Abstract
Prepulse inhibition (PPI) is a measure of sensorimotor gating, a pre-attentional inhibitory brain mechanism that filters extraneous stimuli. PPI is correlated with measures of cognition and executive functioning, and is considered an endophenotype of schizophrenia and other psychiatric illnesses in which patients demonstrate PPI impairments. As a first step towards identifying genes that regulate PPI, we performed a quantitative trait locus (QTL) screen of PPI phenotypes in a panel of mouse chromosome substitution strains (CSS). We identified five CSSs with altered PPI compared to the host C57BL/6J strain: CSS-4 exhibited decreased PPI, whereas CSS-10, -11, -16, and -Y exhibited higher PPI compared to C57BL/6J. These data indicate that A/J chromosomes 4, 10, 11, 16, and Y harbor at least one QTL region that modulates PPI in these CSSs. QTLs for the acoustic startle response were identified on seven chromosomes. Like PPI, habituation of the startle response is also disrupted in schizophrenia, and in the present study CSS-7 and -8 exhibited deficits in startle habituation. Linkage analysis of an F2 intercross identified a highly significant QTL for PPI on chromosome 11 between positions 101.5Mb – 114.4Mb (peak LOD = 4.54). Future studies will map the specific genes contributing to these QTLs using congenic strains and other genomic approaches. Identification of genes that modulate PPI will provide insight into the neural mechanisms underlying sensorimotor gating, as well as the psychopathology of disorders characterized by gating deficits.
Keywords: Sensorimotor gating, Quantitative trait locus, Linkage, Startle, Cognition, Psychopathology, Psychiatric disorders, Behavior, Mouse genetics
Introduction
Sensorimotor gating is a pre-attentional inhibitory brain mechanism that filters extraneous stimuli. Sensorimotor gating can be measured with prepulse inhibition (PPI) (Geyer et al. 2001), a phenomenon in which the startle response induced by a forceful stimulus is reduced by prior presentation of a weak stimulus (prepulse).
PPI is strongly interconnected with psychopathology and cognitive deficits. Decreased PPI has been documented in individuals with a range of psychiatric and neurological disorders (Braff et al. 2001). Although most evidence exists for schizophrenia (Swerdlow et al. 2008), PPI deficits are also reported in schizotypal personality disorder (Cadenhead et al. 1993; Cadenhead et al. 2002), bipolar disorder (Giakoumaki et al. 2007; Perry et al. 2001), and obsessive-compulsive disorder (Hoenig et al. 2005; Swerdlow et al. 1993), among others. Nearly all of these gating disorders also show impaired higher order cognition (Channon et al. 2003; Greisberg & McKay 2003; Kerns et al. 2008; Robinson et al. 2006). Furthermore, PPI deficits are strongly correlated with measures of cognition and executive function in healthy individuals and those with schizophrenia (Bitsios & Giakoumaki 2005; Bitsios et al. 2006; Giakoumaki et al. 2006; Greenwood et al. 2007; Karper et al. 1996; Perry et al. 1999). Habituation of the startle response is also disrupted in schizophrenia (Cadenhead et al. 1999) and may also be related to the observed cognitive deficits.
PPI has a substantial genetic basis, with 32-58% estimated heritability in humans (Anokhin et al. 2003; Greenwood et al. 2007) and 23-48% in inbred mouse strains (Joober et al. 2002; Willott et al. 2003). Numerous quantitative trait loci (QTLs) for PPI have been identified in rodents over the past decade (Hitzemann et al. 2001, 2008; Joober et al. 2003; Liu et al. 2003; Palmer et al. 2003; Petryshen et al. 2005; Watanabe et al. 2007). In recent years, QTL detection in mice has significantly improved due to new analytical methods, genomic resources, and mouse genetic mapping panels (Flint et al. 2005). These include mouse chromosome substitution strains (CSS), utilized in the present study, which offer many advantages over traditional genetic mapping strategies (Belknap 2003). The CSS panel we examined was created from a host C57BL/6J (B6) strain and a donor A/J strain, such that each CSS has a uniform B6 genetic background with the exception of one chromosome pair substituted from A/J (Nadeau et al. 2000). We and others have used the CSSs to identify QTLs for PPI (Petryshen et al. 2005), anxiety (Singer et al. 2005), fear conditioning (Ponder et al. 2007), motivational behaviors (Laarakker et al. 2008), and other complex traits (Buchner et al. 2008; Hoover-Plow et al. 2006; Nathan et al. 2006; Prows et al. 2007; Singer et al. 2004).
We performed a screen of PPI in the C57BL/6J-ChrA/J CSS panel as a starting point from which to identify PPI QTLs. Identification of genes that regulate PPI will improve knowledge of neural circuits involved in sensorimotor gating and higher cognitive functioning, as well as help delineate the genetic contribution to susceptibility of gating disorders.
Methods
Mice
Generation of the mouse CSS panel has been described previously (Nadeau et al. 2000; Singer et al. 2004). Each CSS line is essentially an inbred strain and was therefore maintained with brother-sister matings. CSSs were obtained by embryo transfer from Case Western Reserve University, with the exception of CSS-1, -4, -5, -7, -10, and -mt (mitochondria), which were purchased from the Jackson Laboratory. The B6 strain was also purchased from the Jackson Laboratory. All mice used for behavioral testing were bred in-house. Mice were weaned between 3-4 weeks of age and housed 2-5 per cage of the same sex. Mice were maintained on a 12 hour light:dark cycle (lights on at 0700 hours) with food and water ad libitum, except during behavioral testing. All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication no. 86-23, 1996) and were approved by the Massachusetts Institute of Technology Committee on Animal Care.
Behavioral Testing
Male mice, 7-9 weeks old, were tested between 10am and 6pm using a startle monitor system (Hamilton-Kinder, San Diego, CA). CSS and B6 mice were tested during the same sessions to allow for within-session comparison of PPI levels. Due to poor breeding, CSS-13 was unavailable for study. A total of 12-25 mice per CSS were tested over multiple test sessions (average 5.3 sessions for each CSS), and 115 B6 mice were tested across all sessions. Mice were habituated to the startle monitor on the two days prior to PPI testing by being placed in the monitor for 3 minutes with a constant 65 decibel (dB) white noise background. PPI testing sessions began with a 3 minute acclimatization period using a 65 dB background white noise that was maintained throughout the session. Mice were exposed to six replicate blocks of trials in pseudo-random order. Each block consisted of one pulse-alone trial of a 120 dB 40 millisecond (msec) white noise burst, 3 trials in which the pulse was preceded by 100 msec by a non-startling 20 msec prepulse of 70 dB, 75 dB, 80 dB or 85 dB intensities, and one null trial (no stimulus presented). We determined that these prepulse intensities do not themselves elicit a startle response in B6 mice (<2% of the startle elicited by a 120 dB pulse, data not shown). The inter-trial interval ranged from 6-8 seconds in pseudo-random order. The maximum response in Newtons (N) within a 65 msec record window was used as the startle amplitude. The startle reflex exhibits habituation (Blumenthal 1997), which was assessed by comparing the magnitude of four 120 dB pulse-alone trials presented at the beginning and end of the session, which were not utilized in PPI calculations. Following examples from the literature (Varty et al. 2000; Martinez et al. 1999), the percent habituation was calculated as 100 – ([mean startle block 2/mean startle block 1] × 100), where block 1 was the first four and block 2 was the last four pulse-alone trials. A subset of the current data (PPI for CSS-2, -16, and -18) has been previously reported (Petryshen et al. 2005).
