Abstract
Importance
Optical coherence tomography (OCT) findings of temporal macular thinning are important in the diagnosis and prognosis of X-linked Alport syndrome (XLAS).
Objectives
To report OCT findings and severity of temporal macular thinning in a cohort with XLAS and to correlate these and other ocular findings with mutation genotype.
Design
Patients with XLAS underwent genotyping for COL4A5 mutations and complete eye examinations with retinal imaging using spectral domain OCT and fundus photography. Temporal macular thinning was calculated from OCT measurements by comparing the ratio of the retinal thickness of the temporal to the nasal subfields with a published normative database.
Setting
University departments of ophthalmology and nephrology.
Participants
Thirty-two patients from 24 families.
Main Outcome and Measures
Temporal thinning index calculated from spectral domain OCT scans.
Results
All study patients had a mutation associated with the X-linked COL4A5 gene. Eleven different mutations were identified. Eleven of 32 patients (34%) expressed the L1649R mutation. Of a total of 63 eyes with available OCT scans, 44 (70%) had severe pathological temporal macular thinning. The L1649R mutation was associated with the least amount of severe temporal macular thinning and later onset of renal failure.
Conclusions and Relevance
Temporal macular thinning is a prominent sign associated with XLAS, suggesting that OCT measurements are essential in the diagnosis and prognosis of the disease. The L1649R mutation in the COL4A5 gene causes a relatively mild form of XLAS characterized by late-onset renal failure and less frequent, severe temporal macular thinning relative to other COL4A5 mutations. The pathological basis for the retinal abnormalities of XLAS remains to be established.
ALPORT SYNDROME (AS) IS A hereditary multisystem disease characterized by hematuria, progressive renal failure, hearing loss, anterior lenticonus, and retinopathy. The kidney, ear, lens, and retina all share similar collagen chains that are mutated in different forms of AS.1-7 Among patients with AS, 85% have the X-linked subtype (XLAS), with a prevalence of 1 in 5000 to 1 in 50 000 individuals.8 More than 400 mutations affecting the X-linked COL4A5 gene, which encodes for the α5 chain in type IV collagen, have been described.9 Contrary to common belief, female heterozygotes with a COL4A5 mutation are not asymptomatic carriers; they nearly always display micro-hematuria, and eventually as many as 40% of them will develop end-stage renal failure.10 The autosomal recessive form results from homozygous or compound heterozygous mutations of the COL4A3 or COL4A4 genes and makes up a much smaller percentage of individuals with AS, although the ocular findings are similar to the X-linked form.6,11 The rare autosomal dominant form occurs with a heterozygous mutation of the COL4A3 or COL4A4 gene and is associated with a low risk for ocular and auditory abnormalities but with a higher risk for renal failure in female patients than XLAS.12
According to the published literature, the most common ocular findings in XLAS are anterior lenticonus and a dot-and-fleck retinopathy with an associated dull macular reflex or “lozenge” sign.6 Other less common ocular abnormalities include posterior polymorphous corneal dystrophy, recurrent corneal epithelial erosions, spontaneous anterior capsular rupture, posterior lenticonus, giant macular holes, and temporal macular thinning.13-20
At present, only 2 other articles have addressed temporal macular thinning as a finding in patients with XLAS. Usui and colleagues19 briefly reported a single case of unexplained bilateral thinning seen on optical coherence tomography (OCT). Savige and coworkers20 reported a study involving 10 patients with XLAS or autosomal recessive AS who had consistently thinned temporal macular regions in both inheritance patterns.
Temporal macular thinning and severity have yet to be correlated with specific mutations. We report a cohort of 32 patients with XLAS who had 11 different genetic mutations, prominent temporal macular thinning, and various retinal pathological findings. We correlate their presence and severity with each mutation type.
