Abstract
The Deepwater Horizon oil rig disaster resulted in crude oil contamination along the Gulf coast in sensitive estuaries. Toxicity from exposure to crude oil can affect populations of fish that live or breed in oiled habitats as seen following the Exxon Valdez oil spill. In an ongoing study of the effects of Deepwater Horizon crude oil on fish, Gulf killifish (Fundulus grandis) were collected from an oiled site (Grande Terre, LA) and two reference locations (coastal MS and AL), and monitored for measures of exposure to crude oil. Killifish collected from Grande Terre had divergent gene expression in the liver and gill tissue coincident with the arrival of contaminating oil, and up-regulation of cytochrome P4501A (CYP1A) protein in gill, liver, intestine and head kidney for over one year following peak landfall of oil (August, 2011) compared to fish collected from reference sites. Furthermore, laboratory exposures of Gulf killifish embryos to field-collected sediments from Grande Terre and Barataria Bay, LA also resulted in increased CYP1A and developmental abnormalities when exposed to sediments collected from oiled sites compared to exposure to sediments collected from a reference site. These data are predictive of population-level impacts in fish exposed to sediments from oiled locations along the Gulf of Mexico coast.
Keywords: CYP1A, Transcriptomics, Immunohistochemistry, development, embryo, oil spill, Deepwater Horizon, Macondo
INTRODUCTION
As a result of the explosion of the Deepwater Horizon oil platform, as much as 700 million liters of crude oil were released from the Macondo well1. Despite cleanup efforts, this oil was widely distributed along shorelines of Louisiana, and to a lesser extent, Mississippi, Alabama, and Florida. During the initial response and cleanup effort, visible oil was reported on the water surface, along beaches, and in marshes as early as May 2010, which coincided with the spawning season for many marine and estuarine fish species. By August 2010, much of the surface oil slick had dissipated or was removed, and visible oil on the beaches and marsh became far less noticeable. However to this date, a considerable amount of weathered oil likely remains deposited in the sediment, serving as a reservoir for persistent exposure to resident species2–4. Since crude oil contains chemicals that are toxic to fish such as polycyclic aromatic hydrocarbons (PAHs), it is probable that residual oil from the Deepwater Horizon oil spill (DHOS) will affect populations of fish living and breeding in contaminated locations.
Fish deaths from toxic acute exposure to PAHs certainly occur, and are often used as a measure of the effects of oil on fish populations5. However, sublethal effects of PAH exposure such as impairment of corticosteroid secretion, immune dysfunction, gill damage, impaired growth and reproduction, and reduced cardio-respiratory capacity are capable of reducing an animal’s ability to effectively respond to environmental stressors4, 6–14. Furthermore, PAHs cause developmental abnormalities in larval fish such as craniofacial defects, edema, reduced size, and cardiovascular defects result in a decrease in fitness that can persist into adulthood, affecting the productivity and survivorship of a population4, 5, 15–17. Multiple studies following the Exxon Valdez oil spill (EVOS) linking crude oil to biological effects in fish were reported, providing benchmarks for the evaluation of remediation efforts and recovery status of affected areas5. Examination of adult fish for biological effects and molecular markers of exposure to oil, along with developmental endpoints in embryos and larval fish both in oiled field sites and in laboratory-based exposures, were predictive of population-level impacts in fish populations4, 5. Few reports have emerged to date using a resident species either in field or laboratory-based bioassays of exposure and effect in areas known to be affected by the DHOS.
This study follows the first report to describe the biological effects in populations of resident killifish exposed in situ to crude oil from the DHOS3. Here we present evidence of exposure to xenobiotics in multiple tissues of adult fish; effects that persist for over one year following the landfall of DHOS oil. We also show developmental defects in embryos exposed to sediments collected from oil-impacted sites in coastal Louisiana in 2010 and 2011. Collectively, the long-term exposure of these fish to persistent PAHs bound in the sediment and the reduction in fitness of developing embryos due to early-life exposure to these sediments could be predictive of persistent population impacts as seen after the EVOS4.
