Abstract
Hyptis pectinata, popularly known in Brazil as “sambacaitá” or “canudinho,” is an aromatic shrub largely grown in the northeast of Brazil. The leaves and bark are used in an infusion for the treatment of throat and skin inflammations, bacterial infections, pain, and cancer. Analogues of rosmarinic acid and flavonoids were obtained from the leaves of Hyptis pectinata and consisted of two new compounds, sambacaitaric acid (1) and 3-O-methyl-sambacaitaric acid (2), and nine known compounds, rosmarinic acid (3), 3-O-methyl-rosmarinic acid (4), ethyl caffeate (5), nepetoidin A (6), nepetoidin B (7), cirsiliol (8), circimaritin (9), 7-O-methylluteolin (10), and genkwanin (11). The structures of these compounds were determined by spectroscopic methods. Compounds 1–5, and 7 were evaluated in vitro against the promastigote form of L. braziliensis, and the ethanol extract. The hexane, ethyl acetate, and methanol-water fractions were also evaluated. The EtOH extract, the hexane extract, EtOAc, MeOH:H2O fractions; and compounds 1, 2 and 4 exhibited antileishmanial activity, and compound 1 was as potent as pentamidine. In contrast, compounds 3, 5, and 7 did not present activity against the promastigote form of L. braziliensis below 100 µM. To our knowledge, compounds 1 and 2 are being described for the first time.
1. Introduction
The Lamiaceae family is cosmopolitan and comprises 236 genera and 7173 species [1]. This group is well known for its essential oils [2], which are rich in terpenoids, especially the subfamily Nepetoideae. In South America, Hyptis is one of the main genera of this subfamily and comprises 280 species. Of these species, 146 are endemic to Brazil [3].
Hyptis pectinata (L.) Poit, subfamily Nepetoideae, which is popularly known in Brazil as “sambacaitá” or “canudinho,” is a widespread, aromatic shrub that is largely grown in the northeast of Brazil [4]; it is an herbaceous plant with aromatic leaves and small bilabial flowers that are clustered into axillary inflorescences [5]. Although there are some reports on the constituents of H. pectinata, those studies [6] mainly focused on the essential oil composition [7]. H. pectinata is particularly used in folk medicine for various conditions, such as rhinopharyngitis, nasal congestion, certain skin diseases [8], gastric disorders, fever [9], and bacterial infections [10]. The leaves and bark are used in an infusion for the treatment of throat and skin inflammations, bacterial infections, pain and cancer [11–13].
The healing effect of H. pectinata suggests that this plant may have antileishmanial action. Leishmaniasis is a major global public health problem, with three million cases annually [14]. American tegumentary leishmaniasis (ATL) is a serious zoonosis and is endemic throughout considerable areas of Latin America [15]. The main clinical forms of ATL are cutaneous leishmaniasis, mucosal or mucocutaneous leishmaniasis, and diffuse cutaneous leishmaniasis. In Brazil, ATL is found in all states and has shown a high incidence over the last 20 years; furthermore, the genetic diversity among Leishmania parasites is great. At least seven Brazilian Leishmania species have been described as the etiological agent of human cutaneous disease, with most cases being caused by Leishmania (Viannia) braziliensis [16–18].
The drugs that are commercially used for the treatment of Leishmaniasis are highly toxic and require hospital monitoring because they may lead to death [19]. In this context, research on natural products for the treatment of leishmaniasis has been encouraged by the (World Health Organization) WHO through the Tropical Diseases Program [20].
The current work led to the isolation of two new compounds, namely sambacaitaric acid (1) and 3-O-methyl-sambacaitaric acid (2) (Figure 1), and nine known compounds from H. pectinata. 1–7 were phenylpropanoids, and 8–11 were flavonoids (Figures 2 and 3). The EtOH extract; the hexane, EtOAc, and MeOH:H2O fractions; and compounds 1–5 and 7 were evaluated in vitro against the promastigote form of L. braziliensis.
Figure 1.
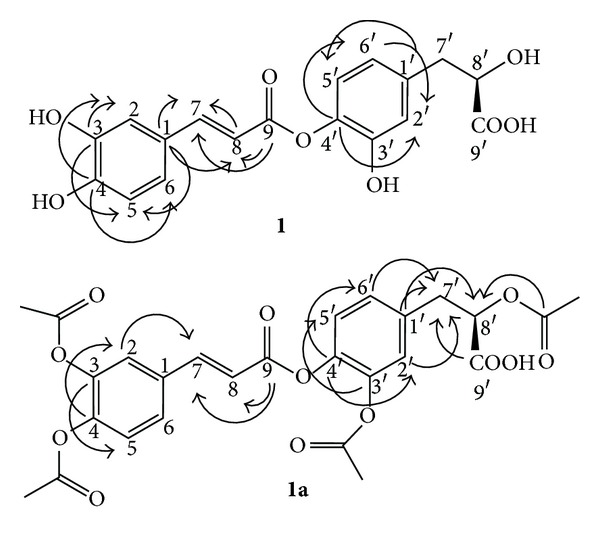
Key HMBC correlations of compounds 1 and 1a.
Figure 2.
