Abstract
Children with autism often suffer from sleep disturbances, and compared to age-matched controls, have decreased melatonin levels, as indicated by urine levels of the primary melatonin metabolite, 6-sulfatoxymelatonin (6-SM). We therefore investigated the relationship between 6-SM levels and sleep architecture in children with autism spectrum disorders (ASD). Twenty-three children, aged 4–10 years, completed two nights of polysomnography and one overnight urine collection for measurement of urinary 6-SM excretion rate. Parents completed the Children’s Sleep Habits Questionnaire. We found that higher urinary 6-SM excretion rates were associated with increased N3 sleep, decreased N2 sleep, and decreased daytime sleepiness. The results warrant further examination to examine the effects of supplemental melatonin on sleep architecture and daytime sleepiness.
Keywords: 6-Sulfatoxymelatonin, 6-SM, Sleep stages, Children’s Sleep Habits Questionnaire, Parental Concerns Questionnaire, Polysomnography
Introduction
Autism spectrum disorders (ASD) are neurodevelopmental disorders with an estimated prevalence of 1:110 children (Autism and Developmental Disabilities Monitoring Network 2009). The ASDs are characterized by delayed development or deficits in social and communication skills, restricted interests, and stereotyped behaviors (American Psychiatric Association 2000). Compared to age-matched controls and other developmental disorders, sleep disturbances are more common in children with ASD with an estimated prevalence of 44–83% (Richdale 1999). Both subjective data from parental questionnaires and sleep diaries, as well as objective data from polysomnograms and actigraphy, demonstrate that difficulty falling and/or maintaining sleep are the most common of these sleep problems (Honomichl et al. 2002; Malow et al. 2006; Richdale 1999; Wiggs and Stores 2004; Williams et al. 2004). In ASD individuals of all ages, sleep difficulties have been associated with intensified symptoms of autism, co-morbidities such as mood disorders and seizures, and family stress (Allik et al.2006; Malow 2004; Schreck et al. 2004). Therefore, there has been great interest in improving these sleep disturbances in individuals with ASD.
Melatonin is a neurohormone most notable for its role in regulating circadian and seasonal rhythms. Although there is evidence that melatonin is synthesized in multiple areas of the human body, including the bone marrow, gut, skin, platelets, and retina, most of the body’s melatonin is synthesized and secreted by the pineal gland (Cagnacci 1996; Champier et al. 1997; Conti et al. 2000; Slominski et al.2008). Melatonin secretion is regulated by the endogenous circadian clock, the suprachiasmatic nucleus, and entrained by daily light–dark cycles with increased production occurring during darkness (Cardinali and Pevet 1998). With longer nights and thus periods of darkness in the winter, it is tempting to assume that increased melatonin secretion occurs in the dark season (fall and winter), and less is secreted in the light season (spring and summer). Multiple studies have found that daytime levels of melatonin are higher in the dark season compared to the light season (Bojkowski and Arendt 1988; Kauppila et al. 1987; Ronnberg et al. 1990). However, as opposed to daytime levels, conclusions on how season affects melatonin’s nocturnal secretion are inconsistent, possibly due to the effect of artificial light (Beck-Friis et al. 1984; Kauppila et al. 1987; Luboshitzky et al. 1998; Sivan et al. 2001).
In the arena of ASD research, melatonin originally drew interest as a possible contributor for autism under the theory that hypersecretion of melatonin could result in a cascade of dysfunctional neurohormonal effects in the pineal–hypothalamic–pituitary–adrenal axis (Chamberlain and Herman 1990). However, 24-h monitoring of serum melatonin levels revealed that ASD individuals have lower nocturnal serum melatonin levels compared to controls (Brzezinski et al. 2005; Kulman et al. 2000; Melke et al.2008; Nir et al. 1995). Similarly, nocturnal excretion of urinary 6-sulfatoxymelatonin, the primary metabolite of melatonin, is low compared to controls (Tordjman et al.2005). These studies, however, have not related the level of endogenous nocturnal melatonin or the melatonin metabolite to subjective or objective measures of sleep.
The gold standard for objective measurement of sleep is the polysomnogram (PSG) which includes monitoring of electroencephalographic (EEG) waveforms. Based on characteristic EEG wave morphology, amplitude and frequency, a determination of whether a person is awake or asleep, and what stage of sleep a person is in can be made. There are four stages of sleep. They are non rapid eye movement (N1, N2, and N3) and rapid eye movement (REM) sleep (Iber et al. 2007). On a PSG, in addition to measuring the proportions of each of these sleep stages, parameters such as sleep latency (time to fall asleep) and sleep efficiency (proportion of the PSG the person was asleep) can be measured. In a comparison of polysomnographic measures in children with ASD who were “good” or “poor sleepers” (based on parent report) and typically developing children, sleep latency was the PSG parameter that was statistically significant with longer sleep latencies seen in the children with ASD who were poor sleepers (Goldman et al. 2009).
