Abstract
The retinoid cycle is a series of biochemical reactions within the eye that is responsible for synthesizing the chromophore, 11-cis retinal, for visual function. The chromophore is bound to G-protein coupled receptors, opsins, within rod and cone photoreceptor cells forming the photosensitive visual pigments. Integral to the sustained function of photoreceptors is the continuous generation of chromophore by the retinoid cycle through two separate processes, one that supplies both rods and cones and another that exclusively supplies cones. Recent findings such as RPE65 localization within cones and the pattern of distribution of retinoid metabolites within mouse and human retinas have challenged previous proposed schemes. This review will focus on recent findings regarding the transport of retinoids, the mechanisms by which chromophore is supplied to both rods and cones, and the metabolism of retinoids within the posterior segment of the eye.
Keywords: visual cycle, retinoid, photoreceptors, opsin, visual pigment, retinal
1. Introduction
Retinoid cycling and metabolism within the eye have been studied extensively for decades. Indeed, George Wald received a Nobel Prize in 1967 for his recognition that the photoreceptive proteins in the eye are “chromoproteins” containing the essential 11-cis vitamin A retinaldehyde (11-cis retinal; see Figure 1 for retinoid structures) as its chromophore (Wald, 1935, 1951). Research has focused on elucidating the mechanisms by which 11-cis retinal is synthesized, transported between the photoreceptors and the retinal pigment epithelium (RPE), and incorporated within the rods and cones, as well as the process by which the product of pigment photobleaching, all-trans retinal, is metabolized. Although much is known and will be discussed in detail within this review, numerous mechanisms still remain to be clarified. Our recent findings that RPE65, the proposed isomerase in the RPE, is present within cones (Tang et al., 2011a; Tang et al., 2011b), and the unexpected distribution of the retinoid metabolite N-retinylidene-N-retinylethanolamine (A2E) ((Ablonczy et al., 2012a) and see Fig. 12) in the human RPE pose challenges to previously accepted mechanisms.
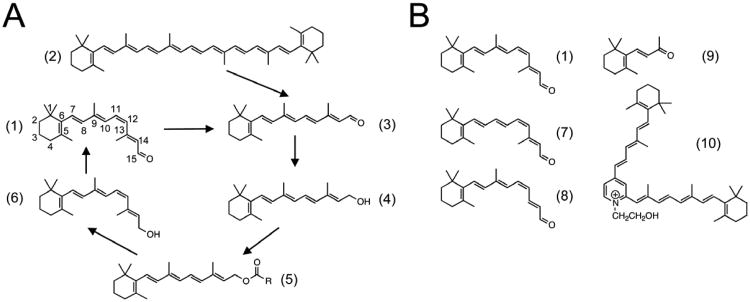
Figure 1. Chemical structures of retinoids involved in vision or used in characterizing opsin structure and function.
(A) Synthesis of 11-cis retinal from β-carotene and recycling of all-trans retinal. (B) Structures of retinoids. 1 = 11-cis retinal; 2 = β-carotene; 3 = all-trans retinal; 4 = all-trans retinol; 5 = all-trans retinylβ-ester (R represents fatty acid); 6 = 11-cis retinol; 7 = 9-demethyl 11-cis retinal; 8 = 13-demethyl 11-cis retinal; 9 = β-ionone; 10 = A2E.
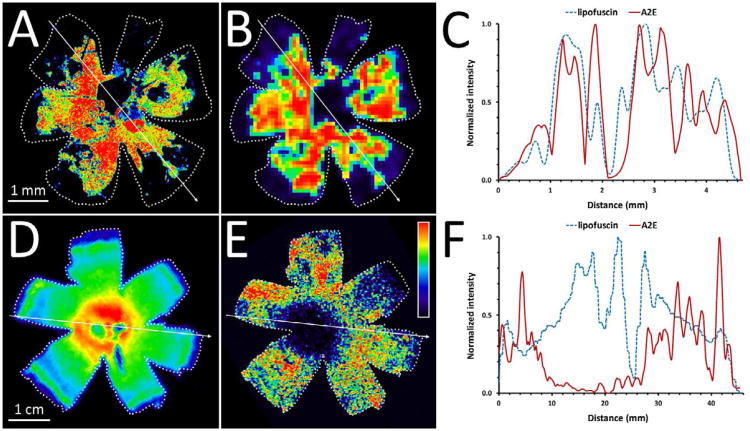
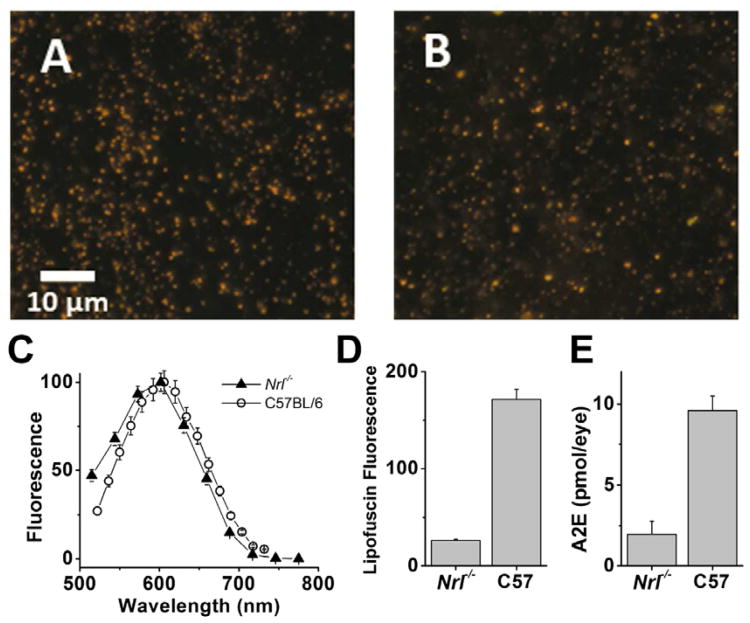
Figure 12. Lipofuscin fluorescence and A2E MALDI images in murine and human eyes.
The spatial distribution of lipofuscin fluorescence (A) and A2E (B) in the right eye of a 6-month-old Abca4-/- mouse. C, cross-sectional profiles along the arrows indicated in the previous panels show a good correlation between lipofuscin and A2E. The fluorescence image was collaged from individual fields taken with a fluorescent microscope (λexc = 488 nm). The MALDI image of A2E was collected at 150 mm resolution. Bar = 1 mm. The spatial distributions of lipofuscin fluorescence (D) and A2E (E) in a 94-year right human eye. F, cross-sectional profiles along the arrows indicated in the previous panels show that lipofuscin (which is central) does not correlate with A2E (which is peripheral). The fluorescence image was acquired in a Xenogen IVIS 200 bioluminescence imaging system (λexc = 450-490 nm, λem = 575 - 650 nm) at a final resolution of 500 μm. The MALDI image of A2E was collected at 300 μm resolution. Bar = 1 cm. The intensity is coded by the indicated false color scale. The images are oriented: dorsal – top; ventral – bottom; nasal – left; temporal – right.
This review will focus on recent findings regarding the transport of retinoids, the mechanisms by which visual pigments are regenerated, and retinoid metabolism within the retina and RPE. Key to the function of visual pigments is the supply of the essential chromophore, 11-cis retinal. There is growing evidence to show that two separate retinoid cycles exist, one that supplies both rods and cones with chromophore (classic visual cycle) and another that specifically supplies cones (cone visual cycle), possibly as a supplemental source. Furthermore, studies regarding retinoid analogues have shown that the binding constraints of the various rod and cone opsins are quite different. Contrary to early dogma, cones are not exclusively dependent upon 11-cis retinal for visual pigment formation, but can also utilize the alcohol form 11-cis retinol. Finally, we will discuss the fate of the retinoid as it is released from the opsin after the absorption of light as well as its regeneration back to the 11-cis form. This area is currently of great interest due to the toxic nature of the metabolites and the implications for further understanding and treatment of retinal degenerative diseases.
2. Visual pigments and chromophore binding
2.1 Visual photoreceptors
The retina is the anatomical component of the eye responsible for encoding light into neuronal signals for vision, a process collectively known as phototransduction. Photoreceptors are highly specialized neurosensory cilia within the retina that initiate this cascade of events and are classified into two major families, rods and cones, to mediate low- and bright-light vision, respectively. Humans are more reliant upon cones to carry out daily functions, but the health of both families of photoreceptors is essential for normal vision, as massive death of rods leads to cone degeneration (Hicks and Sahel, 1999; John et al., 2000). The highest concentration of cones within the human retina is found in the macula, the region that is responsible for sharp central vision, while rods compose the majority of photoreceptor cells in the surrounding (peripheral) retina.
Photoreceptors are structurally compartmentalized into regions located within distinct layers of the outer retina (Figure 2; for review, see (Mustafi et al., 2009)) and make synaptic associations with secondary neurons such as bipolar cells in the outer plexiform layer (OPL). The nuclei of the photoreceptor cells are located in the outer nuclear layer (ONL); the majority of organelles involved in cellular metabolism are present in the inner segment (IS); and the phototransduction components reside in the outer segment (OS). In rod-dominant retinas such as in mice and humans, rods comprise over 94% of the total photoreceptor population (Carter-Dawson and LaVail, 1979). The majority of the rod phototransduction proteins are associated with disc membranes isolated within the OS, whereas cone phototransduction proteins are embedded in the OS plasma membrane that is arranged into stacked invaginations (Arikawa et al., 1992). This open OS structure allows cones to have a much larger surface area in the OS region than rods, facilitating rapid exchange of retinoids and ions between the cell and its surrounding environment (Fu and Yau, 2007).
Figure 2. Mouse retina and photoreceptor cell morphology.
(A) The retina is located in the posterior portion of the eyecup (left) and is organized into multiple distinct layers (right). (B) Schematic of rod and cone photoreceptor cells. RPE = retinal pigment epithelium; OS = outer segment; IS = inner segment; ONL = outer nuclear layer; INL = inner nuclear layer; IPL = inner plexiform layer; GCL = ganglion cell layer.
2.2 Characteristics of visual pigments
Light-sensitivity in the human eye is mediated by the rod pigment rhodopsin and three cone pigments, all of which contain a protein component (opsin) and the chromophore 11-cis retinal. Rod and cone opsins are members of the super-family of G-protein-coupled receptors (Shichida and Imai, 1998). The ligand (chromophore) for opsins is covalently linked to the strictly conserved lysine residue in the seventh transmembrane helix to form the visual pigment. The chromophore is the same for all of mammalian visual pigments, and thus it is the interaction between the protein and 11-cis retinal that gives each pigment its distinct spectral properties. There have been extensive studies on defining the various residues that control the “tuning” of the visual pigments, that is the control of the absorption properties of those visual pigments (Hunt et al., 2009).
11-cis Retinal serves as an inverse agonist to the opsins (Ala-Laurila et al., 2009) resulting in a reduction of the photoreceptor resting current. Light isomerizes the 11-cis chromophore to an all-trans form (Figure 1), leading to a conformational change of the opsin to an intermediate Metarhodopsin II (Meta II) that activates its G-protein, transducin. The pigment remains in an active conformation for some time, but further transducin activation is inhibited by phosphorylation of the opsin C-terminal tail and subsequent arrestin binding. Eventually, all-trans retinal is released from the opsin, the bound arrestin dissociates, and the protein is dephosphorylated. To fully deactivate the receptor and prepare it to respond to another photon, the opsin binds additional 11-cis retinal to regenerate visual pigment. The sources of chromophore can come from the RPE as well as Müller cells, at least for cone photoreceptors (see below).
High sequence homology exists among the vertebrate opsins. For human opsins, there is roughly 40-45% sequence homology, except for red and green cone opsins, which are 96% homologous (Figure 3). Phylogenetic analyses of opsin sequences demonstrate that opsins can be divided into 5 classes correlating roughly with absorption properties (Ebrey and Koutalos, 2001; Yokoyama, 2000). Rhodopsin falls into the RH1 class of opsins; the human blue cone opsin is one form of a short wavelength-sensitive (SWS1) opsin that includes pigments that absorb maximally from about 350 to 440 nm; and the human red and green cone opsins are both medium/long wavelength-sensitive (M/LWS) opsins, which account for their high sequence identity. The other two classes of opsins are not found in mammalian retinas and are a rhodopsin-like blue-shifted opsin (RH2) and a second class of short wavelength-sensitive pigments (SWS2).