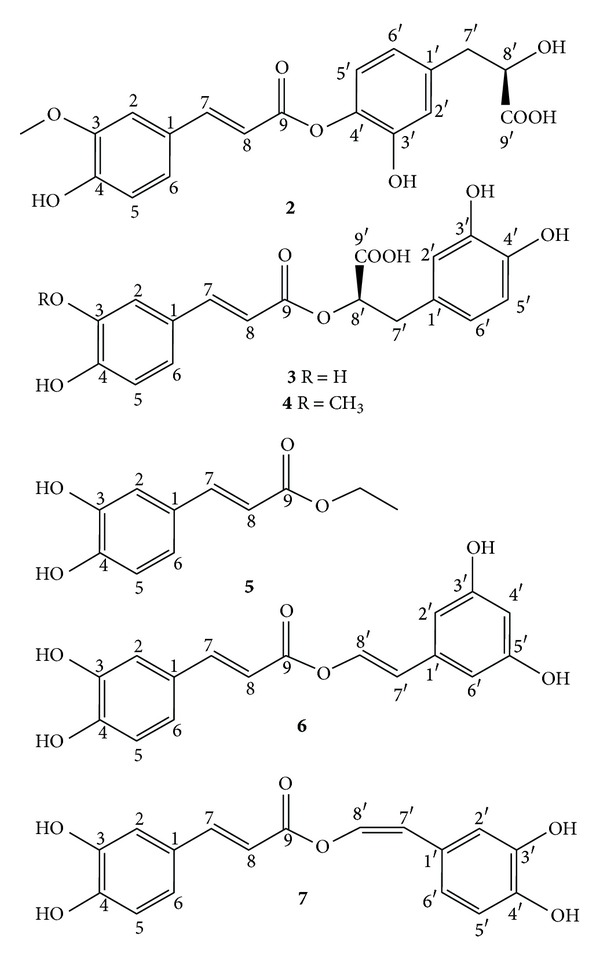
Chemical structures of compounds (2–7) isolated from H. pectinata.
Figure 3.
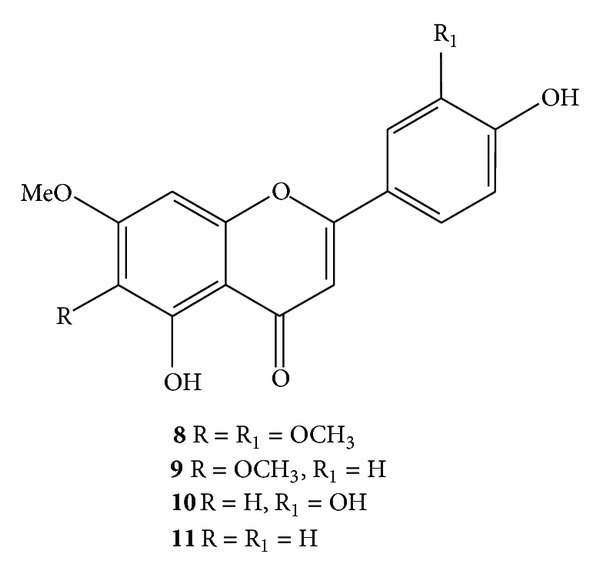
Chemical structures of compounds (8–11) isolated from H. pectinata.
2. Materials and Methods
2.1. General
The infrared absorption spectra were recorded in KBr pellets using a Varian 640 FT-IR spectrophotometer with a PIKE ATR accessory operating in the 4000–400 cm−1 range. The LC-ESI-MS was performed in negative electrospray mode using an Esquire 3000 Plus (Bruker), and the HRESIMS was conducted using a MicroTOF (Bruker). Silica gel 60 F254 (Merck) for TLC plates. Sephadex LH-20 (Sigma) was employed for gel permeation chromatography. 1H and 13C NMR spectra were obtained using a Bruker DRX 500 (500 MHz for 1H and 125 MHz for 13C) and Bruker DPX300 (300 MHz for 1H and 75 MHz for 13C) in DMSO-d 6. The CD was recorded with a Jasco J-515 CD spectrometer. The optic rotation was determined in a KRUESS OPTRONIC spectrometer. All solvents used are of commercial HPLC grade.
2.2. Plant Material
The leaves of Hyptis pectinata were collected in Garanhuns city, State of Pernambuco, Brazil, from April to July 2010. A voucher specimen is deposited at the Instituto de Pesquisa Agropecuária (IPA), Pernambuco, Brazil.
2.3. Extraction and Isolation
The plant material was successively extracted with EtOH to obtain 7.0 g of dry extract. This extract was dissolved in MeOH : H2O (1 : 1) and successively fractionated with hexane and EtOAc. A portion of the EtOAc fraction (3.5 g) was subjected to chromatography on a Sephadex LH-20 column with methanol as the mobile phase. Compounds 1 (44.2 mg), 2 (54.0 mg), 3 (97.9 mg), 4 (26.0 mg), 5 (24.9 mg), 6 (20.0 mg), 7 (28.3 mg), 8 (22.4 mg), 9 (14.0 mg), 10 (11.0 mg), and 11 (7.4 mg) were then purified by semipreparative HPLC on a Luna Phenomenex RP-18 column (21 mm × 250 mm × 5 μm) and detected at 320 nm at a flow rate of 16 mL/min using a mobile phase of H2O (A) and methanol (B) with the following pattern: from 0–10 min, 40–60% B; to 25 min, 80% B; and to 28 min, 100% B. The purity of the compounds was examined via analytical HPLC with diode array detection.