In the last decade, the main focus of melatonin research in ASD has been its use in the treatment of insomnia, defined as difficulty initiating and maintaining sleep. Open label and small randomized, placebo-controlled double-blind crossover trials of melatonin have shown improvement of insomnia in children with ASD, decreasing sleep latencies by about half, although polysomnography was not used as a measure of sleep in these studies (Andersen et al.2008; Garstang and Wallis 2006; Giannotti et al. 2006; Jan et al. 2000; Paavonen et al. 2003; Wirojanan et al. 2009). The sparse studies that have examined the impact of exogenous melatonin on sleep have focused on adult controls and involved small numbers of subjects (Brzezinski et al. 2005). Furthermore, the results have been inconsistent in terms of findings regarding sleep architecture (Brzezinski et al. 2005).
In this study, we examined the relationship between nocturnal melatonin metabolite levels (measured as nocturnal urine 6-sulfatoxymelatonin (6-SM) excretion rates) and sleep architecture in children with ASD. Several features of our work are unique: (1) polysomnography was employed as an objective measure of sleep, (2) a validated questionnaire was used as a subjective, parental report of sleep, and (3) our sample consisted of a well-defined cohort of children with ASD who were medication-free, seizure-free, without intellectual disability, and whose parentally-reported sleep problems ranged from none to severe sleep disruption.
Methods
Participants
This study was approved by the Vanderbilt University Institutional Review Board. All questionnaires and procedures were completed after informed consent was obtained from the parents of participants and assent was obtained from the child. Between October 3, 2005 and November 28, 2007, 24 children (23 boys and 1 girl) with ASD met study criteria and successfully completed overnight urinary 6-SM sampling. This sample was drawn from a larger sample of 30 children with ASD who were undergoing comprehensive sleep testing. The 6 children who did not complete the overnight urinary 6-SM sampling had nocturnal enuresis and were excluded from the study. Sleep data from this larger sample has been published previously (Goldman et al. 2009), but did not include analysis of urinary 6-SM excretion rate findings. Participants were recruited from Vanderbilt subspecialty clinics and surrounding community clinics.
The study criteria included: (1) clinical diagnosis of ASD (American Psychiatric Association 2000) confirmed by the Autism Diagnostic Observation Scale and the Autism Diagnostic Interview-Revised; (2) age 4–10 years; (3) no history of epilepsy or intellectual disability; (4) not taking any psychotropic medications at the time of the study; and (5) a score of ≥85 on the Peabody Picture Vocabulary Test-III (PPVT-III) which is a measure of receptive language function with a score < 85 being two standard deviations from the mean.
The Parental Concerns Questionnaire (PCQ), a validated measure of parental concerns in ASD (McGrew et al.2007), was used to define the degree of sleep disturbance within our study population. It includes one question asking parents to rate the degree of sleep disturbance in their child as no, mild, moderate, or a severe concern.
Sleep Measures
Children’s Sleep Habits Questionnaire (CSHQ)
Parents completed the CSHQ, a survey designed to screen children for the most common pediatric sleep diagnoses. The components of the questionnaire can be grouped into eight subscales representing common domains of pediatric sleep disorders (bedtime resistance, sleep onset delay, sleep duration, sleep anxiety, night wakings, parasomnias, sleep–disordered breathing, and daytime sleepiness). The CSHQ has been validated in children 2–10 years of age (Goodlin-Jones et al. 2008; Owens et al. 2000) and has been used extensively in autism as well as in typically developing children (Couturier et al. 2005; Goodlin-Jones et al. 2008; Malow et al. 2006). Higher scores on the CSHQ indicate a higher frequency of a sleep problem.
