins C, Bangsberg DR. Multiple measures reveal antiretroviral adherence successes and challenges in HIV-infected Ugandan children. PLoS One. 2012;7:e36737. doi: 10.1371/journal.pone.0036737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karcher H, Omondi A, Odera J, Kunz A, Harms G. Risk factors for treatment denial and loss to follow-up in an antiretroviral treatment cohort in Kenya. Trop Med Intern Health. 2007;12:687–694. doi: 10.1111/j.1365-3156.2007.01830.x. [DOI] [PubMed] [Google Scholar]
- 17.van Dijk JH, Sutcliffe CG, Munsanje B, Sinywimaanzi P, Hamangaba F, Thuma PE, et al. HIV-infected children in rural zambia achieve good immunologic and virologic outcomes two years after initiating antiretroviral therapy. PLoS One. 2011;6 doi: 10.1371/journal.pone.0019006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iroha E, Esezobor CI, Ezeaka C, Temiye EO, Akinsulie A. Adherence to antiretroviral therapy among HIV-infected children attending a donor-funded clinic at a tertiary hospital in Nigeria. Afr J AIDS Res. 2010;9:25–30. doi: 10.2989/16085906.2010.484543. [DOI] [PubMed] [Google Scholar]
- 19.Carlucci JG, Kamanga A, Sheneberger R, Shepherd BE, Jenkins CA, Spurrier J, et al. Predictors of adherence to antiretroviral therapy in rural Zambia. J Acquir Immune Defic Syndr. 2008;47:615–622. doi: 10.1097/QAI.0b013e318165dc25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ochieng-Ooko V, Ochieng D, Sidle JE, Holdsworth M, Wools-Kaloustian K, Siika AM, et al. Influence of gender on loss to follow-up in a large HIV treatment programme in western Kenya. Bull World Health Organ. 2010;88:681–688. doi: 10.2471/BLT.09.064329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramadhani HO, Thielman NM, Landman KZ, Ndosi EM, Gao F, Kirchherr JL, et al. Predictors of incomplete adherence, virologic failure, and antiviral drug resistance among HIV-infected adults receiving antiretroviral therapy in Tanzania. Clin Infect Dis. 2007;45:1492–1498. doi: 10.1086/522991. [DOI] [PubMed] [Google Scholar]
- 22.Weiser SD, Tsai AC, Gupta R, Frongillo EA, Kawuma A, Senkungu J, et al. Food insecurity is associated with morbidity and patterns of healthcare utilization among HIV-infected individuals in a resource-poor setting. AIDS. 2012;26:67–75. doi: 10.1097/QAD.0b013e32834cad37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data–or tears: an application to educational enrollments in states of India. Demography. 2001;38:115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 24.Jani JV, De Schacht C, Jani IV, Bjune G. Risk factors for incomplete vaccination and missed opportunity for immunization in rural Mozambique. BMC Public Health. 2008;8:161. doi: 10.1186/1471-2458-8-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoeps A, Gabrysch S, Niamba L, Sie A, Becher H. The effect of distance to health-care facilities on childhood mortality in rural Burkina Faso. Am J Epidemiol. 2011;173:492–498. doi: 10.1093/aje/kwq386. [DOI] [PubMed] [Google Scholar]
- 26.Rahaman MM, Aziz KM, Munshi MH, Patwari Y, Rahman M. A diarrhea clinic in rural Bangladesh: influence of distance, age, and sex on attendance and diarrheal mortality. Am J Public Health. 1982;72:1124–1128. doi: 10.2105/ajph.72.10.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feikin DR, Nguyen LM, Adazu K, Ombok M, Audi A, Slutsker L, et al. The impact of distance of residence from a peripheral health facility on pediatric health utilisation in rural western Kenya. Trop Med Int Health. 2009;14:54–61. doi: 10.1111/j.1365-3156.2008.02193.x. [DOI] [PubMed] [Google Scholar]
- 28.Brinkhof MW, Dabis F, Myer L, Bangsberg DR, Boulle A, Nash D, et al. Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bull World Health Organ. 2008;86:559–567. doi: 10.2471/BLT.07.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mermin J, Ekwaru JP, Were W, Degerman R, Bunnell R, Kaharuza F, et al. Utility of routine viral load, CD4 cell count, and clinical monitoring among adults with HIV receiving antiretroviral therapy in Uganda: randomised trial. BMJ. 2011;343:d6792. doi: 10.1136/bmj.d6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Byakika-Tusiime J, Oyugi JH, Tumwikirize WA, Katabira ET, Mugyenyi PN, Bangsberg DR. Adherence to HIV antiretroviral therapy in HIV plus Ugandan patients purchasing therapy. Int J STD AIDS. 2005;16:38–41. doi: 10.1258/0956462052932548. [DOI] [PubMed] [Google Scholar]
- 32.Kirsten I, Sewangi J, Kunz A, Dugange F, Ziske J, Jordan-Harder B, et al. Adherence to combination prophylaxis for prevention of mother-to-child-transmission of HIV in tanzania. PLoS One. 2011;6 doi: 10.1371/journal.pone.0021020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munseri PJ, Talbot EA, Mtei L, Fordham von Reyn C. Completion of isoniazid preventive therapy among HIV-infected patients in Tanzania. Int J Tuberc Lung Dis. 2008;12:1037–1041. [PubMed] [Google Scholar]
- 34.Haynes R, Jones AP, Sauerzapf V, Zhao H. Validation of travel times to hospital estimated by GIS. Int J Health Geogr. 2006;5:40. doi: 10.1186/1476-072X-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phibbs CS, Luft HS. Correlation of travel time on roads versus straight line distance. Med Care Res Rev. 1995;52:532–542. doi: 10.1177/107755879505200406. [DOI] [PubMed] [Google Scholar]