The acoustic startle response (ASR) was defined as the mean startle amplitude of the six pulse-alone trials. Mice with negative PPI (i.e., higher startle following prepulse) at three or more prepulse intensities were excluded from the analyses. The total number of mice removed prior to analysis represented less than 1% of our total sample (3 out of 595 mice). For each prepulse intensity, the mean startle amplitude across the six replicate trials was calculated. PPI was defined as the percent reduction in the ASR when preceded by the prepulse compared to the ASR alone using the formula 100 * (1 – [mean startle amplitude with prepulse/ASR]). Higher PPI therefore corresponds to greater reduction of the ASR by the prepulse. PPI measures of each strain were determined for each of the four prepulse intensities (70, 75, 80, and 85 dB). Activity within the startle monitors was assessed by the null trials and was similar among all mice, indicating there were no effects of activity level on PPI.
Identification of CSS lines with ASR, habituation, and PPI variation
All statistical analyses were performed using SPSS 12.0. Comparison of CSS and B6 strains was performed using standard guidelines (Belknap 2003). The donor A/J strain was not tested, as comparison of CSSs to A/J is not informative for identifying chromosomes harboring QTLs, as each CSS differs from A/J at all but one chromosome. For all CSSs and B6, a main effect of strain on ASR was tested by one-way ANOVA, and on PPI by a repeated measures ANOVA using the PPI data at the four prepulse intensities. Posthoc analyses were performed to identify specific CSS with PPI differences compared to B6 using Dunnett's method. For the present study of 21 CSS (average of 22.7 mice per CSS) compared to 115 B6 mice (∼4.5:1 ratio of B6 to CSS), the genome-wide significance threshold was p = 0.003 and suggestive threshold was p = 0.05 (Belknap 2003). The same significance thresholds were applied in the posthoc analyses. Percent habituation of the ASR was analyzed by one-way ANOVA, and specific CSSs that differed from B6 were identified using Dunnett's posthoc comparisons.
Each CSS and B6 comparison provided 50% power to detect a QTL that contributes 18% of the trait variance (i.e., proportion of trait variance due to the QTL, v2) and 80% power to detect a QTL that contributes 26% variance, at the genome-wide significance threshold (Belknap 2003). As QTLs have larger effect sizes in a CSS versus B6 comparison than in traditional approaches, the above variances are equivalent to 6% and 9% variance, respectively, of a trait with a heritability of 0.4 and no dominance in an F2 cross of two inbred strains (Belknap 2003).
B6 mice used for comparison to the CSS were phenotyped throughout the study to enable examination of technical or environmental changes, as well as phenotypic drift. A main effect of test session on ASR and PPI was tested by ANOVA (p = 0.05). Additional tests for phenotypic drift in ASR and PPI were performed by ANOVA using B6 mice generated from breeding pairs purchased at the end of the study and compared to B6 mice tested throughout the study.
QTL mapping
Identification of PPI QTL on chromosome 11 was performed using MAPMAKER/QTL (Lander et al. 1987) similar to our previous study (Petryshen et al. 2005). Briefly, F2 intercross mice were generated by mating female CSS-11 mice to male B6 mice to generate F1 progeny that were heterosomic B6 and A/J for chromosome 11, followed by brother-sister intercrossing. A total of 129 male F2 intercross mice were tested for PPI at 70- to 85 dB prepulse intensities using the same behavioral protocol for PPI as stated above. The sample had 100% power to detect the chromosome 11 PPI QTL (alpha = 0.05 one-tailed), which was estimated to explain 28% of the variance in the CSS-11 80 dB prepulse PPI phenotype, using the methods of Belknap (2003). In each test session, B6 mice and CSS-11 mice were also tested to confirm the PPI phenotype of CSS-11 observed in our original sample. Mice were genotyped for 49 chromosome 11 SNPs (∼ 0.5 cM density) selected from the NCBI dbSNP database (http://www.ncbi.nlm.nih.gov/SNP/). Genotyping was performed using the Sequenom MassArray mass spectrometry system as described previously (Sklar et al. 2002). Genotype data from 43 SNPs met the following quality control criteria and were utilized for QTL analyses: (1) duplicate samples had identical genotypes, (2) SNPs were polymorphic in the F2 intercross, and (3) >85% of genotypes were obtained (note that MAPMAKER/QTL imputes missing genotype data by maximum likelihood estimation). Marker genetic distances were estimated from the intercross data and spanned a 91cM region. Additive, dominant, and recessive parametric models were tested. The chromosome-wide empirical significance threshold was determined by permutations (N = 10,000) of parametric linkage analyses using the observed PPI measures randomly assigned to the intercross mice, with the LOD score surpassed by 5% of the permuted analyses (LOD = 2.8) taken as the chromosome-wide threshold corresponding to a chromosome-wide p ≤ 0.05.
Results
CSSs with Significantly Altered ASR
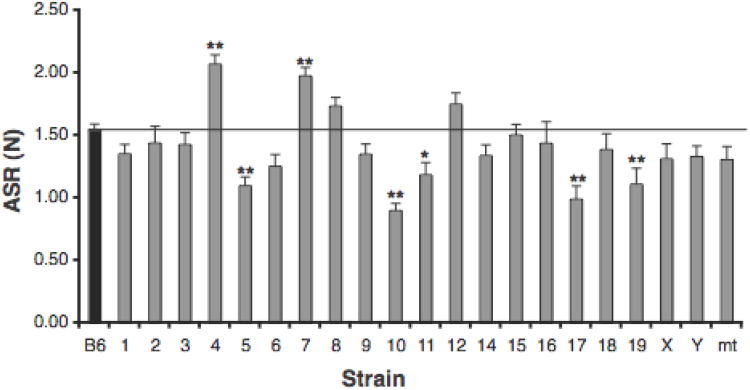
Mean ASR was calculated for each CSS and compared to the mean ASR of B6 (Table 1 and Figure 1). ANOVA detected a significant effect of strain [F(21,570) = 9.864, p < 0.001]. Posthoc analyses comparing each CSS to B6 detected significant or suggestive evidence for QTLs responsible for elevated ASR in CSS-4 and -7 and for decreased ASR in CSS-5, -10, -11, -17, and -19.
Table 1.
ASR and PPI for each chromosome substitution strain and the host B6 strain.
