Abstract
Inducible Heat Shock Protein 70 (HSP70i) is a protein regulated by stress that protects cells from undergoing apoptosis. Such proteins are marvelously well conserved throughout evolution, which has placed them in the spotlight for helping to understand the intriguing relationship between infection and immunity. In the presence of stress proteins, dendritic cells (DCs) will sense this alarm signal and respond by recruiting immune cells of different plumage to fit the occasion. In times of stress, melanocytes will secrete antigen bound HSP70i to act as an alarm signal in activating DCs, that comes equipped with an address of origin to drive the autoimmune response in vitiligo. Here we pose that if the autoimmune response is funneled through HSP70i, then blocking the stress protein from activating DCs can lend new treatment opportunities for vitiligo.
Keywords: autoimmune, dendritic cell, stress, heat shock protein, depigmentation, mouse model, T cell
Vitiligo is an organ-specific autoimmune disease of the skin
Vitiligo is a skin disorder presenting with progressive depigmentation and affecting 0.5% of the world population (1). Depigmentation is due to the loss of melanocytes from the epidermis (2). Genetic studies support the involvement of abnormalities affecting immune function in vitiligo (3). T cell infiltrates are observed in perilesional skin of patients with active vitiligo (4). Melanocyte-reactive T cells are relatively abundant among peripheral T cells from patients with active disease (5). T cells isolated from vitiligo skin are cytotoxic towards melanocytes (6,7). Thus vitiligo is primarily T cell mediated, although humoral responses may also contribute to disease development (8).
Intrinsic abnormalities were found in vitiligo melanocytes, including dilated ER profiles, mitochondrial abnormalities and abnormal melanosome compartmentalization (9), possibly rendering the cells increasingly sensitive to stress (10). Patients consider stress a precipitating factor for their disease (11) and known stressors, including bleaching phenols, UV irradiation and mechanical injury, invoke a Koebner phenomenon in about half of patients (12). In terms of emotional stress, obsession and phobia has been correlated with autoimmune markers in vitiligo (13). In ‘occupational vitiligo’ individuals develop disease in response to bleaching phenols in the workplace (14). These can cause oxidative stress in the skin. Several lines of evidence support an association between oxidative stress and vitiligo (15). This has culminated in the development of pseudocatalase (PC-KUS) treatment for vitiligo, which has unfortunately shown limited efficacy in the clinic (16,17). Gene expression analysis revealed upregulated IL-6 and IL-8 expression by melanocytes in response to bleaching agents (18). Thus stress can cause micro-inflammation and support recruitment of an immune infiltrate to the skin, reflecting the connection between stress and autoimmunity. In summary, vitiligo is a T cell mediated autoimmune disease precipitating under stress.
The immune response presents a mirror image of that found in melanoma
Antigens recognized by T cells infiltrating vitiligo skin were known targets for T cells infiltrating melanoma tumors (19). These antigens are predominantly expressed in melanosomes which bear functional resemblance to lysosomes (20). Including a melanosomal trafficking signal enhances immunogenicity of non-melanosomal proteins (21,22). Thus, localization likely contributes to the immunogenicity of melanosomal proteins. A resemblance between immune reactivity in vitiligo and melanoma is supported by leukoderma in melanoma patients with detectable immune responses to their tumor. In fact, depigmentation is a positive prognostic factor in melanoma (23). Unfortunately the immune response rarely clears tumors, whereas robust immunity directed towards the same antigens is a hallmark of vitiligo. A lack of regulatory T cells infiltrating vitiligo skin compared to their abundance in melanoma contributes to such differences (24,25). In fact, autoimmune destruction of melanocytes following Treg depletion in tumor challenged mice is required for generating effective anti-tumor responses (26).
Treatments under development to boost anti-tumor immunity in melanoma include vaccines based on heat shock protein 70 (HSP70) fusion proteins (27). HSP70 is included in vaccines as a chaperone protein, immunogenic in its own right (28, 29) and functioning as an immune adjuvant as described below.
Inducible HSP70 can mediate immune responses
Cells under stress halt mainstream protein synthesis in favor of heat shock protein and/or glucose regulated protein synthesis (30, 31). In the ER this can activate the unfolded protein response (UPR), upregulating heat shock proteins (32). The UPR has been implicated in many diseases including vitiligo (18). This finding is congruent with dilated ER profiles reported for vitiligo melanocytes, potentially regulated by VIT1/FBXO11 (33,34). Within the cell, stress proteins bind preexisting proteins, promoting autophagy to avert cellular apoptosis (35). This function can have implications for vitiligo (36). Cell derived stress protein fractions can also ignite immune responses specific to the proteins and peptides they chaperone and thus, to the originating host cells (37). Among larger heat shock proteins, inducible HSP70 is unique for its secretion from live cells as a chaperokine (38). Other stress proteins likely gain access to the extracellular milieu only after necrotic cell death (39). The unique secretory property of HSP70i may be ascribed at least in part to its cellular location, associated in part with melanosomes (40). HSP70i is exported by live cells through the endo-lysosomal pathway (41). A rise in intracellular calcium serves as a signal for exocytosis for several cell types (42). In this setting DCs are provided with antigenic peptides from live cells for processing and presentation to T cells (43). HSP70i can also stimulate proliferation and cytotoxicity of natural killer (NK) cells (43), and enhance leukotriene secretion by mast cells (44, 45). Moreover, HSP70i induces maturation and type-1 polarizing cytokine production by dendritic cells (DCs) and stimulates cross priming of T cells (46), and importantly, breaks tolerance and induces autoimmune tissue destruction in mice (47).