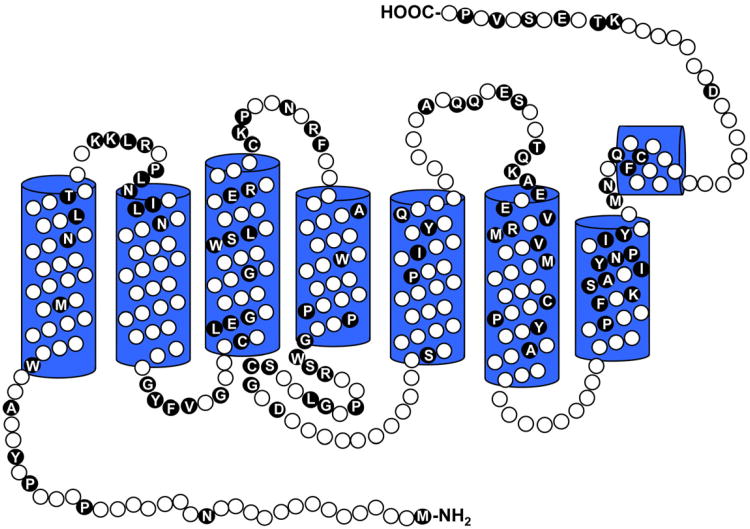
Figure 3. Sequence identity among the human rod and cone opsins.
Opsin secondary structure is represented in two-dimensions based on alignment with the known structure of bovine rhodopsin. Residues that are identical between the human rod and human red, green, and blue cone opsins are denoted as black circles with the conserved amino acid residues identified.
A structural feature that has received some attention is the palmitoylation of the opsins. Rhodopsins are modified at two adjacent cysteines in the C-terminal region, by fatty acid acylation, the majority of which is palmitoylation. Human M/LWS cone opsins have no cysteines at corresponding positions. SWS2 cone opsins have a single corresponding cysteine, and partial palmitoylation of salamander SWS2 cone opsin has been shown (Ablonczy et al., 2006). Human SWS1 cone opsins have one corresponding cysteine, but no acylation of that cysteine was observed for mouse or salamander variants (Ablonczy et al., 2006). Several studies have shown that removal of the palmitate groups on rhodopsin has little effect on the functional properties except, surprisingly, that excessive light greatly accelerates retinal degeneration compared to wild-type animals (Maeda et al., 2010; Wang et al., 2005).
2.3 Opsin specificity and photoreceptor type
In non-mammalian retinas, opsins are not necessarily unique to a single type of photoreceptor. The salamander green rod has been shown to have the same SWS2 opsin as the blue cone of that species (Ma et al., 2001). Interestingly, the two cells express distinct G-protein transducin alpha-subunits: rod alpha-transducin in green rods and cone alpha-transducin in blue-sensitive cones, demonstrating that this opsin can activate both transduction processes.
The question has been addressed as to if the class to which the opsin belongs controls the physiology of the receptor. From studies where the human rod RH1 pigment has been expressed in Xenopus M/L cones, and the human or salamander M/L cone pigment has been expressed in Xenopus rods, it has been clearly demonstrated that the respective cells have the same amplification and kinetics with either the rod or the cone opsin, thus eliminating the possibility that the signaling properties of the cell are controlled by the particular opsin that is expressed (Kefalov et al., 2003).
A further confounding issue is that cones can express more than one opsin. This was first shown for the guppy (Archer and Lythgoe, 1990) and the salamander (Makino and Dodd, 1996). Further studies have shown that several species of rodents, notably the well characterized and genetically-modified mouse, contain a mixture of the two cone opsins with a well-defined dorsal- ventral gradient across the retina, even in the wild-type animals (Applebury et al., 2000; Lukáts et al., 2005).
2.4 Chromophore binding to visual pigments
Despite the similarities of the opsins, there are key differences between rhodopsin and the cone pigments. The rates of sensitivity recovery (Perry and McNaughton, 1991) and visual pigment regeneration (Schnapf et al., 1990) are greater in cones than in rods (Baylor et al., 1979; Baylor et al., 1984). Recent in vitro studies of retinal release from cone opsins after bleaching compared to rhodopsin have shown that cone pigments release retinal at a much faster rate (Chen et al., 2012b; Das et al., 2004; Hunt et al., 2009). In addition, 11-cis retinal is generally not as tightly bound in cone pigments as it is in rhodopsin. Hydroxylamine, which can disrupt the Schiff base linkage of 11-cis retinal by forming an oxime, has greater accessibility to the chromophore in the dark in most cone pigments than in rhodopsin (Fukada et al., 1990; Wald et al., 1955). Furthermore, chromophore has been demonstrated to be exchanged in the dark with cone pigments, but not rhodopsin (Crescitelli, 1984; Matsumoto et al., 1975). The loss of chromophore was also shown to take place in cones as well as in expressed and purified cone pigments; however, this did not occur with rods and rhodopsin (Kefalov et al., 2005).
Studies using retinoid analogues have been instrumental in demonstrating that cone opsins are quite different from one another, both in their ability to bind to various analogues and in the effect of the analogue on the conformation of the proteins, as measured by their ability to activate transducin and thus activate the phototransduction process. These properties have been thoroughly studied in the salamander, with both in vitro studies of transducin activation and single-cell experiments measuring pigment formation and activation (Ala-Laurila et al., 2009; Isayama et al., 2006). Table 1 summarizes a portion of these data. Studies with 11-cis 9-demethyl retinal, where a single methyl group is lacking, have been particularly informative. Salamander M/LWS cone pigments generated with 11-cis 9-demethyl retinal resulted in a longer-lived Meta II intermediate (i.e., slower release of all-trans retinal from the opsin) (Das et al., 2004). The other two salamander cone pigments (SWS1 and SWS2) did not display any alterations in activation or deactivation rates with 11-cis 9-demethyl retinal (Das et al., 2004). These results suggested that the 9-methyl group of retinal was critical in the fast deactivation of only M/LWS cone pigments. Consistent with these results, studies have demonstrated that a prolonged light-activated salamander red cone occurs in isolated salamander red cone cells regenerated with 11-cis 9-demethyl retinal (Estevez et al., 2006; Estevez et al., 2009).
Table 1. Analogue pigments with salamander opsins.
Salamander opsins were expressed in COS-1 cells and pigments generated and purified as described by Das et al. (Das et al., 2004).
| Retinal | Red rod (RH1) | Green rod/blue cone (SWS2) | Red cone (M/LWS) | UV cone (SWS1) |
|---|---|---|---|---|
| 11-cis retinal† | 500 nm | 432 nm | 560 nm | 356 nm |
| 11-cis 9-demethyl retinal† | 468 nm (stable meta I) | 412 nm | 509 nm (stable meta II) | 357 nm |
| 11-cis 13-demethyl retinal‡ | 500 nm | 432 nm | No pigment | ND |
Data from Das et al. (2004);
unpublished data from Kono and Crouch although a spectrum of 11-cis 13-demethyl retinal in bovine RH1 (Nelson et al., 1970) and an action spectrum of salamander rods regenerated with the same analogue have been shown to have the same absorption maximum (Corson et al., 2000). ND = not determined.
Compounds representing a fraction of the structure of retinal have been known for some years to fit into the binding site of rhodopsin and to inhibit the binding of the native chromophore (Matsumoto and Yoshizawa, 1975; Nelson et al., 1970). Unexpectedly, it was found that such small molecules could also act as agonists or inverse agonists to opsins (Kono and Crouch, 2010, 2011). The first observations were made using salamander rods and cones with measurements on isolated photoreceptors (Isayama et al., 2006; Jin et al., 1993; Kefalov et al., 1999). Table 2 presents results from in vitro studies on human opsins. The use of these small molecules is being explored as a means to control cone degeneration observed in situations where the supply of 11-cis retinal has been compromised.
Table 2. Effects of ligands on human opsin activity.
Activity is defined as the effect on the respective opsin's ability to activate transducin in a ligand-dependent manner as previously described (Kono and Crouch, 2011).
| Compound | Structure | red rod opsin (RH1) | activity blue cone opsin (SWS1) | red/green cone opsin (M/LSW) |
|---|---|---|---|---|
| 1,3 dimethylcyclohexane |

|
inverse agonist§ | inverse agonist§ | inverse agonist† |
| β-ionone |
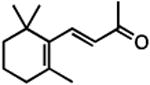
|
agonist§ | agonist§ | inverse agonist† |
| 9-cis C17 aldehyde |

|
agonist§ | ND | Inverse agonist† |
| 11-cis retinol |

|
agonist‡ | inverse agonist‡ | inverse agonist‡ |
| 11-cis retinal |
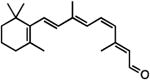
|
inverse agonist‡ | inverse agonist‡ | inverse agonist‡ |
2.5 Generation of the visual pigment chromophore
Intrinsic to the ability of rods and cones to detect light is the availability of chromophore (Figure 1). The stable form of vitamin A (all-trans retinol) is an essential fat-soluble vitamin acquired through the dietary consumption of either β-carotene from plants or retinyl-esters from animal products. After β-carotene is absorbed by the intestinal lumen, it is symmetrically cleaved by β,β-carotene 15,15′-monooxygenase into two molecules of all-trans retinal and eventually reduced to all-trans retinol by retinoid dehydrogenases (RDHs) (Redmond et al., 2001; von-Lintig and Vogt, 2000). Retinyl-esters are composed of all-trans retinol bound to a fatty acid through an ester linkage that must be hydrolyzed for absorption to occur (Rigtrup and Ong, 1992). The most common ester found in the retina is retinyl-palmitate (Trehan et al., 1990). The generation of 11-cis retinal and retinol has been the subject of much study and debate. Current data suggest there are two systems for producing these critical retinoids, the classic visual cycle and the cone visual cycle, both of which are discussed below.
3. Retinoid processing for the rod photoreceptors
3.1 Classic visual cycle
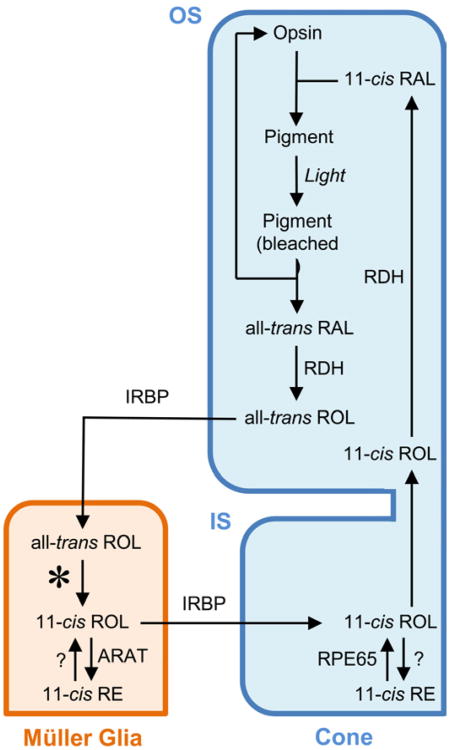
Generation of 11-cis retinal from all-trans retinol occurs in the RPE in a process referred to as the classic visual cycle (Figure 4) (Travis et al., 2007). After absorption of light by visual pigments, 11-cis retinal is destroyed through isomerization to the all-trans configuration. To regenerate 11-cis retinal, all-trans retinal is released from opsin and reduced to all-trans retinol by the combined action of RDH8 and RDH12 (Chen et al., 2012a). All-trans retinol is transported to the RPE where it is esterified by lecithin retinol acyl transferase (LRAT) (Batten et al., 2004). This retinyl-ester is the substrate for the proposed isomerohydrolase, RPE65 (Moiseyev et al., 2003). RPE65 converts all-trans retinyl-ester to 11-cis retinol (Jin et al., 2005; Moiseyev et al., 2005; Redmond et al., 2005), which is then oxidized to 11-cis retinal and transported to photoreceptors to regenerate visual pigments to complete the “visual cycle” (see Section 3.2). The shuttling of all-trans retinol from the photoreceptors to the RPE and 11-cis retinal from the RPE to the photoreceptors is presumably aided by a retinoid binding protein, interphotoreceptor retinoid binding protein (IRBP) (see Section 3.3) (Edwards and Adler, 1994, 2000). Both rods and cones apparently receive 11-cis retinal from the RPE, and we are referring to this as the classic visual cycle to distinguish it from a more recently identified cone visual cycle involving the Müller cells that appears to be exclusive to cones (see Section 4).
Figure 4. Classic visual cycle.
Retinoids are metabolized and shuttled between the RPE (purple) and both rod and cone OS (depicted generically in green). RAL = retinal; ROL = retinol; RDH = retinol dehydrogenase; LRAT = lecithin:retinol acyltransferase.
3.2 Role of RPE65
RPE65 is an abundant protein in the RPE that was first identified and cloned from the RPE by the Redmond group (Hamel et al., 1993). This 61 kDa protein undergoes post-translational modification with palmitates to facilitate association with membranes (Takahashi et al., 2006) and is homologous to beta-carotene oxygenases (Redmond et al., 2001). A crystal structure for RPE65 has been determined at 2.14 Å resolution (Kiser et al., 2009). Studies are underway in several laboratories probing the RPE65 mechanism of action.