| Strain | ASR | 70 dB PPI | 75 db PPI | 80 db PPI | 85 db PPI | N |
|---|---|---|---|---|---|---|
| B6 | 1.55 ± 0.04 | 13.6 ± 1.3 | 24.1 ± 1.2 | 34.6 ± 1.3 | 39.6 ± 1.3 | 115 |
| CSS-1 | 1.35 ± 0.08 | 10.4 ± 2.1 | 25.1 ± 2.6 | 38.3 ± 2.7 | 43.6 ± 2.6 | 25 |
| CSS-2 | 1.44 ± 0.13 | 10.0 ± 2.2 | 20.2 ± 2.3 | 30.2 ± 2.2 | 35.6 ± 2.8 | 24 |
| CSS-3 | 1.43 ± 0.10 | 11.4 ± 1.8 | 22.0 ± 2.0 | 30.7 ± 2.3 | 33.2 ± 2.1 | 25 |
| CSS-4 | 2.07 ± 0.08*** | 9.8 ± 1.8 | 17.6 ± 1.9 | 26.0 ± 2.1 | 27.3 ± 2.6 | 25 |
| CSS-5 | 1.10 ± 0.07*** | 11.9 ± 2.1 | 24.4 ± 2.8 | 38.0 ± 3.0 | 40.8 ± 2.5 | 25 |
| CSS-6 | 1.25 ± 0.10 | 14.7 ± 2.1 | 28.2 ± 2.4 | 37.4 ± 2.7 | 36.8 ± 3.2 | 25 |
| CSS-7 | 1.98 ± 0.07*** | 15.6 ± 2.5 | 28.9 ± 2.8 | 37.2 ± 2.8 | 41.6 ± 3.0 | 25 |
| CSS-8 | 1.74 ± 0.07 | 10.7 ± 2.0 | 20.9 ± 2.6 | 31.5 ± 2.7 | 35.6 ± 2.7 | 25 |
| CSS-9 | 1.35 ± 0.08 | 16.6 ± 2.7 | 29.1 ± 2.3 | 41.7 ± 2.7 | 44.9 ± 2.2 | 25 |
| CSS-10 | 0.90 ± 0.06*** | 24.0 ± 2.6 | 42.0 ± 2.7 | 51.5 ± 2.8 | 48.3 ± 3.0 | 25 |
| CSS-11 | 1.19 ± 0.10** | 21.8 ± 2.6 | 40.8 ± 3.1 | 58.7 ± 3.6 | 56.6 ± 3.8 | 25 |
| CSS-12 | 1.75 ± 0.09 | 13.4 ± 2.2 | 26.0 ± 2.5 | 37.0 ± 2.8 | 43.8 ± 3.0 | 25 |
| CSS-14 | 1.34 ± 0.09 | 10.6 ± 4.0 | 20.7 ± 3.5 | 29.7 ± 2.4 | 36.7 ± 3.6 | 20 |
| CSS-15 | 1.51 ± 0.08 | 12.9 ± 1.7 | 22.6 ± 2.1 | 32.2 ± 2.2 | 37.2 ± 2.0 | 25 |
| CSS-16 | 1.44 ± 0.17 | 24.0 ± 3.1 | 35.4 ± 4.0 | 52.7 ± 4.4 | 57.2 ± 2.7 | 12 |
| CSS-17 | 0.99 ± 0.10*** | 12.5 ± 3.4 | 29.3 ± 4.5 | 44.1 ± 3.4 | 40.9 ± 3.9 | 17 |
| CSS-18 | 1.39 ± 0.13 | 12.8 ± 1.8 | 22.5 ± 2.0 | 32.4 ± 2.0 | 38.1 ± 2.4 | 18 |
| CSS-19 | 1.11 ± 0.13** | 13.3 ± 2.7 | 25.3 ± 2.3 | 35.0 ± 2.9 | 39.6 ± 3.3 | 18 |
| CSS-X | 1.31 ± 0.12 | 14.3 ± 1.7 | 25.1 ± 2.3 | 36.5 ± 2.4 | 42.9 ± 2.9 | 20 |
| CSS-Y | 1.33 ± 0.08 | 17.6 ± 2.5 | 32.6 ± 3.1 | 47.7 ± 2.5 | 53.4 ± 2.7 | 25 |
| CSS-mt | 1.31 ± 0.10 | 12.9 ± 2.4 | 28.3 ± 3.0 | 40.9 ± 3.1 | 41.8 ± 2.5 | 23 |
Acoustic startle response (ASR, Newtons) and prepulse inhibition (PPI, percent) are presented as mean +/− SEM for each strain. CSSs that differed from B6 in ASR are indicated by asterisks denoting significance levels:
p < 0.05,
p < 0.01,
p < 0.003.
CSSs that differed from B6 in repeated measures ANOVA of the PPI data at the four prepulse intensities are highlighted in bold text. CSS-10, 11, 16 and Y exhibited significantly higher PPI than B6 (p< 0.003). CSS-4 exhibited lower PPI than B6 at a statistically suggestive level (p < 0.05). The sample size (N) for each strain is also presented.
Figure 1.
Acoustic startle response (ASR) of B6 and CSS strains. Mean (+SEM) ASR in Newtons to a 120-db white noise burst. Several CSSs exhibited higher (CSS-4 and -7) or lower (CSS-5, -10, -11, -17, -19) ASR when compared to B6. Significance levels: *p< 0.05, **p< 0.003.
CSSs with Significantly Altered Habituation
B6 mice exhibited significant habituation of startle reactivity as assessed by two independent blocks of four 120 dB pulse-alone trials presented at the beginning and end of PPI testing (mean startle amplitude ± SEM was 1.51N ± 0.05 for block 1, and 1.28N ± 0.05 for block 2; percent habituation = 10.92 ± 3.1). Analysis of the B6 and CSS strains revealed a significant difference in percent habituation between strains [F(21, 539) = 2.775, p < 0.001]. Posthoc analyses revealed that only CSS-7 (percent habituation = -15.38 ± 7.5; p < 0.01) and CSS-8 (percent habituation = -13.69 ± 7.6; p < 0.02) significantly differed from B6 in that they did not exhibit habituation of startle between blocks 1 and 2.
CSSs with Significantly Altered PPI
Analyses of PPI of all CSSs and B6 using the data from the four prepulse levels tested (70, 75, 80, and 85 dB) detected a genome-wide significant effect of strain [F(63,1707) = 2.828, p < 0.001]. Posthoc analyses comparing each CSS to B6 across all PPI levels detected significant evidence for QTLs responsible for elevated PPI in CSS-10, -11, -16, and -Y (p < 0.003) and suggestive evidence for decreased PPI in CSS-4 (p < 0.05; Table 1 and Figure 2).
Figure 2.
Prepulse inhibition (PPI) of B6 and CSS strains. Mean (+SEM) PPI calculated as the percent reduction in the ASR by the: a) 70 dB prepulse, b) 75 dB prepulse, c) 80 dB prepulse, and d) 85 dB prepulse. Higher PPI corresponds to greater reduction in the ASR. Across all prepulse levels, CSS-4 exhibited lower (suggestive) PPI than B6, and CSS-10, -11, -16, and -Y exhibited significantly higher PPI than B6. Significance levels are presented based on post-hoc comparisons of B6 and CSSs at each prepulse level: *p< 0.05, **p< 0.003.
Relationship between ASR and PPI
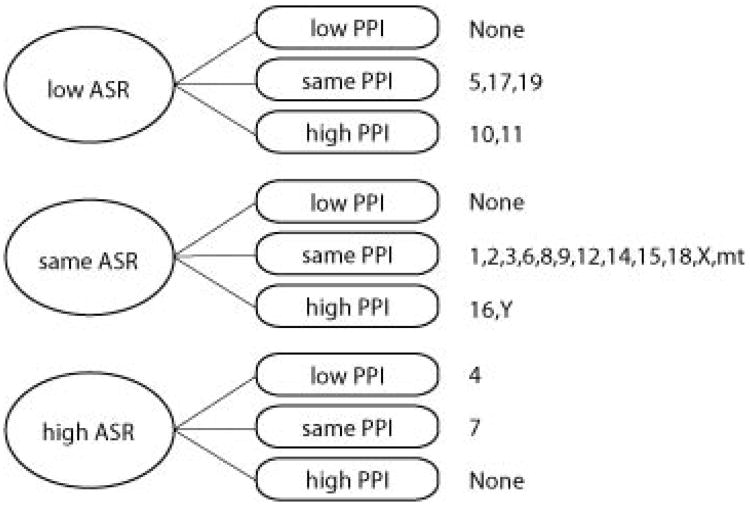
Pearson's correlations were calculated between ASR and PPI at each prepulse intensity for the B6 strain (Table 2). PPI levels were highly correlated across all prepulse intensities (r2 = 0.678 to 0.830, p < 0.001). ASR was negatively associated with PPI at moderate levels (r2 = −0.164 to −0.358 across the four PPI prepulse intensities). Both of these findings are consistent with previous reports (Csomor et al. 2008; Joober et al. 2002; Paylor & Crawley 1997). The correlations between ASR and PPI in the CSSs as a group were nearly identical to B6 mice (r2 = −0.167 to −0.344, p < 0.01; Supplementary Table 1). As the CSSs vary genetically, these data suggest that ASR and PPI do not share a strong genetic component, in which case the correlations would be expected to be greater than in B6 mice. This is supported by comparison of the ASR and PPI results for the CSS panel (Figure 4) suggest that ASR and PPI are relatively independent traits. Of those CSSs that significantly differ from B6, the CSSs are mostly evenly distributed between those that differ in ASR (either lower or higher than B6) but not PPI, those that differ in PPI but not ASR, and those that differ in both traits. However, there was considerable variation in the ASR-PPI correlations within individual CSSs. Only CSS-1, -5, -7, -9, -11, and –mt exhibited significant negative correlations between ASR and PPI for at least one prepulse level (p < 0.05), whereas the remaining CSSs did not show significant ASR-PPI correlations (Supplementary Table 1).