A causative role of HSP70 in autoimmune disease remains controversial (48, 49). However, in vitiligo HSP70 plays a central, non-redundant role in precipitating disease (50). Most studies identify the C-terminal, substrate binding region of the HSP70 protein as the region dictating immune reactivity, which may in turn be modulated by bound antigen (51, 52). The C-terminus of stress proteins is thus likely important for stimulating DCs (53). Subtle sequence differences may define immune activation versus tolerization, as microbial HSP70 was shown to suppress inflammation in several studies (54, 55). The outcome of immune responses was not reported to depend upon the maturation stage of recipient antigen presenting cells. However, the separate identification of immune stimulatory and immune suppressive regions within the C-terminus of HSP70 (56) suggests that receptor binding affects the prevailing consequences of HSP70 exposure.
Several surface receptors were implicated in mediating the effects of extracellular HSP70, including CD91, TLR-2, CD14/TLR4, CCR5, and scavenger receptors (57, 58, 59, 60, 61). Interestingly, elevated surface expression of HSP70 on circulating lymphocytes was reported for vitiligo patients (62). HSP70-induced inflammatory killing of melanocytes confers immunological memory against tumor cells, and may thus enhance autoimmune responses to melanocytes as well (63). The ability of HSP70 to chaperone antigenic moieties and to activate a specific, T cell-mediated immune response is exploited in anti-tumor vaccines (64, 65). Thus HSP70 is a likely contributor to autoimmune reactivity.
HSP70 is involved in trafficking and degradation of lysosomal proteins
The constitutive form of HSP70, HSPA8, reroutes cytosolic proteins otherwise destined for proteasomal degradation to the lysosome (66). Proteins rerouted for lysosomal degradation are linearized by a lysosomal membrane complex involving HSP70, then transferred to LAMP-2a molecules forming a pore in the lysosomal membrane (67). Once inside the lysosome, proteins again encounter HSP70 (lyHSP70) (68), to safeguard entering resident lysosomal proteins from inadvertent degradation. In rheumatoid arthritis, autoimmune reactivity was assigned in part to the process whereby HSP70 chaperones proteins into lysosomes (69). HSP70 safeguards lysosomal integrity, protecting against conditions of oxidative stress (70). When misfolded proteins are no longer remedied by autophagy, loss of lysosomal integrity contributes to programmed necrosis (71). Disrupted autophagy may also occur in vitiligo (72). Consequently, HSP70 and its co-chaperones (particularly CHIP) appear as gatekeepers defining the proportion of proteins undergoing proteasomal degradation and MHC class I antigen presentation, or lysosomal degradation (73). In cells expressing MHC class II molecules, lysosomes are a source of peptides to be presented in the context of such MHC class II molecules, thus HSP70 helps segregate class I and class II destinations (74,75).
Besides professional antigen presenting cells, resident tissue cells can express MHC class II molecules under exceptional circumstances. For melanocytes, these circumstances are met in melanoma, vitiligo and Vogt-Kayanagi-Harada syndrome (76, 77). Melanosomes engage in melanosome-endosome fusion and antigen processing (78). Mutations in constitutive HSP70 have been implicated in disruption of the endosomal/lysosomal compartment (79). Interestingly, overexpression of HSP70i in melanoma cells inhibited melanin production (80). Overall the presence of HSP70 in melanosomes, potentially involved in trafficking of melanosomal proteins, has not been thoroughly investigated. Yet the exceptional immunogenicity of melanosomes can likely be ascribed in part to melanocyte specific melanosomal proteins presented in the context of MHC class II molecules by melanocytes and melanoma cells (22).
The HSP70’s are a complex family of proteins with specific household tasks
The HSP70 family is composed of at least 17 highly related genes on chromosomes 1, 5, 6, 9, 11, 14 and 21 in humans, encoding constitutively expressed and inducible proteins (81). The common denominator is expression induced by elevated temperatures (heat shock) of proteins with an approximate molecular weight of 70 kDa (66–78 kDa) (82). Three functional domains have been assigned: an N-terminal ATPase domain of approximately 44 kD (~350 aa), an 18 kD substrate binding domain (~150 aa) and a 10 kD C terminal domain (~100 aa) responsible for binding chaperone cofactors (83). Family members serve as chaperones, guiding intracellular proteins to respective organelle targets (84). In this function HSP70 facilitates folding, binding and translocation of proteins (85). Loci encoding the HSP70 family were named HSPA1 through HSPA14 (81). Canonical HSP70 isoforms are functionally redundant, with the main differences found in their spatio-temporal expression (86). The localization of individual gene products will vary from nuclear/cytoplasmic (A1/HSP72/Hsp70i, and A8/ HSP73/HSC70) to ER (5/BiP/GRP78) and mitochondrial (9/GRP75/PBP74) (82). HSP70 will bind to CD40 by means of its upstream ATPase domain, coinciding with the binding site of chaperone cofactor Hip, stabilizing the ADP state of HSP70 to facilitate peptide binding (87). Substrate specificity may be defined by J protein cofactor binding (88).
A chaperokine function was assigned mainly to inducible HSPA1A (41). The constitutively expressed isoforms are considered important for cellular housekeeping, whereas inducible isoforms offer protection from stress (81, 89). Enhanced secretion of HSP70i by live cells was observed in response to IFN-γ (90), important for vitiligo development (91). Gene products protecting cells from the consequences of heat shock are well conserved, and homologues are found across species (92).
HSP70 is a star player in anti-tumor vaccines and treatment of autoimmune disease
The chaperone function of HSP70, supporting uptake and processing of antigens by DCs renders the molecule an ideal adjuvant in anti-tumor vaccines (93). DNA encoding HSP70i-antigen fusion proteins was included in vaccines to melanoma (94). Such applications frequently use mycobacterial HSP70 (95). For anti-cancer vaccines, the use of xenogeneic stress proteins has the added advantage that nucleotide variations render the resulting protein increasingly immunogenic (mycobacterial and mouse HSP70 are approximately 50% homologous), whereas either version can bind peptides and proteins. Meanwhile murine cell lines will bind human HSP70 and vice versa (96).