Polysomnography (PSG)
Objective sleep data were obtained on each subject using PSG. These studies were performed in the Sleep Research Core of the Vanderbilt Clinical Research Center (CRC). A digital system (Nihon-Kohden USA, Foothill Ranch, CA) was used to acquire PSG data. PSG monitoring included video, EEG sensors, right and left electro-oculographic (EOG) sensors, nasal pressure transducer and thermistor as measures of airflow, submental electromyography (EMG), right and left anterior tibialis EMG, ECG, pulse oximetry, and respiratory inductance plethysmography as indications of respiratory effort. To minimize anxiety related to the testing procedure, families were given a brochure describing the PSG procedures, the child was hooked up while engaged in preferred activities, and a parent stayed with the child during the testing. Two consecutive nights of polysomnography were obtained so that PSG data would not be affected by a first night effect from difficulty sleeping in an unfamiliar environment. No sedative-hypnotics were used. Start and end times of the PSG were similar to the subject’s routine sleep onset and wake time.
Data collected from overnight PSG were scored and analyzed using commercially available software (Polysmith Acquisition and Review Software, Version 4.0.17.0, Neurotronics, Inc.). One registered PSG technologist who was blinded to the subject’s classification and whether night 1 or 2 of PSG was being analyzed scored the PSGs for sleep stages, respiratory events (apneas and hypopneas), and periodic limb movements. Scoring was done according to standardized pediatric scoring (Iber et al. 2007). Scored studies were then reviewed by a sleep physician who was a Diplomate of the American Board of Sleep Medicine.
Sleep variables of interest that were obtained from the PSG included the following: total sleep time, percentage of total sleep time spent in each stage of sleep (N1, N2, N3 and REM), sleep latency, sleep efficiency, and wake time after sleep onset (WASO). Total sleep time was calculated as the sum of all epochs scored as sleep reported in minutes. Sleep latency was defined as the time from “lights off” to the first epoch of sleep reported in minutes. Sleep efficiency was calculated as the ratio of total sleep time to the total time between “lights off” to “lights on.” WASO was calculated as the sum of all epochs scored as wake during the total sleep time and was reported in minutes.
Biological Sample Collection
Overnight Urinary 6-Sulfatoxymelatonin (6-SM)
The pineal gland synthesizes and secretes melatonin into the circulatory system predominantly at night; however, measuring serum melatonin levels during sleep requires phlebotomy, a procedure poorly tolerated by many children with ASD. The primary metabolite of melatonin is 6-SM which is excreted in the urine. Urinary 6-SM excretion rates have been found to be a reliable reflection of both salivary and serum melatonin concentrations (Nowak et al.1987), and measurement of overnight urinary 6-SM excretion rates requires collection of only one urinary void. By measuring overnight urinary 6-SM excretion rates, we avoided invasive measures such as phlebotomy, placing an intravenous catheter, and using a urinary catheter, all of which may have disturbed a child’s sleep and thus could have introduced significant error into objective sleep measures.
First morning urinary voids were collected from the participants on the morning after the second night of PSG recording. The subject number, protocol number, date, time of the last void before bed, time of the first morning void, and the calculated hours between the last void before bed and the first morning void were recorded. Upon collection, the urine samples were stored in plastic sample containers and refrigerated. The CRC Core Laboratory personnel retrieved, processed the samples, and aliquotted the urine into the appropriate polypropylene storage tubes. These tubes were then placed in a −20°C freezer. The urine samples were thawed, mixed by inversion, and centrifuged at 2,000 rpm for 10 min. Urinary 6-SM levels were detected via the commercially available Buhlmann 6-sulfatoxymelatonin ELISA kit (ALPCO, Windham, NH). The lower detection limit for 6-SM in this assay is 0.8 ng/mL. The urinary 6-SM level was divided by the hours of collection to obtain a urinary 6-SM excretion rate (μg/h).
Statistical Analyses
Given the small sample size, Spearman (nonparametric) correlation was used to assess the associations between 6-SM excretion rate and averaged sleep parameters measured by polysomnography (sleep parameters were averaged to decrease a first night effect resulting from the child sleeping in a novel environment). As age (higher melatonin levels in younger children) and season (higher melatonin levels in the fall and winter) can influence melatonin levels, a secondary analysis was done to assess the association between 6-SM and sleep parameters while adjusting for age and seasonal effects. To do this, we used a linear regression model with the sleep parameter as the outcome, 6-SM level as the main independent variable, and age and season as the covariate variables. Analyses were performed using SAS Version 9.1.3 (SAS Institute Inc. Cary, NC).
Results
Study Population
The mean age of the sample was 5.7 ± 1.9 years. Our sample of ASD children exhibited heterogeneity in terms of parental sleep concerns. On the Parental Concerns Questionnaire, 9 children were classified as having no or mild parental sleep concerns while 15 children were classified as having moderate or severe parental sleep concerns. The mean nocturnal urinary 6-SM excretion rate was 1.15 ± 0.85 μg/h. Overnight urinary 6-SM excretion rates in our population of ASD children were comparable to the urinary 6-SM excretion rates previously reported in ASD children by Tordjman et al. (2005) (mean nocturnal urinary 6-SM excretion rate was 0.75 ± 0.11 μg/h), confirming that urinary 6-SM excretion rates are low in children with ASD (Table 1).