Table 2.
Correlations among ASR and PPI at various prepulse intensities in the B6 strain.
| ASR | 70 dB PPI | 75 dB PPI | 80 dB PPI | 85 dB PPI | |
|---|---|---|---|---|---|
| ASR | - | −0.164 | −0.232* | −0.358*** | −0.268** |
| 70 dB PPI | - | - | 0.678*** | 0.685*** | 0.699*** |
| 75 dB PPI | - | - | - | 0.717*** | 0.746*** |
| 80 dB PPI | - | - | - | - | 0.830*** |
| 85 dB PPI | - | - | - | - | - |
Pearson's correlations were calculated using mean values for acoustic startle response (ASR) and prepulse inhibition (PPI) at 70, 75, 80, and 85 dB prepulse intensities for B6 mice only. Significance levels:
p<0.05,
p<0.01,
p<0.001.
Figure 4.
Summary of ASR and PPI results in the CSS panel. The CSSs are categorized into those that had significantly lower or higher ASR, or no significant difference (same ASR) compared to B6, as well as significantly lower or higher PPI, or no significant difference (same PPI) compared to B6.
Longitudinal Analyses
Analysis of B6 data collected throughout the study did not detect a significant effect of test session on ASR [F(4,95) < 1.0] or PPI [F(5,110) < 1.0], indicating that there were no substantiated technical or environmental changes, or no significant phenotypic drift in the colony, during the course of the study. Lack of phenotypic drift was confirmed by comparison of a random subset of B6 mice tested throughout the study (N = 35) to an independent set of B6 mice (N = 33) bred within our colony from mice obtained from The Jackson Laboratory at the end of the study. No significant difference in PPI was detected between the two sets of mice [F(3,198) = 1.430, p > 0.1]. A trend towards lower ASR was found in the group tested at the study end (B6 throughout study ASR=1.48 ± 0.07; B6 at end of study ASR = 1.29 ± 0.07; F(1,66) = 3.786, p = 0.056).
QTL mapping identifies a significant PPI locus on chromosome 11
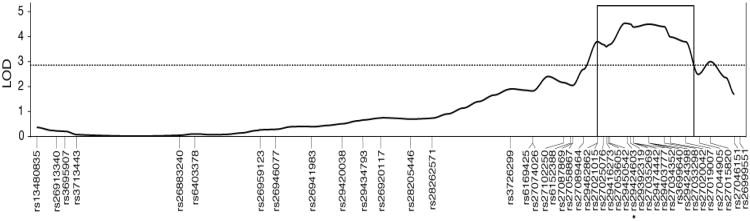
We performed genetic F2 intercross mapping to identify the PPI locus on chromosome 11 indicated by our finding of significantly elevated PPI in CSS-11 compared to B6 (Table 1 and Figure 2). Analysis of CSS-11 and B6 detected a highly significant effect of strain on PPI across all four prepulse intensities (CSS-11, N = 53; B6, N = 57; F(3,3.961), p = 0.009). Post hoc analyses indicated that the result was driven by the 75dB prepulse data (B6 mean = 27.5 ± 1.8; CSS-11 mean = 33.5 ± 2.1; ANOVA p = 0.031) and the 80dB prepulse data (B6 mean = 41.0 ± 2.1; CSS-11 mean = 48.1 ± 2.2; ANOVA p = 0.023). For this reason, and because the 80dB PPI data had the most normal distribution and thus was most suitable for parametric testing, we selected the 80dB PPI data for our initial linkage analysis. Single locus parametric analysis of the F2 intercross mice using 43 chromosome 11 SNPs identified a highly significant QTL (peak LOD = 4.54 at rs29424603, Figure 3) that surpassed the chromosome-wide significance threshold (LOD = 2.8). According to the recommendations of Manichaikul et al. (2006), we utilized a 1.8-LOD support interval to define the QTL region between markers rs29462862 and rs27044905 from chr11:101,475,308 – 114,442,851 (NCBI Build 37 genome assembly). Subsequent analyses of the 70, 75, and 85dB prepulse intensity PPI data supported linkage to the same chromosome 11 interval (peak LODs = 2.0, 4.9, and 4.3, respectively, at rs29424603). QTL analysis of the ASR phenotype did not detect significant linkage to chromosome 11 (peak LOD = 1.44 at rs27021015).
Figure 3.
Chromosome 11 F2 intercross parametric linkage analysis results. Chromosome 11 SNPs (N = 43) were tested for linkage with PPI at the 80 dB prepulse intensity in 129 F2 intercross mice. The single locus model multipoint LOD curve is indicated by the solid line. The 1-LOD confidence interval of the QTL is indicated by the boxed region. The chromosome-wide significance threshold (LOD = 2.8) is indicated by the dotted line. The most significant SNP (peak LOD = 4.54) is indicated by an asterisk.
Analyses of the PPI data at all four prepulse intensities indicated that the PPI QTL fit a recessive inheritance model in which A/J harbored the high PPI allele. This was apparent in comparison of the 80 dB PPI phenotype of F2 mice grouped according to genotype at the peak marker rs29424603, which differed significantly between genotypes [F(2,117) = 11.11, p < 0.001]. Posthoc analyses indicated that the AB heterozygote mice did not significantly differ from B6 allele homozygotes (mean PPI = 37.9 ± 1.9 and 33.9 ± 3.0, respectively; p > 0.1), whereas A/J allele homozygotes had significantly higher PPI than either group (mean PPI = 54.1 ± 3.7; p < 0.001 versus AB or B6).
Discussion
We performed a genetic screen in the C57BL/6J-ChrA/J CSS mouse panel as a starting point to identify genes that control sensorimotor gating. QTLs regulating PPI, ASR, or startle habituation were detected on numerous chromosomes. An advantage of the CSS approach is that effect sizes are typically larger in a CSS versus host strain comparison than in inbred F2 crosses, thereby greatly enhancing QTL detection (Belknap 2003; Shao et al. 2008).