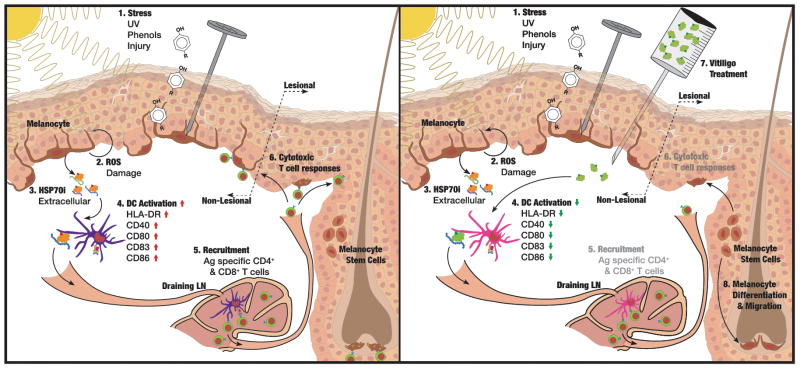
An intriguing relationship exists between anti-tumor immunity and autoimmunity in melanoma versus vitiligo (97). This ultimately prompted our studies into the involvement of heat shock proteins in vitiligo after HSPs were implicated in anti-tumor immunity (98). Whereas vaccines supporting the role of HSP70 in anti-tumor immunity will benefit melanoma patients, blocking HSP70 from perpetuating autoimmune responses can benefit vitiligo patients. Stress proteins are suited to serve as the ‘molecular funnel’ channeling environmental stress into an autoimmune response targeting melanocytes these events. Initial studies supporting this hypothesis involved studying differential expression of heat shock proteins among non-lesional and lesional vitiligo skin samples (76). Subsequently, it was shown that heat shock proteins induced DCs to assume a cytotoxic profile, attacking cells expressing TGF receptor family molecules, including stressed melanocytes (99). Heat shock protein overexpressing melanocytes can support immune response to differentiation antigens (63). Overexpression of HSP70i likewise led to accelerated and progressive vitiligo (48). As other stress proteins can activate DCs, these may likewise supports vitiligo development. Thus vitiligo-prone mice expressing a transgenic T cell receptor were gene gun vaccinated with HSP70i-encoding DNA to show that this stress protein was sufficient to cause disease, whereas it proved impossible to ignite vitiligo in models knockout for HSP70i (50). Thus HSP70i is necessary and sufficient to precipitate disease. This prompted the identification of a molecular region responsible for activating DCs, with the objective of blocking these events and potentially interfering with vitiligo development. Aligning the human HSP70i molecule with a microbial peptide mediating inflammation after microbial infection, a homologous region was selected for site directed mutagenesis and mutant molecules were introduced into vitiligo mouse models. A mutation was selected based on its location, likely to interfere with DC activation without affecting activation or substrate binding (100). Interestingly, mutant HSP70iQ435A -encoding DNA was able to reverse the inflammatory phenotype of DCs, prevent infiltration of melanocyte reactive T cells to the skin and avert depigmentation (100). The general strategy is outlined in Fig. 1.
Fig. 1. Translating stress to the skin into an autoimmune response to melanocytes.
Under [1], melanocytes are exposed to stressors that [2] compromise their physiology and lead to generation of ROS. [3] Melanocytes secrete/release HSP70 (in part chaperoning melanocyte-specific antigens) which [4] activates DC that migrate to skin-draining lymph nodes to [5] recruit CD4 and, particularly, CD8 T cells which [6] kill remaining melanocytes by the perforin/granzyme pathway. We propose to [7] apply mutant HSP70iQ435A to block HSP70 from activating and perpetuating autoimmunity and associated depigmentation, allowing [8] melanocyte stem cells to differentiate and migrate to depigmented areas of the skin during repigmentation.
This leaves several important and interesting questions to be addressed. Can human patients be treated with a DNA vaccine introduced to the skin? Will it affect the development of other autoimmune diseases, or cancer? The responses HSP70i will ignite are directed towards the antigens chaperoned by the stress protein. Thus, muted responses may likewise depend on the peptides bound to the (mutant) stress protein, and may be less dependent on the stress protein (if any) engaged in disease precipitation. When stimulating anti-tumor responses or interfering with responses to self, such substrate specificity or the source of the heat shock protein can help selectively support therapeutic effects. Another selective approach makes use of the exclusive surface expression of HSP70 family members by tumors. This renders the extracellular portion amenable to targeting by antibodies, and antibodies to HSP70 can be therapeutic in cancer (29).
Taken together, targeting HSP70i is a promising approach towards the treatment of vitiligo. Mutant HSP70iQ435A both prevented and reversed the depigmentation process in different mouse models prone to vitiligo development. Future studies will show how best to translate findings to a clinical trial to determine the safety and efficacy of mutant HSP70 treatment in vitiligo patients.