METHODS
PATIENTS
The University of Utah institutional review board approved the prospective collection of data for this study. Thirty-two patients with genetically confirmed XLAS consented and were included in this study (Table 1). The cohort consisted of 17 female and 15 male patients with XLAS; the patients ranged in age from 11 to 85 years. One patient permitted OCT imaging of just 1 eye, so 63 total eyes were included for the corresponding calculations. Mutation analysis was performed by various methods as described previously,21 and serum creatinine levels were measured for those patients who did not already have renal failure. All patients underwent slitlamp evaluation and dilated fundus biomicroscopy for lenticonus and retinal changes associated with XLAS by an ophthalmologist who specialized in retinal diseases (P.S.B.). The ophthalmologist sought confirmatory consultations with experienced anterior segment colleagues when equivocal lenticular changes were present. Anterior segment and fundus photographs were obtained to document findings.
Table 1.
Stratification and Incidence of COL4A5 Mutations Among Patients in the Cohorta
| COL4A5 Mutations | No. of Patients | No. of Families |
|---|---|---|
| L1649R | 11 | 7 |
| C1564S | 7 | 5 |
| 3528+T | 2 | 1 |
| G1060X | 2 | 2 |
| G576S | 2 | 1 |
| 4887del4 | 2 | 2 |
| K1097X | 2 | 2 |
| del ex2 | 1 | 1 |
| G635D | 1 | 1 |
| 4A5/A6del | 1 | 1 |
| G96A | 1 | 1 |
Mutations are described in the Results section.
TEMPORAL MACULAR THINNING
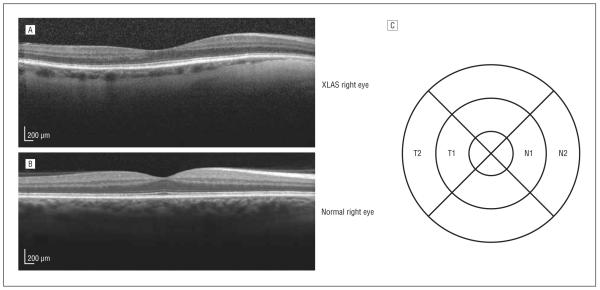
Spectral domain OCT (Spectralis; Heidelberg Engineering) was performed in a high-resolution mode on all patients to assess macular thickness changes. Each nasal and temporal subfield of the standard 6-mm macular OCT grid with 1-, 3-, and 6-mm-diameter circles centered on the fovea (Figure 1) was labeled as N1 (inner nasal), N2 (outer nasal), T1 (inner temporal), or T2 (outer temporal). The thinning of the temporal macula relative to the nasal macula (temporal thinning index [TTI]) was calculated using the following formula:
Because Spectralis OCT measurements are highly reproducible between instruments, and because we obtained a relative thinning measurement, we calculated the TTI and its standard deviations for a healthy population of 50 subjects (described by Grover and colleagues22), who then served as our reference population. Their healthy population was divided into 3 groups of individuals younger than 41, 41 to 60, and older than 60 years, and we further subdivided the TTI into 3 categories of normal physiological thinning (<1 SD of the mean), moderate pathological thinning (1-2 SDs above the mean), and severe pathological thinning (>2 SDs above the mean) (Table 2).
Figure 1.
Spectral domain optical coherence tomography (OCT) findings and evaluation method. A, Pathological temporal macular thinning of a right eye with X-linked Alport syndrome (XLAS) in an OCT image. B, Normal physiological thinning in a right eye in an OCT image. C, Corresponding grid with subfields at 1-, 3-, and 6-mm diameter-circles labeled T1 (inner temporal), T2 (outer temporal), N1 (inner nasal), and N2 (outer nasal) to represent those areas of the retina. The formula used to determine the temporal thinning index is given in the Temporal Macular Thinning subsection of the Methods section.
Table 2.