Significant evidence was found for PPI QTLs on chromosomes 10, 11, 16, and Y in which the A/J allele increases PPI on the B6 background. Chromosome 4 had suggestive evidence for a QTL in which the A/J allele reduced PPI. The four autosomal PPI QTLs are supported by independent studies, suggesting that our loci represent bona fide genes that regulate gating. Several PPI QTLs are reported on chromosome 11 from crosses derived from B6 and either the A/J (Joober et al. 2003), C3H/He (Watanabe et al. 2007), or DBA/2J strains (Hitzemann et al. 2001, 2008). These include a QTL located ∼2Mb centromeric to our locus in which the A/J allele elevates PPI over B6 (Joober et al. 2003), as for our QTL, suggesting the two loci represent the same gene. We previously mapped two PPI QTLs on chromosome 16, Eppiq1 and Eppiq2 (Mouse Genome Database; http://www.informatics.jax.org/), using the same CSS linkage approach as this study (Petryshen et al. 2005). Support for these QTLs comes from selectively bred PPI mouse lines (Hitzemann et al. 2008) and a B6.A recombinant congenic panel (Joober et al. 2003). QTLs reported on chromosomes 4 and 10 (Hitzemann 2001; Watanabe et al. 2007) may or may not correspond to the loci we detected, as we have not mapped the intervals. PPI QTLs have been reported on other rodent chromosomes (Hitzemann et al. 2001, 2008; Joober et al. 2003; Liu et al. 2003; Palmer et al. 2003; Watanabe et al. 2007; Webb et al. 2009), suggesting that our QTLs are a subset of numerous loci controlling sensorimotor gating.
We also identified QTLs for ASR on chromosomes 4 and 7 (increased ASR versus B6) and on chromosomes 5, 10, 11 (suggestive), 17, and 19 (decreased ASR). The chromosomes 5, 10, 11, and 19 loci are supported by other studies (Hitzemann 2001; Liu et al. 2003). The lower response to acoustic startle of CSS-10 may be due to the age-related hearing loss loci ahl1/mdfw or ahl4 on chromosome 10, in which the A/J alleles are associated with hearing impairment (Noben-Trauth et al. 1997; Zheng et al. 2008). The Deafness dn locus on chromosome 19 (Keats et al. 1995) is probably not responsible for the lower startle of CSS-19, as hearing loss was not detected in this strain (Feng et al. 2000). Genes and QTLs affecting hearing exist on other chromosomes where we found ASR QTLs (http://www.informatics.jax.org/searches/allele_report.cgi?phenotypes=“age+related+hearing+loss”+OR+deafness), however it is unknown whether the A/J or B6 stains carry alleles associated with hearing loss.
QTLs for both PPI and ASR were found on chromosomes 4, 10 and 11, suggesting there may be genes regulating a neural mechanism common to both traits. Alternatively, separate genetic factors may be responsible, as suggested by the relatively low correlations between ASR and PPI in our and previous studies (Logue et al. 1997; McCaughran et al. 1999; Paylor & Crawley 1997). Furthermore, rodent and human studies have established that PPI is regulated by dopaminergic, serotonergic, cholinergic and glutamatergic forebrain processes, including the medial prefrontal cortex, hippocampus, striatum, thalamus, and basolateral amygdala (Geyer et al. 2001; Swerdlow et al. 2001). In contrast, the ASR neural circuitry is considerably less complex, consisting of a few synaptic connections linking the auditory nerve, cochlear nuclei, caudal pontine reticular nucleus, and motor neurons mediating motor output (Davis et al. 1982; Koch & Schnitzler 1997). The minor overlap between ASR and PPI neural circuits suggests that these processes are primarily governed by independent factors. Future linkage analyses are required to determine whether the PPI and ASR QTLs common to chromosomes 4, 10, or 11 represent the same or autonomous loci.
We also investigated habituation of the startle response, which like PPI is disrupted in individuals with schizophrenia (Cadenhead et al. 1999). CSS-7 and -8 exhibited significant habituation deficits compared to B6, suggesting that genes modulating habituation exist on the corresponding chromosomes. Genes contributing to habituation appear to be dissociable from those relevant to ASR and PPI, as the majority of CSSs with ASR and/or PPI phenotypes had normal startle habituation.
Many CSSs exhibited significantly lower or higher PPI levels than B6. As PPI is reported to be similar in the A/J and B6 strains (Bullock et al. 1997; Logue et al. 1997; Willott et al. 2003), our data suggest that A/J alleles of PPI genes operate differently when introgressed into the B6 background. Such transgressive effects have been previously documented for PPI (Hitzemann 2001) and other complex traits (Ponder et al. 2007; Shockley & Churchill 2006; Singer et al. 2004). These findings support the CSS approach as a feasible method to map complex traits that do not substantially differ between the parental strains. In other words, it is not critical to observe marked phenotypic differences between the parental strains in order to detect QTLs in a CSS panel.
It should be noted that we have observed very low ASR in A/J mice (data not shown) that is unlikely due to impaired hearing of the acoustic stimulus, as the mice also had reduced startle to a tactile air puff stimulus. Low ASR in A/J has been documented by other researchers (Varty et al. 2001), although not consistently (Logue et al. 1997; Paylor & Crawley 1997; Willott et al. 2003). It is therefore possible that a new mutation has arisen in some A/J populations. The potential impact on our results is unknown, however, as we do not know whether the donor A/J mice of the CSSs had the low startle phenotype.
Our finding of elevated PPI in the CSS-Y strain compared to B6 is intriguing. Several Y chromosome genes are expressed in mouse and human brain (Vawter et al. 2004; Xu et al. 2002). These include Usp9y and Ube1y, which function in ubiquitin-proteasome degradation that is causal in several neurological disorders (Jiang & Beaudet 2004), and Smcy/Jarid1d, whose X chromosome homolog Smcx functions in neuronal survival and dendritic development (Iwase et al. 2007). It is unknown whether these genes regulate sensorimotor gating, although their neural functions are in line with such a hypothesis. While only male mice were included in the present study, the Y chromosome gene implicated by our data could contribute to the observed sex differences in PPI, in addition to female sex hormones that are reported to have a role (Ison & Allen 2007; Kumari et al. 2008; Swerdlow et al. 1993).
QTL mapping identified a highly significant locus on chromosome 11 that is specific for PPI and not ASR. The QTL interval is over 10Mb in size and contains over 100 genes, including many with biological functions or phenotypes that make them strong candidates for the PPI gene (or genes) in this QTL. For example, reduced angiotensin converting enzyme (Ace) levels in mice causes resistance to dopamine-mediated disruption of PPI (van den Buuse et al. 2005). Also, transgenic mice carrying a coding mutation in the microtubule-associated protein tau (Mapt) gene have impaired PPI (Taniguchi et al. 2005). According to the Mouse Phenome Database (http://www.jax.org/phenome), 21 genes in the interval contain potentially functional non-synonymous coding SNPs between the B6 and A/J strains. These include regulator of G-protein signaling 9 (Rgs9) that functions in dopaminergic signaling and is enriched in striatum (Kovoor et al. 2005), the membrane protein palmitoylated 3 (Mpp3) MAGUK-like protein that regulates the serotonin 5HT2C receptor calcium response (Gavarini et al. 2006), granulin (Grn) which is implicated in neuronal survival (Baker et al. 2006, Cruts et al. 2006), the neurofilament glial fibrillary acidic protein (Gfap) that functions in myelination (Liedtke et al. 1996), and sidekick homolog 2 (Sdk2) which mediates synaptic connectivity of developing neurons (Yamagata et al. 2002). Clearly, refinement of the chromosome 11 QTL interval is required before initiating functional studies of positional candidate genes. Indeed, a key advantage of the CSS approach is that it facilitates QTL fine-mapping because congenic strain generation requires few crosses due to the homogeneous B6 background, in contrast to the numerous crosses required for traditional inbred strain QTL mapping.
The PPI deficits documented in psychiatric disorders provide impetus to identify genes that control sensorimotor gating, as they may also contribute to disease risk and pathology. Our study identified several mouse chromosomes containing PPI QTLs that provide a foundation for selecting candidate genes for functional studies to determine their roles in gating. Furthermore, CSSs with altered PPI can be assessed for cognition and other behavioral phenotypes commonly found in psychiatric illnesses where PPI deficits exist. Thus, the present results will facilitate delineation of the specific genetic contributions to sensorimotor gating, and the role of these genes in other phenotypes associated with psychiatric disease.