MATERIALS AND METHODS
Sampling of adult fish
Adult Gulf killifish, an abundant, non-migratory baitfish, were collected using wire minnow traps during four trips between May 2010 and August 2011, a time frame that would have coincided with the peak spawning period of this species in the field (Table S1)3. The first three trips in 2010, as described in Whitehead et al., 20123, consisted of five sites identified as unoiled reference sites and a sixth site (Grand Terre Island, LA) directly impacted by contaminating oil. Here we utilize remaining tissues from that study collected at two of the five reference sites, Bay St. Louis, MS (BSL) and Bayou La Batre, AL (BLB), and the oiled location, Grande Terre Island, LA (GT) on trip one (T1) prior to oiling, on trip two (T2) at the peak of oiling, and on trip three (T3) after peak oiling. Additional tissues were collected from GT during a fourth trip (T4) in August 2011, one year after T3.
Transcriptomics
Gill tissues were excised immediately from five field-captured male fish of reproductive age (>6 cm), preserved in RNA-later (Ambion, Inc.), and stored at −20° C until nucleic acid extraction. Microarray data collection was as reported in Whitehead et al., 20123. Briefly, total RNA was extracted using Trizol reagent (Invitrogen; Life Technologies Corp.), antisense RNA (aRNA) was prepared (Ambion Amino Allyl Messageamp II aRNA amplification kit), then aRNA coupled with Alexa Fluor dyes (Alexa Fluor 555 and 647; Molecular Probes), and hybridized to custom oligonucleotide microarray slides (Agilent eArray design ID 027999). The microarray was designed from F. heteroclitus expressed sequences and includes probes for 6800 target sequences. This same microarray platform was previously used in studies of PCB exposures in F. heteroclitus18, in a field study of the liver response to the DHOS in F. grandis3, and a laboratory study of gill and liver responses to weathered Louisiana crude oil in F. grandis (A.W., unpublished data).
Raw data were sequentially normalized by lowess, mixed model analysis, and quantile methods in JMP Genomics (SAS, Inc.), then log2 transformed. Statistical analysis was performed using mixed models in JMP Genomics, where main effects were specified as "time" (including our 4 sampling time-points) and "site" (including our 3 field sites), including an interaction term. Five biological replicates were included within each treatment. A transcriptional response that was different between sites throughout the oiling event was identified by statistically significant (p<0.01) time-by-site interaction. Gene ontology enrichment analysis was performed using David Bioinformatics Resources19, and gene interaction network analysis was performed using Ingenuity Pathway Analysis software (Ingenuity Systems, Inc.).
Immunohistochemistry
All tissues were processed as described in Whitehead et al., 20123 for immunohistochemical visualization of protein abundance and distribution of cytochrome P4501A (CYP1A), a marker of exposure to AhR-active PAHs, using monoclonal antibody (mAB) C10-712, 20, 21. Tissues adhered to poly-l-lysine coated slides were probed with mAb C10-7 using Vectastain ABC immunoperoxidase system (Vector Laboratories). CYP1A was visualized with NovaRed (Vector Laboratories), and counter-stained with Hematoxylin QS (Vector Laboratories). Gill tissues from T1, T2, and T3 were previously used in Whitehead et al., 20123. Slides from these previously used tissues were produced and imaged alongside newly processed liver, intestine, and head kidney tissues from the same fish (see above).
Sediment collection and embryo exposures
Sediment was collected from GT on June 16, 2010, coincident with peak oiling, and approximately one year later on August 28, 2011, when much of the visible oil had dissipated (Table S2). A second location was sampled on August 16, 2011 on a small island in South Wilkinson Bay, LA (WB) where visible oiling was reported from July 2010 to December 2012 by the National Oceanographic and Atmospheric Administration (NOAA) during the Shoreline Cleanup and Assessment Technique (SCAT) surveys (www.gomex.erma.noaa.gov). A third location was chosen in the north of Bay Sansbois (NBS) on August 3, 2011 where no oil was reported by SCAT surveys and this sample served as our non-oiled control sediment (see below).
Sediments were collected using a stainless steel sediment sampler (WILDCO®). Approximately 10cm sediment cores were removed along the shoreline every 0.5 m, and combined and mixed in an aluminum container, then aliquoted into 950 ml amber glass bottles with polytetrafluoroethylene (PTFE) lined lids. Samples were stored on ice for transport to Louisiana State University. Sediments collected in 2010 were stored at −20° C until collection of 2011 sediments, when they were stored with the later at 4° C until use.
Sediment analytical chemistry
Analytical chemistry of sediments used in embryo exposures was conducted as reported in Whitehead et al., 20123. Briefly, sediments were solvent-extracted and extracts analyzed by gas chromatography interfaced with a mass spectrometer. Spectral data were analyzed by Chemstation Software (Agilent Technologies, Inc.).