TTI Calculations by Age Group for 50 Normal Eyesa
| TTI by Age Group |
|||
|---|---|---|---|
| <41 y | 41-60 y | >60 y | |
| Physiological temporal thinning, mean TTI in a healthy cohort |
4.94 | 4.38 | 4.77 |
| Threshold for moderate temporal thinning, mean TTI + 1 SD |
6.60 | 6.33 | 6.54 |
| Threshold for severe temporal thinning, mean TTI + 2 SD |
8.25 | 8.28 | 8.31 |
Abbreviation: TTI, temporal thinning index.
Data are obtained from normative optical coherence tomography thickness data reported by Grover et al.22 Temporal macular thinning in patients with Alport syndrome was categorized as physiological (TTI, <1 SD of the mean for normal eyes), moderate (TTI, 1-2 SDs above the mean for normal eyes), and severe (TTI >2 SDs above the mean for normal eyes). TTI is given in the Temporal Macular Thinning subsection of the Methods section.
GENOTYPE-PHENOTYPE MATCHING
Mutations in the COL4A5 gene (OMIM 303630) were identified and confirmed by mapping of restriction sites, reverse transcriptase–polymerase chain reaction, denaturing gel electrophoresis, ribonuclease protection assays, allele-specific oligonucleotide probe tests, polymerase chain reaction with direct DNA sequencing, and multiplex genomic polymerase chain reaction–single-stranded conformational polymorphism in different families. References to these methods are given in a prior publication.21 Phenotypic ocular findings and history of renal transplant were correlated with genotype. Ocular findings included temporal macular thinning, lenticonus, pseudophakia, dot-and-fleck retinopathy, the lozenge sign, and lamellar macular hole. We used this information to determine the incidence per genotype as a function of the total number of eyes in the study.
RESULTS
All male and female patients in our cohort had XLAS with 1 of the following 11 COL4A5 mutations: L1649R, C1564S, G576S, G635D, G96A (missense), 3528+T (insertion), G1060X, K1097X (nonsense), 4887del4, del ex2 (deletion), and 4A5/A6del (large deletion). The L1649R mutation constituted the largest group of 11 patients (34%) in the cohort (Table 1). Pathological temporal macular thinning was an overwhelmingly prominent finding in this group of patients, with 44 eyes (70%) having severe thinning and 7 eyes (11%) having moderate thinning. The remaining 12 eyes (19%) were all within 1 SD of the normative mean. The lozenge sign was the second most common finding, occurring in 14 eyes (19%). The sign was not necessarily associated with dot-and-fleck retinopathy, which occurred in only 8 eyes (13%). Severe or moderate temporal macular thinning was not directly associated with the presence or absence of the lozenge sign or of dot-and-fleck retinopathy. Evidence of lamellar macular holes was present in 2 eyes (3%) on OCT studies. Pseudophakia and lenticonus were uncommon findings, occurring in 2 eyes (3%) and 1 eye (2%), respectively. We found no correlation between ocular findings and renal function, as assessed from serum creatinine values; however, renal transplantation was reported in 9 patients (28%).
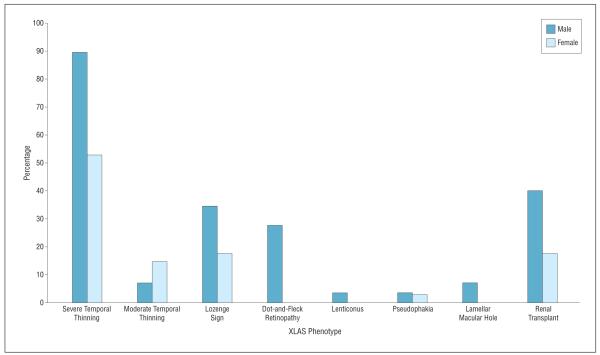
Phenotypic comparison by sex, shown in Figure 2, exhibited prominent findings in male patients in all categories except for moderate temporal thinning. Ocular abnormalities were generally more noteworthy in male (n = 29) than in female eyes (n = 34), including severe temporal macular thinning (26 [90%] vs 18 [53%]), the lozenge sign (10 [34%] vs 2 [6%]), dot-and-fleck retinopathy (8 [28%] vs 0), and lenticonus (1 [3%] vs 0). More male patients (6 of 15 [40%]) underwent a renal transplant than did female patients (3 of 17 [18%]). These phenotypic findings are consistent with X-linked inheritance of the COL4A5 mutation, in which female patients are less affected than male patients.