2.4. In Vitro Activity against Leishmania braziliensis
Promastigotes of L. braziliensis (MHOM/BR/87/BA125) were obtained from Dr. Valéria de Matos Borges at the Gonçalo Moniz Research Center. The parasites were maintained in vitro in Schneider's medium supplemented with 10% FBS and 2% human urine. Stock solutions of the EtOH extract; the hexane, EtOAc, and MeOH:H2O fractions; and compounds 1–5 and 7 from H. pectinata, as well as pentamidine (the reference leishmanicidal drug), were prepared in DMSO immediately before use. The cytotoxicities of the extract, fractions, and compounds against the promastigotes were determined. Stationary phase L. braziliensis promastigotes were plated in 96-well vessels (Nunc) at 1 × 105 cells per well in Schneider's medium supplemented with 10% FBS and 2% human urine. Each compound solution was added at increasing concentrations (0.001–100 μg/mL for the extract and fractions; 0.001–100 μM for the compounds). Cells were also cultured in a medium without compounds and vehicle (basal growth control) or with DMSO 0.1% (vehicle control). After 48 h, the extracellular load of L. braziliensis promastigotes was estimated by counting the promastigotes in Schneider's medium with a CELM automatic cell counter (model CC530) [21].
3. Results and Discussion
Upon extraction and fractionation, the leaves of Hyptis pectinata yielded compounds 1–11 (Figures 1–3). Compounds 1 and 2 were identified as new compounds and as rosmarinic acid analogues, based on the detailed NMR analysis described below (Table 1). Compound 1 was obtained as a yellowish, amorphous powder, and its optical rotation was [α]D = +30 (c 0.001, MeOH). Its molecular formula was deduced to be C18H16O8 by HRESIMS, which showed a molecular ion peak [M-H]+ at m/z 359.0759 (Calcd m/z for C18H15O8, 359.0761). The UV spectrum exhibited signals at 322, 296, and 239 nm, and the IR spectrum showed signals at 3435, 1628, 1524, and 1405 cm−1. The 1H and 13C NMR spectra of 1 were similar to those of rosmarinic acid.
Table 1.
1H (300 MHz) and 13C NMR (75 MHz) spectroscopic data for 1 and 1a (DMSO-d 6, δ in ppm).
| 1 | 1a | |||||||
|---|---|---|---|---|---|---|---|---|
| Position | δ C | δH | 2JCH | 3JCH | δC | δ H | 2JCH | 3 J CH |
| 9′ | 172.1 | 172.5 | H-8′ | H-7′ | ||||
| 9 | 166.2 | H-8 | H-7 | 166.3 | H-8 | H-7 | ||
| 4 | 148.4 | H-5 | H-2, H-6 | 149.4 | H-2 | |||
| 3 | 145.81 | H-2 | H-5 | 138.53 | H-2 | H-5 | ||
| 4′ | 144.9 | H-5′ | H-2′, H-6′ | 141.8 | H-2′, H-6′ | |||
| 3′ | 143.5 | 141.0 | H-2′ | H-5′ | ||||
| 1′ | 129.9 | H-5′ | 134.8 | H-7′ | H-8′ | |||
| 1 | 125.6 | H-7 | H-5, H-8 | 127.4 | H-2, H-6, H-7 | H-5, H-8 | ||
| 7 | 144.34 | 7.34 (d, 16.0) | H-6 | H-2, H-8 | 145.3 | 7.56 (d, 16.0) | ||
| 6 | 120.8 | 6.92 (dd, 8.5; 2,0) | H-2, H-7 | 126.6 | 7.24 (sl) | |||
| 6′ | 119.7 | 6.48 (dd, 8.0; 2.0) | 127.6 | 7.14 (sl) | H-7′ | |||
| 2′ | 116.6 | 6.66 (d, 2.0) | H-6′ | 124.2 | 7.16 (s) | H-2′, H-7′ | ||
| 5 | 115.9 | 6.74 (dd, 8.5; 2,0) | 117.9 | 6.32 (d, 8.0) | ||||
| 5′ | 115.4 | 6.59 (dd, 8.5; 2,0) | 117.1 | 7.11 (m) | ||||
| 2 | 114.9 | 7.03 (d, 2.0) | 123.38 | 7.11 (m) | H-7 | |||
| 8 | 114.9 | 6.18 (d, 16.0) | H-7 | 115.3 | 6.29 (d, 16.0) | |||
| 8′ | 75.9 | 4.85 (m) | 72.3 | 5.4 (m) | H-8′ | |||
| 7′ | 37.2 | 3.01 (m), 2.74 (m) | 36.6 | 3.24 (m) | ||||
| OCOCH3 | 168.3–169.1 | |||||||
| OCOCH3 | 20.7–20.9 | 2.26–2.34 (s) | ||||||