In 2005, three groups independently concluded that RPE65 was the isomerohydrolase, most probably acting in complex with other retinyl-binding protein (s) and dehydrogenase(s), responsible for converting all-trans retinyl-ester to 11-cis retinol within the RPE (Jin et al., 2005; Moiseyev et al., 2005; Redmond et al., 2005). The mechanism of action is still unclear, but recent studies support a carbocation mechanism (Redmond et al., 2010) as previously proposed (McBee et al., 2000). Surprisingly, the RPE65-based complex is not specific for the 11-cis isomer, but may be driven by a mass action effect of the binding protein(s) (Redmond et al., 2010). Mutations to RPE65 are associated with Type 2 Leber congenital amaurosis (LCA2), an early onset congenital blindness that reduces or abolishes the innate synthesis of 11-cis retinal (Harris EW, 2001; Marlhens et al., 1997). Mice whose RPE65 gene has been knocked out (Rpe65-/-) do not generate 11-cis retinal (Redmond et al., 1998) and exhibit rapid cone loss and cone opsin mistrafficking (Fan et al., 2008; Tang et al., 2010; Znoiko et al., 2005). Gene therapy studies on mouse (Pang et al., 2006) and canine (Acland et al., 2001; Acland et al., 2005) models, as well as LCA2 patients (Bainbridge et al., 2008; Maguire et al., 2008; Maguire et al., 2009), have shown that inserting a functional gene for RPE65 into RPE cells holds great promise for restoring visual function.
3.3 Role of IRBP in rods
IRBP is the most abundant soluble protein in the sub-retinal space (Gonzalez-Fernandez, 2003) and is known to bind retinoids (Edwards and Adler, 1994; Pepperberg et al., 1993). In vitro data from several laboratories have shown that IRBP facilitates clearance of all-trans retinol from photoreceptor cells and uptake of 11-cis retinal from the RPE (Edwards and Adler, 2000; Jones et al., 1989). In addition, IRBP preserves the isomeric state of retinoids in the sub-retinal space (Crouch et al., 1992). The specific role of IRBP is still not clear. Knocking out IRBP in mice (Irbp-/-) did not affect recovery of sensitivity by the rods as might have been expected if IRBP were critical for the transport of 11-cis retinal to photoreceptors (Palczewski et al., 1999; Ripps et al., 2000); however, Irbp-/- mice do exhibit rod degeneration (Liou et al., 1998), indicating that IRBP's absence is not benign.
4. Retinoid processing for the cone photoreceptors
4.1 Cone visual cycle
Recent evidence suggests the existence of a second retinoid visual cycle within the vertebrate retina functioning in parallel with the classic visual cycle to specifically support rapid cone pigment regeneration (Figure 5; for reviews, see (Muniz et al., 2007; Wang and Kefalov, 2011; Wolf, 2004)). Salamander cones maintained within an isolated retina free of the RPE recover light sensitivity after bleaching; however, this recovery disappears in bleached cones disassociated from the retina (Wang et al., 2009). Previous work in the isolated frog retina has also implicated the regeneration of 11-cis retinal by the retina independent of the RPE (Goldstein, 1968; Hood and Hock, 1973).
Figure 5. Cone visual cycle.
Retinoids are metabolized and shuttled between the Müller glia (orange) and cones (blue). Currently, the isomerase of the Müller glia remains unknown (depicted as *). ARAT = Acyl CoA:retinol acyltransferase.
Isomerization of all-trans retinol has been proposed to occur in the Müller cells through an RPE65-independent manner. Cultured chicken Müller cells were shown to convert all-trans retinol to all-trans and 11-cis palmitates and 11-cis retinol, similar to reactions in the classical visual cycle of the RPE (Das et al., 1992). Recently, the Travis group identified the presence of dihydroceramide desaturase-1 (DES1) in Müller cells and reported that this protein mediates a direct isomerization of all-trans retinol to 11-cis retinol (Kaylor et al., 2012). In vitro studies using the expressed protein demonstrated that DES1 has isomerase activity. Interestingly, like RPE65 (Redmond et al., 2010), DES1 is not specific in forming 11-cis retinol, but also generates 13-cis and 9-cis retinol as well. Müller cells contain CRBP and CRALBP, both of which are retinoid binding proteins found in the RPE, with the latter preferentially binding 11-cis retinoids (Bunt-Milam and Saari, 1983; Eisenfeld et al., 1985). Zebrafish express two forms of CRALBP, one in the RPE and another in the Müller cell. Knocking down the expression of CRALBP in the Müller cells of zebrafish larvae by using antisense morpholinos results in a substantial decrease in 11-cis retinal and reduction in light sensitivity, supporting the key role of this protein (Collery et al., 2008; Fleisch et al., 2008). DES1 is reported to bind CRALBP, supporting its role as an isomerase (Kaylor et al., 2012). Unlike the LRAT-dependent retinol esterification in the RPE, microsomes of chicken retina generated 11-cis retinyl-ester from all-trans retinol through a mechanism requiring palm-CoA as an acyl donor, suggesting this isomerase is dependent on acyl CoA-retinol acyltransferase (ARAT) (Muniz et al., 2009). Within the intact isolated salamander retina, lesioning Müller cells with a gliotoxin inhibited the ability of bleached cones to recover light sensitivity (Wang et al., 2009).
Thus, Müller cells are capable of converting all-trans retinol to 11-cis retinol similar to what is observed in the RPE with the exception of the final oxidation reaction to generate 11-cis retinal. It has been shown that cones are capable of carrying out 11-cis retinol oxidation, whereas rods cannot. Bleached cones, but not bleached rods, were first demonstrated to regenerate resting dark current and light-sensitivity after the addition of 11-cis retinol in single salamander cell electrophysiological recordings (Jones et al., 1989). More recently, it has been shown directly that 11-cis retinol can indeed regenerate cone pigments within the cells by microspectrophotometry (Ala-Laurila et al., 2009). Further demonstration of oxidase activity in cones was shown in eyecups of mice that lacked endogenous 11-cis retinal from which 11-cis retinal could be generated after incubation with 11-cis retinol (Parker et al., 2011). Interestingly, 11-cis retinol is now known to act as an agonist to rod opsin, activating the rod opsins in a light-independent manner (Kono et al., 2008). The compound was originally proposed to be toxic to bleached rods due to increased desensitization, but now has been shown to be unable to form pigment in rods (Ala-Laurila et al., 2009).
Müller cells and cones contain the necessary machinery for completion of the cone visual cycle. By not performing the final oxidation step in the Müller cells, 11-cis retinol becomes a source of chromophore usable only by cones. Using suction electrodes to mask the OS of isolated salamander rods and cones, 11-cis retinal presented to the cell bodies of bleached photoreceptors restored light sensitivity in cones, but had no effect on rods (Jin et al., 1994). This finding is particularly interesting considering the topology of Müller cell processes, as they make numerous associations with cones at this region (Sarantis and Mobbs, 1992; Wang et al., 2009). These data suggest that the site of entry of the chromophore may be different for rods and cones. A cone-specific visual cycle involving the Müller cells (Kaylor et al., 2012) has been proposed and an isomerase identified which is localized in Müller cells. In the RPE, RPE65 is the accepted isomerohydrolase that converts all-trans retinyl-ester to 11-cis retinol (Jin et al., 2005; Moiseyev et al., 2005; Redmond et al., 2005). There is no evidence for the presence of RPE65 within Müller cells; however, the absence of RPE65 abolishes the generation of 11-cis retinoids in the mammalian eye (Redmond et al., 1998). In Rpe65-/- mice, the absence of 11-cis retinal also results in rapid degeneration of cones (Rohrer et al., 2005; Znoiko et al., 2005). RPE65 has been shown to be essential in cone function of the neural retina leucine zipper knockout (Nrl-/-) mouse (Wenzel et al., 2007), a model for a pure-cone retina (Mears et al., 2001). The level of 11-cis retinal generated by the cone visual cycle synthesis appears to be insufficient to promote cone survival. Data to support this come from Lrat-/- mouse studies (Fan et al., 2008; Zhang et al., 2008), where the lack of esterification of all-trans retinol in the RPE, the substrate for RPE65-dependent isomerization (Moiseyev et al., 2003), leads to a similar degeneration of cones as observed in the Rpe65-/- mouse.
4.2 RPE65 within cones
4.2.1 Identification of RPE65 in cones
RPE65 has been well-documented to be abundant in the RPE of many species (Hamel et al., 1993; Tsilou et al., 1997) and was originally proposed to be specific to that tissue. However, the mRNA of RPE65 has been reported in the iris (Kociok et al., 1998), and the protein has been found in transformed kidney cells (Ma et al., 1999), human epidermis (Hinterhuber et al., 2004), and non-melanocytic skin tumors (Hinterhuber et al., 2005). We have localized RPE65 to cones although, interestingly, there does not appear to be a common location within cones for RPE65 among the cones of different species that we have studied.
RT-PCR analysis of isolated salamander photoreceptors first demonstrated the presence of RPE65 mRNA in cones, but not rods (Ma et al., 1998). Using a polyclonal anti-RPE65 antibody generated from a synthetic peptide corresponding to residues 150-164 of bovine RPE65 (NFITKVNPETLETIK) (Redmond and Hamel, 2000), we showed localization of RPE65 in the OS of cones of the wild-type mice (Figure 6) (Tang et al., 2011b). Further support for the existence of RPE65 in mouse cones comes from the Nrl-/- mouse, which contains elevated levels of RPE65 within the retina when compared to the wild-type mouse (Wenzel et al., 2007). Immunohistology showed RPE65 to be present in the OS of these cone-like photoreceptors (Figure 6, B-C) (Boyer et al., 2012b). Interestingly, the level of RPE65 within cones across different strains of mice appears to be inversely related to the level of RPE65 present within the RPE (Tang et al., 2011b). This last finding is likely the basis of some controversy over the presence of RPE65 in cones (summarized in (Tang et al., 2011b)) as RPE65 is highest in the C57BL/6 mice (low levels of RPE65 in the RPE) and undetectable by immunostaining in BALB/c and 129Sv mouse eyecups where RPE65 is high in the RPE. Furthermore, the affinity of the antibody proved to be critical for its immunodetection (Tang et al., 2011b).
Figure 6. RPE65 in mouse cones.
(A) Cross section of C57BL6 mouse eyecup shows RPE65 staining in both the RPE and the cone OS. (RPE65 = green; SWS1 opsin = red; DAPI = blue) (B) RPE65 is localized within rosettes in the ONL of Nrl-/- mouse retina. (RPE65 = green; PI = propidium iodide = red) (C) RPE65 co-localizes with SWS1-opsin, a marker for cone OS, within the rosette of the Nrl-/- mouse retina as shown by yellow. (RPE65 = green; SWS1 opsin = red).
We have recently found RPE65 to also be localized in the OS of human cones (Figure 7) (Tang et al., 2011a). The RPE65 antibody labels the red/green cones in a punctate appearance with the majority of the fluorescence between the IS/OS interface and in an apical pattern extending along the length of the OS. In humans, RPE65 appears to be selectively expressed in the OS regions of cones that detect green/red light, but not in blue cones (Tang et al., 2011a). This may be due to a requirement of green and red cones for a supplemental source of retinoids for rapid chromophore turnover, as they are the only subtypes that appear within the fovea. Further studies are required to explore these findings.
Figure 7. RPE65 in human cone photoreceptors.

Frozen section from temporal peripheral area of a human retina immunostained for RPE65 shows the protein is localized within the cone OS of the human retina. Images shown (A) composite of transmitted light and RPE65 fluorescence, (B) composite of RPE65 and propidium iodide fluorescence and (C) RPE65 fluorescence alone Trans = transmitted light; RPE65 = green; PI = propidium iodide = red.
While much of the data gathered about RPE65 in cones come from the rod-dominant mouse and human retina, little is known about the presence of the protein in cones of the cone-dominant retina. We have conducted a preliminary evaluation of RPE65 staining in cones of the cone-dominant Anolis lizard and found, surprisingly, that RPE65 is localized to the IS, specifically within the paraboloid (Figure 8). This structure is known to be composed of smooth ER and glycogen (Amemiya, 1975; Braekevelt, 1990); however the implications for this varied appearance of RPE65 compared to cones of the rod-dominant retinas will require further investigation.
Figure 8. RPE65 in lizard cone photoreceptors.
(A-B) Cross-section of lizard retina stained with Hematoxylin/Eosin (H&E) or Periodic Acid Schiff (PAS) shows cones with large IS and tapered OS, as previously described (Makaretz and Levine, 1980). (A′-B′) Higher magnification show the paraboloid structure (*). (C) Diagram of lizard cone shows various compartments. (D) RPE65 staining is present within the paraboloid of IS using DAB-color development. (E) Rabbit IgG serves as control. (F-G) Immunofluorescence shows RPE65 present in both RPE and cone IS. (RPE65 = green; DAPI = blue).