Table 1.
Description of study population
| Subject no. | Age (years) | Gender | Autism spectrum diagnosis |
Medications | Medical/surgical history |
|---|---|---|---|---|---|
| 1 | 6.08 | M | PDD-NOS | None | None |
| 2 | 4.03 | M | Autistic disorder | None | None |
| 3 | 5.02 | M | PDD-NOS | Zyrtec | None |
| 4 | 6.09 | M | Autistic disorder | None | Chronic diarrhea |
| 5 | 6.10 | M | Autistic disorder | None | None |
| 6 | 5.02 | M | Autistic disorder | None | None |
| 7 | 9.00 | M | Autistic disorder | None | None |
| 8 | 9.07 | M | PDD-NOS | Allergen drops, vitamin supplements |
Seasonal allergies |
| 9 | 4.03 | M | Autistic disorder | Singulair, zyrtec | Seasonal allergies |
| 10 | 5.00 | M | PDD-NOS | Flonase | Seasonal allergies |
| 11 | 4.05 | M | Autistic disorder | None | None |
| 12 | 6.04 | M | Autistic disorder | None | None |
| 13 | 8.00 | M | Autistic disorder | Claritin | None |
| 14 | 4.00 | M | PDD-NOS | Clarinex | Seasonal allergies |
| 15 | 5.09 | M | Autistic disorder | Miralax, multivitamin | None |
| 16 | 4.00 | M | Autistic disorder | None | None |
| 17 | 10.08 | M | Autistic disorder | Multivitamin | Mild asthma |
| 18 | 7.09 | M | Autistic disorder | Nasonex, zyrtec | Seasonal allergies, tonsillectomy/adenoidectomy |
| 19 | 4.09 | M | Autistic disorder | None | Seasonal allergies |
| 20 | 4.09 | M | Autistic disorder | Singulair, vitamin B6 complex | Seasonal allergies, tonsillectomy/adenoidectomy |
| 21 | 4.02 | M | Autistic disorder | Multivitamin, calcium supplement | Seasonal allergies |
| 22 | 8.01 | M | Asperger syndrome | None | Tonsillectomy/adenoidectomy |
| 23 | 5.00 | F | Autistic disorder | Multivitamin | None |
| 24 | 4.00 | M | PDD-NOS | Multivitamin | None |
PDD-NOS, Pervasive developmental disorder-not otherwise specified
Polysomnography
Twenty-three of the 24 children recruited to this study completed two consecutive nights of PSG. One child was able to tolerate only one night of the PSG. No participants had obstructive sleep apnea. Increased nocturnal urinary 6-SM excretion rates were associated with an increased percentage of N3 sleep (r = 0.45; p = 0.033, Fig. 1) and a decreased percentage of N2 sleep (r = −0.57; p = 0.005, Fig. 2). Urinary 6-SM excretion rates and percentages of N1 and REM sleep, sleep latency, sleep efficiency, total sleep time, or wake time after sleep onset were not significantly associated.
Fig. 1.
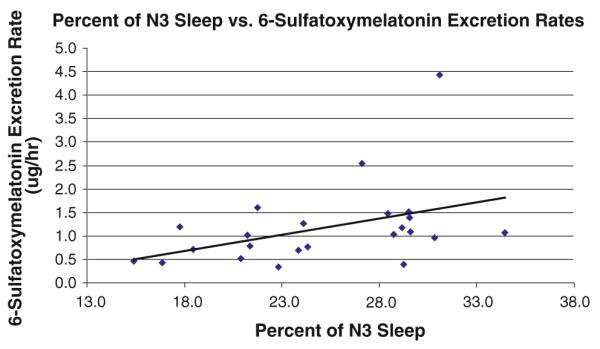
Higher rates of urinary 6-sulfatoxymelatonin excretion were associated with increased percentages of N3 sleep
Fig. 2.