In the 1H-NMR spectrum of 1, two sets of ABX proton signals at (δ 7.03, d, J = 2.0 Hz; 6.74, d, J = 8.5 Hz; 6.92, dd, J = 8.5, 2.0 Hz) and (δ 6.66, d, J = 2.0 Hz; 6.59, d, J = 8.5 Hz; 6.48, dd, J = 8.5, 2.0 Hz) and two olefinic proton signals at δ 7.34 (d, J = 17.0 Hz) and 6.18 (d, J = 17.0 Hz) were observed. In the aliphatic region, there were three proton signals at δ 4.85 (m), 3.01 (m), and 2.74 (m). The 13C-NMR spectrum showed 18 carbon signals. In the heteronuclear multiple bond correlation (HMBC) spectrum, the H-7 proton signal (δ 7.34) was long range coupled with aromatic carbons at δ 125.58 (C-1), 114.23 (C-2), and 119.4 (C-6), an olefinic carbon at δ 114.90 (C-8) and the carbonyl carbon at δ 166.22 (C-9) (Figure 1). The absolute configuration of 1 was determined by CD spectroscopy. Because the chiral center and its immediate environment are identical to those of rosmarinic acid 3 (Figure 4), one would expect a similar CD spectrum if the configuration around C-8′ in 3 was the same as in 1. On the basis of these observations, the structure of compound (1) was established to be isoferuloyl-4′-(3′-hydroxyphenyl)-(8′R)-lactic acid, and the compound was named sambacaitaric acid.
Figure 4.
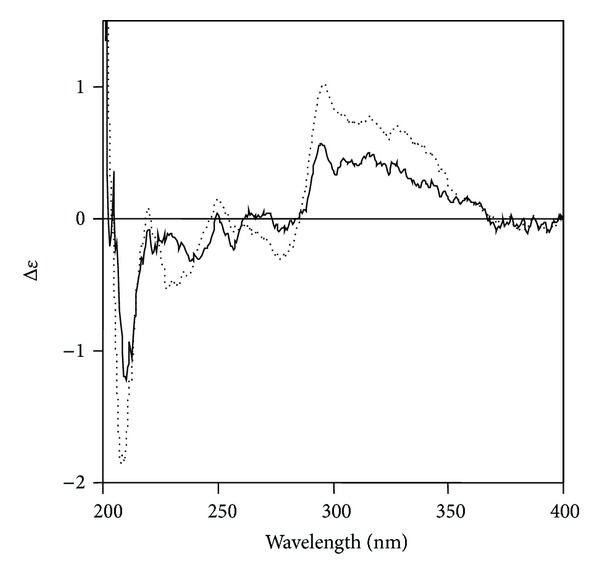
CD spectra of 1 (dotted line) and 3 (solid line).
The sambacaitaric acid (1) was treated with Ac2O/pyridine to yield the peracetyl derivative (1a). The 1H and 13C NMR spectral data of 1a, obtained through the analysis of extensive 1D and 2D NMR experiments (Figure 1 and Table 1), was also used to confirm the postulated structure of 1. Electrospray ionization mass spectroscopy (ESI-MS) of 1a showed the [M-H]+ at m/z 527, corresponding to the molecular formula C26H24O12.
Compound 2 was obtained as a yellowish, amorphous powder and showed positive optical rotation, [α]D = +10 (c 0.1, MeOH). Its molecular formula was deduced to be C19H18O8, which produced the [M-H]+ peak at m/z 373. The UV spectrum exhibited signal at 340 nm and the IR spectrum showed signals at 3420, 1680, and 1607 cm−1. The 1H and 13C NMR spectra were similar to those of 1 with the addition of a methoxyl group on the 3 position. Thus, the structure of this new compound was established as 3-O-methyl-sambacaitaric acid.
The known compounds were identified from the spectroscopic data (UV, IR, ESIMS, and NMR) to be rosmarinic acid (3), 3-O-methyl-rosmarinic acid (4) [22], ethyl caffeate (5) [23], nepetoidin A (6), nepetoidin B (7) [24], cirsiliol (8), [25] circimaritin (9) [26], 7-O-methylluteolin (10) [27], and genkwanin (11) [28].
Regarding compounds 6 and 7 (nepetoidins A and B, resp.), according to Grayer et al. [29], the presence of this pair of caffeic acid esters is chemotaxonomically significant for distinguishing the Nepetoideae from the other subfamilies of Lamiaceae and related families.
This is the first occurrence of flavonoid 8 (cirsiliol) in the Hyptis genus; however, it has also been found in the Labiateae family in the Sideritis, Stachys, Teucrium and Rosmarinus genera. According to Tomás-Barberán and wollenweber [30], these compounds are externally located and are dissolved in a terpenoid matrix, and they have been found in larger amounts in species that grow in xeric habitats. Flavonoids 9 (circimaritin) and 11 (genkwanin) were isolated from Hyptis fasciculata, a native species of Brazil, Argentina, and Uruguay [31]. 10 (7-O-methylluteolin) was reported for the first time in the genus Hyptis.