4.2.2 Role of RPE65 within cones
Unlike in the RPE, the role of RPE65 in cones remains unclear. As isomerization of the retinal/retinol does not occur within the cones, RPE65 is not functioning as an isomerase within the cone. It is possible that RPE65 has a role as a retinol binding protein or perhaps as a hydrolase. Because processes from the Müller glia make numerous associations with the cone cell body (Uehara et al., 1990), and 11-cis retinol is thought to be delivered to this region of the cell (Wang et al., 2009), a retinoid binding protein within cones would be useful as new opsins are being synthesized. The cone-derived 661W cells (Tan et al., 2004) are known to have retinol esterase activity (Kanan et al., 2008), and we found that 11-cis retinol can be esterified in these cells (Tang et al., 2011a). The role of RPE65 may be to bind the ester and mediate the ester hydrolysis, perhaps occurring at the OS interface at the connecting cilium in the mouse which could explain the localization of the protein in this region of the mouse cone (Tang et al., 2011b). RPE65, in a role as a retinoid binding protein, may shuttle 11-cis retinol along the OS, thus explaining the punctate appearance of RPE65 immunostaining in the apical regions of the mouse cone OS (Tang et al., 2011b). Higher resolution imaging with techniques such as immuno-electron microscopy will be critical to determining the exact subcellular localization of RPE65 within cones. Another possibility is that retinol binding function of RPE65 may have a role in the 11-cis retinol oxidation by RDHs (Hemati et al., 2005; Kiser et al., 2009). We have found that mouse strains with higher levels of cone RPE65 are more efficient at synthesizing cone pigments than those with less of the protein (Tang et al., 2011b). We thus propose that RPE65 is acting as a retinol binding protein, and the distribution within the cones of various species may vary depending on the localization of the hydrolysis/oxidation reactions. The design of animal models that specifically lack the cone RPE65 while the RPE65 remains intact within the RPE and vice versa is needed to address these questions. Thus, the role of RPE65 in cones remains unresolved, but there is no evidence for this protein acting as an isomerase in the cone.
4.3 Role of IRBP in cone pigment regeneration
The role of IRBP for shuttling 11-cis retinol from Müller cells to cones is unclear. Its involvement in promoting the cone visual cycle is supported by evidence that IRBP binds 11-cis retinol endogenously (Saari et al., 1985), is co-localized with Müller cell processes surrounding the cone cell body (Uehara et al., 1990), and is found in high concentrations around the cones (Carter-Dawson and Burroughs, 1992a, 1992b). Cones in Irbp-/- mice were found to be retinoid-deficient under photopic conditions in two studies (Parker et al., 2009), leading to the proposal that 11-cis retinol supplies are disrupted in the absence of IRBP. Using isolated Nrl-/-Rpe65-/-retinas, IRBP was shown to deliver 11-cis retinol for oxidation in cones and to enhance the efficiency of the oxidation reaction (Parker et al., 2011). The isoform of RPE65 appears to affect the phenotype of Irbp-/- mice as Jin et al. (Jin et al., 2009) reported cones in Irbp-/- mice with the Leu450 variant of RPE65 also had reduced outer segment length, partially mislocalized cone opsin, and underwent progressive degeneration. Parker et al. (Parker et al., 2009) found a reduction in cone ERG responses in Irbp-/- mice having the Met450 variant of RPE65 but observed no change in cone density or cone opsin levels and distribution. The studies are in agreement that retinoid deficiency is a likely cause for the impaired cone function in the absence of IRBP. As IRBP protects the isomeric state of 11-cis retinol, even in the presence of light (Crouch et al., 1992), the protein is likely to have a critical role in the delivery of 11-cis retinol to cones and to facilitate cone function.
5. Products of retinoid metabolism
There is a constant supply of all-trans retinol from the blood stream and from the supplies of retinyl ester stored in the RPE. STRA6, the recently identified membrane receptor for plasma retinol binding protein (RPB), mediates the cellular uptake of trans retinol into the RPE (Kawaguchi et al., 2007; Sun, 2012).An additional supply of all-trans retinol comes from the stores of the retinyl esters within the RPE itself. There are two sources of retinoids resulting from the visual process: the all-trans retinal generated from the absorption of light by the visual pigments and the 11-cis retinal linked to unbleached visual pigments. Under light conditions, the all-trans retinal produced from the absorption of light is assumed to be reduced to all-trans retinol and then transported to the RPE where it is stored as the ester. The portion of visual pigments that are not bleached will contain the 11-cis retinal and could contribute a minor source of retinoids to the visual process. The rod photoreceptors are phagocytized by the RPE at a rate of about 10% per day for humans (Anderson et al., 1978). Much less is known about phagocytosis of the cones; however, as the cone pigments only represent 1-5% of the total visual pigment, this retinoid source is likely to be minor in rod-dominant mammalian eyes.
5.1 Reduction of all-trans retinal
Due to its relation to the toxicity of non-reduced all-trans retinal, the formation of lipofuscin precursors, and the recycling of the retinoid chromophore, the reduction of all-trans retinal to retinol has been examined in detail. Imaging the fluorescence of retinol has permitted the study of its formation kinetics in isolated photoreceptors from several vertebrate species, including salamander (Ala-Laurila et al., 2006; Tsina et al., 2004), frog (Chen et al., 2005; Wu et al., 2007), gecko (Kolesnikov et al., 2007), and mouse (Blakeley et al., 2011; Chen et al., 2009). Figure 9 shows the kinetics of retinol formation after bleaching in mouse rod photoreceptors and in the cone-like photoreceptors from the Nrl-/- mouse retina. In Nrl-/- photoreceptors as well as cones, formation of retinol after bleaching occurs more rapidly than in rods. This is in agreement with the faster release of retinal from the photoactivated visual pigment, and the higher retinol dehydrogenase activity in cones (Miyazono et al., 2008). It would allow cone photoreceptors to recycle the chromophore faster than rods, so they can support faster visual pigment regeneration. However, the faster formation of retinol in cones is not simply due to the earlier availability of all-trans retinal because of its faster release from photoactivated visual pigment. In the salamander retina, blue-sensitive cones and green rods contain the same visual pigment, SWS2. The kinetics of retinol formation, however, are faster in the cone than in the rod (Ala-Laurila et al., 2006), underlining the importance of the higher retinol dehydrogenase activity in the former.
Figure 9. Kinetics of all-trans retinol formation in the outer segments of single isolated photoreceptors from C57BL/6 and Nrl-/- mouse retinas.
Retinas obtained from (A) C57BL/6 or (B) Nrl-/- mice. The insets show dark-adapted isolated photoreceptors: left panel = the bright field; middle = fluorescence images before bleaching; and the right panel = after bleaching for (A)30 min and (B) 33 sec. Note the appearance of retinol fluorescence. Scale bars = 5 μm. Data re-plotted from (Chen et al., 2009).
5.2 Retinoids and lipofuscin formation
In both rod and cone photoreceptors, photoactivation of the visual pigment occurs through the photoisomerization of the 11-cis retinal to the all-trans form. In both types of photoreceptors, all-trans retinal released from the photoactivated pigment is reduced to all-trans retinol within the OS (Figure 10). The reduction requires NADPH (Futterman et al., 1970) and, at the same time, achieves both the removal of retinal and initiation of the recycling of all-trans retinal back to the 11-cis form. Retinal is a reactive aldehyde and a photosensitizer (Delmelle, 1978; Krasnovsky and Kagan, 1979; Matsumoto et al., 1975; Rozanowska and Sarna, 2005), and its toxicity may play a role in several diseases of the retina.
Figure 10. Reactions that generate and eliminate all-trans retinal in the photoreceptor outer segment.
all-trans Retinal generated on decay of the photo-bleached pigment (see Figs. 4 and 5) can be reduced to all-trans retinol or react with an amine such as phosphatidylethanolamine to generate precursors to A2E.
All-trans retinal can form a Schiff base with amine groups, including the one on phosphatidylethanolamine, a major lipid component of OS membranes (Fliesler and Anderson, 1983). A second molecule of all-trans retinal can then react with the Schiff base, forming bis-retinoids, such as A2PE (Ben-Shabat et al., 2002b; Liu et al., 2000). A2PE is a precursor of A2E, the best characterized among the bis-retinoids. The release and removal of all-trans retinal that occur as a part of the light detection process are thought to be associated with the formation of lipofuscin (Katz and Redmond, 2001; Katz and Robinson-Jr, 2002; Sparrow et al., 2010), the fluorescent pigment that accumulates with age in the RPE lysosomal compartment. Lipofuscin and A2E can accumulate in the RPE of both rod-dominant and cone-dominant retinas, as shown by recent studies on the Nrl-/- mouse (Figure 11) (Boyer et al., 2012b).
Figure 11. Lipofuscin and A2E in the RPE from a rod-dominant and a cone-dominant retina.
(A) C57BL/6 mouse, (B) Nrl-/- mouse, (C) Fluorescence emission spectra of lipofuscin granules, (D) Total lipofuscin fluorescence levels in the RPE of 12-month-old mice, (E) Total A2E levels in the RPE of 12-month-old mice. Nrl-/- mice were used as a cone-dominant model. True color RPE fluorescence micrographs (490 nm excitation). Data re-plotted from (Boyer et al., 2012b).
There are several lines of evidence in support of the relation between retinal and lipofuscin. First, lipofuscin levels are greatly decreased in mice that lack RPE65 (Katz and Redmond, 2001), indicating that lipofuscin formation requires the generation of 11-cis retinal. Second, bis-retinoids constitute the major extractable component of lipofuscin (Kim et al., 2007; Sparrow et al., 2012). Third, lipofuscin accumulation requires the phagocytosis of the photoreceptor OS by the RPE, and in the absence of phagocytosis, retinoid-derived fluorescent material accumulates in the photoreceptors (Katz et al., 1987). Finally, all-trans retinal can form A2PE in rod OS membranes. This includes the formation of A2PE from all-trans retinal added exogenously to membranes (Liu et al., 2000), as well as from retinal released endogenously from rhodopsin following bleaching (Ben-Shabat et al., 2002b). Taken together, these pieces of evidence have led to the proposal that bis-retinoid precursors of lipofuscin, such as A2PE, form in the photoreceptor OS from all-trans retinal that is escaping reduction to retinol (Katz and Redmond, 2001; Katz and Robinson-Jr, 2002; Sparrow et al., 2010). Subsequently, bis-retinoids enter the RPE through the daily phagocytosis of photoreceptor outer segments (Young, 1967), ending up in the lysosomal compartment where they accumulate in the form of lipofuscin. Consistent with this notion, mice that lack retinol dehydrogenases, the enzymes that oxidize all-trans retinol to retinal show increased A2E accumulation in the RPE (Chrispell et al., 2009; Maeda et al., 2005).
5.3 A2E formation from 11-cis retinal
While it is clear that retinoids are involved in lipofuscin, recent evidence shows 11-cis retinal itself may be a major source of lipofuscin and A2E (Boyer et al., 2012a). These results are based on the finding that mice raised in the dark have the same levels of lipofuscin and lipofuscin precursors as animals raised in cyclic light. Furthermore, direct addition of 11-cis retinal to rod photoreceptors results in the generation of lipofuscin precursors in the absence of light. This surprising result challenges the existing notion that A2E and lipofuscin arise from all-trans retinal generated from the bleaching of the visual pigments. These data indicate that 11-cis retinal may have a major role in events resulting in retinal degeneration.
5.4 Distribution of A2E in the RPE
The accumulation of lipofuscin is one of the most striking features of the aging human eye (Delori et al., 1995; Feeney, 1978; Wing et al., 1978). Through interaction with blue light, lipofuscin was proposed to contribute to the development of age-related macular degeneration (Birnbach et al., 1994; Holz et al., 2001; Roberts et al., 2002). The exact composition of lipofuscin is not known (Ng et al., 2008; Sparrow et al., 2010); however, bis-retinoids are a major component of lipofuscin. More than 20 bis-retinoids have been identified in the last two decades (Sparrow et al., 2010), but the best-studied bis-retinoid is still A2E (Eldred and Lasky, 1993; Parish et al., 1998; Sakai et al., 1995). There are numerous studies suggesting a toxic role for A2E to the RPE, particularly in its oxidized form (Kim et al., 2008; Wang et al., 2006). A detoxifying role for A2E itself has been suggested as it removes the highly toxic free all-trans retinal (Maeda et al., 2011; Maeda et al., 2009a; Roberts et al., 2002). The inhibition of A2E formation through the limitation of normal retinoid processing has become a primary target of ocular pharmaceutics.