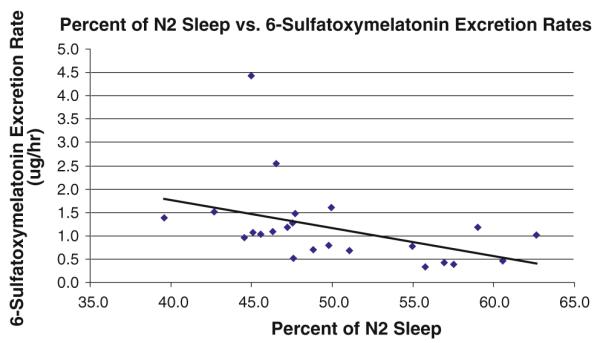
Higher rates of urinary 6-sulfatoxymelatonin excretion were associated with decreased percentages of N2 sleep
In a secondary analysis, we adjusted for the effects of age and season (see Statistical Analysis section). Nocturnal urinary 6-SM excretion rates remained marginally associated with percentage of N3 sleep (p = 0.06) and N2 sleep (p = 0.06). Neither age nor season alone were associated with the percentage of N2 or N3 sleep.
CSHQ
Completed CSHQs were obtained from 21 of 24 participants. Nocturnal urinary 6-SM excretion rates were inversely correlated with the daytime sleepiness subscale, with higher 6-SM excretion rates associated with less daytime sleepiness (r = −0.466; p = 0.03). No significant relationship was found with any other CSHQ subscale or the CSHQ total score. After adjusting for age and season, higher 6-SM excretion rates remained associated with less daytime sleepiness (p = 0.04). Neither age nor season alone were associated with daytime sleepiness.
Discussion
In this study, we related measurements of overnight urinary 6-SM, the primary metabolite of endogenous melatonin, to both subjective and objective measures of sleep in children with ASD. We confirmed prior work showing that 6-SM levels were overall low in children with ASD, and that children with ASD exhibited variability in 6-SM levels. Our work is unique in relating 6-SM levels to sleep architecture in these children. We found that 6-SM levels were positively associated with percentage of N3 sleep while being negatively associated with percentage of N2 sleep. Once age and season were controlled for, the associations remained marginally significant. Greater significance was likely not achieved due to the small sample size. We also found that 6-SM levels were negatively correlated with daytime sleepiness on the CSHQ.
Although it is tempting to conclude that melatonin promotes deeper levels of sleep, as well as ameliorates daytime sleepiness, as this is an association study, we cannot assume causation. It is also possible that children who exhibit deeper levels of sleep are less sleepy during the day, and are producing more urinary 6-SM independently of their sleep (e.g., related to the severity of their autism or other factors that influence 6-SM production, sleep, and sleepiness). Furthermore, although urinary overnight 6-SM has been shown to be an excellent measure of overnight plasma melatonin levels (Nowak et al. 1987), it is possible that our findings of higher 6-SM levels represent increased nocturnal metabolism of melatonin, rather than increased nocturnal production of melatonin. Future studies elucidating activity of enzymes converting melatonin to 6-SM, including CYP1A2, will be necessary for better definition of our results.
One of the strengths of our study is that our cohort of children were medication-free, seizure-free, and without intellectual disability. Our findings; however, cannot necessarily be generalized to the broader population of children with ASD. Another shortcoming of our study was that we sampled urinary 6-SM over the entire night, rather than in several hour increments. Choosing to sample urinary 6-SM over the entire night had the benefit of minimizing sleep disruption in these children and also avoided the use of catheters, an invasive procedure, in this population. Although overnight sampling of urinary 6-SM levels does provide an estimate of plasma melatonin levels (Graham et al. 1998), measuring urinary 6-SM over many hours does not adequately reflect phase shifts in the circadian rhythm (Nowak et al. 1987). Of note, we did not find associations between urinary 6-SM excretion rates and sleep parameters such as sleep latency. This may have reflected the fact that we did not obtain information on the specific segments of sleep but rather we captured more of an overall profile of the child’s sleep. In future work, we plan to obtain repeated blood specimens of melatonin to obtain phase information that may better reflect sleep latency and other sleep parameters.
Interestingly, a study on children with Asperger’s Disorder showed improvement in daytime behavioral measures when sleep latency on actigraphy was improved with melatonin administration (Paavonen et al. 2003). Due to melatonin’s popularity as a sleep aid in individuals with insomnia, it might be hypothesized that the improvement in daytime behavioral measures is due to longer sleep time. However, subjects in this study did not show a significant change in sleep duration (Paavonen et al. 2003). This raises the question of whether the improvement in daytime behavior with nocturnal melatonin administration may be related to melatonin’s effects on the proportion of specific sleep stages. Future studies appear warranted to study the effects of supplemental melatonin on sleep architecture, daytime sleepiness, and other aspects of daytime behavior.