Supplementary Material
Supplementary Table 1: Correlations between ASR and PPI at each prepulse intensity in the B6 and CSS strains.
Pearson's correlations were calculated using mean values for acoustic startle response (ASR) and prepulse inhibition (PPI) at 70, 75, 80, and 85 dB prepulse intensities for each CSS and for all CSSs as a group. Correlations for B6 mice presented in Table 2 are included for comparison. Significance levels (2-tailed): *p<0.05, **p<0.01.
Acknowledgments
This research was supported in whole or in part by National Institutes of Health grants R21 MH071673 (PS) and RR12305 (JHN and AEBH), USPHS Award R01 MH0703930 (RPH), the Stanley Medical Research Institute (TLP), Young Investigator Awards from the National Alliance for Research on Schizophrenia and Depression (TLP), and a Canadian Institutes of Health Research Postdoctoral Fellowship (TLP). The authors wish to thank Dr. Mark Daly and Andrew Kirby for analytical assistance and Skye Waggoner for technical assistance.
References
- Anokhin AP, Heath AC, Myers E, Ralano A, Wood S. Genetic influences on prepulse inhibition of startle reflex in humans. Neurosci Lett. 2003;353:45–48. doi: 10.1016/j.neulet.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, Mcgowan E, Mann D, Boeve B, Feldman H, Hutton M. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–9. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- Belknap JK. Chromosome substitution strains: some quantitative considerations for genome scans and fine mapping. Mamm Genome. 2003;14:723–732. doi: 10.1007/s00335-003-2264-1. [DOI] [PubMed] [Google Scholar]
- Bitsios P, Giakoumaki SG. Relationship of prepulse inhibition of the startle reflex to attentional and executive mechanisms in man. Int J Psychophysiol. 2005;55:229–241. doi: 10.1016/j.ijpsycho.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Bitsios P, Giakoumaki SG, Theou K, Frangou S. Increased prepulse inhibition of the acoustic startle response is associated with better strategy formation and execution times in healthy males. Neuropsychologia. 2006;44:2494–2499. doi: 10.1016/j.neuropsychologia.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD. Prepulse inhibition decreases as startle reactivity habituates. Psychophysiology. 1997;34:446–450. doi: 10.1111/j.1469-8986.1997.tb02388.x. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Buchner DA, Burrage LC, Hill AE, Yazbek SN, O'Brien WE, Croniger CM, Nadeau JH. Resistance to diet-induced obesity in mice with a single substituted chromosome. Physiological genomics. 2008;35:116–122. doi: 10.1152/physiolgenomics.00033.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock AE, Slobe BS, Vazquez V, Collins AC. Inbred mouse strains differ in the regulation of startle and prepulse inhibition of the startle response. Behavioral neuroscience. 1997;111:1353–1360. doi: 10.1037//0735-7044.111.6.1353. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Geyer MA, Braff DL. Impaired startle prepulse inhibition and habituation in patients with schizotypal personality disorder. The American journal of psychiatry. 1993;150:1862–1867. doi: 10.1176/ajp.150.12.1862. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Carasso BS, Swerdlow NR, Geyer MA, Braff DL. Prepulse inhibition and habituation of the startle response are stable neurobiological measures in a normal male population. Biol Psychiatry. 1999;45:360–364. doi: 10.1016/s0006-3223(98)00294-7. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Light GA, Geyer MA, McDowell JE, Braff DL. Neurobiological measures of schizotypal personality disorder: defining an inhibitory endophenotype? The American journal of psychiatry. 2002;159:869–871. doi: 10.1176/appi.ajp.159.5.869. [DOI] [PubMed] [Google Scholar]
- Channon S, Pratt P, Robertson MM. Executive function, memory, and learning in Tourette's syndrome. Neuropsychology. 2003;17:247–254. doi: 10.1037/0894-4105.17.2.247. [DOI] [PubMed] [Google Scholar]
- Cruts M, Gijselinck I, Van Der Zee J, Engelborghs S, Wils H, Pirici D, Rademakers R, Vandenberghe R, Dermaut B, Martin JJ, Van Duijn C, Peeters K, Sciot R, Santens P, De Pooter T, Mattheijssens M, Van Den Broeck M, Cuijt I, Vennekens K, De Deyn PP, Kumar-Singh S, Van Broeckhoven C. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–4. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- Csomor PA, Yee BK, Vollenweider FX, Feldon J, Nicolet T, Quednow BB. On the influence of baseline startle reactivity on the indexation of prepulse inhibition. Behav Neurosci. 2008;122:885–900. doi: 10.1037/0735-7044.122.4.885. [DOI] [PubMed] [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci. 1982;2:791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Zheng J, Bennett WP, Heston LL, Jones IR, Craddock N, Sommer SS. Five missense variants in the amino-terminal domain of the glucocorticoid receptor: no association with puerperal psychosis or schizophrenia. Am J Med Genet. 2000;96:412–417. doi: 10.1002/1096-8628(20000612)96:3<412::aid-ajmg33>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Flint J, Valdar W, Shifman S, Mott R. Strategies for mapping and cloning quantitative trait genes in rodents. Nature reviews. 2005;6:271–286. doi: 10.1038/nrg1576. [DOI] [PubMed] [Google Scholar]
- Gavarini S, Becamel C, Altier C, Lory P, Poncet J, Wijnholds J, Bockaert J, Marin P. Opposite effects of psd-95 and mpp3 pdz proteins on serotonin 5-hydroxytryptamine2c receptor desensitization and membrane stability. Mol Biol Cell. 2006;17:4619–31. doi: 10.1091/mbc.E06-03-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Giakoumaki SG, Bitsios P, Frangou S. The level of prepulse inhibition in healthy individuals may index cortical modulation of early information processing. Brain research. 2006;1078:168–170. doi: 10.1016/j.brainres.2006.01.056. [DOI] [PubMed] [Google Scholar]
- Giakoumaki SG, Roussos P, Rogdaki M, Karli C, Bitsios P, Frangou S. Evidence of Disrupted Prepulse Inhibition in Unaffected Siblings of Bipolar Disorder Patients. Biological psychiatry. 2007;62:1418–1422. doi: 10.1016/j.biopsych.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Braff DL, Light GA, Cadenhead KS, Calkins ME, Dobie DJ, Freedman R, Green MF, Gur RE, Gur RC, Mintz J, Nuechterlein KH, Olincy A, Radant AD, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, et al. Initial heritability analyses of endophenotypic measures for schizophrenia: the consortium on the genetics of schizophrenia. Archives of general psychiatry. 2007;64:1242–1250. doi: 10.1001/archpsyc.64.11.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greisberg S, McKay D. Neuropsychology of obsessive-compulsive disorder: a review and treatment implications. Clin Psychol Rev. 2003;23:95–117. doi: 10.1016/s0272-7358(02)00232-5. [DOI] [PubMed] [Google Scholar]