To evaluate and compare the leishmanicidal profile of H. pectinata, the EtOH extract; the hexane, EtOAc, and MeOH:H2O fractions; and the compounds isolated in major quantities (1–5 and 7) were evaluated in vitro against the promastigote form of L. braziliensis. The maximum effect and the IC50 value (the concentration of sample causing 50% reduction in survival/viability of the parasites) were used as the parameters for antileishmanial activity (Table 2).
Table 2.
Effect of extract, fractions, and compounds isolated from H. pectinata against promastigotes of L. braziliensis.
| Treatment | IC50 a (concentration ± S.E.M.) | Maximum effect (% ± S.E.M.) |
|---|---|---|
| Pentamidine | 0.9 ± 0.03 µM/0.3 ± 0.01 µg/mL | 93.5 ± 0.7** |
| EtOH extract | 0.7 ± 0.1 µg/mL | 91.6 ± 2.5** |
| MeOH : H2O fraction | 3.9 ± 1.5 µg/mL | 61.5 ± 1.2** |
| AcOEt fraction | 0.4 ± 0.1 µg/mL | 81.5 ± 5.9** |
| Hexane fraction | 0.2 ± 0.1 µg/mL | 90.0 ± 3.6** |
| 1 | 6.9 ± 0.7 µM/2.5 ± 0.04 µg/mL | 56.0 ± 0.8** |
| 2 | >100 µM/>36.0 µg/mL | 48.8 ± 1.7** |
| 3 | >100 µM/>36.0 µg/mL | NA |
| 4 | 5.4 ± 0.8 µM/2.0 ± 0.3 µg/mL | 69.1 ± 2.7** |
| 5 | >100 µM/>20.8 µg/mL | NA |
| 7 | >100 µM/>31.4 µg/mL | NA |
Data are reported as the mean ± S.E.M. Differences with a *P value < 0.05 were considered significant relative to the 0.1% DMSO group.
aIC50 is the concentration required to give 50% mortality, calculated by linear regression analysis from the Kc values at the concentrations employed.
NA: the compound is not active.
The EtOH extract; the hexane, EtOAc, and MeOH:H2O fractions; and compounds 1, 2, and 4 exhibited antileishmanial activity, with maximum effects of 91.6 ± 2.5, 90.0 ± 3.6, 81.5 ± 5.9, 61.5 ± 1.2, 56.0 ± 0.8, 48.8 ± 1.7, and 69.1 ± 2.7%, respectively. Moreover, the EtOH extract (IC50 = 0.7 ± 0.1 μg/mL), the EtOAc fraction (IC50 = 0.4 ± 0.1 μg/mL), the hexane fraction (IC50 = 0.2 ± 0.1 μg/mL), compound 1 (IC50 = 6.9 ± 0.7 μM/2.5 ± 0.04 μg/mL), and compound 4 (IC50 = 5.4 ± 0.8 μM/2.0 ± 0.3 μg/mL) were as potent as pentamidine (which has an efficacy of 93.5 ± 0.7% and IC50 = 0.9 ± 0.03 μM/0.3 ± 0.01 μg/mL). In contrast, compounds 3, 5, and 7 did not present activity against the promastigote form of L. braziliensis below 100 μM.
Several polyphenols with promising antileishmanial effects have been reported [32, 33]. Concerning the structure-activity relationship, it would appear that methoxylation of sambacaitaric acid on C3 diminished the efficacy and potency. However, the absence of the methoxyl group on the 3 position in rosmarinic acid (3) abolished the leishmanicidal activity against the promastigote form of L. braziliensis. Moreover, Radtke [34] demonstrated that rosmarinic acid did not show selective toxicity when tested against the promastigote stages of the other Leishmania species (L. major, L. donovani, L. guyanensis, and L. killicki) but did exhibit moderate antileishmanial activity against intracellular amastigotes. Although the caffeic acid esters assessed in this study (5 and 7) have not shown leishmanicidal activity against the promastigotes of L. braziliensis [35] they showed that other caffeic acid esters (1-methylbutyl caffeate, 1′-methylhexyl caffeate and 1′-methyloctyl caffeate) were active against the axenic amastigote forms of L. amazonensis, with IC50 values of 2.0 ± 0.1, 10.0 ± 0.4 and 1.8 ± 0.1 μM, respectively.
Sambacaitaric acid (1), [α]D = +30 (c 0.001, MeOH), IR (KBr) ν max: 3435 (OH), 1658 (C=O), 1524, (C=C from aromatic rings). 1H NMR (DMSO-d 6, 300 MHz, see Table 1), 13C NMR (DMSO-d 6, 75 MHz, see Table 1). HRESIMS (negative mode) m/z 359.0761 [M-H]+ (C18H15O8).