Embryo collection
Male and female Gulf killifish were collected from Cocodrie, Louisiana (29.254175°, – 90.663559°) and held in a 1500 l recirculating tank containing 10ppt artificially-formulated seawater (AFS) (Instant Ocean). Embryos were collected on Spawntex spawning mats (Aquatic Ecosystems), which were placed in the tanks at sundown, and removed one hour after sunrise. Eggs were removed and held in a 950 ml Pyrex® dish until use.
Sediment exposures
Laboratory mesocosms were prepared by first saturating sediments with 10 ppt AFS prior to adding 150 × 5 ml of this mixture to graduated 950 ml Pyrex® dishes. This mixture was then overlaid with 75 ml AFS. Suspended sediment was allowed to settle for one hour prior to insertion of sampling baskets containing embryos (see below).
Sampling baskets were constructed using virgin polytetrafluoroethylene (PTFE) pipe stock (4B Plastics, Baton Rouge, LA) and PTFE mesh with 250 µm openings (Macmaster-Carr). Two interlocking rings were machined (4B Plastics, Baton Rouge, LA) and fitted together to clamp PTFE mesh tightly across the rings to create a filter basket that rested on the sediment for the duration of the exposure, and elevated the embryos approximately 5 mm above the sediment-water interface.
At the start of experiments, 20–54 embryos were randomly distributed to each PTFE basket, which was positioned on top of sediment within a Pyrex® dish. Four mesocosms were created for GT sediments from 2010 (N=146), five from GT sediments from 2011 (N=132), three from WB sediments collected in 2011 (N=60), and five from NBS sediments collected in 2011 (N=132). Mesocosms were placed on an orbital shaker at 29 RPM to simulate tidal and wind movement of water, without causing an observable increase in turbidity. Animals were kept at room temperature (20–22° C) on a natural light cycle. Hatching of Gulf killifish embryos typically occurs between days 10–14 post fertilization at this temperature, so embryos were observed daily for mortality and hatching for 21 days prior to termination of an experiment. Every other day, 25 ml of water was removed from the mesocosms and replaced with fresh 10ppt AFS. Preliminary experiments using reference sediments and this high water to biomass ratio showed no increase in dissolved oxygen, ammonia, or pH when water was replaced every second day. On day eight, the filter baskets were briefly removed from the mesocosms and placed under a stereomicroscope, where the heartbeats of eight embryos per treatment were measured. Each day, newly-hatched larvae were preserved in either Z-Fix buffered zinc formalin (Ameresco) for histological processing as above, or in RNAlater (Ambion, Inc.) for future genomics work. Additional exposures using NBS 2011, WB 2011, and GT 2010 sediments were conducted under identical conditions to measure larval length at hatch. Our supply of GT sediment from 2011 was depleted at the time of these exposures, thus GT 2011 sediment was not included in this test. Larval length at hatch was measured using Zeiss AxioVision 4.8 software on a Zeiss Lumar® stereomicroscope. Embryonic heartbeat, mortality, hatching success, and larval length were evaluated using XLSTAT ver. 2012.6.08, which were compared using the Kruskal-Wallis test, followed by Dunn’s pairwise comparison. Cumulative daily hatching success was analyzed using generalized linear mixed models (GLMM) with the GLIMMIX procedure (SAS, Inc.) to compare daily hatching between treatment and day.
Imaging
Microscope slides were observed and imaged on a Nikon Eclipse 80i compound microscope using a Nikon DS-Fi1 camera and NIS-Elements BR 3.10 software. Images were balanced globally in Photoshop CS3 (Adobe) for levels using the curves tool for white balance or the levels function.