Figure 2.
Phenotypic comparison by sex. Ocular findings are reported as the percentage of total eyes for each sex. Renal transplants are reported as the percentage of patients for each sex. XLAS indicates X-linked Alport syndrome.
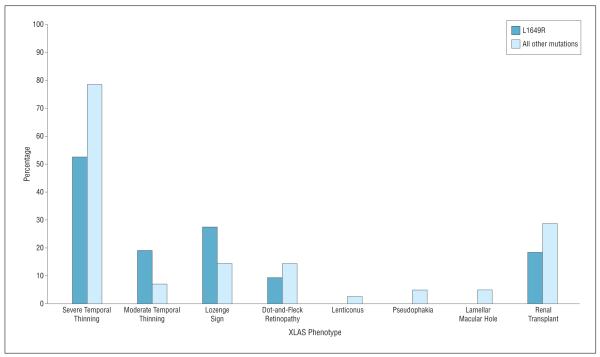
All mutations were associated with pathological temporal macular thinning; however, patients with L1649R had less severe temporal macular thinning compared with other mutations as a whole (11 of 21 [52%] vs 33 of 42 [79%]) (Figure 3). Dot-and-fleck retinopathy also occurred less frequently among the L1649R group patients (2 of 22 [9%] vs 6 of 42 [14%]), but moderate retinal thinning and the lozenge sign were more common in the L1649R group. Severe temporal macular thinning was found in all enrolled eyes with the rare 3528+T, G1060X, G576S, del ex2, and G635D mutations. The presence of dot-and-fleck retinopathy and the lozenge sign were highly associated with the 3528+T mutation. Otherwise, dot-and-fleck retinopathy and the lozenge sign were fairly uncommon in the total cohort (8 of 63 [13%] and 12 of 63 [19%], respectively). As expected, we found high right and left eye concordance in patients with severe and moderate temporal macular thinning.
Figure 3.
Phenotypic comparison between the L1649R and the other 10 mutations. Ocular findings are reported as the percentage of total eyes. Renal transplants are reported as the percentage of patients. XLAS indicates X-linked Alport syndrome.
Renal failure requiring a transplant occurred in all of our patients with 4887del4, G635D, and G96A mutations. Renal transplant was less common among patients with the L1649R genotype and occurred at an older mean age of 52 years (range, 50-54 years) compared with the mean age of renal transplant for all other mutations together of 35 years (range, 18-54 years), consistent with the relatively mild renal phenotype caused by this mutation.21
COMMENT
The most noted ocular findings in AS in the published literature are dot-and-fleck retinopathy and lenticonus.1,23 Among male patients with XLAS, 30% to 50% have been reported to have anterior lenticonus, and the reported incidence is at least 50% in female patients with the autosomal recessive form.8 Other than cataract and lenticonus, the ocular findings in XLAS tend to have no effect on vision. A decrease in vision due to lenticonus can be corrected with cataract surgery, although difficulty in performing the capsulorrhexis owing to the increased elasticity and tenuous nature of the thin capsular bag described in XLAS has been reported.24-27
The dot-and-fleck retinopathy tends to spare the perifoveal area and can range from a few dots in the temporal macula to a densely packed arrangement of dots forming an annulus around the perifovea, resulting in a dull macular reflex or lozenge sign.6 These dots appear to form curvilinear streaks that follow the nerve fiber layer.19
Temporal macular thinning has been described in a single case study by Usui and coworkers19 and in a study of 10 patients by Savige and colleagues.20 Macular changes are not a common finding; however, in our cohort, most eyes were found to have temporal macular thinning, specifically, 70% with severe thinning and another 11% with moderate thinning. Male patients were affected the most with severe thinning compared with female patients (90% vs 53%). Other ocular abnormalities and renal failure were also more prevalent among the male patients, which is consistent with X-linked inheritance (Figure 2).