No immunological techniques are suitable for determining the spatial localization of A2E, and therefore the spatial relationship of A2E and lipofuscin has been hard to establish. The Bernstein laboratory utilized 8 mm tissue punches on human RPE and surprisingly found no correlation with the lipofuscin distribution reported by others (Bhosale et al., 2009; Wing et al., 1978). We have recently developed a method for the localization of bis-retinoid utilizing a mass spectrometry-based technique, MALDI imaging (Grey et al., 2011). In this method, mass spectra are collected over the surface, allowing the relative levels of individual molecular ions (such as A2E) to be measured and visualized across the entire tissue. This information is directly comparable to fluorescence measurements; therefore, the spatial distributions of individual molecular ions (such as A2E, its oxides, precursors, and degradation products) can be determined and correlated with the measured fluorescence.
The distributions of A2E and its oxides were determined in three murine models, Abca4-/- mice (with a rapid accumulation of A2E (Mata et al., 2000)), Sv129 mice (the base strain for the Abca4-/-), and C57BL/6 mice (Grey et al., 2011). In the murine RPE, we found a marked agreement between the MALDI images of A2E and the fluorescence of lipofuscin in the RPE. Age and the higher accumulation of A2E in the Abca4-/- model increased the correlation of lipofuscin and A2E (Figure 12, A&B). The normalized profiles of relative lipofuscin fluorescence and A2E quantity along a cross-section of an eye (Figure 12, C) reinforce these observations. In vitro studies had demonstrated up to nine oxygens could be detected on A2E exposed to oxidizing conditions (Ben-Shabat et al., 2002b); however, the tissue profiling studies only detected A2E with an addition of a maximum of 3 oxygens. The distribution of oxidized forms of A2E, which are proposed to be more harmful than A2E itself (Ben-Shabat et al., 2002a), followed closely that of A2E but at much reduced levels. Although the quantity of A2E changed with age and strain, the fraction of oxidized A2E to unoxidized A2E was constant (0.17 ± 0.03 oxygen per A2E).
Post mortem human eyes from fetal age to 98 years have also been analyzed in a similar manner (Ablonczy et al., 2012b). Contrary to the murine models, the distribution of A2E in the human eyes did not correlate with lipofuscin fluorescence (Figure 12, D-F), regardless of age. Our measurements of lipofuscin fluorescence reinforced prior data (central distribution of lipofuscin which increases with age, see (Wing et al., 1978)). However, A2E was most abundant in the far periphery (50-100-fold more than in the center), independent of age. These findings are in agreement with the data obtained from the tissue punch study (Bhosale et al., 2009), and demonstrate that lipofuscin fluorescence is not an adequate monitor of A2E in the human eye.
6. Therapeutic use of retinoids and retinoid-like compounds
Diseases resulting in visual impairment arising from the disrupting retinoid metabolism have been recognized for some years and excellent reviews cover this area (Travis et al., 2007). Night blindness resulting from vitamin A deficiency was identified as the result of the rod photoreceptors lacking chromophore, (Dowling, 1964) and indeed many of these studies led to the initial findings in retinoid cycling. There has been recent interest in the use of retinoids and small molecules mimicking retinoids as therapeutic agents acting as inhibitors of the visual cycle as a means of preventing the accumulation of the lipofuscin that occurs in Stargardt's disease and macular degeneration (Golczak et al., 2008; Maiti et al., 2006; Palczewski, 2010). Primary amines, which can sequester all-trans retinal, have been shown to exert a protective role against light-induced damage in mouse models of retina degeneration (Maeda et al., 2011). Furthermore, successful use of small molecules and retinoid derivatives to ameliorate the cone degeneration reported in animal models of LCA2 have been reported (Maeda et al., 2009b; Rohrer et al., 2005). The small molecules have the advantage in not being light sensitive and also in not forming photoreactive pigments with the opsins (Fan et al., 2011). Methods of sustained delivery of the retinoids have also been developed for the potential use of these hydrophobic agents (Tang et al., 2010). The use of these retinoid-like compounds as therapeutic agents is an active area of investigation.
7. Conclusions and Future Directions
Although our understanding of the retinoid visual cycle has progressed significantly throughout the last decade, several recent observations show there are still many questions to be addressed. RPE65 has been shown to be present in cones; however, the role of RPE65 within this cell type remains unknown. How does its presence fit in with what is known about the cone visual cycle? As RPE65 in cones does not act as an isomerase, is its role that of a retinoid binding protein? Cone opsins have been shown to mislocalize in the absence of the native chromophore, but the mechanism responsible for this has yet to be deduced. What is the process by which cone opsins are trafficked, and why does the absence of chromophore inhibit this process? Recent studies suggest IRBP has a role in cone pigment regeneration, but the details are still unclear. The assumption that A2E and lipofuscin precursors arise from all-trans retinal generated by light has been challenged as 11-cis retinal is found to generate the same precursors independent of light. Finally, why does the posterior pole of the human eye exhibit strong fluorescence without the presence of significant levels of A2E? Currently, much interest lies within limiting retinoid metabolism to prevent the accumulation of bis-retinoids. The lack of correlation between A2E and lipofuscin in the human eye clearly shows that further study is needed. New analytical imaging techniques, experiments focused on the novel cone visual cycle and transduction mechanisms, animal models which more closely model the human retina, and experimental investigation of the human retina are needed to address these poorly understood areas to further understand the retinal degenerative diseases affecting human health and well-being.
Acknowledgments
This study was supported in part by National Institutes of Health Grants R01 EY04939 (RKC), R01 EY014850 (YK), R01 EY019515 (MK), R01 EY019065 (ZA), R21 EY020661 (ZA/RKC), and C06 RR015455 (Medical University of South Carolina), Lions Clubs of South Carolina (MK), Foundation Fighting Blindness (RKC), and an unrestricted grant (Department of Ophthalmology, Medical University of South Carolina) from Research to Prevent Blindness (RPB), the RPB Senior Scientific Investigator Award (RKC), and the RPB Medical Student Research Fellowship (PHT).
Footnotes
Any conflict of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ablonczy Z, Gutierrez DB, Grey AC, Schey KL, Crouch RK. Molecule-specific imaging and quantitation of A2E in the RPE. Adv Exp Med Biol. 2012a;723:75–81. doi: 10.1007/978-1-4614-0631-0_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ablonczy Z, Higbee D, Hanneken AM, Schey KL, Koutalos Y, Crouch RK. Linking retinoids to clinical patterns of AMD. Invest Ophthalmol Vis Sci. 2012b ARVO E-Abstract 6477. [Google Scholar]
- Ablonczy Z, Kono M, Knapp DR, Crouch RK. Palmitylation of cone opsins. Vision Res. 2006;46:4493–4501. doi: 10.1016/j.visres.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, Pearce-Kelling SE, Anand V, Zeng Y, Maguire AM, Jacobson SG, Hauswirth WW, Bennett J. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- Acland GM, Aquirre GD, Bennett J, Aleman TS, Cideciyan AV, Bennicelli J, Dejneka NS, Pearce-Kelling SE, Maguire AM, Palczewski K, Hauswirth WW, Jacobson SG. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12:1072–1082. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ala-Laurila P, Cornwall MC, Crouch RK, Kono M. The action of 11-cis retinol on cone opsins and intact cone photoreceptors. J Biol Chem. 2009;284:16492–16500. doi: 10.1074/jbc.M109.004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ala-Laurila P, Kolesnikov AV, Crouch RK, Tsina E, Shukolyukov SA, Govardovskii VI, Koutalos Y, Wiggert B, Estevez ME, Cornwall MC. Visual cycle: Dependence of retinol production and removal on photoproduct decay and cell morphology. J Gen Physiol. 2006;128:153–169. doi: 10.1085/jgp.200609557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amemiya T. Electron microscopic and cytochemical study on paraboloid glycogen of the accessory cone of the chick retina. Histochemistry. 1975;43:185–192. doi: 10.1007/BF00492446. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Fisher SK, Steinberg RH. Mammalian cones: disc shedding, phagocytosis, and renewal. Invest Ophthalmol Vis Sci. 1978;17:117–133. [PubMed] [Google Scholar]
- Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, Kage K, Kryzstolik MG, Lyass LA, Robbins JT. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27:513–523. doi: 10.1016/s0896-6273(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Archer SN, Lythgoe JN. The visual pigment basis for cone polymorphism in the guppy, Poecilia reticulata. Vision Res. 1990;30:225–233. doi: 10.1016/0042-6989(90)90038-m. [DOI] [PubMed] [Google Scholar]
- Arikawa K, Molday LL, Molday RS, Williams DS. Localization of Peripherin/Rds in the disk membranes of cone and rod photoreceptors - relationship to disk membrane morphogenesis and retinal degeneration. J Cell Biol. 1992;116:659–667. doi: 10.1083/jcb.116.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, Bhattacharya SS, Thrasher AJ, Fitzke FW, Carter BJ, Rubin GS, Moore AT, Ali RR. Effect of gene therapy on visual function in leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Batten ML, Imanishi Y, Maeda T, Tu DC, Moise AR, Bronson D, Possin D, Van-Gelder RN, Baehr W, Palczewski K. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem. 2004;279:10422–10432. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Lamb TD, Yau KW. The membrane current of single rod outer segments. J Physiol. 1979;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Nunn BJ, Schnapf JL. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol. 1984;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shabat S, Itagaki Y, Jockusch S, Sparrow JR, Turro NJ, Nakanishi K. Formation of a nonaoxirane from A2E, a lipofuscin fluorophore related to macular degeneration, and evidence of singlet oxygen involvement. Angew Chem Int Engl. 2002a;41:814–817. doi: 10.1002/1521-3773(20020301)41:5<814::aid-anie814>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Ben-Shabat S, Parish CA, Vollmer HR, Itagaki Y, Fishkin N, Nakanishi K, Sparrow JR. Biosynthetic studies of A2E, a major fluorophore of retinal pigment epithelial lipofuscin. J Biol Chem. 2002b;277:7183–7190. doi: 10.1074/jbc.M108981200. [DOI] [PubMed] [Google Scholar]
- Bhosale P, Serban B, Bernstein PS. Retinal carotenoids can attenuate formation of A2E in the retinal pigment epithelium. Arch Biochem Biophys. 2009;483:175–181. doi: 10.1016/j.abb.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbach CD, Järveläinen M, Possin DE, Milam AH. Histopathology and immunocytochemistry of the neurosensory retina in fundus flavimaculatus. Ophthalmology. 1994;101:1211–1219. doi: 10.1016/s0161-6420(13)31725-4. [DOI] [PubMed] [Google Scholar]
- Blakeley LR, Chen C, Chen CK, Chen J, Crouch RK, Travis GH, Koutalos Y. Rod outer segment retinol formation is independent of Abca4, arrestin, rhodopsin kinase, and rhodopsin palmitylation. Invest Ophthalmol Vis Sci. 2011;52:3483–3491. doi: 10.1167/iovs.10-6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer NP, Higbee D, Currin MB, Blakeley LR, Chen C, Ablonczy Z, Crouch RK, Koutalos Y. Lipofuscin and N-retinylidene-N-retinylethanolamine (A2E) accumulate in the retinal pigment epithelium in the absence of light exposure: their origin is 11-cis retinal. J Biol Chem. 2012a doi: 10.1074/jbc.M111.329235. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer NP, Tang PH, Higbee D, Ablonczy Z, Crouch RK, Koutalos Y. Lipofuscin and A2E accumulate with age in the retinal pigment epithelium of Nrl-/- mice. Photochem Photobiol. 2012b doi: 10.1111/j.1751-1097.2012.01127.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braekevelt CR. Retinal photoreceptor fine structure in the mallard duck (Anas platyrhynchos) Histol Histopathol. 1990;5:123–131. [PubMed] [Google Scholar]