Supplementary Material
Acknowledgments
All authors assisted in drafting and critical review of the manuscript. JME has contributed the figure. This study is supported by NIH RO1 AR54749 to CLP
References
- 1.Krüger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol. 2012;51:1206–1212. doi: 10.1111/j.1365-4632.2011.05377.x. [DOI] [PubMed] [Google Scholar]
- 2.Le Poole IC, van den Wijngaard RMJGJ, Westerhof W, Dutrieux RP, Das PK. Presence or absence of melanocytes in vitiligo lesions: An immunohistochemical investigation. J Invest Dermatol. 1993;100:816–822. doi: 10.1111/1523-1747.ep12476645. [DOI] [PubMed] [Google Scholar]
- 3.Jin Y, Birlea SA, Fain PR, Gowan K, Riccardi SL, Holland PJ, Mailloux CM, Sufit AJ, Hutton SM, Amadi-Myers A, Bennett DC, Wallace MR, McCormack WT, Kemp EH, Gawkrodger DJ, Weetman AP, Picardo M, Leone G, Taïeb A, Jouary T, Ezzedine K, van Geel N, Lambert J, Overbeck A, Spritz RA. Variant of TYR and autoimmunity susceptibility loci in generalized vitiligo. N Engl J Med. 2010;362:1686–1697. doi: 10.1056/NEJMoa0908547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Poole IC, van den Wijngaard RMJGJ, Westerhof W, Das PK. Presence of T cells and macrophages in inflammatory vitiligo skin parallels melanocyte disappearance. Am J Pathol. 1996;148:1219–1228. [PMC free article] [PubMed] [Google Scholar]
- 5.Ogg GS, Rod Dunbar P, Romero P, Chen JL, Cerundolo V. High frequency of skin-homing melanocyte-specific cytotoxic T lymphocytes in autoimmune vitiligo. J Exp Med. 1998;188:1203–1208. doi: 10.1084/jem.188.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wankowicz-Kalinska A, Le Poole C, van den Wijngaard R, Storkus WJ, Das PK. Melanocyte- specific immune response in melanoma and vitiligo: two faces of the same coin? Pigment Cell Res. 2003;16:254–260. doi: 10.1034/j.1600-0749.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 7.van den Boorn JG, Konijnenberg D, Dellemijn TA, van der Veen JP, Bos JD, Melief CJ, Vyth- Dreese FA, Luiten RM. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Invest Dermatol. 2009;129:2220–2232. doi: 10.1038/jid.2009.32. [DOI] [PubMed] [Google Scholar]
- 8.Kemp EH, Emhemad S, Akhtar S, Watson PF, Gawkrodger DJ, Weetman AP. Autoantibodies against tyrosine hydroxylase in patients with non-segmental (generalised) vitiligo. Exp Dermatol. 2011;20:35–40. doi: 10.1111/j.1600-0625.2010.01181.x. [DOI] [PubMed] [Google Scholar]
- 9.Boissy RE, Liu YY, Medrano EE, Nordlund JJ. Structural aberration of the rough endoplasmic reticulum and melanosome compartmentalization in long-term cultures of melanocytes from vitiligo patients. J Invest Dermatol. 1991;97:395–404. doi: 10.1111/1523-1747.ep12480976. [DOI] [PubMed] [Google Scholar]
- 10.Le Poole IC, Yang F, Brown TL, Cornelius J, Babcock GF, Das PK, Boissy RE. Altered Gene Expression in Melanocytes Exposed to 4-Tertiary Butyl Phenol (4-TBP): Upregulation of the A2b Adenosine Receptor. A2bR J Invest Dermatol. 1999;113:725–731. doi: 10.1046/j.1523-1747.1999.00756.x. [DOI] [PubMed] [Google Scholar]
- 11.Cedercreutz K, Denman CJ, Klarquist J, Vaitla R, Boissy RE, Westerhof W, Hernandez C, Le Poole IC. Vitiligo etiology and treatment: parameters derived from a patient survey. J Dermatol Nurses Assoc. 2010;2:265–272. [Google Scholar]
- 12.van Geel N, Speeckaert R, de Wolf J, Bracke S, Chevolet I, Brochez, Lambert J. Clinical significance of Koebner phenomenon in vitiligo. Br J Dermatol. 2012;167:1017–1024. doi: 10.1111/j.1365-2133.2012.11158.x. [DOI] [PubMed] [Google Scholar]
- 13.Moretti S, Arunachalam M, Colucci R, Pallanti S, Kline JA, Berti S, Lotti F, Lotti T. Autoimmune markers in vitiligo patients appear correlated with obsession and phobia. J Eur Acad Dermatol Venereol. 2012;26:861. doi: 10.1111/j.1468-3083.2011.04171.x. [DOI] [PubMed] [Google Scholar]
- 14.Boissy RE, Manga P. On the etiology of contact/occupational vitiligo. Pigment Cell Res. 2004;17:208–214. doi: 10.1111/j.1600-0749.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- 15.Laddha NC, Dwivedi M, Mansuri MS, Gani AR, Ansarullah M, Ramachandran AV, Dalai S, Begum R. Vitiligo: interplay between oxidative stress and immune system. Exp Dermatol. 2013;22:245–250. doi: 10.1111/exd.12103. [DOI] [PubMed] [Google Scholar]
- 16.Schallreuter KU, Krueger C, Wurfel BA, Panske A, Wood JM. From basic research to the bedside: efficacy of topical treatment with pseudolcatalase PC-KUS in 71 children with vitiligo. Int J Dermatol. 2008;47:743–753. doi: 10.1111/j.1365-4632.2008.03660.x. [DOI] [PubMed] [Google Scholar]
- 17.Bakis-Petsoglou S, Le Guay JL, Wittal R. A randomized, double-blinded, placebo-controlled trial of pseudocatalase cream and narrowband ultraviolet B in the treatment of vitiligo. Br J Dermatol. 2009;161:910–917. doi: 10.1111/j.1365-2133.2009.09252.x. [DOI] [PubMed] [Google Scholar]
- 18.Toosi S, Orlow SJ, Manga P. Vitiligo-inducing phenols activate the unfolded protein response in melanocytes resulting in upregulation of IL6 and IL8. J Invest Dermatol. 2012;132:2601–2609. doi: 10.1038/jid.2012.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das PK, van den Wijngaard RMJGJ, Wankowicz-Kaoinska A, Le Poole IC. A symbiotic concept of autoimmunity and tumor immunity: lessons from vitiligo. Trends Immunol. 2001;22:130–136. doi: 10.1016/s1471-4906(00)01844-5. [DOI] [PubMed] [Google Scholar]