Among the various mutations in our cohort, L1649R was the most common mutation affecting the COL4A5 gene. As previously reported,28 this mutation was associated with a mild form of XLAS and later onset of renal transplant (mean age, 52 years) compared with all other mutations as a whole (mean age, 35 years). Patients with the L1649R mutation also showed comparatively less ocular involvement. Other less common COL4A5 mutations, namely, C1564S, G576S, G1060X, 3528+T, del ex2, K1097X, and 4887del4, were associated with higher rates of severe temporal macular thinning compared with the L1649R-affected eyes (79% vs 52%) (Figure 3).
As a systemic disease, XLAS mainly affects the kidneys, ears, and eyes because of the presence of basement membrane abnormalities resulting from mutations in the COL4A5 gene. The phenotypic changes vary in type and severity. Colville and colleagues6 recently described the importance of the retinal lozenge sign as a diagnostic finding for AS, which carries prognostic value in determining early-onset renal failure if present. They reported that nearly 40% of their patients had the lozenge sign. The lozenge sign was a less prominent finding in our study, occurring in only 22% of total eyes. Dot-and-fleck retinopathy was even less common, with only 13% of all eyes affected. Severe and moderate temporal macular thinning, however, were very common among our cohort, as noted in the Results section, and did not correlate with a lozenge sign or with dot-and-fleck retinopathy. Anterior lenticonus was an extremely uncommon finding in our cohort, affecting only 2% of eyes, although another 3% were pseudophakic, presumably because of lenticonus.
In distinction to the published literature, our study found that anterior lenticonus, the lozenge sign, and dot-and-fleck retinopathy were not prominent diagnostic findings for XLAS. Temporal macular thinning was not associated directly with these ocular findings and occurred in their absence. Moreover, our study showed that temporal macular thinning occurs frequently in patients with XLAS and may correlate better with diagnosis and severity of disease. At present, no evidence indicates that temporal macular thinning causes any clinically significant visual disturbances. When we tested visual fields and conducted macular microperimetry and multifocal electroretinography in a few of our patients with XLAS, we were unable to detect any abnormalities that correlated with temporal macular thinning.
A recent study showed that the retinal pigment epithelium and choriocapillaris basal laminae contain α3α4α5 type IV collagen networks. These basal laminae were not markedly thinned, lamellated, or irregular in a single donor eye from an individual with a history of AS.20 In contrast, the retinal inner limiting membrane (ILM), which was also documented by these same investigators to contain an α3α4α5 type IV collagen network in unaffected individuals, was markedly thinned in AS cases according to OCT findings. Because XLAS-associated mutations in the COL4A5 gene commonly result in the loss of the α3α4α5 type IV collagen heterotrimer associated with thinning and lamination of affected basal laminae,24,29,30 we can reasonably assume that the primary defect leading to retinopathy is likely manifest primarily by the retinal ILM. However, the type IV collagen composition of the retinal ILM in XLAS cases is not known.
All 11 mutations observed in this study were associated with temporal macular thinning. Thinning was more severe in cases with the 3528+T, G1060X, G576S, del ex2, and G635D variants and less severe, in general, in cases with the L1649R variant. We speculate that the 11 variants identified herein likely affect the α3 through α5 type IV collagen network in the ILM and that the defective ILM is involved in the retinal thinning. However, 2 of the less severe mutations, L1649R and C1564S, occur in C-type lectinlike domains and a bone morphogenetic protein 4–binding domain, whereas the more severe mutations lie in regions not associated with any known functions.31
Further studies will be required to determine how defects in the ILM cause regional thinning of the temporal macula and whether retinas of affected individuals are thinned from birth. Because the ILM mediates attachment of the Müller cells to the underlying vitreous, one can speculate that the temporal thinning phenomenon may be related to the postnatal development of the macula, to tractional vitreoretinal forces, and/or to aberrant Müller cell adhesion. Others have suggested that Müller cells, in combination with vitreous traction, may be involved in this topographical retinopathy and the occurrence of macular holes that are a common feature of XLAS.20 These thoughts are purely speculative, however; the nature of temporal retinal thinning must await morphological assessment of XLAS eyes.