- Bunt-Milam AH, Saari JC. Immunocytochemical localization of two retinoid-binding proteins in vertebrate retina. J Cell Biol. 1983;97:703–712. doi: 10.1083/jcb.97.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Dawson L, Burroughs M. Interphotoreceptor retinoid-binding protein in the cone matrix sheath. Electron microscopic immunocytochemical localization. Invest Ophthalmol Vis Sci. 1992a;33:1584–1588. [PubMed] [Google Scholar]
- Carter-Dawson L, Burroughs M. Interphotoreceptor retinoid-binding protein in the Golgi apparatus of monkey foveal cones. Electron microscopic immunocytochemical localization. Invest Ophthalmol Vis Sci. 1992b;33:1589–1594. [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J Comp Neurol. 1979;188:245–262. doi: 10.1002/cne.901880204. [DOI] [PubMed] [Google Scholar]
- Chen C, Blakeley LR, Koutalos Y. Formation of all-trans retinol after visual pigment bleaching in mouse photoreceptors. Invest Ophthalmol Vis Sci. 2009;50:3589–3595. doi: 10.1167/iovs.08-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Thompson DA, Koutalos Y. Reduction of all-trans retinal in vertebrate rod photoreceptors requires the combined action of RDH8 and RDH12. J Biol Chem. 2012a doi: 10.1074/jbc.M112.354514. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Tsina E, Cornwall MC, Crouch RK, Vijayaraghavan S, Koutalos Y. Reduction of All-trans Retinal to All-trans Retinol in the Outer Segments of Frog and Mouse Rod Photoreceptors. Biophys J. 2005;88:2278–2287. doi: 10.1529/biophysj.104.054254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MH, Kuemmel C, Birge RR, Knox BE. Rapid retinal release from a cone visual pigment following photoactivation. Biochemistry. 2012b doi: 10.1021/bi201522h. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispell JD, Feathers KL, Kane MA, Kim CY, Brooks M, Khanna R, Kurth I, Hübner CA, Gal A, Mears AJ, Swaroop A, Napoli JL, Sparrow JR, Thompson DA. Rdh12 activitiy and effects on retinoid processing in the murine retina. J Biol Chem. 2009;284:21468–21477. doi: 10.1074/jbc.M109.020966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collery R, McLoughlin S, Vendrell V, Finneagan J, Crabb JW, Saari JC, Kennedy BN. Duplication and divergence of zebrafish CRALBP genes uncovers novel role for RPE- and Muller-CRALBP in cone vision. Invest Ophthalmol Vis Sci. 2008;49:3812–3820. doi: 10.1167/iovs.08-1957. [DOI] [PubMed] [Google Scholar]
- Corson DW, Kefalov VJ, Cornwall MC, Crouch RK. Effect of 11-cis 13-demethylretinal on phototransduction in bleach-adapted rod and cone photoreceptors. J Gen Physiol. 2000;116:283–297. doi: 10.1085/jgp.116.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescitelli F. The gecko visual pigment: the dark exchange of chromophore. Vision Res. 1984;24:1551–1553. doi: 10.1016/s0042-6989(84)80004-8. [DOI] [PubMed] [Google Scholar]
- Crouch RK, Hazard ES, Lind T, Wiggert B, Chader G, Corson DW. Interphotoreceptor retinoid-binding protein and alpha-tocopherol preserve the isomeric and oxidation state of retinol. Photochem Photobiol. 1992;56:251–255. doi: 10.1111/j.1751-1097.1992.tb02154.x. [DOI] [PubMed] [Google Scholar]
- Das J, Crouch RK, Ma JX, Oprian DD, Kono M. Role of the 9-methyl group of retinal in cone visual pigments. Biochemistry. 2004;43:5532–5538. doi: 10.1021/bi036097u. [DOI] [PubMed] [Google Scholar]
- Das SR, Bhardwaj N, Kjeldbye H, Gouras P. Muller cells of chicken retina synthesize 11-cis-retinol. Biochem J. 1992;285:907–913. doi: 10.1042/bj2850907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmelle M. Retinal sensitized photodynamic damage to liposomes. Photochem Photobiol. 1978;28:357–360. doi: 10.1111/j.1751-1097.1978.tb07718.x. [DOI] [PubMed] [Google Scholar]
- Delori FC, Staurenghi G, Arend O, Dorey CK, Goger DG, Weiter JJ. In vivo measurement of lipofuscin in Stargardt's disease--Fundus flavimaculatus. Invest Ophthalmol Vis Sci. 1995;36:2327–2331. [PubMed] [Google Scholar]
- Dowling JE. Nutritional and inherited blindness in the rat. Exp Eye Res. 1964;3:348–356. doi: 10.1016/s0014-4835(64)80042-7. [DOI] [PubMed] [Google Scholar]
- Ebrey T, Koutalos Y. Vertebrate photoreceptors. Prog Retin Eye Res. 2001;20:49–94. doi: 10.1016/s1350-9462(00)00014-8. [DOI] [PubMed] [Google Scholar]
- Edwards RB, Adler AJ. Exchange of retinol between IRBP and CRBP. Exp Eye Res. 1994;59:161–170. doi: 10.1006/exer.1994.1094. [DOI] [PubMed] [Google Scholar]
- Edwards RB, Adler AJ. IRBP enhances removal of 11-cis retinaldehyde from isolated RPE membranes. Exp Eye Res. 2000;70:235–245. doi: 10.1006/exer.1999.0781. [DOI] [PubMed] [Google Scholar]
- Eisenfeld AJ, Bunt-Milam AH, Saari JC. Localization of retinoid-binding proteins in developing rat retina. Exp Eye Res. 1985;41:299–304. doi: 10.1016/s0014-4835(85)80020-8. [DOI] [PubMed] [Google Scholar]
- Eldred GE, Lasky MR. Retinal age pigments generated by self-assembling lysosomotropic detergents. Nature. 1993;361:724–726. doi: 10.1038/361724a0. [DOI] [PubMed] [Google Scholar]
- Estevez ME, Ala-Laurila P, Crouch RK, Cornwall MC. Turning cones off: the role of the 9-methyl group of retinal in red cones. J Gen Physiol. 2006;128:671–685. doi: 10.1085/jgp.200609630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez ME, Kolesnikov AV, Ala-Laurila P, Crouch RK, Govardovskii VI, Cornwall MC. The 9-methyl group of retinal is essential for rapid Meta II decay and phototransduction quenching in red cones. J Gen Physiol. 2009;134:137–150. doi: 10.1085/jgp.200910232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Crouch RK, Kono M. Light prevents exogenous 11-cis retinal from maintaining cone photoreceptors in chromophore-deficient mice. Invest Ophthalmol Vis Sci. 2011;52:2412–2416. doi: 10.1167/iovs.10-6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Rohrer B, Frederick JM, Baehr W, Crouch RK. RPE65-/- and LRAT-/- mice: comparable models of Leber Congenital Amaurosis. Invest Ophthalmol Vis Sci. 2008;49:2384–2389. doi: 10.1167/iovs.08-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney L. Lipofuscin and melanin of human retinal pigment epithelium. Fluorescence, enzyme cytochemical, and ultrastructural studies. Invest Ophthalmol Vis Sci. 1978;17:583–600. [PubMed] [Google Scholar]
- Fleisch VC, Schonthaler HB, von-Lintig J, Neuhauss SC. Subfunctionalization of a retinoid-binding protein provides evidence for two parallel visual cycles in the cone-dominant zebrafish retina. J Neurosci. 2008;28:8208–8216. doi: 10.1523/JNEUROSCI.2367-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliesler SJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Prog Lipid Res. 1983;22:79–131. doi: 10.1016/0163-7827(83)90004-8. [DOI] [PubMed] [Google Scholar]
- Fu Y, Yau KW. Phototransduction in mouse rods and cones. Pflugers Arch. 2007;454:805–819. doi: 10.1007/s00424-006-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada Y, Okano T, Shichida Y, Yoshizawa T, Trehan A, Mead D, Denny M, Asato AE, Liu RS. Comparative study on the chromophore binding sites of rod and red-sensitive cone visual pigments by use of synthetic retinal isomers and analogues. Biochemistry. 1990;29:3133–3140. doi: 10.1021/bi00464a033. [DOI] [PubMed] [Google Scholar]
- Futterman S, Hendrickson A, Bishop PE, Rollins MH, Vacano E. Metabolism of glucose and reduction of retinaldehyde in retinal photoreceptors. J Neurochem. 1970;17:149–156. doi: 10.1111/j.1471-4159.1970.tb02195.x. [DOI] [PubMed] [Google Scholar]
- Golczak M, Maeda A, Bereta G, Maeda T, Kiser PD, Hunzelmann S, von-Lintig J, Blaner WS, Palczewski K. Metabolic basis of visual cycle inhibition by retinoid and nonretinoid compounds in the vertebrate retina. J Biol Chem. 2008;283:9543–9554. doi: 10.1074/jbc.M708982200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein EB. Visual pigments and the early receptor potential of the isolated frog retina. Vision Res. 1968;8:953–963. doi: 10.1016/0042-6989(68)90070-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Fernandez F. Interphotoreceptor retinoid-binding protein--an old gene for new eyes. Vision Res. 2003;43:3021–3036. doi: 10.1016/j.visres.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Grey AC, Crouch RK, Koutalos Y, Schey KL, Ablonczy Z. Spatial localization of A2E in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2011;52:3926–3933. doi: 10.1167/iovs.10-7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel CP, Tsilou E, Pfeffer BA, Hooks JJ, Detrick B, Redmond TM. Molecular cloning and expression of RPE65, a novel retinal pigment epithelium-specific microsomal protein that is post-transcriptionally regulated in vitro. J Biol Chem. 1993;268:15751–15757. [PubMed] [Google Scholar]
- Harris EW. Leber's congenital amaurosis and RPE65. Int Ophthalmol Clin. 2001;41:73–82. doi: 10.1097/00004397-200101000-00008. [DOI] [PubMed] [Google Scholar]
- Hemati N, Feathers KL, Chrispell JD, Reed DM, Carlson TJ, Thompson DA. RPE65 surface epitopes, protein interactions, and expression in rod- and cone-dominant species. Mol Vis. 2005;11:1151–1165. [PubMed] [Google Scholar]
- Hicks D, Sahel J. The implications of rod-dependent cone survival for basic clinical research. Invest Ophthalmol Vis Sci. 1999;40:3071–3074. [PubMed] [Google Scholar]
- Hinterhuber G, Cauza K, Brugger K, Dingelmaier-Hovorka R, Horvat R, Wolff K, Foedinger D. RPE65 of retinal pigment epithelium, a putative receptor molecule for plasma retinol-binding protein, is expressed in human keratinocytes. J Inves Dermatol. 2004;122:406–413. doi: 10.1046/j.0022-202X.2004.22216.x. [DOI] [PubMed] [Google Scholar]
- Hinterhuber G, Cauza K, Dingelmaier-Hovorka R, Diem E, Horvat R, Wolff K, Foedinger D. Expression of RPE65, a putative receptor for plasma retinol-binding protein, in nonmelanocytic skin tumours. Br J Dermatol. 2005;153:785–789. doi: 10.1111/j.1365-2133.2005.06769.x. [DOI] [PubMed] [Google Scholar]
- Holz FG, Bellman C, Staudt S, Schütt F, Völcker HE. Fundus autofluorescence and development of geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2001;42:1051–1056. [PubMed] [Google Scholar]
- Hood DC, Hock PA. Recovery of cone receptor activity in the frog's isolated retina. Vision Res. 1973;13:1943–1951. doi: 10.1016/0042-6989(73)90065-5. [DOI] [PubMed] [Google Scholar]
- Hunt DM, Carvalho LS, Cowing JA, Davies WL. Evolution and spectral tuning of visual pigments in birds and mammals. Philos Trans R Soc Lond B Biol Sci. 2009;364:2941–2955. doi: 10.1098/rstb.2009.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isayama T, Chen Y, Kono M, Degrip WJ, Ma JX, Crouch RK, Makino CL. Differences in the pharmacological activation of visual opsins. Vis Neurosci. 2006;23:899–908. doi: 10.1017/S0952523806230256. [DOI] [PubMed] [Google Scholar]
- Jin J, Crouch RK, Corson DW, Katz BM, MacNichol EF, Cornwall MC. Noncovalent occupancy of the retinal-binding pocket of opsin diminishes bleaching adaptation of retinal cones. Neuron. 1993;11:513–522. doi: 10.1016/0896-6273(93)90155-k. [DOI] [PubMed] [Google Scholar]
- Jin J, Jones GJ, Cornwall MC. Movement of retinal along cone and rod photoreceptors. Vis Neurosci. 1994;11:389–399. doi: 10.1017/s0952523800001735. [DOI] [PubMed] [Google Scholar]
- Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122:449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Li S, Nusinowitz S, Lloyd M, Hu J, Radu RA, Bok D, Travis GH. The role of interphotoreceptor retinoid-binding protein on the translocation of visual retinoids and function of cone photoreceptors. J Neurosci. 2009;29:1486–1495. doi: 10.1523/JNEUROSCI.3882-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John SK, Smith JE, Aguirre GD, Milam AH. Loss of cone molecular markers in rhodopsin-mutant human retinas with retinitis pigmentosa. Mol Vis. 2000;6:204–215. [PubMed] [Google Scholar]