- 20.Raposo G, Marks MS. Melanosomes—dark organelles enlighten endosomal transport. Nat Rev Mol Cell Biol. 2007;8:786–797. doi: 10.1038/nrm2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S, Bartido S, Yang G, Qin J, Moroi Y, Panageas KS, Lewis JJ, Houghton AN. A role for a melanosome transport signal in accessing the MHC class II presentation pathway in eliciting CD4+ T cell responses. J Imunol. 1999;163:5820–5826. [PubMed] [Google Scholar]
- 22.Robila V, Ostankovitch M, Altrich-Vanlith ML, Theos AC, Drover S, Marks MS, Restifo N, Engelhard VH. MHC class II presentation of gp100 epitopes in melanoma cells requires the function of conventional endosomes and is influenced by melanosomes. J Immunol. 2008;181:7843–7852. doi: 10.4049/jimmunol.181.11.7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quaglino P, Marenco F, Osella-Abate S, Capello N, Ortoncelli M, Salomone B, Fierro MT, Savoia P, Bernengo MG. Vitiligo is an independent favourable prognositic factor in stage III and IV metastatic melanoma patients: results form a single-institution hospital-based observational study. Ann Oncol. 2010;21:409–414. doi: 10.1093/annonc/mdp325. [DOI] [PubMed] [Google Scholar]
- 24.Turk MJ, Guevara-Patiño JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–782. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klarquist J, Denman CJ, Hernandez C, Wainwright DA, Strickland FM, Overbeck A, Mehrotra S, Nishimura MI, Le Poole IC. Reduced skin homing by functional Treg in vitiligo. Pigment Cell Melanoma Res. 2010;23:276–286. doi: 10.1111/j.1755-148X.2010.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byrne KT, Cote AL, Zhang P, Stainberg SM, Guo Y, Allie R, Zhang W, Ernstoff MS, Usherwood EJ, Turk MJ. Autoimmune melanocyte desctruction is required for roust CD8+ memory T cell responses to mouse melanoma. J Clin Invest. 2011;121:1797–1809. doi: 10.1172/JCI44849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qu P Ma, Zhang JH, Huang XM, Yang XJ, Yan-Fang XWS. A novel DNA vaccine constructed by heat shock protein 70 and melanoma antigen-encoding gene 3 against tumorigenesis. Indian J Exp Biol. 2010;48:436–443. [PubMed] [Google Scholar]
- 28.Faure O, Graff-Dubois S, Alves PM, Cornet S, Duffour MT, Scardino A, Gross DA, Miconnet I, Saldeco M, Chouaib S, Lemonnier FA, Abastado JP, Kosmatopoulos K. Induction of multiple CD8+ T cell responses against the inducible Hsp70 employing an HSP70 oligoepitope peptide. Oncol Rep. 2007;17:679–685. [PubMed] [Google Scholar]
- 29.Stangl S, Gahrmann M, Riegger J, Kuhs K, Riederer I, Sievert W, Hube K, Mocikat R, Dressel R, Kremmer E, Pockley AG, Friedrich L, Vigh L, Skerra A, Multhoff G. Targeting membrane heat-shock protein 70 (Hsp70) on tumors by cmHsp70.1 antibody. Proc Natl Acad Sci. 2011;108:733–738. doi: 10.1073/pnas.1016065108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welch WJ. Heat shock proteins functioning as molecular chaperones: their roles in normal and stressed cells. Philos Trans R Soc Lond B Biol Sci. 1993;339:327–333. doi: 10.1098/rstb.1993.0031. [DOI] [PubMed] [Google Scholar]
- 31.Määttänen P, Gehring K, Bergeron JJ, Thomas DY. Protein quality control in the ER: the recognition of misfolded proteins. Semin Cell Dev Biol. 2012;21:500–11. doi: 10.1016/j.semcdb.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Gardner BM, Pincus D, Gotthardt K, Gsllagher CM, Walter P. Endoplasmatic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perpect Biol. 2013 doi: 10.1101/cshperspect.a013169. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Poole IC, Sarangarajan R, Zhao Y, Stennett LS, Brown TL, Sheth P, Miki T, Boissy RE. ‘VIT1’, a novel gene associated with vitiligo. Pigment Cell Res. 2001;14:475–484. doi: 10.1034/j.1600-0749.2001.140608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guan C, Lin F, Zhou M, Hong W, Fu L, Xu A. The role of VIT1/FBXO11 in the regulation of apoptosis and tyrosinase export from endoplasmic reticulum in cultured melanocytes. Int J Mol Med. 2010;26:57–65. doi: 10.3892/ijmm_00000435. [DOI] [PubMed] [Google Scholar]
- 35.Benbrook DM, Long A. Integration of autophagy, proteasomal degradation, unfolded protein response and apoptosis. Exp Oncol. 2012;34:286–297. [PubMed] [Google Scholar]
- 36.Elassiuty YE, Klarquist J, Speiser J, Yousef RM, El Refaee AA, Hunter NS, Shaker OG, Gundeti M, Nieuweboer-Krobotova L, Le Poole IC. Heme oxygenase-1 expression protects melanocytes from stress-induced cell death: implications for vitiligo. Exp Dermatol. 2011;20:496–501. doi: 10.1111/j.1600-0625.2010.01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calderwood SK, Stevenson MA, Murshid A. Heat shock proteins, autoimmunity, and cancer treatment. Autoimmune Dis. 2012;2012:486069. doi: 10.1155/2012/486069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vega VL, Rodríguez-Silva M, Frey T, Gehrmann M, Diaz JC, Steinem C, Multhoff G, Arispe N, De Maio A. Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J Immunol. 2008;180:4299–4307. doi: 10.4049/jimmunol.180.6.4299. [DOI] [PubMed] [Google Scholar]
