Abstract
The present work aimed to analyze the alkaloid content of the ethanolic extract of Tabernaemontana catharinensis (Apocynaceae family) and its fractions as well as to evaluate their antioxidant and anticholinesterasic activities. The analyses of the ethanolic extract of T. catharinensis by mass spectrometry allowed identifying the presence of the alkaloids 16-epi-affinine, coronaridine-hydroxyindolenine, voachalotine, voacristine-hydroxyindolenine, and 12-methoxy-n-methyl-voachalotine, as well as an alkaloid with m/z 385.21 whose spectrum suggests a derivative of voacristine or voacangine. The extract and its alkaloid rich fractions showed antioxidant activity, especially those that contain the alkaloid m/z 385.21 or 16-epi-affinine with DPPH scavenging activity (IC50) between 37.18 and 74.69 μg/mL. Moreover, the extract and its fractions exhibited anticholinesterasic activity, particularly the fractions characterized by the presence of 12-methoxy-n-methyl-voachalotine, with IC50 = 2.1 to 2.5 μg/mL. Fractions with 16-epi-affinine combined good antioxidant (IC50 = 65.59 to 74.69 μg/mL) and anticholinesterasic (IC50 = 7.7 to 8.3 μg/mL) activities, representing an option for further studies aimed at treating neurodegenerative diseases.
1. Introduction
Alzheimer's disease is a progressive neurodegenerative disorder related to genetic predisposition and characterized by the presence of neurofibrillary tangles, formation of extracellular deposition of β-amyloid peptide, oxidative stress, increased production of superoxide radicals, and reduced neurotransmitter levels [1, 2]. The treatments available to combat the symptoms of this condition are complex and include increasing acetylcholine levels in brain regions such as frontal cortex and hippocampus and possibly preventing neuronal degeneration with antioxidants [3].
Natural products derived from plants represent a viable alternative for discovering new potentially active substances. Tabernaemontana catharinensis ADC is a small tree of the Apocynaceae family currently found in Brazil, Argentina, Uruguay, Paraguay, and Bolivia [4]. The genus Tabernaemontana has evoked interest due to the important biologic activity of its extracts, particularly, antimicrobial [5, 6], anti-tumoral [7], antioxidant [8], anti-cholinesterasic [9], and anti-inflammatory [10] activities, most of which have been associated with indole alkaloids. Although several biological activities of T. catharinensis extracts have been reported, few substances with anticholinesterase activity able to minimize damage caused by oxidative stress have been described in T. catharinensis. The association of these properties may represent an alternative for the control of neurodegenerative disorders as Alzheimer's disease.
In view of the foregoing, the present work aimed to evaluate the content of the ethanolic extract obtained from the aerial parts of T. catharinensis as well as to examine the in vitro antioxidant and anticholinesterase activity of the extract and its main fractions and to relate biological activities to the identified compounds.
2. Materials and Methods
Twenty samples of Tabernaemontana catharinensis were collected in Santo Angelo, RS, Brazil (28°27′59′′S, 54°29′37′′W) in November 2008. The plants were identified by professor Ronaldo A. Wasum and deposited in the herbarium of Universidade de Caxias do Sul (HUCS 34038–34057/guia 1669).
After the removal of inflorescences, the terminal regions of aerial parts (leaves and branches) were dried in a greenhouse with forced air circulation at a temperature of 30°C for 4 days. The dry material was grinded in a Willey TE 650 grinder mill and stored in container protected from light for subsequent analysis.
2.1. Methods of Extraction and Purification
The triturated vegetal material underwent extraction with a Soxhlet apparatus using ethanol as extraction solvent (10 mL ethanol/g) for 12 hours at a temperature of nearly 70°C. The ethanolic extract was concentrated in a rotary evaporator at reduced pressure until complete removal of the solvent.
According to the methodology described by Guida et al. [11], 150 mL of hydrochloric acid at 2% were added to each 10 grams of dry extract and extracted with 200 mL of chloroform (fraction named B1). The acid aqueous phase was alkalinized with sodium hydroxide 10% until pH 7.8 and extracted with 200 mL of chloroform (fraction B2). The aqueous phase was alkalinized until pH 11.2 with solution of sodium hydroxide 10% and extracted with 200 mL of chloroform (fraction B3) (Figure 1).
Figure 1.
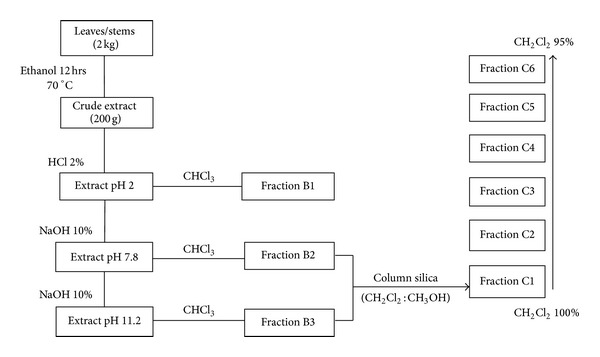
Diagram of fractionation of Tabernaemontana catharinensis extract.
Fractions B1, B2, and B3 were monitored by thin-layer chromatography (TLC) using aluminium chromatoplates precoated with silica gel F254 0.2 mm (Merck) with eluent system CH2Cl2 : MeOH (93 : 7). Bands were visualized under ultraviolet light (254 and 365 nm) after staining with Dragendorff's reagent.