Very few investigations have been conducted assessing the ocular genotype-phenotype correlation in XLAS. The strengths of our study include a large cohort of patients with XLAS who underwent complete genotyping, which allows us to gain insight into the presence and severity of the ocular abnormalities and to correlate them with the severity of systemic involvement, namely, renal failure and age at onset. This study also shows the prominence of macular thinning among patients with XLAS, but it should not be considered pathognomonic because temporal macular thinning has also been reported in patients with proliferative sickle cell retinopathy and idiopathic macular telangiectasia.32,33 The much lower number of patients with individual mutations other than L1649R represents a limitation to our study that precludes extensive statistical analyses.
In conclusion, temporal macular thinning is highly associated with XLAS and is more common than dot-and-fleck retinopathy, the lozenge sign, or anterior lenticonus, which often have been considered to be pathognomonic of the disease. We find OCT testing to be extremely helpful in the diagnosis of XLAS, and an abnormal TTI may be a biomarker of severe disease that culminates in early-onset renal failure. Optical coherence tomography is quick, painless, noninvasive, relatively inexpensive, and operator independent, so it is particularly well suited for evaluation of suspected XLAS in female patients because nephrologists are often reluctant to perform renal biopsies in minimally symptomatic women.
Acknowledgments
Funding/Support: This study was supported by grant T35 HL007744 from the National Institutes of Health and by Research to Prevent Blindness.
Footnotes
Author Contributions: Drs Ahmed, Kamae, and Bernstein had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest Disclosures: None reported.
REFERENCES
- 1.Colville DJ, Savige J. Alport syndrome: a review of the ocular manifestations. Ophthalmic Genet. 1997;18(4):161–173. doi: 10.3109/13816819709041431. [DOI] [PubMed] [Google Scholar]
- 2.Kashtan CE. Alport syndrome: an inherited disorder of renal, ocular, and cochlear basement membranes. Medicine (Baltimore) 1999;78(5):338–360. doi: 10.1097/00005792-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Heidet L, Gubler MC. The renal lesions of Alport syndrome. J Am Soc Nephrol. 2009;20(6):1210–1215. doi: 10.1681/ASN.2008090984. [DOI] [PubMed] [Google Scholar]
- 4.Zehnder AF, Adams JC, Santi PA, et al. Distribution of type IV collagen in the cochlea in Alport syndrome. Arch Otolaryngol Head Neck Surg. 2005;131(11):1007–1013. doi: 10.1001/archotol.131.11.1007. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen CE. Lenticonus anterior and Alport’s syndrome. Acta Ophthalmol (Copenh) 1978;56(4):518–530. doi: 10.1111/j.1755-3768.1978.tb01365.x. [DOI] [PubMed] [Google Scholar]
- 6.Colville D, Wang YY, Tan R, Savige J. The retinal “lozenge” or “dull macular reflex” in Alport syndrome may be associated with a severe retinopathy and early-onset renal failure. Br J Ophthalmol. 2009;93(3):383–386. doi: 10.1136/bjo.2008.142869. [DOI] [PubMed] [Google Scholar]