- 39.Strbo N, Podack ER. Secreted heat shock protein 96-Ig: an innovative vaccine approach. Am J Reprod Immunol. 2008;59:407–416. doi: 10.1111/j.1600-0897.2008.00594.x. [DOI] [PubMed] [Google Scholar]
- 40.Chi A, Valencia JC, Hu ZZ, Watabe H, Yamaguchi H, Mangini NJ, Huang H, Canfield VA, Cheng KC, Yang F, Abe R, Yamagishi S, Shabanowitz J, Hearing VJ, Wu C, Appella E, Hunt DF. Proteomic and bioinformatic characterization of the biogenesis and function of melanosomes. J Proteome Res. 2006;5:3135–3144. doi: 10.1021/pr060363j. [DOI] [PubMed] [Google Scholar]
- 41.Mambula SS, Calderwood SK. Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J Immunol. 2006;177:7849–7857. doi: 10.4049/jimmunol.177.11.7849. [DOI] [PubMed] [Google Scholar]
- 42.Johnson JD, Fleshner M. Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukoc Biol. 2006;79:425–434. doi: 10.1189/jlb.0905523. [DOI] [PubMed] [Google Scholar]
- 43.Nicchitta CV. Re-evaluating the role of heat-shock protein-peptide interactions in tumour immunity. Nat Rev Immunol. 2003;3:427–432. doi: 10.1038/nri1089. [DOI] [PubMed] [Google Scholar]
- 44.Multhoff G. Hyperthermia classic commentary: Activation of natural killer cells by heat shock protein 70: Gabriele Multhoff, International Journal of Hyperthermia, 2002: 18: 576–585. Int J Hyperthermia. 2009;25:176–179. doi: 10.1080/02656730902835672. [DOI] [PubMed] [Google Scholar]
- 45.Mortaz E, Redegeld FA, Dunsmore K, Odoms K, Wong HR, Nijkamp FP, Engels F. Stimulation of cysteinyl leukotreine production in mast cells by heat shock and acetylsalicylic acid. Eur J Paracol. 2007;561:214–219. doi: 10.1016/j.ejphar.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 46.Kammerer R, Stober D, Riedl P, Oehninger C, Schirmbeck R, Reimann J. Noncovalent association with stress protein facilitates cross-priming of CD8+ T cells to tumor cell antigens by dendritic cells. J Immunol. 2002;168:108– 117. doi: 10.4049/jimmunol.168.1.108. [DOI] [PubMed] [Google Scholar]
- 47.Millar DG, Garza KM, Odermatt B, Elford AR, Ono N, Li Z, Ohashi PS. HSP70 promotes antigen-presenting cell function and converts T-cell tolerance to autoimmunity in vivo. Nat Med. 2003;9:1469–1476. doi: 10.1038/nm962. [DOI] [PubMed] [Google Scholar]
- 48.Denman CJ, McCracken J, Hariaharan V, Klarquist J, Oyarbide-Valencia K, Guevara-Patino JA, Le Poole IC. HSP70i accelerates depigmentation in a mouse model of autoimmune vitiligo. J Invest Dermatol. 2008;128:2041–2048. doi: 10.1038/jid.2008.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wachstein J, Tischer S, Figueiredo C, Limbourg A, Falk C, Immenshuh S, Blascyk R, Eiz-Vesper B. HSP70 enhances immunosuppressive function of CD4(+)CD25(+)FoxP3(+) T regulatory cells and cytotoxicity in CD4(+) CD25(−) T cells. PLoS One. 2012;7:51747. doi: 10.1371/journal.pone.0051747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mosenson JA, Zloza A, Klarquist J, Barfuss AJ, Guevara-Patino JA, Le Poole IC. HSP70i is a critical component of the immune response leading to vitiligo. Pigment Cell Melanoma Res. 2012;25:88–98. doi: 10.1111/j.1755-148X.2011.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wheeler DS, Dunsmore KE, Denenberg AG, Muething L, Poynter SE, Wong HR. Biological activity of truncated C-terminus human heat shock protein 72. Immunol Lett. 2011;135:173–179. doi: 10.1016/j.imlet.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spiering R, van der Zee R, Wagenaar J, canEden W, Broere F. Mycobacterial and mouse HSP70 have immune-modulatory effects on dendritic cells. Cell Stess Chaperones. 2012 doi: 10.1007/s12192-012-0397-4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang H, Huang W. Fusion proteins of Hsp70 with tumor-associated antigen acting as a potent tumor vaccine and the C-terminal peptide-binding domain of Hsp70 being essential in inducing antigen-independent antitumor response in vivo. Cell Stress Chaperones. 2006;11:216–226. doi: 10.1379/CSC-191R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Motta A, Schmitz C, Rodriguez L, Ribeiro F, Teixeira C, Detanico T, Bonan C, Zwickey H, Bonorino C. Mycobacterium tuberculosis heat-shock protein 70 impairs maturation of dendritic cells from bone marrow precursors, induces interleukin-10 production and inhibits T-cell proliferation in vitro. Immunology. 2007;121:462–472. doi: 10.1111/j.1365-2567.2007.02564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borges TJ, Wieten L, van Herwijnen MJ, Broere F, van der Zee R, Bonorino C, van Eden W. The anti-inflammatory mechanisms of HSP70. Front Immunol. 2012;3:95. doi: 10.3389/fimmu.2012.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, Whittal T, McGowan E, Younson J, Kelly C, Bergmeier LA, Singh M, Lehner T. Identification of stimulating and inhibitory epitopes within the heat shock protein 70 molecule that modulate cytokine production and maturation of dendritic cells. J Immunol. 2005;174:3306–3316. doi: 10.4049/jimmunol.174.6.3306. [DOI] [PubMed] [Google Scholar]
- 57.Th riault JR, Adachi H, Calderwood SK. Role of scavenger receptors in the binding and internalization of heat shock protein 70. J Immunol. 2006;177:8604–8611. doi: 10.4049/jimmunol.177.12.8604. [DOI] [PubMed] [Google Scholar]