Fractions B2 and B3 had similar chromatographic profiles by TLC and were gathered. The mixture of these fractions underwent column chromatography using Merck silica gel (0.06–0.2) as a support and dichloromethane, dichloromethane with increasing percentages of methanol and methanol as eluent, according to Gower et al. [12]. A total of 14 fractions of 100ml each were collected and monitored by TLC, using the same procedure previously described and gathered according to the similarities observed, resulting in 6 fractions (C1–C6).
2.2. Identification of Alkaloids by Mass Spectrometry
The extract and fractions of T. catharinensis were dissolved in a solution of 50% (v/v) chromatographic grade acetonitrile (Tedia, Fairfield, OH, USA), acidified with 0.1% formic acid. The solutions were individually infused directly into the electrospray ionization (ESI) source by means of a syringe pump (Harvard Apparatus) at a flow rate of 10 μL min−1. Electrospray ionization mass spectrometry (ESI(+)-MS) and tandem ESI(+)-MS/MS were acquired using a hybrid high resolution and high accuracy (5 μL/L) Orbitrap XL mass spectrometer (Thermo Fisher Scientific) under the following conditions: capillary and cone voltages were set to +3500 V and +40 V, respectively, with a desolvation temperature of 100°C. For ESI(+)-MS/MS, the energy for the collision-induced dissociations (CID) was optimized for each component. Diagnostic ions in different fractions were identified by the comparison of their ESI(+)-MS/MS dissociation patterns with those of compounds identified in previous studies. For data acquisition and processing, Xcalibur software (Thermo Fisher Scientific) was used. The data were collected in the m/z range of 70–700, providing the resolution of 50,000 (FWHM) at m/z 200. No important ions were observed below m/z 200 or above m/z 650.
2.3. Determination of Antioxidant Activity
The antioxidant activity was measured by 2,2-diphenyl-1-picrylhydrazyl radical (DPPH●) scavenging activity [13]. Extract and fractions (B1–B3, C1–C6) of T. catharinensis (200 μL) were added to Tris-HCl buffer 100 mM (pH 7.4, 800 μL) and 1 mL DPPH● (500 μM dissolved in ethanol). The tubes were stored in the dark at room temperature for 20 min and absorbance was measured at 517 nm. Samples, blanks, and positive controls (ascorbic acid) were assessed in triplicate. Results were expressed as IC50, that is, the amount of the extract needed to scavenge 50% of DPPH●.
2.4. Acetylcholinesterase Inhibitory Activity (AChE) and Evaluation of Kinetic Parameters
Acetylcholinesterase inhibitory activity was determined from the assay described by Ellman et al. [14]. Acetylthiocholine iodide (ATCI) and 5,5′dithiobis-(2-nitrobenzoic acid) (DTNB) were used as solvent and reagent for activity determination. Experiments used acetylcholinesterase from electric eel (Electrophorus electricus), Sigma C2888. In 96-well microplates, the reaction mixture containing 185 μL of sodium phosphate buffer (concentration 0.1 M) pH 8.0, 5 μL of DTNB (concentration 0.3 mM), 5 μL of ATCI (concentration 0.5 Mm), and 5 μL of solution were stored at a temperature of 25°C. Hydrolysis of ATCI initiated with the addition of 20 μL of enzyme suspension (concentration 0.032 U/mL) and then microplates were read at 405 nm every 30 s for 2 min. Samples, blanks, and positive controls (galantamine) were assessed in triplicate. The results were expressed as IC50, that is, the concentration required to inhibit 50% enzymatic activity.
The same methodology was used to evaluate kinetic parameters, except for DTNB content (concentration 1 mM) and multiple ATCI concentrations (0.5, 0.6, 0.9, 1.35, 1.8, and 3 mM) [15, 16]. Negative and positive controls (galantamine 0.2 and 1 μg/mL) and fraction C6 (0.25, 1 and 100 μg/mL) were assessed in triplicate. The values obtained were graphed according to the Michaelis-Menten and Lineweaver-Burk models.
2.5. Statistical Analysis
Results underwent statistical analysis by analysis of variance and Tukey's post test (P ≤ 0.05) using SPSS16.0 software for Windows (SPSS Inc., Chicago, IL, USA).
3. Results and Discussion
ESI(+)-MS analysis of the raw extract of T. catharinensis revealed the presence of chemicals with molecular weight ranging from 203 to 487 (Figure 2). The results obtained in this study, also considering previous reports of the genus Tabernaemontana [17, 20], indicated the presence of the following alkaloids: 16-epi-affinine (m/z 325.1911), coronaridine-hydroxyindolenine (m/z 355.2019), voachalotine (m/z 367.2020), voacristine-hydroxyindolenine (m/z 401.2072), 12-methoxy-n-methyl-voachalotine (m/z 411.2280), and of an unidentified compound with m/z 385.2125, whose fragmentation profile suggested a derivative of voacristine or voacangine.
Figure 2.