- Jones GJ, Crouch RK, Wiggert B, Cornwall MC, Chader GJ. Retinoid requirements for recovery of sensitivity after visual-pigment bleaching in isolated photoreceptors. Proc Natl Acad Sci U S A. 1989;86:9606–9610. doi: 10.1073/pnas.86.23.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanan Y, Kasus-Jacobi A, Moiseyev G, Sawyer K, Ma JX, al-Ubaidi MR. Retinoid processing in cone and Müller cell lines. Exp Eye Res. 2008;86:344–354. doi: 10.1016/j.exer.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz ML, Eldred GE, Robison WG., Jr Lipofuscin autofluorescence: evidence for vitamin A involvement in the retina. Mech Ageing Dev. 1987;39:81–90. doi: 10.1016/0047-6374(87)90088-1. [DOI] [PubMed] [Google Scholar]
- Katz ML, Redmond TM. Effect of Rpe65 knockout on accumulation of lipofuscin fluorophores in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2001;42:3023–3030. [PubMed] [Google Scholar]
- Katz ML, Robinson WG., Jr What is lipofuscin? Defining characteristics and differentiation from other autofluorescent lysosomal storage bodies. Arch Gerontol Geriatr. 2002;34:169–184. doi: 10.1016/s0167-4943(02)00005-5. [DOI] [PubMed] [Google Scholar]
- Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- Kaylor JJ, Yuan Q, Cook J, Sarfare S, Makshanoff J, Miu A, Kim A, Kim P, Habib S, Roybal CN, Xu T, Nusinowitz S, Travis GH. Identification of DES1 as a vitamin A isomerase in Müller glia cells of the retina. Nat Chem Biol. 2012 doi: 10.1038/nchembio.1114. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalov VJ, Cornwall MC, Crouch RK. Occupancy of the chromophore binding site of opsin activates visual transduction in rod photoreceptors. J Gen Physiol. 1999;113:491–503. doi: 10.1085/jgp.113.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalov VJ, Estevez ME, Kono M, Goletz PW, Crouch RK, Cornwall MC, Yau KW. Breaking the covalent bond - a pigment property that contributes to desensitization in cones. Neuron. 2005;46:879–890. doi: 10.1016/j.neuron.2005.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalov VJ, Fu Y, Marsh-Armstrong N, Yau KW. Role of visual pigment properties in rod and cone phototransduction. Nature. 2003;425:526–531. doi: 10.1038/nature01992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SR, Jang YP, Jockusch S, Fishkin NE, Turro NJ, Sparrow JR. The all-trans-retinal dimer series of lipofuscin pigments in retinal pigment epithelial cells in a recessive Stargardt disease model. Proc Natl Acad Sci U S A. 2007;104:19273–19278. doi: 10.1073/pnas.0708714104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SR, Jockusch S, Itagaki Y, Turro NJ, Sparrow JR. Mechanisms involved in A2E oxidation. Exp Eye Res. 2008;86:975–982. doi: 10.1016/j.exer.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser PD, Golczak M, Lodowski DT, Chance MR, Palczewski K. Crystal structure of native RPE65, the retinoid isomerase of the visual cycle. Proc Natl Acad Sci U S A. 2009;106:17325–17330. doi: 10.1073/pnas.0906600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kociok N, Heppekausen H, Schraermeyer U, Esser P, Thumann G, Grisanti S, Heimann K. The mRNA expression of cytokines and their receptors in cultured iris pigment epithelial cells: a comparison with retinal pigment epithelial cells. Exp Eye Res. 1998;67:237–250. doi: 10.1006/exer.1998.0517. [DOI] [PubMed] [Google Scholar]
- Kolesnikov AV, Ala-Laurila P, Shukolyukov SA, Crouch RK, Wiggert B, Estevez ME, Govardovskii VI, Cornwall MC. Visual cycle and its metabolic support in gecko photoreceptors. Vision Res. 2007;47:363–374. doi: 10.1016/j.visres.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Kono M, Crouch RK. In vitro assays of rod and cone opsin activity: retinoid analogs as agnoists and inverse agonists. Methods Mol Biol. 2010;652:85–94. doi: 10.1007/978-1-60327-325-1_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono M, Crouch RK. Probing human red cone opsin activity with retinal analogues. J Nat Prod. 2011;74:391–394. doi: 10.1021/np100749j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono M, Goletz PW, Crouch RK. 11-cis and all-trans retinols can activate rod opsin: rational design of the visual cycle. Biochemistry. 2008;47:7567–7571. doi: 10.1021/bi800357b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnovsky AA, Kagan VE. Photosensitization and quenching of singlet oxygen by pigments and lipids of photoreceptor cells of the retina. FEBS Lett. 1979;108:152–154. doi: 10.1016/0014-5793(79)81198-9. [DOI] [PubMed] [Google Scholar]
- Liou GI, Peachey NS, Matragoon S, Wei S, Blaner WS, Wang Y, Liu C, Gottesman ME, Ripps H. Early onset photoreceptor abnormalities induced by targeted disruption of the interphotoreceptor retinoid-binding protein gene. J Neurosci. 1998;18:4511–4520. doi: 10.1523/JNEUROSCI.18-12-04511.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Itagaki Y, Ben-Shabat S, Nakanishi K, Sparrow JR. The biosynthesis of A2E, a fluorophore of aging retina, involves the formation of the precursor, A2-PE, in the photoreceptor outer segment membrane. J Biol Chem. 2000;275:29354–29360. doi: 10.1074/jbc.M910191199. [DOI] [PubMed] [Google Scholar]
- Lukáts A, Szabó A, Röhlich P, Vigh B, Szél A. Photopigment coexpression in mammals: comparative and developmental aspects. Histol Histopathol. 2005;20:551–574. doi: 10.14670/HH-20.551. [DOI] [PubMed] [Google Scholar]
- Ma J, Znoiko S, Othersen KL, Ryan JC, Das J, Isayama T, Kono M, Oprian DD, Corson DW, Cornwall MC, Cameron DA, Harosi FI, Makino CL, Crouch RK. A visual pigment expressed in both rod and cone photoreceptors. Neuron. 2001;32:451–461. doi: 10.1016/s0896-6273(01)00482-2. [DOI] [PubMed] [Google Scholar]
- Ma JX, Xu L, Othersen DK, Redmond TM, Crouch RK. Cloning and localization of RPE65 mRNA in salamander cone photoreceptor cells. Biochim Biophys Acta. 1998;1443:255–261. doi: 10.1016/s0167-4781(98)00221-8. [DOI] [PubMed] [Google Scholar]
- Ma JX, Zhang D, Laser M, Brownlee NA, Re GG, Hazen-Martin DJ, Redmond TM, Crouch RK. Identification of RPE65 in transformed kidney cells. FEBS Lett. 1999;452:199–204. doi: 10.1016/s0014-5793(99)00606-7. [DOI] [PubMed] [Google Scholar]
- Maeda A, Golczak M, Chen Y, Okano K, Kohno H, Shiose S, Ishikawa K, Harte W, Palczewski G, Maeda T, Palczewski K. Primary amines protect against retinal degeneration in mouse models of retinopathies. Nat Chem Biol. 2011;8:170–178. doi: 10.1038/nchembio.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A, Maeda T, Golczak M, Chou S, Desai A, Hoppel CL, Matsuyama S, Palczewski K. Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J Biol Chem. 2009a;284:15173–15183. doi: 10.1074/jbc.M900322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A, Maeda T, Imanishi Y, Kuksa V, Alekseev A, Bronson JD, Zhang H, Zhu L, Sun W, Saperstein DA, Rieke F, Baehr W, Palczewski K. Role of photoreceptor-specific retinol dehydrogenase in the retinoid cycle in vivo. J Biol Chem. 2005;280:18822–18832. doi: 10.1074/jbc.M501757200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A, Okano K, Park PS, Lem J, Crouch RK, Maeda T, Palczewski K. Palmitoylation stabilizes unliganded rod opsin. Proc Natl Acad Sci U S A. 2010;107:8428–8433. doi: 10.1073/pnas.1000640107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Cideciyan AV, Maeda A, Golczak M, Aleman TS, Jacobson SG, Palczewski K. Loss of cone photoreceptors caused by chromophore depletion is partially prevented by the artificial chromophore pro-drug, 9-cis-retinyl acetate. Hum Mol Genet. 2009b;18:2277–2287. doi: 10.1093/hmg/ddp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire A, Simonelli F, Pierce EA, Pugh EN, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, Rossi S, Lyubarsky A, Arruda VR, Konkle B, Stone E, Sun J, Jacobs J, Dell'Osso L, Hertle R, Ma JX, Redmond TM, Zhu X, Hauck B, Zelenaia O, Shindler KS, Maguire MG, Wright JF, Volpe NJ, McDonnell JW, Auricchio A, High KA, Bennett J. Safety and efficacy of gene transfer for leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, Mingozzi F, Bennicelli JL, Ying GS, Rossi S, Fulton A, Marshall KA, Banfi S, Chung DC, Morgan JI, Hauck B, Zelenaia O, Zhu X, Raffini L, Coppieters F, De-Baere E, Shindler KS, Volpe NJ, Surace EM, Acerra C, Lyubarsky A, Redmond TM, Stone E, Sun J, McDonnell JW, Leroy BP, Simonelli F, Bennett J. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597–1605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti P, Kong J, Kim SR, Allikmets R, Rando RR. Small molecule RPE65 antagonists limit the visual cycle and prevent lipofuscin formation. Biochemistry. 2006;45:852–860. doi: 10.1021/bi0518545. [DOI] [PubMed] [Google Scholar]
- Makaretz M, Levine RL. A light microscopic study of the bifoveate retina in the lizard Anolis carolinensis: general observations and convergence ratios. Vision Res. 1980;20:679–686. doi: 10.1016/0042-6989(80)90092-9. [DOI] [PubMed] [Google Scholar]
- Makino CL, Dodd RL. Multiple visual pigments in a photoreceptor of the salamander retina. J Gen Physiol. 1996;108:27–34. doi: 10.1085/jgp.108.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlhens F, Bareil C, Griffoin JM, Zrenner E, Amalric P, Eliaou C, Liu SY, Harris E, Redmond TM, Arnaud B, Claustres M, Hamel CP. Mutations in RPE65 cause Leber's congenital amaurosis. Nat Genet. 1997;17:139–141. doi: 10.1038/ng1097-139. [DOI] [PubMed] [Google Scholar]
- Mata NL, Weng J, Travis GH. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc Natl Acad Sci U S A. 2000;97:7154–7159. doi: 10.1073/pnas.130110497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Tokunaga F, Yoshizawa T. Accessibility of the iodopsin chromophore. Biochim Biophys Acta. 1975;404:300–308. doi: 10.1016/0304-4165(75)90337-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Yoshizawa T. Existence of a beta-ionone ring-binding site in the rhodopsin molecule. Nature. 1975;258:523–526. doi: 10.1038/258523a0. [DOI] [PubMed] [Google Scholar]
- McBee JK, Kuksa V, Alvarez R, de-Lera AR, Prezhdo O, Haeseleer F, Sokal I, Palczewski K. Isomerization of all-trans-retinol to cis-retinoids in bovine retinal pigment epithelial cells: dependence on the specificity of retinoid-binding proteins. Biochemistry. 2000;39:11370–11380. doi: 10.1021/bi001061c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears AJ, Kondo M, Swain PK, Takada Y, Bush RA, Saunders TL, Sieving PA, Swaroop A. Nrl is required for rod photoreceptor development. Nat Genet. 2001;29:447–452. doi: 10.1038/ng774. [DOI] [PubMed] [Google Scholar]
- Miyazono S, Shimauchi-Matsukawa Y, Tachibanaki S, Kawamura S. Highly efficient retinal metabolism in cones. Proc Natl Acad Sci U S A. 2008;105:16051–16056. doi: 10.1073/pnas.0806593105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci U S A. 2005;102:12413–12418. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseyev G, Crouch RK, Goletz P, Oatis JJ, Redmond TM, Ma JX. Retinyl esters are the substrate for isomerohydrolase. Biochemistry. 2003;42:2229–2238. doi: 10.1021/bi026911y. [DOI] [PubMed] [Google Scholar]
- Muniz A, Betts BS, Trevino AR, Buddavarapu K, Roman R, Ma JX, Tsin AT. Evidence for two retinoid cycles in the cone-dominant chicken eye. Biochemistry. 2009;48:6854–6863. doi: 10.1021/bi9002937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniz A, Villazana-Espinoza ET, Hatch AL, Trevino SG, Allen DM, Tsin AT. A novel cone visual cycle in the cone-dominant retina. Exp Eye Res. 2007;85:175–184. doi: 10.1016/j.exer.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafi D, Engel AH, Palczewski K. Structure of cone photoreceptors. Prog Retin Eye Res. 2009;28:289–302. doi: 10.1016/j.preteyeres.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R, deRiel JK, Kropf A. 13-desmethyl rhodopsin and 13-desmethyl isorhodopsin: visual pigment analogues. Proc Natl Acad Sci U S A. 1970;66:531–538. doi: 10.1073/pnas.66.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KP, Gugiu B, Renganathan K, Davies MW, Gu X, Crabb JS, Kim SR, Rozanowska MB, Bonilha VL, Rayborn ME, Salomon RG, Sparrow JR, Boulton ME, Hollyfield JG, Crabb JW. Retinal pigment epithelium lipofuscin proteomics. Mol Cell Proteomics. 2008;7:1397–1405. doi: 10.1074/mcp.M700525-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K. Retinoids for treatment of retinal diseases. Trends Pharmacol Sci. 2010;31:284–295. doi: 10.1016/j.tips.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, Van-Hooser JP, Garwin GG, Chen J, Liou GI, Saari JC. Kinetics of visual pigment regeneration in excised mouse eyes and in mice with a targeted disruption of the gene encoding interphotoreceptor retinoid-binding protein or arrestin. Biochemistry. 1999;38:12012–12019. doi: 10.1021/bi990504d. [DOI] [PubMed] [Google Scholar]