- 58.Aneja R, Odoms K, Dunsmore K, Shanley TP, Wog HR. Extracellular heat shock protein-70 induces endotoxin tolerance in THP-1 cells. J Immunol. 2006;177:7184–7192. doi: 10.4049/jimmunol.177.10.7184. [DOI] [PubMed] [Google Scholar]
- 59.Floto RA, MacAry PA, Boname JM, Mien TS, Kampmann B, Hair JR, Huey OS, Houben EN, Pieters J, Day C, Oehlmann W, Singh M, Smith KG, Lehner PJ. Dendritic cell stimulation by mycobacterial Hsp70 is mediated through CCR5. Science. 2006;314:454–458. doi: 10.1126/science.1133515. [DOI] [PubMed] [Google Scholar]
- 60.Qazi KR, Oehlmann W, Singh M, Lopez MC, Fernandez C. Microbial heat shock protein 70 stimulatory properties have different TLR requirements. Vaccine. 2007;25:1096–1103. doi: 10.1016/j.vaccine.2006.09.058. [DOI] [PubMed] [Google Scholar]
- 61.Fischer N, Haug M, Kwok WW, Kalbacher H, Wernet D, Dannecker GE, Holzer U. Involvement of CD91 and scavenger receptors in Hsp70–facilitated activation of human antigen-specific CD4+ memory T cells. Eur J Immunol. 2010;40:986–997. doi: 10.1002/eji.200939738. [DOI] [PubMed] [Google Scholar]
- 62.Frediani T, Lucarelli S, Frediani S, Frati C. The role of heat shock proteins in vitiligo: deviation of cytotoxic response. J Dermatol Sci. 2005;37:114–117. doi: 10.1016/j.jdermsci.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 63.Sanchez-Perez L, Kottke T, Daniels GA, Diaz RM, Thompson J, Pulido J, Melcher A, Vile RG. Killing of normal melanocytes, combined with heat shock protein 70 and CD40L expression, cures large established melanomas. J Immunol. 2006;177:4168–4177. doi: 10.4049/jimmunol.177.6.4168. [DOI] [PubMed] [Google Scholar]
- 64.Fletchner JB, Cohane KP, Mehta S, Slusarewicz P, Leonard AK, Barber BH, Levey DL, Andjelic S. High affinity interactions between peptides and heat shock protein 70 augment CD8+ T-cell lymphocyte immune responses. J Immunol. 2006;177:1017–1027. doi: 10.4049/jimmunol.177.2.1017. [DOI] [PubMed] [Google Scholar]
- 65.Abkin SV, Pankratova KM, Komarova EY, Guzhova IV, Margulis BA. Hsp70 chaperone-based gel composition as a novel immunotherapeutic anti-tumor tool. Cell Stress Chaperones. 2012 doi: 10.1007/s12192-012-0391-x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Terlecky SR. Hsp70s and lysomal proteolysis. Experientia. 1994;50:1021–1025. doi: 10.1007/BF01923456. [DOI] [PubMed] [Google Scholar]
- 67.Agarraberas FA, Dice JF. A molecular chaperone complex at the lysosomal membrane is required for protein translation. J Cell Sci. 2001;114:2491–2499. doi: 10.1242/jcs.114.13.2491. [DOI] [PubMed] [Google Scholar]
- 68.Agarraberas FA, Terlecky SR, Dice JF. An intralysosomal hsp70 is required for a selective pathway of lysosomal protein degradation. J Cell Biol. 1997;137:825–834. doi: 10.1083/jcb.137.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Auger I, Roudier J. Interaction between HSP73 and HLA-DRB1*0401: implications for the development of rheumathoid arthiritis. Immunol Res. 2005;31:261–266. doi: 10.1385/IR:31:3:261. [DOI] [PubMed] [Google Scholar]
- 70.Nylandsted J, Gyrd-Hansen M, Danielewicz A, Fehrenbacher N, Lademann U, Hoyer Hansen M, Weber E, Multhoff G, Rohde M, Jaatela M. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J Exp Med. 2004;200:425–435. doi: 10.1084/jem.20040531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamashima T. Hsp701 and related lysosomal factors for necrotic neuronal death. J Neurochem. 2012;120:477–494. doi: 10.1111/j.1471-4159.2011.07596.x. [DOI] [PubMed] [Google Scholar]
- 72.van den Boorn JG, Picavet DI, van Swieten PF, van Veen HA, Konijnenberg D, van Veelen PA, van Capel T, de Jong EC, Reits EA, Drijfhout JW, Bos JD, Meleif CJ, Luiten RM. Skin-depigmenting agent monobenzome induces potent T-cell autoimmunity towards pigmented cells by tyrosinase haptenation and melanosomal autophagy. J Invest Dermatol. 2001;131:1240–1251. doi: 10.1038/jid.2011.16. [DOI] [PubMed] [Google Scholar]
- 73.Shin Y, Klucken J, Patterson C, Hyman BT, McLean PJ. The co-chaperone carboxylterminus of HSP70-interacting protein (CHIP) mediates alpha-synuclein degradation decisions between proteasomal and lysomal pathways. J Biol Chem. 2005;280:23727–23734. doi: 10.1074/jbc.M503326200. [DOI] [PubMed] [Google Scholar]
- 74.Zhou D, Li Y, Lott JM, Hinslop AD, Canaday DH, Brutkiewicz RR, Blum JS. Lamp-2a facilitates MHC class II presentation of cytoplasmic antigens. Immunity. 2005;22:571–581. doi: 10.1016/j.immuni.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 75.Nedelkovska H, Robert J. Hsp72 mediates stronger antgen-dependent non-classical MHC class Ib anti-tumor reponses than Hsc73 in Xenopus laevis. Cancer Immun. 2013;13:4. [PMC free article] [PubMed] [Google Scholar]
- 76.Le Poole IC, Luiten RM. Autoimmune etiology of generalized vitiligo. Curr Dir Autoimmun. 2008;10:227–243. doi: 10.1159/000131485. [DOI] [PubMed] [Google Scholar]
- 77.Damico FM, Bezerra FT, Silva GC, Gasparin F, Yamamoto JH. New insights into Vogt-Koyanagi-Harada disease. Arq Bras Ortalmol. 2009;72:413–420. doi: 10.1590/s0004-27492009000300028. [DOI] [PubMed] [Google Scholar]
- 78.Marks MS, Theos AC, Raposo G. Melanosomes and MHC class II antigen-processing compartments: a tinted view of intracellular trafficking and immunity. Immunol Res. 2003;27:409–426. doi: 10.1385/IR:27:2-3:409. [DOI] [PubMed] [Google Scholar]