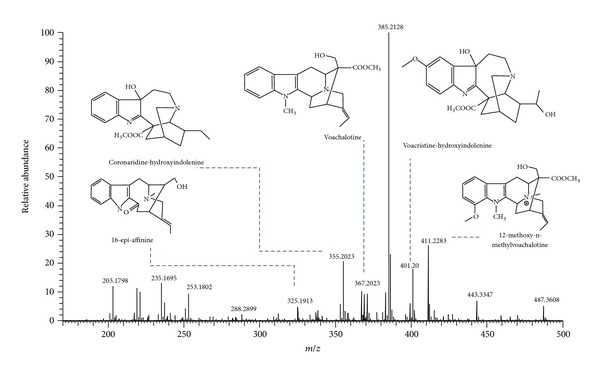
Electrospray positive spectra of T. catharinensis extract and structures of identified alkaloids compounds.
Table 1 lists the compounds identified in the raw extract of T. catharinensis by ESI(+)-MS as well as the analyses of the main precursor ions by ESI(+)-MS/MS. Indole alkaloids were characterized by the important ability to stabilize positive charges, provided by the nitrogen atom and by the presence of carboxyl groups that can be ruptured in situations of ionization [23]. These characteristics can be evidenced in Figure 3, in which, through ion isolation and fragmentations, it was possible to confirm the chemical structure of the affinisine compound.
Table 1.
Chemical composition of T. catharinensis extract by ESI(+)-MS e ESI(+)-MS/MS.
| Entry | Precursor ion m/z |
Fragmentation ms/ms (%) [MS2] {MS3} |
Identification | Elem. comp. | Diff. ppm | Fragmentation pathways | References |
|---|---|---|---|---|---|---|---|
| 1 | 309.1963 | 291.1864(35); 202.1232(5); 158.0968(17); 138.0917(16) | Affinisine | C20H24N2O | 0.646 | 291.1864[M-OH]; 202.1232[M-C7H8N]; 158.0968[M-C9H12NO] |
[9, 17] |
|
| |||||||
| 2 | 325.1918 | 308.1891(15); 307.1814(13) [289.1706(4); 265.1700(100) {234.1281(11); 122.0966(8)}176.1071(5)]; 265.1706(5); 152.1074(12) |
16-epi-Affinine | C20H24N2O2 | 2.460 | 308.1891[M-OH]; 265.1700[M-C2H3O2]; 152.1074[M-C11H10NO] | [18] |
|
| |||||||
| 3 | 353.1866 | 322.1443(9); 321.1606(33), [293.1654(65), 275.1548(22) 264.1388(100), 221.1078(24), 211.0870(65), 183.0921(98)] 290.1184(6) |
Vobasine | C21H24N2O3 | 1.981 | 322.1443[M-C2H6]; 293.1654[M-C2H4O2] 275.1548[M-C2H6O3]; 264.1388[M-C3H7NO2] |
[19] |
|
| |||||||
| 4 | 355.2023 | 337.1920(100) [305.1656(60); 277.1706(21); 216.1024(8); 144.0811(9)]; 323.1762(7); 305.1657(60) [290.1418(22); 277.1704(100) {207.0921(10)}; 235.0869(67); 184.0760(36); 174.0916(33)] | Coronaridine-hydroxyindolenine | C21H26N2O3 | 1.970 | 337.1920[M-OH]; 323.1762[M-CH3O] 305.1657[M-CH4O2]; 277.1704[M-C2H5O3] | [20] |
|
| |||||||
| 5 | 367.2023 | 337.1921(100) [305.1655(28); 172.1125(16); 158.0967(34)]; 335.1765(7); 305.1658(23); 172.1126(31); 166.0868(5); 158.0969(7) | Voachalotine | C22H26N2O3 | 1.906 | 337.1921[M-CH3OH]; 305.1655[M-C2H4O2] 172.1125[M-C12H12NO3]; 158.0969[M-C11H15NO3] |
[21] |
|
| |||||||
| 6 | 385.2128 | 367.2024(100), [335.1761(100), {307.1812(40), 265.0977(21), 214.0867(9), 174.0917(15)}; 307.1810(100) 246.1130(19); 174.0918(10)]; 335.1765(20); 307.1815(7) | Derivative of voacristine or voacangine | n.d | |||
|
| |||||||
| 7 | 401.2075 | 383.1975(100) [365.1867(100); {333.1604(20); 201.1026(26)} 351.1711(21); 337.1553(21); 201.0976(23)]; 365.1867(62); [351.1711(21); 337.1553(21); 333.1605(7); 323.1760(8); 201.1027(23)]; 351.1715(7); 201.1029(6) |
Voacristine- hydroxyindolenine |
C22H28N2O5 | 1.246 | 383.1975[M-OH]; 365.1867[M-O2H4] 351.1711[M-CH7O2]; 333.1604[M-CH7O3] 201.1027[M-C10H15O4] |
[17] |
|
| |||||||
| 8 | 411.2283 | 381.2181(100); 349.1917(100); [334.1681(16); 321.1966(10); 317.1654(15); 266.1544(35); {251.1309(21); 237.1151(44)}]; 200.1076(100); [185.0839(14); 169.0890(12)]; 180.1024(25) | 12-Methoxy-n-methyl-voachalotine | C24H31N2O4 | 1.215 | 381.2181[M-CH4O]; 349.1917[M-C2H6O2] 334.1681[M-C3H8O2]; 321.1966[M-C3H7O3] 200.1076[M-C11H15NO3] | [22] |
|
| |||||||
| 9 | 415.2126 | ni | |||||
ni: not identificated.
Figure 3.