- Pang JJ, Chang B, Kumar A, Nusinowitz S, Noorwez SM, Li J, Rani A, Foster TC, Chiodo VA, Doyle T, Li H, Malhotra R, Teusner JT, McDowell JH, Min SH, Li Q, Kaushal S, Hauswirth WW. Gene therapy restores vision-dependent behavior as well as retinal structure and function in a mouse model of RPE65 Leber congenital amaurosis. Mol Ther. 2006;13:565–572. doi: 10.1016/j.ymthe.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Parish CA, Hashimoto M, Nakanishi K, Dillon J, Sparrow JR. Isolation and one-step preparation of A2E and iso-A2E, fluorophores from human retinal pigment epithelium. Proc Natl Acad Sci U S A. 1998;95:14609–14613. doi: 10.1073/pnas.95.25.14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker RO, Fan J, Nickerson JM, Liou GI, Crouch RK. Normal cone function requires the interphotoreceptor retinoid binding protein. J Neurosci. 2009;29:4616–4621. doi: 10.1523/JNEUROSCI.0063-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker RO, Wang JS, Kefalov VJ, Crouch RK. Interphotoreceptor retinoid-binding protein as the physiologically relevant carrier of 11-cis-retinol in the cone visual cycle. J Neurosci. 2011;31:4714–4719. doi: 10.1523/JNEUROSCI.3722-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepperberg DR, Okajima TL, Wiggert B, Ripps H, Crouch RK, Chader GJ. Interphotoreceptor retinoid-binding protein (IRBP): Molecular biology and physiological role in the visual cycle of rhodopsin. Mol Neurobiol. 1993;6:61–85. doi: 10.1007/BF02780609. [DOI] [PubMed] [Google Scholar]
- Perry RJ, McNaughton PA. Response properties of cones from the retina of the tiger salamander. J Physiol. 1991;433:561–587. doi: 10.1113/jphysiol.1991.sp018444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond TM, Gentleman S, Duncan T, Yu S, Wiggert B, Grantt E, Jr, C FX. Identification, expression, and substrate specificity of a mammalian beta-carotene 15,15′-dioxygenase. J Biol Chem. 2001;276:6560–6565. doi: 10.1074/jbc.M009030200. [DOI] [PubMed] [Google Scholar]
- Redmond TM, Hamel CP. Genetic analysis of RPE65: from human disease to mouse model. Methods Enzymol. 2000;316:705–724. doi: 10.1016/s0076-6879(00)16758-8. [DOI] [PubMed] [Google Scholar]
- Redmond TM, Poliakov E, Kuo S, Chander P, Gentleman S. RPE65, visual cycle retinol isomerase, is not inherently 11-cis-specific: support for a carbocation mechanism of retinol isomerization. J Biol Chem. 2010;285:1919–1927. doi: 10.1074/jbc.M109.027458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, Gentleman S. Mutation of key residues of Rpe65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc Natl Acad Sci U S A. 2005;102:13658–13663. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma JX, Crouch RK, Pfeifer K. RPE65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- Rigtrup KM, Ong DE. A retinyl ester hydrolase activity intrinsic to the brush border membrane of rat small intestine. Biochemistry. 1992;31:2920–2926. doi: 10.1021/bi00126a011. [DOI] [PubMed] [Google Scholar]
- Ripps H, Peachey NS, Xu X, Nozell SE, Smith SB, Liou GI. The rhodopsin cycle is preserved in IRBP “knockout” mice despite abnormalities in retinal structure and function. Vis Neurosci. 2000;17:97–105. doi: 10.1017/s095252380017110x. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Kukielczak BM, Hu DN, Miller DS, Bilski P, Sik RH, Motten AG, Chignell CF. The role of A2E in prevention or enhancement of light damage in human retinal pigment epithelial cells. Photochem Photobiol. 2002;75:184–190. doi: 10.1562/0031-8655(2002)075<0184:troaip>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Rohrer B, Lohr HR, Humphries P, Redmond TM, Seeliger MW, Crouch RK. Cone opsin mislocalization in Rpe65-/- mice: a defect that can be corrected by 11-cis retinal. Invest Ophthalmol Vis Sci. 2005;46:3876–3882. doi: 10.1167/iovs.05-0533. [DOI] [PubMed] [Google Scholar]
- Rozanowska M, Sarna T. Light-induced damage to the retina: role of rhodopsin chromophore revisited. Photochem Photobiol. 2005;81:1305–1330. doi: 10.1562/2004-11-13-IR-371. [DOI] [PubMed] [Google Scholar]
- Saari JC, Teller DC, Crabb JW, Bredberg L. Properties of an interphotoreceptor retinoid-binding protein from bovine retina. J Biol Chem. 1985;260:195–201. [PubMed] [Google Scholar]
- Sakai T, Furukawa T, Iwanari H, Oka C, Nakano T, Kawaichi M, Honjo T. Loss of immunostaining of the RBP-J kappa transcription factor F9 cell differentiation induced by retinoic acid. J Biochem. 1995;118:621–628. doi: 10.1093/oxfordjournals.jbchem.a124955. [DOI] [PubMed] [Google Scholar]
- Sarantis M, Mobbs P. The spatial relationship between Müller cell processes and the photoreceptor output synapse. Brain Res. 1992;584:299–304. doi: 10.1016/0006-8993(92)90909-s. [DOI] [PubMed] [Google Scholar]
- Schnapf JL, Nunn BJ, Meister M, Baylor DA. Visual transduction in cones of the monkey Macaca fascicularis. J Physiol. 1990;427:681–713. doi: 10.1113/jphysiol.1990.sp018193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichida Y, Imai H. Visual pigment: G-protein-coupled receptor for light signals. Cell Mol Life Sci. 1998;54:1299–1315. doi: 10.1007/s000180050256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow JR, Gregory-Roberts E, Yamamoto K, Blonska A, Ghosh SK, Ueda K, Zhou J. The bisretinoids of retinal pigment epithelium. Prog Retin Eye Res. 2012;31:121–135. doi: 10.1016/j.preteyeres.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow JR, Wu Y, Kim CY, Zhou J. Phospholipid meets all-trans retinal: the making of RPE bisretinoids. J Lipid Res. 2010;51:247–261. doi: 10.1194/jlr.R000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H. Membrane receptors and transporters involved in the function and transport of vitamin A and its derivatives. Biochim Biophys Acta. 2012;1821:99–112. doi: 10.1016/j.bbalip.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Moiseyev G, Chen Y, Ma JX. The roles of three palmitoylation sites of RPE65 in its membrane association and isomerohydrolase activity. Invest Ophthalmol Vis Sci. 2006;47:5191–5196. doi: 10.1167/iovs.06-0614. [DOI] [PubMed] [Google Scholar]
- Tan E, Ding XQ, Saadi A, Agarwal N, Naash MI, Al-Ubaidi MR. Expression of cone-photoreceptor-specific antigens in a cell line derived from retinal tumors in transgenic mice. Invest Ophthalmol Vis Sci. 2004;45:764–768. doi: 10.1167/iovs.03-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang PH, Buhusi MC, Ma JX, Crouch RK. RPE65 is present in human green/red cones and promotes photopigment regeneration in an in vitro cone cell model. J Neurosci. 2011a;31:18618–18626. doi: 10.1523/JNEUROSCI.4265-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang PH, Fan J, Goletz PW, Wheless L, Crouch RK. Effective and sustained delivery of hydrophobic retinoids to photoreceptors. Invest Ophthalmol Vis Sci. 2010;51:5958–5964. doi: 10.1167/iovs.10-5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang PH, Wheless L, Crouch RK. Regeneration of photopigment is enhanced in mouse cone photoreceptors expressing RPE65 protein. J Neurosci. 2011b;31:10403–10411. doi: 10.1523/JNEUROSCI.0182-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu Rev Pharmocol Toxicol. 2007;47:1–44. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trehan A, Cañada FJ, Rando RR. Inhibitors of retinyl ester formation also prevent the biosynthesis of 11-cis retinol. Biochemistry. 1990;29:309–312. doi: 10.1021/bi00454a001. [DOI] [PubMed] [Google Scholar]
- Tsilou E, Hamel CP, Yu S, Redmond TM. RPE65, the major retinal pigment epithelium microsomal membrane protein, associates with phospholipid liposomes. Arch Biochem Biophys. 1997;346:21–27. doi: 10.1006/abbi.1997.0276. [DOI] [PubMed] [Google Scholar]
- Tsina E, Chen C, Koutalos Y, Ala-Laurila P, Tsacopoulos M, Wiggert B, Crouch RK, Cornwall MC. Physiological and microfluorometric studies of reduction and clearance of retinal in bleached rod photoreceptors. J Gen Physiol. 2004;124:429–443. doi: 10.1085/jgp.200409078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara F, Matthes MT, Yasumura D, LaVail MM. Light-evoked changes in the interphotoreceptor matrix. Science. 1990;248:1633–1636. doi: 10.1126/science.2194288. [DOI] [PubMed] [Google Scholar]
- von-Lintig J, Vogt K. Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving beta-carotene to retinal. J Biol Chem. 2000;275:11915–11920. doi: 10.1074/jbc.275.16.11915. [DOI] [PubMed] [Google Scholar]
- Wald G. Carotenoids and the visual cycle. J Gen Physiol. 1935;19:351–371. doi: 10.1085/jgp.19.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald G. The chemistry of rod vision. Science. 1951;113:287–291. doi: 10.1126/science.113.2933.287. [DOI] [PubMed] [Google Scholar]
- Wald G, Brown PK, Smith PH. Iodopsin. J Gen Physiol. 1955;38:623–681. doi: 10.1085/jgp.38.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JS, Estevez ME, Cornwall MC, Kefalov VJ. Intra-retinal visual cycle required for rapid and complete cone dark adaptation. Nat Neurosci. 2009;12:295–302. doi: 10.1038/nn.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JS, Kefalov VJ. The cone-specific visual cycle. Prog Retin Eye Res. 2011;30:115–128. doi: 10.1016/j.preteyeres.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Keller LM, Dillon J, Gaillard ER. Oxidation of A2E results in the formation of highly reactive aldehydes and ketones. Photochem Photobiol. 2006;82:1251–1257. doi: 10.1562/2006-04-01-RA-864. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wen XH, Ablonczy Z, Crouch RK, Makino CL, Lem J. Enhanced shutoff of phototransduction in transgenic mice expressing palmitoylation-deficient rhodopsin. J Biol Chem. 2005;280:24293–24300. doi: 10.1074/jbc.M502588200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel A, von-Lintig J, Oberhauser V, Tanimoto N, Grimm C, Seeliger MW. RPE65 is essential for the function of cone photoreceptors in NRL-deficient mice. Invest Ophthalmol Vis Sci. 2007;48:534–542. doi: 10.1167/iovs.06-0652. [DOI] [PubMed] [Google Scholar]
- Wing GL, Blanchard GC, Weiter JJ. The topography and age relationship of lipofuscin concentration in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1978;17:601–607. [PubMed] [Google Scholar]
- Wolf G. The visual cycle of the cone photoreceptors of the retina. Nutr Rev. 2004;62:283–286. doi: 10.1111/j.1753-4887.2004.tb00053.x. [DOI] [PubMed] [Google Scholar]
- Wu Q, Blakeley LR, Cornwall MC, Crouch RK, Wiggert BN, Koutalos Y. Interphotoreceptor retinoid-binding protein is the physiologically relevant carrier that removes retinol from rod photoreceptor outer segments. Biochemistry. 2007;46:8669–8679. doi: 10.1021/bi7004619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S. Molecular evolution of vertebrate visual pigments. Prog Retin Eye Res. 2000;19:385–419. doi: 10.1016/s1350-9462(00)00002-1. [DOI] [PubMed] [Google Scholar]
- Young RW. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967;33:61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Fan J, Li S, Karan S, Rohrer B, Palczewski K, Frederick JM, Crouch RK, Baehr W. Trafficking of membrane-associated proteins to cone photoreceptor outer segments requires the chromophore 11-cis-retinal. J Neurosci. 2008;28:4008–4014. doi: 10.1523/JNEUROSCI.0317-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Znoiko SL, Rohrer B, Lu K, Lohr HR, Crouch RK, Ma JX. Downregulation of cone-specific gene expression and degeneration of cone photoreceptors in the Rpe65-/- mouse at early ages. Invest Ophthalmol Vis Sci. 2005;46:1473–1479. doi: 10.1167/iovs.04-0653. [DOI] [PubMed] [Google Scholar]