RESULTS AND DISCUSSION
Genome expression analysis
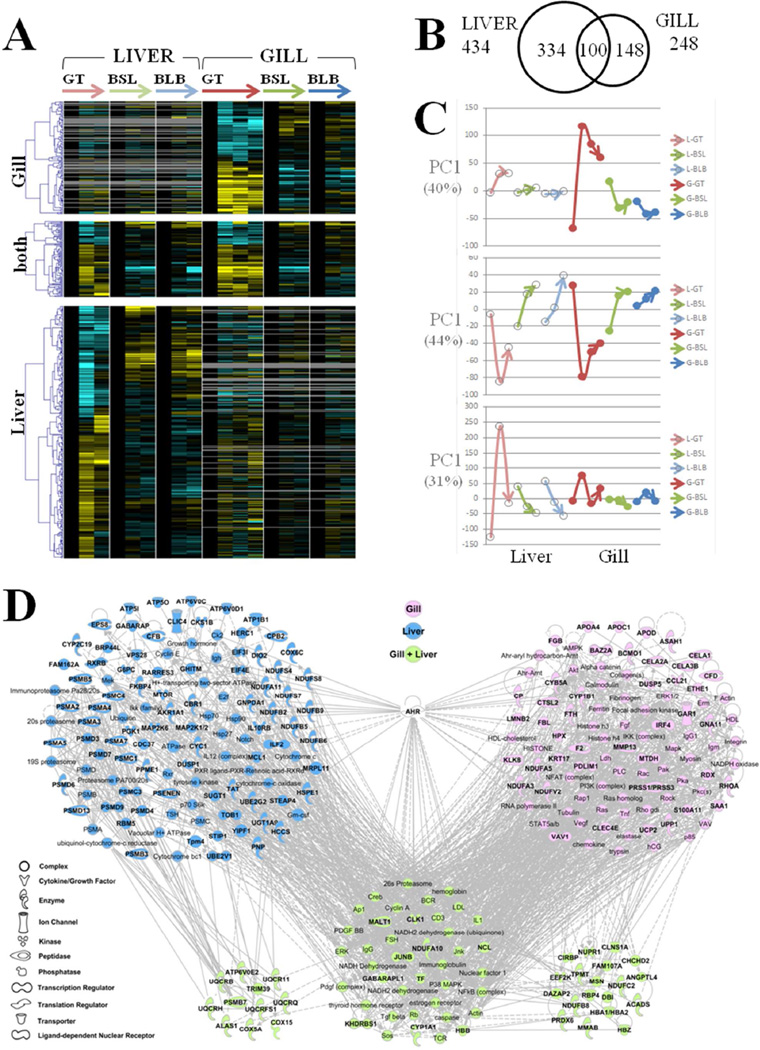
In gill, 374 genes were divergently expressed between field sites throughout the time-course of the oiling event (significant time-by-site interaction; p<0.01) (gene expression data are archived under the EBI Array-Express accession number E-MTAB-1622 - see Supporting Information Microarray Excel file for results of statistical analyses for each gene). Of these, the vast majority (94%) were different in their expression between the oil-exposed site (GT) relative to the two reference sites (BSL and BLB). That is, the response at the oiled GT site was the outlier for 94% of the genes that showed site-dependent expression; whereas, for only 6% of these genes, the outlier was one of the reference sites. The departure in expression at GT from other field sites occurred primarily at the second sampling time-point (Figure 1A and 1C). This indicates that divergence in genome expression is tightly associated with the timing and location of major oiling events in the field. This is consistent with the divergence in genome expression in the liver, which was also tightly coupled with the timing and location of oil contamination (Figure 1 and Whitehead et al., 20123).
Figure 1.
A–D. Transcriptomics. Patterns of expression for the genes that showed site-dependent expression throughout the oiling event (genes with significant time-by-site interaction). (A) Clusters of genes with gill-specific (top cluster), liver-specific (bottom cluster), and common (middle cluster) expression response between tissues, where columns are mean expression for a treatment, rows are genes, and color of cells indicate fold up-regulation (yellow) or down-regulation (blue) relative to pre-oil controls per site. Columns are organized by consecutive time-points within sites, where sites are Grand Terre (GT), Bay St. Louis (BSL), and Bayou La Batre (BLB). Three consecutive time-points are pre-oil, peak oil, and post-oil (left to right) in 2010. Gills were sampled at a fourth time-point at the GT site one year later (August 2011). (B) Venn diagram indicating number of oil-associated genes expressed per tissue. (C) Plots of principal component 1 (PC1) from principal components analysis of the trajectory of transcriptome change through time and between sites for liver (open symbols) and gill (closed symbols). Red, green, and blue represent the trajectories of time-course response (base through head of arrows represent first through last sampling times) at sites GT, BSL, and BLB, respectively. Top, middle, and bottom panels represent genes that were gill-specific, common to both tissues, and liver-specific, respectively, mirroring the clusters represented in the heatmap. In brackets is the proportion of variation accounted for by PC1. (D) Interaction networks for genes that were gill-specific (pink), liver-specific (blue), or common between tissues (green), in their transcriptional response. Bold symbols represent genes included in the analysis, whereas other genes form connections with one degree of separation. The common (green) set of genes are separated into three clusters, where the middle cluster is equally connected to the gill and liver networks, but the left and right clusters primarily associate with the liver or gill clusters, respectively.