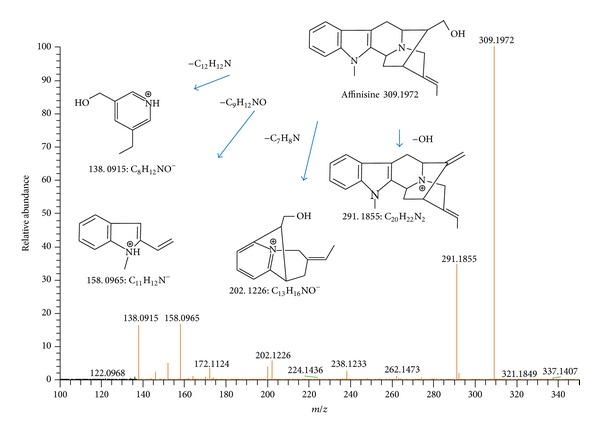
ESI(+)-MS/MS of affinisine.
The fractions obtained from the dry extract were analyzed by ESI(+)-MS (Figure 4). Fractions B2 and B3 (0.4% of the initial material) had similar chromatographic profiles by TLC and showed significant differences in the spectrum obtained by ESI(+)-MS. Fractions C1 and C2 were characterized by the presence of the possible derivative of voacristine or voacangine (m/z 385.2134). On the other hand, fractions C3 and C4 had a higher peak corresponding to 16-epi-affinine (m/z 325.1911) as well as an unidentified compound with m/z 311.1769. Fractions C5 and C6, in turn, had 12-methoxy-n-methyl-voachalotine (m/z 411.2171) as the most intense peak in the spectrum.
Figure 4.
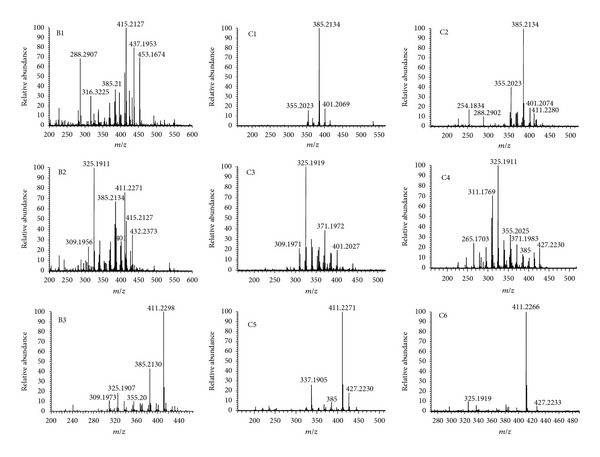
2 Electrospray positive spectra of fractions B1–B3, C1–C6.
Of the compounds identified in the raw extract of T. catharinensis, affinisine was previously described in T. catharinensis extracts [24] as well as in extracts from T. buchtieni [25], T. fuchsiaefolia [17], and T. histrix [9]. In turn, 16-epi-affinine was identified in T. catharinensis, T. latea, T. pachysiphon and T. psychotrifolia [18], and T. fuchsiaefolia [22]. Vobasine is present in several species of the genus Tabernaemontana, including T. catharinensis [21]. Coronaridine-hydroxyindolenine, in turn, was identified in T. catharinensis extracts [20] and was also found in T. divaricata [26], T. buchtieni [25], and T. heyneana [27]. Voachalotine and 12-methoxy-n-methyl-voachalotine were detected in extracts from T. catharinensis [20] and T. fuchsiaefolia [17], among other species of the genus. Voacristine-hydroxyindolenine, in turn, was described in T. apoda and T. dichotoma [21], in T. buchtieni [25], and also in T. heyneana [27].
Thus, generally speaking, the compounds identified in the raw ethanolic extract of T. catharinensis are consistent with previous studies on this vegetal species. However, the major indole alkaloids, voacangine, and coronaridine, described in branch extracts obtained by supercritical CO2 [28, 29] and in leaf ethanolic extracts [29], were not detected in the present research. The absence of these compounds may be attributed to environmental and genetic differences in the evaluated material, or to the analysis methods used, since these authors employed liquid chromatographic/mass spectrometric (LC/MS) and gas chromatography/mass spectrometry (GC/MS), respectively. It is worth highlighting that coronaridine-hydroxyindolenine and a possible derivative of voacristine or voacangine were detected in the raw extract and in some of its fractions.
The extract of T. catharinensis and its fractions were evaluated with regard to their DPPH● scavenging activity, a method routinely used and recognized due to its practicality and rapidity [30, 31]. According to the results shown in Table 2, the raw extract exhibited DPPH● scavenging activity with IC50 of 313.46 μg/mL. Fraction B1, which had low content of alkaloids according to the chromatographic analysis, exhibited lower antioxidant activity than the raw extract, with IC50 of 1.590.00 μg/mL.
Table 2.