Acknowledgments
Support provided by the National Alliance for Autism Research/Autism Speaks Foundation.
Contributor Information
Roberta M. Leu, Sleep Disorders Division, Department of Neurology, Vanderbilt University School of Medicine, Medical Center North, Room A-0118, 1161 21st Avenue South, Nashville, TN 37232-2551, USA
Liya Beyderman, Sleep Disorders Division, Department of Neurology, Vanderbilt University School of Medicine, Medical Center North, Room A-0118, 1161 21st Avenue South, Nashville, TN 37232-2551, USA.
Emmanuel J. Botzolakis, Medical Scientist Training Program, Vanderbilt University School of Medicine, Nashville, TN, USA
Kyla Surdyka, Sleep Disorders Division, Department of Neurology, Vanderbilt University School of Medicine, Medical Center North, Room A-0118, 1161 21st Avenue South, Nashville, TN 37232-2551, USA.
Lily Wang, Department of Biostatistics, Vanderbilt University School of Medicine, Nashville, TN, USA.
Beth A. Malow, Sleep Disorders Division, Department of Neurology, Vanderbilt University School of Medicine, Medical Center North, Room A-0118, 1161 21st Avenue South, Nashville, TN 37232-2551, USA; Vanderbilt Kennedy Center for Human Development, Vanderbilt University, Nashville, TN, USA
References
- Allik H, Larsson JO, Smedje H. Insomnia in school-age children with Asperger syndrome or high-functioning autism. BMC Psychiatry. 2006;6:18. doi: 10.1186/1471-244X-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th edition. text revision American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Andersen IM, Kaczmarska J, McGrew SG, Malow BA. Melatonin for insomnia in children with autism spectrum disorders. Journal of Child Neurology. 2008;23(5):482–485. doi: 10.1177/0883073807309783. [DOI] [PubMed] [Google Scholar]
- Autism and Developmental Disabilities Monitoring Network. United States, 2006 Prevalence of autism spectrum disorders. MMWR Surveillance Summaries. 2009;58(1):57–66. [PubMed] [Google Scholar]
- Beck-Friis J, von Rosen D, Kjellman BF, Ljunggren JG, Wetterberg L. Melatonin in relation to body measures, sex, age, season and the use of drugs in patients with major affective disorders and healthy subjects. Psychoneuroendocrinology. 1984;9(3):61–277. doi: 10.1016/0306-4530(84)90005-2. [DOI] [PubMed] [Google Scholar]
- Bojkowski CJ, Arendt J. Annual changes in 6-sulphatoxymelatonin excretion in man. Acta Endocrinologica. 1988;117(4):470–476. doi: 10.1530/acta.0.1170470. [DOI] [PubMed] [Google Scholar]
- Brzezinski A, Vangel MG, Wurtman RJ, Norrie G, Zhdanova I, Ben-Shushan A, et al. Effects of exogenous melatonin on sleep: A meta-analysis. Sleep Medicine Reviews. 2005;9(1):41–50. doi: 10.1016/j.smrv.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Cagnacci A. Melatonin in relation to physiology in adult humans. Journal of Pineal Research. 1996;21(4):200–213. doi: 10.1111/j.1600-079x.1996.tb00287.x. [DOI] [PubMed] [Google Scholar]
- Cardinali DP, Pevet P. Basic aspects of melatonin action. Sleep Medicine Reviews. 1998;2(3):175–190. doi: 10.1016/s1087-0792(98)90020-x. [DOI] [PubMed] [Google Scholar]
- Chamberlain RS, Herman BH. A novel biochemical model linking dysfunctions in brain melatonin, proopiomelanocortin peptides, and serotonin in autism. Biological Psychiatry. 1990;28(9):773–793. doi: 10.1016/0006-3223(90)90513-2. [DOI] [PubMed] [Google Scholar]
- Champier J, Claustrat B, Besancon R, Eymin C, Killer C, Jouvet A, et al. Evidence for tryptophan hydroxylase and hydroxy-indol-O-methyl-transferase mRNAs in human blood platelets. Life Sciences. 1997;60(24):2191–2197. doi: 10.1016/s0024-3205(97)00234-8. [DOI] [PubMed] [Google Scholar]
- Conti A, Conconi S, Hertens E, Skwarlo-Sonta K, Markowska M, Maestroni JM. Evidence for melatonin synthesis in mouse and human bone marrow cells. Journal of Pineal Research. 2000;28(4):193–202. doi: 10.1034/j.1600-079x.2000.280401.x. [DOI] [PubMed] [Google Scholar]