Gene ontology categories that were significantly enriched within the set of oiling-associated genes in gill include "response to wounding", "inflammatory response", "acute phase response", and "cytochrome P450". PCBs are mechanistically-related to the toxic components of oil (PAHs), insofar as toxicity is largely mediated through the aryl hydrocarbon receptor (AhR) signaling pathway22. Genes that are PCB dose-responsive in killifish18 are significantly enriched within this set of oil-associated genes in the gill (p<0.01, Fisher's exact test). Up-regulation of a canonical set of genes is diagnostic of activation of the AhR pathway. Gene targets of the activated AhR pathway are among the genes that have oil-associated expression, including CYP1A1, CYP1B1, GCHFR, CYB5, and NUPR1. Among the top five biological functions implicated by network and pathway analyses for gill and gill-only genes (Figure 1D, pink + green genes) were “dermatological diseases”, “immunological disease”, and “cancer”. Acute phase response signaling was also implicated (p<0.0001) among the top canonical pathways for gill tissue-specific genes (Figure 1D, pink genes). Inflammatory signaling is becoming increasingly recognized as an important mechanism mediating the toxic effects of AhR agonists23. The molecular mechanism of AhR ligand-activated inflammation is cell-type specific23, where the inflammatory response is facilitated by cytokines (e.g., TNF) and chemokines, both of which are implicated in the gill-specific gene cluster (Figure 1D). Such AhR activation can mediate immune modulation24, which may increase health risks for animals encountering persistent pathogen challenge in the wild. These patterns of expression provide clear evidence that killifish were exposed to the toxic components of oil, and that gill is a sensitive target of such exposure.
Patterns of liver gene expression (from Whitehead et al., 20123) were compared with patterns in gill to uncover expression responses that were unique between tissues, and common between tissues. More genes were diagnostic of the oiling event in the liver than in the gill (434 versus 248 respectively (Figure 1A and 1B)). However, the degree of up- or down-regulation of genes in response to the oiling event was more dramatic in gill than in liver tissue (Figure 1C). Most of the genes that showed statistically significant divergence in expression only in the liver showed the same trend in gill (Figure 1A, bottom panel), though the reciprocal pattern (correlation between liver and gill patterns for genes with significant response only in the gill) was not apparent (Figure 1A, top panel). This more dramatic transcriptional response in the gill may be reflective of this organ's direct contact with the contaminated external environment. In contrast, the liver is not in direct contact with the environment, and its attenuated response relative to the gill may be reflective of mechanisms of chemical uptake at epithelia and the complex internal dynamics metabolism.
Immunohistochemistry of adult fish sampled in situ
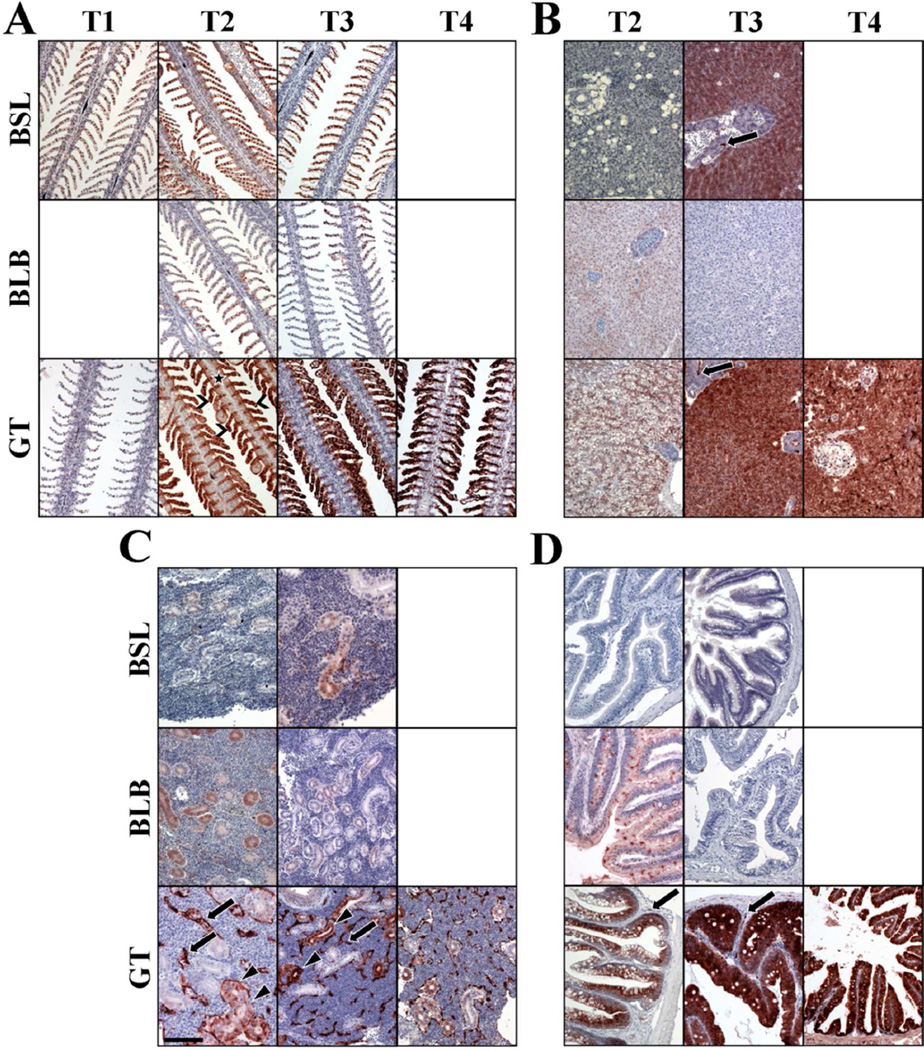