Antioxidant and anticholinesterasic activity of T. catharinensis extract and fractions.
| Samples | DPPH-IC50 (μg/mL) | AChE inhibition-IC50 (μg/mL) |
|---|---|---|
| Extract | 313.46 ± 0.5b | 261.55 ± 9.1b |
| Organic/aqueus fractions | ||
| B1 | 1590.00 ± 1.4a | 458.40 ± 8.2a |
| B2 | 60.75 ± 0.5e | 18.35 ± 1.4d |
| B3 | 67.28 ± 0.6e | 9.0 ± 0.4e |
| c. column fractions | ||
| C1 | 94.92 ± 0.05d | 17.35 ± 1.4d |
| C2 | 37.18 ± 0.1f | 91.22 ± 7.1c |
| C3 | 74.69 ± 0.9e | 7.71 ± 0.1e |
| C4 | 65.59 ± 0.3e | 8.34 ± 0.6e |
| C5 | 230.25 ± 0.1c | 2.50 ± 0.1f |
| C6 | 249.61 ± 0.9c | 2.10 ± 0.1f |
| Ascorbic acid | 20.13 ± 0.45g | — |
| Galantamine | — | 0.2 ± 0.05g |
Different letters correspond to values statistically different by analysis of variance (ANOVA) and Tukey's post hoc test, for P ≤ 0.05, for each activity.
Fractions C1 and C2, which had similar spectra, with the presence of a possible derivative of voacristine or voacangine, showed a significant difference in their antioxidant activities. We suggest that the higher activity observed in fraction C2 may be attributed to other unidentified compounds or to its content of coronaridine-hydroxyindolenine (Figure 4). On the other hand, fractions C3 and C4, characterized by the presence of 16-epi-affinine and of an unidentified compound with m/z 311.1769, exhibited similar antioxidant activity, close to that of fractions B2 and B3. The lower antioxidant activity among the fractions obtained by chromatographic separation was observed in fractions C5 and C6, which were characterized by the presence of 12-methoxy-n-methyl-voachalotine.
The antioxidant activity of the raw extract from the aerial parts of T. catharinensis was lower than that observed in root ethanolic extracts of this species (IC50 = 100 μg/mL) [32] but higher than that identified in ethanolic extracts of T. heyneana leaves (IC50 = 537 μg/mL) [33].
Indole alkaloids have been previously described as exhibiting antioxidant activity [32, 34]. Studies showed that reactive oxygen species uptake activity in indole derivatives could be related to the exceptional redox property of the indole ring, which is especially conferred by the indole nitrogen [35]. Among the indole alkaloids present in Tabernaemontana, antioxidant activity has been identified for isovoacristine-hydroxyindolenine and voacangine [32]. On the other hand, variations in the content of coronaridine and voacangine in T. catharinensis extracts did not change in vitro antioxidant potential [8].
The results for the inhibitory activity of the extract of T. catharinensis and its fractions on acetylcholinesterase (AChE) enzyme using Ellman's method [14] can also be observed in Table 2. The raw extract had the ability of inhibiting AChE enzyme (IC50 = 261.55 μg/mL). This antiacetylcholinesterase activity was previously reported in the ethanolic extract of Tabernaemontana divaricata roots (IC50 = 2.56 μg/mL), in which there has been also reports of selective and reversible enzyme inhibition [36].
Fractions B2 and B3, as well as samples obtained by column fractionation, showed higher values for AChE enzyme inhibition when compared to the value observed for the raw extract. Fractions C5 and C6 were those that obtained better results for AChE inhibition, with IC50 of 2.50 μg/mL and 2.10 μg/mL, respectively. On the other hand, fraction B1 exhibited low anticholinesterase activity (IC50 = 458.40 μg/mL). These results corroborate previous reports that mentioned the anticholinesterase potential of alkaloids found in the genus Tabernaemontana and the possibility of using these compounds for studies involving the treatment of neurodegenerative diseases [9, 24].
Significant enzyme inhibition activities were observed in fractions C3 (IC50 7.71 μg/mL), C4 (IC50 8.34 μg/mL), and B3 (IC50 9.0 μg/mL). These fractions were characterized by the presence of the compounds affinisine, 16-epi-affinine, vobasine, and coronaridine-hydroxyindolenine. In previous reports, affinine and affinisine inhibited AChE enzyme [9, 24]. On the other hand, vobasine and coronaridine-hydroxyindolenine showed low inhibition results at the concentrations evaluated by Zhan et al. [37].
Recent studies related the molecular structure of indole alkaloids to AChE inhibitory capacity, based on compounds similar to coronaridine. It was observed that structures with hydrophobic substituents or electron-donor substituents exhibited higher inhibitory activity when compared to their hydroxylated derivatives [37]. However, these attributes were not observed with isovoacangine and its hydroxilated derivative [9]. Representatives of the Corinantea class such as 12-methoxy-n-methyl-voachalotine, were characterized by exhibiting antiacetylcholinesterase activity [24]. It is worth stressing that this case requires further clarification when relating structure to activity potential, taking into account that n-methyl-voachalotine showed negative results for enzyme inhibition in previous reports [9].
In an attempt of understanding inhibition results for fraction C6, kinetic studies were conducted, which may suggest possible models of interaction between AChE and its inhibitors [38]. It is known so far that AChE, an enzyme belonging to the family of αβ hydrolases, has two main binding sites: an active site (ACS) and an anionic site (PAS) [39]. Figure 5 shows the models of enzyme kinetics and Michaelis-Menten as well as the linear model described by Lineweaver-Burk with different inhibitor concentrations (fraction C6).
Figure 5.