- 7.Gross O, Netzer KO, Lambrecht R, Seibold S, Weber M. Meta-analysis of genotype-phenotype correlation in X-linked Alport syndrome: impact on clinical counselling. Nephrol Dial Transplant. 2002;17(7):1218–1227. doi: 10.1093/ndt/17.7.1218. [DOI] [PubMed] [Google Scholar]
- 8.Tan R, Colville D, Wang YY, Rigby L, Savige J. Alport retinopathy results from “severe” COL4A5 mutations and predicts early renal failure. Clin J Am Soc Nephrol. 2010;5(1):34–38. doi: 10.2215/CJN.01030209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pont-Kingdon G, Sumner K, Gedge F, et al. Molecular testing for adult type Alport syndrome. BMC Nephrol. 2009;17(10):38. doi: 10.1186/1471-2369-10-38. doi:10.1186/1471-2369-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jais JP, Knebelmann B, Giatras I, et al. X-linked Alport syndrome: natural history and genotype-phenotype correlations in girls and women belonging to 195 families: a “European Community Alport Syndrome Concerted Action” study. J Am Soc Nephrol. 2003;14(10):2603–2610. doi: 10.1097/01.asn.0000090034.71205.74. [DOI] [PubMed] [Google Scholar]
- 11.Shaw EA, Colville D, Wang YY, et al. Characterization of the peripheral retinopathy in X-linked and autosomal recessive Alport syndrome. Nephrol Dial Transplant. 2007;22(1):104–108. doi: 10.1093/ndt/gfl607. [DOI] [PubMed] [Google Scholar]
- 12.Marcocci E, Uliana V, Bruttini M, et al. Autosomal dominant Alport syndrome: molecular analysis of the COL4A4 gene and clinical outcome. Nephrol Dial Transplant. 2009;24(5):1464–1471. doi: 10.1093/ndt/gfn681. [DOI] [PubMed] [Google Scholar]
- 13.Teekhasaenee C, Nimmanit S, Wutthiphan S, et al. Posterior polymorphous dystrophy and Alport syndrome. Ophthalmology. 1991;98(8):1207–1215. doi: 10.1016/s0161-6420(91)32152-3. [DOI] [PubMed] [Google Scholar]
- 14.Burke JP, Clearkin LG, Talbot JF. Recurrent corneal epithelial erosions in Alport’s syndrome. Acta Ophthalmol (Copenh) 1991;69(4):555–557. doi: 10.1111/j.1755-3768.1991.tb02041.x. [DOI] [PubMed] [Google Scholar]
- 15.Wilson ME, Jr, Trivedi RH, Biber JM, Golub R. Anterior capsule rupture and subsequent cataract formation in Alport syndrome. J AAPOS. 2006;10(2):182–183. doi: 10.1016/j.jaapos.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Vedantham V, Rajagopal J, Ratnagiri PK. Bilateral simultaneous anterior and posterior lenticonus in Alport’s syndrome. Indian J Ophthalmol. 2005;53(3):212–213. doi: 10.4103/0301-4738.16691. [DOI] [PubMed] [Google Scholar]
- 17.Shah SN, Weinberg DV. Giant macular hole in Alport syndrome. Ophthalmic Genet. 2010;31(2):94–97. doi: 10.3109/13816811003767128. [DOI] [PubMed] [Google Scholar]
- 18.Rahman W, Banerjee S. Giant macular hole in Alport syndrome. Can J Ophthalmol. 2007;42(2):314–315. [PubMed] [Google Scholar]
- 19.Usui T, Ichibe M, Hasegawa S, et al. Symmetrical reduced retinal thickness in a patient with Alport syndrome. Retina. 2004;24(6):977–979. doi: 10.1097/00006982-200412000-00026. [DOI] [PubMed] [Google Scholar]