- 79.Chang HC, Newmyer SL, Hull MJ, Ebersold M, Schmid SL, Mellman I. HSC70 is required for endocytosis and clathrin function in Drosophila. J Cell Biol. 2002;159:477–487. doi: 10.1083/jcb.200205086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoshino T, Matsuda M, Yamashita Y, Takehara M, Fukuya M, Mineda K, Maji D, Ihn H, Adachi H, Sobue G, Funasaka Y, Mizushima T. Suppression of melanin production by expression of HSP70. J Biol Chem. 2010;285:13254–13263. doi: 10.1074/jbc.M110.103051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brocchieri L, Conway de Macario E, Macario AJ. Hsp70 genes in the human genome: Conservation and differentiation patterns predict a wide array of overlapping and specialized functions. BMC Evol Biol. 2008;8:19. doi: 10.1186/1471-2148-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tavaria M, Gabriele T, Kola I, Anderson RL. A hitchhiker’s guide to the human Hsp70 family. Cell Stress Chap. 1996;2:23–28. doi: 10.1379/1466-1268(1996)001<0023:ahsgtt>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lehner T, Wang Y, Whittal T, McGowan E, Kelly CG, Singh M. Functional domains of HSP70 stimulate generation of cytokines and chemokines, maturation of dendritic cells and adjuvanticity. Biochem Soc Trans. 2004;32:629–632. doi: 10.1042/BST0320629. [DOI] [PubMed] [Google Scholar]
- 84.Kriebaumer V, von Loffelholz O, Abell BM. Chaperone receptors: guiding proteins to intracellular compartments. Protoplasma. 2012;249:21–30. doi: 10.1007/s00709-011-0270-9. [DOI] [PubMed] [Google Scholar]
- 85.Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 86.Kabani M, Martineau CN. Multiple hsp70 isoforms in the eukaryotic cytosol: mere redundancy or functional specificity? Curr Genomics. 2008;9:338–248. doi: 10.2174/138920208785133280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. CMLS Cell Mol Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol. 2010;11:579–592. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Daugaard M, Rohde M, Jäättelä M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–3710. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 90.Bausero MA, Gastpar R, Multhoff G, Asea A. Alternative mechanism by which IFN-gamma enhances tumor recognition: active release of heat shock protein 72. J Immunol. 2005;175:2900–2912. doi: 10.4049/jimmunol.175.5.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, Turka LA. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-gamma for autoreactive CD8+ T-cell accumulation in the skin. J Invest Dermatol. 2012;132:1869–1876. doi: 10.1038/jid.2011.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Petitjean C, Moreira D, Lopez-Garcia P, Brochier-Armanet C. Horizontal gene transfer of a chloroplast-derived Dna-J-Fer protein to Thaumarcharchaeota and the evolutionary history of the DnaK chaperone system in Archea. BMC Evol Biol. 2012;12:226. doi: 10.1186/1471-2148-12-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Farzanehpour M, Soleimanjahi H, Hassan ZM, Amanzadeh A, Ghaemi A, Fazeli M. HSP70 modified response against HPV based tumor. Eur Rev Med Pharmaol Sci. 2013;17:228–234. [PubMed] [Google Scholar]
- 94.Choi DH, Woo JK, Choi Y, Seo HS, Kim CW. A novel chimeric DNA vaccine: enhancement of preventive and therapeutic efficacy of DNA vaccine by fusion of mucin 1 to a heat shock protein 70 gene. Mol Med Rep. 2011;4:885–890. doi: 10.3892/mmr.2011.525. [DOI] [PubMed] [Google Scholar]
- 95.Liu G, Yao K, Wang B, Chen Y, Zhou F, Guo Y, Xu J, Shi H. Immunotherapy of Eppstein-Barr virus associates malignancies using mycobacterial HSP70 and LMP2A356–364 epitope fusion protein. Cell Mol Immunol. 2009;6:423–431. doi: 10.1038/cmi.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.MacAry PA, Javid B, Floto RA, Smith KGC, Oehlmann W, Singh M, Lehner PJ. HSP70 peptide binding mutants separate antigen delivery from dendritic cell stimulation. Immunity. 2004;20:95–106. doi: 10.1016/s1074-7613(03)00357-1. [DOI] [PubMed] [Google Scholar]
- 97.Teulings HE, Overkamp M, Ceylan E, Nieuweboer-Krobotova L, Bos JD, Nijsten T, Wolkerstorfer AW, Luiten RM, van der Veen JP. Decreased risk of melanoma and nonmelanoma skin cancer in patients with vitiligo: a survey among 1307 patients and their partners. Br J Dermatol. 2013;168:162–171. doi: 10.1111/bjd.12111. [DOI] [PubMed] [Google Scholar]
- 98.Srivastava PK, Udono H. Heat shock protein-peptide complexes in cancer immunotherapy. Curr Opin Immunol. 1994;6:728–732. doi: 10.1016/0952-7915(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 99.Kroll TM, Bommiasamy H, Boissy RE, Hernandez C, Nickoloff BJ, Mestril R, Le Poole IC. 4-tertiary butyl phenol exposure sensitize human melanocytes to dendritic cell mediated killing: relevance to vitiligo. J Invest Dermatol. 2005;124:79–806. doi: 10.1111/j.0022-202X.2005.23653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mosenson JA, Zloza A, Nieland JD, Garrett-Mayer E, Eby JM, Huelsmann EJ, Kumar P, Denman CJ, Lacek AT, Kohlhapp FJ, Alamiri A, Hughes T, Bines SD, Kaufman HL, Overbeck A, Mehrotra S, Hernandez C, Nishimura MI, Guevara-Patino JA, Le Poole IC. Mutant HSP70 reverses autoimmune depigmentation in vitiligo. Sci Transl Med. 2013;5:174ra28. doi: 10.1126/scitranslmed.3005127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.