3-O-methyl-sambacaitaric acid (2), [α]D = +10 (c 0.001, MeOH), 1H NMR (300 Mz, DMSO): δ 7.35 (1H, d, J = 15.9 Hz, H-7); 7.01 (1H, d, J = 2.1 Hz, H-2), 6.91 (1H, dd, J = 8.1; 2.1 Hz, H-6), 6.73 (1H, d, J = 8.1 Hz, H-5), 6.66 (1H, d, J = 2.1 Hz, H-2′), 6.58 (1H, d, J = 8.1 Hz, H-5′), 6.48 (1H, dd, J = 8.1; 2.1 Hz, H-6′), 6.18 (1H, d, J = 15.9 Hz, H-8), 8.83 (1H, m; H-8′), 3.02 and 2.73 (2H, m, H-7′). 13C NMR (75 Mz, DMSO): δ 172.6 (C-9′), 166.7 (C-9), 148.2 (C-4), 146.3 (C-3), 145.3 (C-3′), 144.5 (C-7), 143.89 (C-4′), 130.5 (C-1′), 126.1 (C-1), 121.3 (C-6), 120.1 (C-6′), 116.9 (C-2′), 116.3 (C-5), 115.8 (5′), 115.5 (C-2), 115.3 (C-8), 76.6 (C-8′), 56.9 (OCH3), 37.8 (C-7′).
Rosmarinic acid (3), [α]D = +10 (c 0.001, MeOH), UV λ max 242, 324. IR (KBr) v max: 3382 (OH), 1697 (C=O), 1606, 1522 (C=C from aromatic rings). LC-ESI-MS (negative mode) m/z 359 [M-H]+ (C18H16O8).
3-O-methyl-rosmarinic acid (4), [α]D = +10 (c 0.001, MeOH), UV λ max 253, 340. IR (KBr) v max: 3394 (OH), 1692 (C=O), 1603, 1520 (C=C from aromatic rings). LC-ESI-MS (negative mode) m/z 373 [M-H]+ (C19H18O8).
Ethyl caffeate (5) UV λ max 283, 337. IR (KBr) v max: 3397 (OH), 1678 (C=O), 1605, 1520 (C=C from aromatic rings). LC-ESI-MS (negative mode) m/z 207 [M-H]+ (C11H12O4).
Nepetoidin A (6) UV λ max 249, 340. IR (KBr) v max: 3418 (OH), 1680 (C=O), 1603, 1520 (C=C from aromatic rings). LC-ESI-MS (negative mode) m/z 313 [M-H]+ (C17H14O6).
Nepetoidin B (7) UV λ max 251, 340. IR (KBr) v max: 3382 (OH), 1701 (C=O), 1604, 1516 (C=C from aromatic rings). LC-ESI-MS (negative mode) m/z 313 [M-H]+ (C17H14O6).
Cirsiliol (8) UV λ max 272, 346. IR (KBr) v max: 3419 (OH), 1650 (C=O), 1600, (C=C from aromatic rings). LC-ESI-MS (negative mode) m/z 329 [M-H]+ (C17H14O7).
Circimaritin (9) UV λ max 274, 336. IR (KBr) v max: 3434 (OH), 1652 (C=O), 1599, (C=C from aromatic rings). LC-ESI-MS (negative mode) m/z 313 [M-H]+ (C17H14O6).
7-O-methylluteolin (10) UV λ max 254, 349. IR (KBr) v max: 3397 (OH), 1664 (C=O), 1601, 1507 (C=C from aromatic rings). LC-ESI-MS (negative mode) m/z 299 [M-H]+ (C16H12O6).
Genkwanin (11) UV λ max 267, 338. IR (KBr) v max: 3445 (OH), 1670(C=O), 1608, 1504 (C=C from aromatic rings). LC-ESI-MS (negative mode) m/z 283 [M-H]+ (C16H12O5).
4. Conclusions
The chemical study of leaves from Hyptis pectinata resulted in the isolation of two new compounds, sambacaitaric acid (1) and 3-O-methyl-sambacaitaric acid (2), and nine known compounds, rosmarinic acid (3), 3-O-methyl-rosmarinic acid (4), ethyl caffeate (5), nepetoidin A (6), nepetoidin B (7), cirsiliol (8), circimaritin (9), 7-O-methylluteolin (10), and genkwanin (11). The EtOH extract, the hexane, EtOAc, and MeOH:H2O fractions; and compounds 1, 2, and 4 exhibited antileishmanial activity; compound 1 was as potent as pentamidine. In contrast, compounds 3, 5, and 7 did not present activity against the promastigote form of L. braziliensis below 100 μM. The activity of the EtOAc fraction can be partially attributed to the isolated compounds 1, 2, and 4.
Conflict of Interest
The authors declare that they have no conflict of interests.
Acknowledgments
The authors thank CNPq (TMSS and CAC research fellowships), CAPES and FACEPE (PRONEM APQ-1232.1.06/10 grant number) for financial support and the CENAPESQ and CETENE analytical centers for the recorded data they kindly provided. TMSS also thanks PRPPG-UFRPE for their kind financial support through a fellowship. The authors also thank Dr. Clecio de Souza Ramos for CD analysis.