- 20.Savige J, Liu J, DeBuc DC, et al. Retinal basement membrane abnormalities and the retinopathy of Alport syndrome. Invest Ophthalmol Vis Sci. 2010;51(3):1621–1627. doi: 10.1167/iovs.08-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bekheirnia MR, Reed B, Gregory MC, et al. Genotype-phenotype correlation in X-linked Alport syndrome. J Am Soc Nephrol. 2010;21(5):876–883. doi: 10.1681/ASN.2009070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grover S, Murthy RK, Brar VS, Chalam KV. Normative data for macular thickness by high-definition spectral-domain optical coherence tomography (Spectralis) Am J Ophthalmol. 2009;148(2):266–271. doi: 10.1016/j.ajo.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Polak BCP, Hogewind BL. Macular lesions in Alport’s disease. Am J Ophthalmol. 1977;84(4):532–535. doi: 10.1016/0002-9394(77)90447-0. [DOI] [PubMed] [Google Scholar]
- 24.Streeten BW, Robinson MR, Wallace R, Jones DB. Lens capsule abnormalities in Alport’s syndrome. Arch Ophthalmol. 1987;105(12):1693–1697. doi: 10.1001/archopht.1987.01060120091033. [DOI] [PubMed] [Google Scholar]
- 25.Kato T, Watanabe Y, Nakayasu K, Kanai A, Yajima Y. The ultrastructure of the lens capsule abnormalities in Alport’s syndrome. Jpn J Ophthalmol. 1998;42(5):401–405. doi: 10.1016/s0021-5155(98)00031-8. [DOI] [PubMed] [Google Scholar]
- 26.Junk AK, Stefani FH, Ludwig K. Bilateral anterior lenticonus: Scheimpflug imaging system documentation and ultrastructural confirmation of Alport syndrome in the lens capsule. Arch Ophthalmol. 2000;118(7):895–897. [PubMed] [Google Scholar]
- 27.John ME, Noblitt RL, Coots SD, Boleyn KL, Ballew C. Clear lens extraction and intraocular lens implantation in a patient with bilateral anterior lenticonus secondary to Alport’s syndrome. J Cataract Refract Surg. 1994;20(6):652–655. doi: 10.1016/s0886-3350(13)80657-8. [DOI] [PubMed] [Google Scholar]
- 28.Barker DF, Pruchno CJ, Jiang X, et al. A mutation causing Alport syndrome with tardive hearing loss is common in the western United States. Am J Hum Genet. 1996;58(6):1157–1165. [PMC free article] [PubMed] [Google Scholar]
- 29.Rumpelt HJ, Langer KH, Schärer K, Straub E, Thoenes W. Split and extremely thin glomerular basement membranes in hereditary nephropathy (Alport’s syndrome) Virchows Arch A Pathol Anat Histol. 1974;364(3):225–233. doi: 10.1007/BF00433075. [DOI] [PubMed] [Google Scholar]
- 30.Arnold W. Considerations on the pathogenesis of the cochleo-renal syndrome [author’s translation] [in German] Acta Otolaryngol. 1980;89(3-4):330–341. doi: 10.3109/00016488009127145. [DOI] [PubMed] [Google Scholar]
- 31.Parkin JD, San Antonio JD, Pedchenko V, Hudson B, Jensen ST, Savige J. Mapping structural landmarks, ligand binding sites, and missense mutations to the collagen IV heterotrimers predicts major functional domains, novel interactions, and variation in phenotypes in inherited diseases affecting basement membranes. Hum Mutat. 2011;32(2):127–143. doi: 10.1002/humu.21401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murthy RK, Grover S, Chalam KV. Temporal macular thinning on spectral-domain optical coherence tomography in proliferative sickle cell retinopathy. Arch Ophthalmol. 2011;129(2):247–249. doi: 10.1001/archophthalmol.2010.357. [DOI] [PubMed] [Google Scholar]
- 33.Maruko I, Iida T, Sugano Y, Ojima A, Oyamada H, Sekiryu T. Demographic features of idiopathic macular telangiectasia in Japanese patients. Jpn J Ophthalmol. 2012;56(2):152–158. doi: 10.1007/s10384-011-0112-5. [DOI] [PubMed] [Google Scholar]