- Couturier JL, Speechley KN, Steele M, Norman R, Stringer B, Nicolson R. Parental perception of sleep problems in children of normal intelligence with pervasive developmental disorders: Prevalence, severity, and pattern. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(8):815–822. doi: 10.1097/01.chi.0000166377.22651.87. [DOI] [PubMed] [Google Scholar]
- Garstang J, Wallis M. Randomized controlled trial of melatonin for children with autistic spectrum disorders and sleep problems. Child: Care, Health and Development. 2006;32(5):585–589. doi: 10.1111/j.1365-2214.2006.00616.x. [DOI] [PubMed] [Google Scholar]
- Giannotti F, Cortesi F, Cerquiglini A, Bernabei P. An open-label study of controlled-release melatonin in treatment of sleep disorders in children with autism. Journal of Autism and Developmental Disorders. 2006;36(6):741–752. doi: 10.1007/s10803-006-0116-z. [DOI] [PubMed] [Google Scholar]
- Goldman SE, Surdyka K, Cuevas R, Adkins K, Wang L, Malow BA. Defining the sleep phenotype in children with autism. Developmental Neuropsychology. 2009;34(5):560–573. doi: 10.1080/87565640903133509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlin-Jones BL, Sitnick SL, Tang K, Liu J, Anders TF. The children’s sleep habits questionnaire in toddlers and preschool children. Journal of Developmental and Behavioral Pediatrics. 2008a;29(2):82–88. doi: 10.1097/dbp.0b013e318163c39a. [DOI] [PubMed] [Google Scholar]
- Goodlin-Jones BL, Tang K, Liu J, Anders TF. Sleep patterns in preschool-age children with autism, developmental delay, and typical development. Journal of the American Academy of Child and Adolescent Psychiatry. 2008b;47(8):930–938. doi: 10.1097/CHI.ObO13e3181799f7c. [DOI] [PubMed] [Google Scholar]
- Graham C, Cook MR, Kavet R, Sastre A, Smith DK. Prediction of nocturnal plasma melatonin from morning urinary measures. Journal of Pineal Research. 1998;24(4):230–238. doi: 10.1111/j.1600-079x.1998.tb00538.x. [DOI] [PubMed] [Google Scholar]
- Honomichl RD, Goodlin-Jones BL, Burnham M, Gaylor E, Anders TF. Sleep patterns of children with pervasive developmental disorders. Journal of Autism and Developmental Disorders. 2002;32(6):553–561. doi: 10.1023/a:1021254914276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson AL, Quan SF, for the American Academy of Sleep Medicine . The AASM manual for scoring of sleep and associated events: Rules, terminology and technical specification. 1st ed American Academy of Sleep Medicine; Westchester, IL: 2007. [Google Scholar]
- Jan JE, Hamilton D, Seward N, Fast DK, Freeman RD, Laudon M. Clinical trials of controlled-release melatonin in children with sleep-wake cycle disorders. Journal of Pineal Research. 2000;29(1):34–39. doi: 10.1034/j.1600-079x.2000.290105.x. [DOI] [PubMed] [Google Scholar]
- Kauppila A, Kivela A, Pakarinen A, Vakkuri O. Inverse seasonal relationship between melatonin and ovarian activity in humans in a region with a strong seasonal contrast in luminosity. The Journal of Clinical Endocrinology and Metabolism. 1987;65(5):823–828. doi: 10.1210/jcem-65-5-823. [DOI] [PubMed] [Google Scholar]
- Kulman G, Lissoni P, Rovelli F, Roselli MG, Brivio F, Sequeri P. Evidence of pineal endocrine hypofunction in autistic children. Neuroendocrinology Letters. 2000;21(1):31–34. [PubMed] [Google Scholar]
- Luboshitzky R, Yanai D, Shen-Orr Z, Israeli E, Herer P, Lavie P. Daily and seasonal variations in the concentration of melatonin in the human pineal gland. Brain Research Bulletin. 1998;47(3):271–276. doi: 10.1016/s0361-9230(98)00105-1. [DOI] [PubMed] [Google Scholar]
- Malow BA. Sleep disorders, epilepsy, and autism. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10(2):122–125. doi: 10.1002/mrdd.20023. [DOI] [PubMed] [Google Scholar]
- Malow BA, Marzec ML, McGrew SG, Wang L, Henderson LM, Stone WL. Characterizing sleep in children with autism spectrum disorders: A multidimensional approach. Sleep. 2006;29(12):1563–1571. doi: 10.1093/sleep/29.12.1563. [DOI] [PubMed] [Google Scholar]