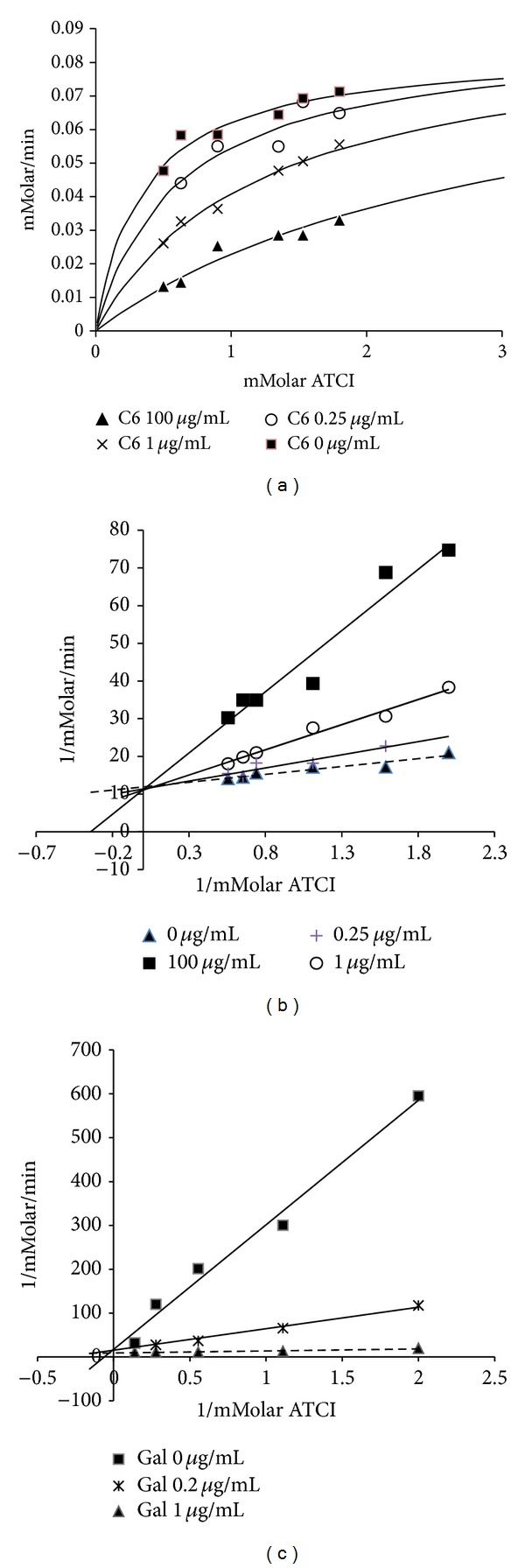
Kinetic study of inhibition of acetylcholinesterase by C6 fraction and galantamine (standard acetylcholinesterase inhibitor). (a) Michaelis-Menten kinetic for C6 fraction, (b) double reciprocal (Lineweaver Burk) plot for C6 fraction, and (c) double reciprocal (Line-weaver Burk) plot for galantamine.
According to the graph that associated varied substrate concentrations (1/mMolar ATCI) with velocities of product formation (1/mMolar/min) (Figure 5(b)), it is possible to find that high substrate concentrations displace the balance of the reaction and favor the formation of the enzyme-substrate complex, thus reducing the likelihood of the inhibitor (C6) to bond to the enzyme active site. This characteristic can be observed when considering the intersection between the reaction with and without the inhibitor on the y-axis. This behavior is typical of competitive inhibitors such as galantamine (Figure 5(c)), as described by Khan et al. [38]. In addition, the maximum velocity of product formation obtained in the absence or presence of C6 inhibitor at different concentrations remained constant (V max = 0.079 mMolar/min), and different K m values were observed in the absence of the inhibitor (K m = 0.28 mMolar ATCI) and in the presence of C6 at a 1 μg/mL concentration (K m = 1.20 mMolar ATCI). This is the first report of a kinetic study involving the model of interaction between AChE and fractions containing indole alkaloids found in Tabernaemontana, suggesting competitive inhibitory action.
Structures with quaternary nitrogen exhibit significant binding activity to aromatic wastes at the AChE active site [40], which justifies the activity of the fraction C6 according to the structural characteristics of the compound 12-methoxy-n-methyl-voachalotine, the most intense peak in C6 spectrum. However, it is worth emphasizing the need of purifying this compound in order to confirm these results. Even so, there is the possibility that the alkaloids described in this paper exhibit also bonding connection to subtypes of receptors, similar to other compounds already evaluated [41]; therefore, in vitro studies are also necessary in order to examine these possible mechanisms.
Given the potential of the extracts of T. catharinensis and their compounds, further investigations should be conducted with the purpose of purifying, identifying, and confirming their biological properties, having in view their therapeutic use.