References
- 1.Harley RM, Atkins S, Budantsev AL, et al. Labiatae. In: Kubitzki K, editor. The Families and Genera of Vascular Plants. New York, NY, USA: Springer; 2004. pp. 167–275. [Google Scholar]
- 2.Raymundo LJRP, Guilhon CC, Alviano DS, et al. Characterisation of the anti-inflammatory and antinociceptive activities of the Hyptis pectinata (L.) Poit essential oil. Journal of Ethnopharmacology. 2011;134(3):725–732. doi: 10.1016/j.jep.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 3.Harley RF, França F, Santos JS. Lamiaceae. Lista de Espécies da Flora do Brasil, Rio de Janeiro, Brazil, 2010, http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB142.
- 4.de Almeida CDFCBR, de Albuquerque UP. Check-list of the family Lamiaceae in Pernambuco, Brazil. Brazilian Archives of Biology and Technology. 2002;45(3):343–353. [Google Scholar]
- 5.Basílio IJLD, Agra MDF, Rocha EA, Leal CKA, Abrantes HF. Estudo farmaco botânico comparativo das folhas de Hyptis pectinata (L.) Poit. e Hyptis suaveolens (L.) Poit. (Lamiaceae) Acta Farmaceutica Bonaerense. 2006;25(4):518–525. [Google Scholar]
- 6.Pereda-Miranda R, Hernández L, Villavicencio MJ, et al. Structure and stereochemistry of pectinolides A-C, novel antimicrobial and cytotoxic 5,6-dihydro-α-pyrones from Hyptis pectinata . Journal of Natural Products. 1993;56(4):583–593. doi: 10.1021/np50094a019. [DOI] [PubMed] [Google Scholar]
- 7.Nascimento PFC, Alviano WS, Nascimento ALC, et al. Hyptis pectinata essential oil: chemical composition and anti-Streptococcus mutans activity. Oral Diseases. 2008;14(6):485–489. doi: 10.1111/j.1601-0825.2007.01405.x. [DOI] [PubMed] [Google Scholar]
- 8.Malan K, Pelissier Y, Marion C, Blaise A, Bessiere JM. The essential oil of Hyptis pectinata . Planta medica. 1988;54(6):531–532. doi: 10.1055/s-2006-962540. [DOI] [PubMed] [Google Scholar]
- 9.Lisboa ACCD, Mello ICM, Nunes RS, et al. Antinociceptive effect of Hyptis pectinata leaves extracts. Fitoterapia. 2006;77(6):439–442. doi: 10.1016/j.fitote.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Rojas A, Hernandez L, Pereda-Miranda R, Mata R. Screening for antimicrobial activity of crude drug extracts and pure natural products from Mexican medicinal plants. Journal of Ethnopharmacology. 1992;35(3):275–283. doi: 10.1016/0378-8741(92)90025-m. [DOI] [PubMed] [Google Scholar]
- 11.Melo GB, Silva RL, Melo VA, et al. Proliferative effect of the aqueous extract of Hyptis pectinata on liver regeneration after partial hepatectomy in rats. Acta Cirurgica Brasileira. 2006;21(1):33–36. doi: 10.1590/s0102-86502006000700008. [DOI] [PubMed] [Google Scholar]
- 12.Bispo MD, Mourão RHV, Franzotti EM, et al. Antinociceptive and antiedematogenic effects of the aqueous extract of Hyptis pectinata leaves in experimental animals. Journal of Ethnopharmacology. 2001;76(1):81–86. doi: 10.1016/s0378-8741(01)00172-6. [DOI] [PubMed] [Google Scholar]
- 13.Arrigoni-Blank MF, Silva-Mann R, Campos DA, et al. Morfological, agronomical and pharmacological characterization of Hyptis pectinata (L.) Poit germplasm. Brazilian Journal Pharmacognosy. 2005;15(4):298–303. [Google Scholar]
- 14.Iwu MM, Jackson JE, Schuster BG. Medicinal plants in the fight against leishmaniasis. Parasitology Today. 1994;10(2):65–68. doi: 10.1016/0169-4758(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 15.Amato VS, Tuon FF, Bacha HA, Neto VA, Nicodemo AC. Mucosal leishmaniasis. Current scenario and prospects for treatment. Acta Tropica. 2008;105(1):1–9. doi: 10.1016/j.actatropica.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Baptista C, Schubach AO, Madeira MF, et al. Leishmania (Viannia) braziliensis genotypes identified in lesions of patients with atypical or typical manifestations of tegumentary leishmaniasis: evaluation by two molecular markers. Experimental Parasitology. 2009;121(4):317–322. doi: 10.1016/j.exppara.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Gomes Rodrigues EH, Felinto de Brito ME, Mendonça MG, et al. Evaluation of PCR for diagnosis of American cutaneous leishmaniasis in an area of endemicity in Northeastern Brazil. Journal of Clinical Microbiology. 2002;40(10):3572–3576. doi: 10.1128/JCM.40.10.3572-3576.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tojal da Silva AC, Cupolillo E, Volpini ÂC, Almeida R, Sierra Romero GA. Species diversity causing human cutaneous leishmaniasis in Rio Branco, state of Acre, Brazil. Tropical Medicine and International Health. 2006;11(9):1388–1398. doi: 10.1111/j.1365-3156.2006.01695.x. [DOI] [PubMed] [Google Scholar]