- McGrew S, Malow BA, Henderson L, Wang L, Song Y, Stone WL. Developmental and behavioral questionnaire for autism spectrum disorders. Pediatric Neurology. 2007;37(2):108–116. doi: 10.1016/j.pediatrneurol.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Melke J, Goubran Botros H, Chaste P, Betancur C, Nygren G, Anckarsater H, et al. Abnormal melatonin synthesis in autism spectrum disorders. Molecular Psychiatry. 2008;13(1):90–98. doi: 10.1038/sj.mp.4002016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir I, Meir D, Zilber N, Knobler H, Hadjez J, Lerner Y. Brief report: Circadian melatonin, thyroid-stimulating hormone, prolactin, and cortisol levels in serum of young adults with autism. Journal of Autism and Developmental Disorders. 1995;25(6):641–654. doi: 10.1007/BF02178193. [DOI] [PubMed] [Google Scholar]
- Nowak R, McMillen IC, Redman J, Short RV. The correlation between serum and salivary melatonin concentrations and urinary 6-hydroxymelatonin sulphate excretion rates: Two non-invasive techniques for monitoring human circadian rhythmicity. Clinical Endocrinology (Oxford) 1987;27(4):445–452. doi: 10.1111/j.1365-2265.1987.tb01172.x. [DOI] [PubMed] [Google Scholar]
- Owens JA, Spirito A, McGuinn M. The children’s sleep habits questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23(8):1043–1051. [PubMed] [Google Scholar]
- Paavonen EJ, Nieminen-von Wendt T, Vanhala R, Aronen ET, von Wendt L. Effectiveness of melatonin in the treatment of sleep disturbances in children with Asperger disorder. Journal of Child and Adolescent Psychopharmacology. 2003;13(1):83–95. doi: 10.1089/104454603321666225. [DOI] [PubMed] [Google Scholar]
- Richdale AL. Sleep problems in autism: Prevalence, cause, and intervention. Developmental Medicine and Child Neurology. 1999;41(1):60–66. doi: 10.1017/s0012162299000122. [DOI] [PubMed] [Google Scholar]
- Ronnberg L, Kauppila A, Leppaluoto J, Martikainen H, Vakkuri O. Circadian and seasonal variation in human preovulatory follicular fluid melatonin concentration. The Journal of Clinical Endocrinology and Metabolism. 1990;71(2):492–496. doi: 10.1210/jcem-71-2-493. [DOI] [PubMed] [Google Scholar]
- Schreck KA, Mulick JA, Smith AF. Sleep problems as possible predictors of intensified symptoms of autism. Research in Developmental Disabilities. 2004;25(1):57–66. doi: 10.1016/j.ridd.2003.04.007. [DOI] [PubMed] [Google Scholar]
- Sivan Y, Laudon M, Tauman R, Zisapel N. Melatonin production in healthy infants: Evidence for seasonal variations. Pediatric Research. 2001;49(1):63–68. doi: 10.1203/00006450-200101000-00015. [DOI] [PubMed] [Google Scholar]
- Slominski A, Tobin DJ, Zmijewski MA, Wortsman J, Paus R. Melatonin in the skin: Synthesis, metabolism and functions. Trends in Endocrinology and Metabolism. 2008;19(1):17–24. doi: 10.1016/j.tem.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Tordjman S, Anderson GM, Pichard N, Charbuy H, Touitou Y. Nocturnal excretion of 6-sulphatoxymelatonin in children and adolescents with autistic disorder. Biological Psychiatry. 2005;57(2):134–138. doi: 10.1016/j.biopsych.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Wiggs L, Stores G. Sleep patterns and sleep disorders in children with autistic spectrum disorders: Insights using parent report and actigraphy. Developmental Medicine and Child Neurology. 2004;46(6):372–380. doi: 10.1017/s0012162204000611. [DOI] [PubMed] [Google Scholar]
- Williams PG, Sears LL, Allard A. Sleep problems in children with autism. Journal of Sleep Research. 2004;13:265–268. doi: 10.1111/j.1365-2869.2004.00405.x. [DOI] [PubMed] [Google Scholar]
- Wirojanan J, Jacquemont S, Diaz R, Bacalman S, Anders TF, Hagerman RJ, et al. The efficacy of melatonin for sleep problems in children with autism, fragile X syndrome, or autism and fragile X syndrome. Journal of Clinical Sleep Medicine. 2009;5(2):145–150. [PMC free article] [PubMed] [Google Scholar]