References
- 1.Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430(7000):631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verri M, Pastoris O, Dossena M, et al. Mitochondrial alterations, oxidative stress and neuroinflammation in Alzheimer disease. International Journal of Immunopathology and Pharmacology. 2012;25(2):345–353. doi: 10.1177/039463201202500204. [DOI] [PubMed] [Google Scholar]
- 3.Youdim MBH, Buccafusco JJ. Multi-functional drugs for various CNS targets in the treatment of neurodegenerative disorders. Trends in Pharmacological Sciences. 2005;26(1):27–35. doi: 10.1016/j.tips.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Matozinhos CN, Konno TUP. Diversidade taxônomica de Apocynaceae na Serra Negra, MG, Brasil. Hoehnea. 2011;38(4):569–596. [Google Scholar]
- 5.Federici E, Palazzino G, Nicoletti M, Galeffi C. Antiplasmodial activity of the alkaloids of Peschiera fuchsiaefolia . Planta Medica. 2000;66(1):93–95. doi: 10.1055/s-0029-1243122. [DOI] [PubMed] [Google Scholar]
- 6.Munoz V, Moretti C, Sauvain M, et al. Isolation of bis-indole alkaloids with antileishmanial and antibacterial activities from Peschiera van heurkii . Planta Medica. 1994;60(5):455–459. doi: 10.1055/s-2006-959531. [DOI] [PubMed] [Google Scholar]
- 7.Mansoor TA, Dewanjee S, Borralho PM, Mulhovo S, Rodrigues CMP, Ferreira MJU. Cytotoxic alkaloids from the roots of Tabernaemontan elegans . Planta Medica. 2012;78(11, article 177) [Google Scholar]
- 8.Pereira CG, Leal PF, Sato DN, Meireles MAA. Antioxidant and antimycobacterial activities of Tabernaemoniana catharinensis extracts obtained by supercritical CO2 + cosolvent. Journal of Medicinal Food. 2005;8(4):533–538. doi: 10.1089/jmf.2005.8.533. [DOI] [PubMed] [Google Scholar]
- 9.Vieira IJC, Medeiros WLB, Monnerat CS, et al. Two fast screening methods (GC-MS and TLC-ChEI assay) for rapid evaluation of potential anticholinesterasic indole alkaloids in complex mixtures. Anais da Academia Brasileira de Ciencias. 2008;80(3):419–426. doi: 10.1590/s0001-37652008000300003. [DOI] [PubMed] [Google Scholar]
- 10.Taesotikul T, Panthong A, Kanjanapothi D, Verpoorte R, Scheffer JJC. Anti-inflammatory, antipyretic and antinociceptive activities of Tabernaemontana pandacaqui Poir . Journal of Ethnopharmacology. 2003;84(1):31–35. doi: 10.1016/s0378-8741(02)00264-7. [DOI] [PubMed] [Google Scholar]
- 11.Guida A, de Battista G, Bargardi S. The antibacterial activity of alkaloids obtained from Tabernaemontana catharinensis A.DC. Ars Pharmaceutica. 2003;44(2):167–173. [Google Scholar]
- 12.Gower AE, Pereira BDS, Marsaioli AJ. Indole alkaloids from Peschiera campestris . Phytochemistry. 1986;25(12):2908–2910. [Google Scholar]
- 13.Youdim MBH, Buccafusco JJ. Multi-functional drugs for various CNS targets in the treatment of neurodegenerative disorders. Trends in Pharmacological Sciences. 2005;26(1):27–35. doi: 10.1016/j.tips.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Ellman GL, Courtney KD, Andres V, Jr., Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology. 1961;7(2):88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 15.Raza A, Saeed A, Ibrar A, Muddassar M, Khan AA, Iqbal J. Pharmacological evaluation and docking studies of 3-thiadiazol and thioxo 1, 2, 4-triazolylcoumarin derivates as cholinesterase inhibitors. ISRN Pharmacology. 2012;2012:11 pages. doi: 10.5402/2012/707932.707932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.López MD, Pascual-Villalobos MJ. Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Industrial Crops and Products. 2010;31(2):284–288. [Google Scholar]
- 17.Zocoler MA, de Oliveira AJB, Sarragiotto MH, Grzesiuk VL, Vidotti GJ. Qualitative determination of indole alkaloids of Tabernaemontana fuchsiaefolia (Apocynaceae) Journal of the Brazilian Chemical Society. 2005;16(6):1372–1377. [Google Scholar]
- 18.van Beek TA, Kuijlaars FLC, Thomassen PHAM, Verpoorte R, Baerheim Svendsen A. Antimicrobially active alkaloids from Tabernaemontana pachysiphon . Phytochemistry. 1984;23(8):1771–1778. [Google Scholar]
- 19.Munigunti R, Mulabagal V, Calderón AI. Screening of natural compounds for ligands to PfTrxR by ultrafiltration and LC-MS based binding assay. Journal of Pharmaceutical and Biomedical Analysis. 2011;55(2):265–271. doi: 10.1016/j.jpba.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 20.Pereira PS, França SC, Oliveira PVA, Breves CMS, Pereira SIVP. Chemical constituents from T. catharinensis root bark: a brief NMR review of índole alkaloids and in vitro cytotoxicity. Quimica Nova. 2008;31:20–24. [Google Scholar]
- 21.van Beek TA, Verpoorte R, Svendsen AB. Tabernaemontana L. (Apocynaceae): a review of its taxonomy, phytochemistry, ethnobotany and pharmacology. Journal of Ethnopharmacology. 1984;10(1):1–156. doi: 10.1016/0378-8741(84)90046-1. [DOI] [PubMed] [Google Scholar]