- Hitzemann R, Malmanger B, Belknap J, Darakjian P, McWeeney S. Short-term selective breeding for high and low prepulse inhibition of the acoustic startle response; pharmacological characterization and QTL mapping in the selected lines. Pharmacol Biochem Behav. 2008;90:525–533. doi: 10.1016/j.pbb.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitzemann RJ, Bell J, Rasmussen E, McCaughran J. Mapping the genes for the acoustic startle response (ASR) and prepulse inhibition of the ASR in the BXD recombinant inbred series: effect of high-frequency hearing loss and cochlear pathology. In: Willott JF, editor. Handbook of mouse auditory research: from behavior to molecular biology. CRC Press; Boca Raton: 2001. pp. 441–455. [Google Scholar]
- Hoenig K, Hochrein A, Quednow BB, Maier W, Wagner M. Impaired prepulse inhibition of acoustic startle in obsessive-compulsive disorder. Biological psychiatry. 2005;57:1153–1158. doi: 10.1016/j.biopsych.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Hoover-Plow J, Shchurin A, Hart E, Sha J, Hill AE, Singer JB, Nadeau JH. Genetic background determines response to hemostasis and thrombosis. BMC Blood Disord. 2006;6:6. doi: 10.1186/1471-2326-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ison JR, Allen PD. Pre- but not post-menopausal female CBA/CaJ mice show less prepulse inhibition than male mice of the same age. Behavioural brain research. 2007;185:76–81. doi: 10.1016/j.bbr.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Iwase S, Lan F, Bayliss P, de la Torre-Ubieta L, Huarte M, Qi HH, Whetstine JR, Bonni A, Roberts TM, Shi Y. The X-linked mental retardation gene SMCX/JARID1C defines a family of histone H3 lysine 4 demethylases. Cell. 2007;128:1077–1088. doi: 10.1016/j.cell.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Jiang YH, Beaudet AL. Human disorders of ubiquitination and proteasomal degradation. Current opinion in pediatrics. 2004;16:419–426. doi: 10.1097/01.mop.0000133634.79661.cd. [DOI] [PubMed] [Google Scholar]
- Joober R, Zarate JM, Rouleau GA, Skamene E, Boksa P. Provisional mapping of quantitative trait loci modulating the acoustic startle response and prepulse inhibition of acoustic startle. Neuropsychopharmacology. 2002;27:765–781. doi: 10.1016/S0893-133X(02)00333-0. [DOI] [PubMed] [Google Scholar]
- Joober R, Zarate JM, Rouleau GA, Skamene E, Boksa P. Reply: Inappropriate choice of the experimental unit leads to a dramatic overestimation of the significance of quantitative trait loci for prepulse inhibition and startle response in recombinant congenic mice. Neuropsychopharmacology. 2003;28:819. doi: 10.1038/sj.npp.1300064. [DOI] [PubMed] [Google Scholar]
- Karper LP, Freeman GK, Grillon C, Morgan CA, 3rd, Charney DS, Krystal JH. Preliminary evidence of an association between sensorimotor gating and distractibility in psychosis. J Neuropsychiatry Clin Neurosci. 1996;8:60–66. doi: 10.1176/jnp.8.1.60. [DOI] [PubMed] [Google Scholar]
- Keats BJ, Nouri N, Huang JM, Money M, Webster DB, Berlin CI. The deafness locus (dn) maps to mouse chromosome 19. Mamm Genome. 1995;6:8–10. doi: 10.1007/BF00350886. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Nuechterlein KH, Braver TS, Barch DM. Executive functioning component mechanisms and schizophrenia. Biological psychiatry. 2008;64:26–33. doi: 10.1016/j.biopsych.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Koch M, Schnitzler HU. The acoustic startle response in rats--circuits mediating evocation, inhibition and potentiation. Behav Brain Res. 1997;89:35–49. doi: 10.1016/s0166-4328(97)02296-1. [DOI] [PubMed] [Google Scholar]
- Kovoor A, Seyffarth P, Ebert J, Barghshoon S, Chen CK, Schwarz S, Axelrod JD, Cheyette BN, Simon MI, Lester HA, Schwarz J. D2 dopamine receptors colocalize regulator of g-protein signaling 9-2 (rgs9-2) via the rgs9 dep domain, and rgs9 knock-out mice develop dyskinesias associated with dopamine pathways. J Neurosci. 2005;25:2157–65. doi: 10.1523/JNEUROSCI.2840-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Aasen I, Papadopoulos A, Bojang F, Poon L, Halari R, Cleare AJ. A comparison of prepulse inhibition in pre- and postmenopausal women and age-matched men. Neuropsychopharmacology. 2008;33:2610–2618. doi: 10.1038/sj.npp.1301670. [DOI] [PubMed] [Google Scholar]
- Laarakker MC, Ohl F, van Lith HA. Chromosomal assignment of quantitative trait loci influencing modified hole board behavior in laboratory mice using consomic strains, with special reference to anxiety-related behavior and mouse chromosome 19. Behav Genet. 2008;38:159–184. doi: 10.1007/s10519-007-9188-6. [DOI] [PubMed] [Google Scholar]
- Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newburg L. MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics. 1987;1:174–181. doi: 10.1016/0888-7543(87)90010-3. [DOI] [PubMed] [Google Scholar]
- Liedtke W, Edelmann W, Bieri PL, Chiu FC, Cowan NJ, Kucherlapati R, Raine CS. Gfap is necessary for the integrity of cns white matter architecture and long-term maintenance of myelination. Neuron. 1996;17:607–15. doi: 10.1016/s0896-6273(00)80194-4. [DOI] [PubMed] [Google Scholar]
- Liu D, Singh RP, Khan AH, Bhavsar K, Lusis AJ, Davis RC, Smith DJ. Identifying loci for behavioral traits using genome-tagged mice. J Neurosci Res. 2003;74:562–569. doi: 10.1002/jnr.10765. [DOI] [PubMed] [Google Scholar]
- Logue SF, Owen EH, Rasmussen DL, Wehner JM. Assessment of locomotor activity, acoustic and tactile startle, and prepulse inhibition of startle in inbred mouse strains and F1 hybrids: implications of genetic background for single gene and quantitative trait loci analyses. Neuroscience. 1997;80:1075–1086. doi: 10.1016/s0306-4522(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Manichaikul A, Dupuis J, Sen S, Broman KW. Poor performance of bootstrap confidence intervals for the location of a quantitative trait locus. Genetics. 2006;174:481–9. doi: 10.1534/genetics.106.061549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez ZA, Ellison GD, Geyer MA, Swerdlow NR. Effects of sustained phencyclidine exposure on sensorimotor gating of startle in rats. Neuropsychopharmacology. 1999;21:28–39. doi: 10.1016/S0893-133X(98)00137-7. [DOI] [PubMed] [Google Scholar]
- McCaughran J, Jr, Bell J, Hitzemann R. On the relationships of high-frequency hearing loss and cochlear pathology to the acoustic startle response (ASR) and prepulse inhibition of the ASR in the BXD recombinant inbred series. Behavior genetics. 1999;29:21–30. doi: 10.1023/a:1021433705004. [DOI] [PubMed] [Google Scholar]
- Nadeau JH, Singer JB, Matin A, Lander ES. Analysing complex genetic traits with chromosome substitution strains. Nat Genet. 2000;24:221–225. doi: 10.1038/73427. [DOI] [PubMed] [Google Scholar]
- Nathan BM, Hodges CA, Supelak PJ, Burrage LC, Nadeau JH, Palmert MR. A quantitative trait locus on chromosome 6 regulates the onset of puberty in mice. Endocrinology. 2006;147:5132–5138. doi: 10.1210/en.2006-0745. [DOI] [PubMed] [Google Scholar]
- Noben-Trauth K, Zheng QY, Johnson KR, Nishina PM. mdfw: a deafness susceptibility locus that interacts with deaf waddler (dfw) Genomics. 1997;44:266–272. doi: 10.1006/geno.1997.4869. [DOI] [PubMed] [Google Scholar]
- Palmer AA, Breen LL, Flodman P, Conti LH, Spence MA, Printz MP. Identification of quantitative trait loci for prepulse inhibition in rats. Psychopharmacology. 2003;165:270–279. doi: 10.1007/s00213-002-1258-0. [DOI] [PubMed] [Google Scholar]