- 19.Mishra J, Saxena A, Singh S. Chemotherapy of leishmaniasis: past, present and future. Current Medicinal Chemistry. 2007;14(10):1153–1169. doi: 10.2174/092986707780362862. [DOI] [PubMed] [Google Scholar]
- 20.García M, Monzote L, Montalvo AM, Scull R. Screening of medicinal plants against Leishmania amazonensis . Pharmaceutical Biology. 2010;48(9):1053–1058. doi: 10.3109/13880200903485729. [DOI] [PubMed] [Google Scholar]
- 21.Rangel H, Dagger F, Hernandez A, Liendo A, Urbina JA. Naturally azole-resistant Leishmania braziliensis promastigotes are rendered susceptible in the presence of terbinafine: comparative study with azole-susceptible Leishmania mexicana promastigotes. Antimicrobial Agents and Chemotherapy. 1996;40(12):2785–2791. doi: 10.1128/aac.40.12.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baba S, Osakabe N, Natsume M, Terao J. Orally administered rosmarinic acid is present as the conjugated and/or methylated forms in plasma, and is degraded and metabolized to conjugated forms of caffeic acid, ferulic acid and m-coumaric acid. Life Sciences. 2004;75(2):165–178. doi: 10.1016/j.lfs.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 23.Xiang MS, Su H, Hu J, Yan Y. Isolation, identification and determination of methyl caffeate, ethyl caffeate and other phenolic compounds from Polygonum amplexicaule var. sinense . Journal of Medicinal Plant Research. 2011;5(9):1685–1691. [Google Scholar]
- 24.Nakanishi T, Nishi M, Inada A, et al. Two new potent inhibitors of xanthine oxidase from leaves of Perilla frutescens britton var. acuta kudo. Chemical and Pharmaceutical Bulletin. 1990;38(6):1772–1774. doi: 10.1248/cpb.38.1772. [DOI] [PubMed] [Google Scholar]
- 25.Abdelshafeek KA, Abdelrahem F, Elwahsh MA, Abdelkhalek IA. Investigation of the flavonoidal constituents and insecticidal activity of Teucrium zanonii. . Pharmacognosy Research. 2009;1(6):410–416. [Google Scholar]
- 26.Masterova I, Uhrin D, Kettmann V, Suchy V. Phytochemical study of Salvia officinalis L. Chemical Papers. 1989;43(6):797–803. [Google Scholar]
- 27.Noro T, Oda Y, Miyase T, Ueno A, Fukushima S. Inhibitors of xanthine oxidase from the flowers and buds of Daphne genkwa . Chemical and Pharmaceutical Bulletin. 1983;31(11):3984–3987. doi: 10.1248/cpb.31.3984. [DOI] [PubMed] [Google Scholar]
- 28.da Silva TMS, de Carvalho MG, Braz-Filho R. Spectroscopy study on structural elucidation of flavonoids from Solanum jabrense Agra & Nee e S. paludosum moric. Quimica Nova. 2009;32(5):1119–1128. [Google Scholar]
- 29.Grayer RJ, Eckert MR, Veitch NC, et al. The chemotaxonomic significance of two bioactive caffeic acid esters, nepetoidins A and B, in the Lamiaceae. Phytochemistry. 2003;64(2):519–528. doi: 10.1016/s0031-9422(03)00192-4. [DOI] [PubMed] [Google Scholar]
- 30.Tomás-Barberán FA, Wollenweber E. Flavonoid aglycones from the leaf surfaces of some Labiatae species. Plant Systematics and Evolution. 1990;173(3-4):109–118. [Google Scholar]
- 31.Isobe T, Doe M, Morimoto Y, Nagata K, Ohsaki A. The anti-Helicobacter pylori flavones in a Brazilian plant, Hyptis fasciculata, and the activity of methoxyflavones. Biological and Pharmaceutical Bulletin. 2006;29(5):1039–1041. doi: 10.1248/bpb.29.1039. [DOI] [PubMed] [Google Scholar]
- 32.Hay A-E, Merza J, Landreau A, et al. Antileishmanial polyphenols from Garcinia vieillardii . Fitoterapia. 2008;79(1):42–46. doi: 10.1016/j.fitote.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Abdel-Hady NM, Dawoud GTM, El-Hela AA, Morsy TA. Interrelation of antioxidant, anticancer and antilieshmania effects of some selected Egyptian plants and their phenolic constituents. Journal of the Egyptian Society of Parasitology. 2011;41(3):785–800. [PubMed] [Google Scholar]
- 34.Radtke OA, Yeap Foo L, Lu Y, Kiderlen AF, Kolodziej H. Evaluation of sage phenolics for their antileishmanial activity and modulatory effects on interleukin-6, interferon and tumour necrosis factor-α-release in RAW 264.7 Cells. Zeitschrift fur Naturforschung C. 2003;58(5-6):395–400. doi: 10.1515/znc-2003-5-618. [DOI] [PubMed] [Google Scholar]
- 35.Cabanillas BJ, le Lamer A-C, Castillo D, et al. Caffeic acid esters and lignans from Piper sanguineispicum . Journal of Natural Products. 2010;73(11):1884–1890. doi: 10.1021/np1005357. [DOI] [PubMed] [Google Scholar]