- 22.Lépine F, Milot S, Zamir L, Morel R. Liquid chromatographic/mass spectrometric determination of biologically active alkaloids in extracts of Peschiera fuschiaefolia . Journal of Mass Spectrometry. 2002;37(2):216–222. doi: 10.1002/jms.277. [DOI] [PubMed] [Google Scholar]
- 23.Silverstein RM. Identificação espectrométrica de compostos orgânicos. 7th edition. Rio de Janeiro, Brazil: LTC; 2006. [Google Scholar]
- 24.Andrade MT, Lima JA, Pinto AC, Rezende CM, Carvalho MP, Epifanio RA. Indole alkaloids from Tabernaemontana australis (Müell. Arg) Miers that inhibit acetylcholinesterase enzyme. Bioorganic and Medicinal Chemistry. 2005;13(12):4092–4095. doi: 10.1016/j.bmc.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 25.Azoug M, Loukaci A, Richard B, et al. Alkaloids from stem bark and leaves of Peschiera buchtieni . Phytochemistry. 1995;39(5):1223–1228. [Google Scholar]
- 26.Rastogi K, Kapil RS, Popli SP. New alkaloids from Tabernaemontana divaricata . Phytochemistry. 1980;19(6):1209–1212. [Google Scholar]
- 27.Srivastava S, Singh MM, Kulshreshtha DK. A new alkaloid and other anti-implantation principles from Tabernaemontana heyneana . Planta Medica. 2001;67(6):577–579. doi: 10.1055/s-2001-16495. [DOI] [PubMed] [Google Scholar]
- 28.Pereira CG, Carvalho JE, Meireles MAA. Anticancer activity of Tabernaemontana catharinensis extract obtained by supercritical fluid extraction. Revista Brasileira de Plantas Medicinais. 2006;8(4):144–149. [Google Scholar]
- 29.Soares DC, Pereira CG, Meireles MAA, Saraiva EMB. Anti-Leishmania amazonensis activity of supercritical CO2 + ethanol extracts from Tabernaemontana catharinensis . Parasitology International. 2007;56(2):135–139. doi: 10.1016/j.parint.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi T, Takamura H, Matoba T, Terao J. HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1-diphenyl-2-picrylhydrazyl. Bioscience, Biotechnology and Biochemistry. 1998;62(6):1201–1204. doi: 10.1271/bbb.62.1201. [DOI] [PubMed] [Google Scholar]
- 31.Cheng Z, Moore J, Yu L. High-throughput relative DPPH radical scavenging capacity assay. Journal of Agricultural and Food Chemistry. 2006;54(20):7429–7436. doi: 10.1021/jf0611668. [DOI] [PubMed] [Google Scholar]
- 32.Santos AK, Magalhães TS, Monte FJ, de Mattos C. Alcalóides iboga de Peschiera affinis atribuição inequívoca dos deslocamentos químicos dos átomos de hidrogênio e carbono e atividade antioxidante. Quimica Nova. 2009;32(7):1834–1838. [Google Scholar]
- 33.Sathishkumar T, Baskar R. Evaluation of antioxidant properties of Tabernaemontana heyneana wall leaves. Indian Journal of Natural Products and Resources. 2012;3(2):197–207. [Google Scholar]
- 34.Fragoso V, Nascimento NCD, Moura DJ, et al. Antioxidant and antimutagenic properties of the monoterpene indole alkaloid psychollatine and the crude foliar extract of Psychotria umbellata Vell. Toxicology In Vitro. 2008;22(3):559–566. doi: 10.1016/j.tiv.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Estevão HS, Carvalho LC, Ferreira LM, Fernandes E, Marques MMB. Analysis of the antioxidant activity of the indole library: cyclic voltammetry versus ROS scavening activity. Tetrahedron Letters. 2011;52:101–106. [Google Scholar]
- 36.Chattipakorn S, Pongpanparadorn A, Pratchayasakul W, Pongchaidacha A, Ingkaninan K, Chattipakorn N. Tabernaemontana divaricata extract inhibits neuronal acetylcholinesterase activity in rats. Journal of Ethnopharmacology. 2007;110(1):61–68. doi: 10.1016/j.jep.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 37.Zhan ZJ, Yu Q, Wang ZL, Shan WG. Indole alkaloids from Ervatamia hainanensis with potent acetylcholinesterase inhibition activities. Bioorganic and Medicinal Chemistry Letters. 2010;20(21):6185–6187. doi: 10.1016/j.bmcl.2010.08.123. [DOI] [PubMed] [Google Scholar]
- 38.Khan MT, Orhan I, Senol FS, et al. Cholinesterase inhibitory activities of some flavonoid derivates and chosen xanthone and their molecular docking studies. Chemico-Biological Interactions. 2009;181(3):383–389. doi: 10.1016/j.cbi.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 39.Dvir H, Silman I, Harel M, Rosenberry TL, Sussman JL. Acetylcholinesterase: from 3D structure to function. Chemico-Biological Interactions. 2010;187(1–3):10–22. doi: 10.1016/j.cbi.2010.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harel M, Schalk I, Ehret-Sabatier L, et al. Quaternary ligand binding to aromatic residues in the active-site gorge of acetylcholinesterase. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(19):9031–9035. doi: 10.1073/pnas.90.19.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.King CHR, Meckler H, Herr RJ, Trova MP, Glick SD, Maisonneuve IM. Synthesis of enantiomerically pure (+)- and (-)-18-methoxycoronaridine hydrochloride and their preliminary assessment as anti-addictive agents. Bioorganic and Medicinal Chemistry Letters. 2000;10(5):473–476. doi: 10.1016/s0960-894x(00)00033-0. [DOI] [PubMed] [Google Scholar]