- Paylor R, Crawley JN. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology. 1997;132:169–180. doi: 10.1007/s002130050333. [DOI] [PubMed] [Google Scholar]
- Perry W, Geyer MA, Braff DL. Sensorimotor gating and thought disturbance measured in close temporal proximity in schizophrenic patients. Archives of general psychiatry. 1999;56:277–281. doi: 10.1001/archpsyc.56.3.277. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Feifel D, Braff DL. Sensorimotor gating deficits in bipolar disorder patients with acute psychotic mania. Biological psychiatry. 2001;50:418–424. doi: 10.1016/s0006-3223(01)01184-2. [DOI] [PubMed] [Google Scholar]
- Petryshen TL, Kirby A, Hammer RP, Jr, Purcell S, O'Leary SB, Singer JB, Hill AE, Nadeau JH, Daly MJ, Sklar P. Two quantitative trait Loci for prepulse inhibition of startle identified on mouse chromosome 16 using chromosome substitution strains. Genetics. 2005;171:1895–1904. doi: 10.1534/genetics.105.045658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponder CA, Munoz M, Gilliam TC, Palmer AA. Genetic architecture of fear conditioning in chromosome substitution strains: relationship to measures of innate (unlearned) anxiety-like behavior. Mamm Genome. 2007;18:221–228. doi: 10.1007/s00335-007-9013-9. [DOI] [PubMed] [Google Scholar]
- Prows DR, Hafertepen AP, Winterberg AV, Gibbons WJ, Jr, Wesselkamper SC, Singer JB, Hill AE, Nadeau JH, Leikauf GD. Reciprocal Congenic Lines of Mice Capture the Aliq1 Effect on Acute Lung Injury Survival Time. Am J Respir Cell Mol Biol. 2007;38:68–77. doi: 10.1165/rcmb.2006-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LJ, Thompson JM, Gallagher P, Goswami U, Young AH, Ferrier IN, Moore PB. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. J Affect Disord. 2006;93:105–115. doi: 10.1016/j.jad.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Shao H, Burrage LC, Sinasac DS, Hill AE, Ernest SR, O'Brien W, Courtland HW, Jepsen KJ, Kirby A, Kulbokas EJ, Daly MJ, Broman KW, Lander ES, Nadeau JH. Genetic architecture of complex traits: large phenotypic effects and pervasive epistasis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19910–19914. doi: 10.1073/pnas.0810388105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockley KR, Churchill GA. Gene expression analysis of mouse chromosome substitution strains. Mamm Genome. 2006;17:598–614. doi: 10.1007/s00335-005-0176-y. [DOI] [PubMed] [Google Scholar]
- Singer JB, Hill AE, Burrage LC, Olszens KR, Song J, Justice M, O'Brien WE, Conti DV, Witte JS, Lander ES, Nadeau JH. Genetic dissection of complex traits with chromosome substitution strains of mice. Science. 2004;304:445–448. doi: 10.1126/science.1093139. [DOI] [PubMed] [Google Scholar]
- Singer JB, Hill AE, Nadeau JH, Lander ES. Mapping quantitative trait loci for anxiety in chromosome substitution strains of mice. Genetics. 2005;169:855–862. doi: 10.1534/genetics.104.031492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar P, Gabriel SB, McInnis MG, Bennett P, Lim YM, Tsan G, Schaffner S, Kirov G, Jones I, Owen M, Craddock N, DePaulo JR, Lander ES. Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. Mol Psychiatry. 2002;7:579–593. doi: 10.1038/sj.mp.4001058. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Benbow CH, Zisook S, Geyer MA, Braff DL. A preliminary assessment of sensorimotor gating in patients with obsessive compulsive disorder. Biological psychiatry. 1993;33:298–301. doi: 10.1016/0006-3223(93)90300-3. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology. 2008;199:331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T, Doe N, Matsuyama S, Kitamura Y, Mori H, Saito N, Tanaka C. Transgenic mice expressing mutant (N279K) human tau show mutation dependent cognitive deficits without neurofibrillary tangle formation. FEBS Lett. 2005;579:5704–5712. doi: 10.1016/j.febslet.2005.09.047. [DOI] [PubMed] [Google Scholar]
- van den Buuse M, Zheng TW, Walker LL, Denton DA. Angiotensin-converting enzyme (ACE) interacts with dopaminergic mechanisms in the brain to modulate prepulse inhibition in mice. Neuroscience letters. 2005;380:6–11. doi: 10.1016/j.neulet.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Varty GB, Paulus MP, Braff DL, Geyer MA. Environmental enrichment and isolation rearing in the rat: effects on locomotor behavior and startle response plasticity. Biol Psychiatry. 2000;47:864–873. doi: 10.1016/s0006-3223(99)00269-3. [DOI] [PubMed] [Google Scholar]
- Varty GB, Walters N, Cohen-Williams M, Carey GJ. Comparison of apomorphine, amphetamine and dizocilpine disruptions of prepulse inhibition in inbred and outbred mice strains. European Journal of Pharmacology. 2001;424:27–36. doi: 10.1016/s0014-2999(01)01115-3. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Evans S, Choudary P, Tomita H, Meador-Woodruff J, Molnar M, Li J, Lopez JF, Myers R, Cox D, Watson SJ, Akil H, Jones EG, Bunney WE. Gender-specific gene expression in post-mortem human brain: localization to sex chromosomes. Neuropsychopharmacology. 2004;29:373–384. doi: 10.1038/sj.npp.1300337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, Toyota T, Owada Y, Hayashi T, Iwayama Y, Matsumata M, Ishitsuka Y, Nakaya A, Maekawa M, Ohnishi T, Arai R, Sakurai K, Yamada K, Kondo H, Hashimoto K, Osumi N, Yoshikawa T. Fabp7 maps to a quantitative trait locus for a schizophrenia endophenotype. PLoS Biol. 2007;5:e297. doi: 10.1371/journal.pbio.0050297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb BT, McClay JL, Vargas-Irwin C, York TP, van den Oord EJ. In silico whole genome association scan for murine prepulse inhibition. PLoS ONE. 2009;4:e5246. doi: 10.1371/journal.pone.0005246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott JF, Tanner L, O'Steen J, Johnson KR, Bogue MA, Gagnon L. Acoustic startle and prepulse inhibition in 40 inbred strains of mice. Behav Neurosci. 2003;117:716–727. doi: 10.1037/0735-7044.117.4.716. [DOI] [PubMed] [Google Scholar]
- Xu J, Burgoyne PS, Arnold AP. Sex differences in sex chromosome gene expression in mouse brain. Human molecular genetics. 2002;11:1409–1419. doi: 10.1093/hmg/11.12.1409. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Weiner JA, Sanes JR. Sidekicks: Synaptic adhesion molecules that promote lamina-specific connectivity in the retina. Cel. 2002;110:649–60. doi: 10.1016/s0092-8674(02)00910-8. [DOI] [PubMed] [Google Scholar]
- Zheng QY, Ding D, Yu H, Salvi RJ, Johnson KR. A locus on distal chromosome 10 (ahl4) affecting age-related hearing loss in A/J mice. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2007.12.011. Epub Feb 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Correlations between ASR and PPI at each prepulse intensity in the B6 and CSS strains.
Pearson's correlations were calculated using mean values for acoustic startle response (ASR) and prepulse inhibition (PPI) at 70, 75, 80, and 85 dB prepulse intensities for each CSS and for all CSSs as a group. Correlations for B6 mice presented in Table 2 are included for comparison. Significance levels (2-tailed): *p<0.05, **p<0.01.