Immunolocalization of CYP1A in gill, liver, intestine, and head kidneys from fish collected in situ from three locations (GT, BSL, BLB) during peak oiling, one month after peak oiling, and one year after peak oiling (Table S1) reveals a pattern of CYP1A protein expression indicative of exposure to crude oil at GT. GT fish collected after the landfall of oil (T2–T4) had elevated CYP1A protein within the interlamellar region of the filament and in the pillar cells of the lamellae compared to fish gills collected prior to oiling (T1), or fish collected from the reference sites (Figure 2A and Whitehead et al., 20123). Increased hyperplasia along the filamental and lamellar epithelia of the gill was also present in GT fish post-oil compared to fish from GT pre-oil and reference sites. Liver tissues from GT fish collected during T3 and T4 also had elevated CYP1A protein, in contrast to fish collected during T2, when CYP1A was less abundant (Figure 2B) compared to fish collected from reference sites. Distribution and abundance of CYP1A protein in the head kidney (Figure 2C) and intestine (Figure 2D) also suggest exposure to PAHs in crude oil in GT fish collected after the arrival of oil (T2–T4), when compared to fish from reference sites. CYP1A expression, as found pervasively in the vascular endothelial cells of intestine and head kidney of GT fish, is a hallmark of AhR pathway activation2, 20, 25. Head kidney tissues also had increased CYP1A protein localized in the tubular epithelial cells, while intestinal epithelial cells were heavily stained in all GT fish collected after the arrival of oil, in contrast to the near absence of staining in head kidney and intestine from fish collected from BSL and BLB. CYP1A expression was detected in some reference fish tissues, consistent with the endogenous CYP1A protein expression in most tissues associated with diverse biological functions20, 26. The AhR can be activated by multiple anthropogenic and natural environmental stressors and endogenous AhR ligands to induce the expression of CYP1A. However, CYP1A expression is far greater when the AhR is activated by xenobiotic ligands and the relative increase in CYP1A protein distributed throughout multiple tissues in GT fish is consistent with exposure to PAHs coincident with the arrival of contaminating oil (Figure 2)23.
Figure 2.
A–D. Distribution of CYP1A protein (burgundy staining) in gills, liver, head kidneys, and intestines from adult killifish collected from Bay St. Louis (BSL), Bayou La Batre (BLB), and Grande Terre (GT) at four time points (T1–T4) beginning prior to landfall of oil in May 2010 (T1), at the peak of oiling in June 2010 (T2), after peak oiling in August 2010 (T3), and one year after landfall of oil in August 2011 at GT only (T4). (A) Increase in CYP1A in the gill lamellae (chevrons) and in the epithelia of the interlamellar regions of the gill filament (star) as well as an increase in hyperplasia in the gill lamellae and in the interlamellar regions of the gill filaments. (B) Livers from GT showed the highest expression of CYP1A at T3 and T4 time points, and an increase in CYP1A was observed in BSL fish at T3. (C) Increased staining of epithelial cells of the kidney tubules (arrow heads) and an increase in CYP1A-positive vascular endothelial cells were found in GT fish. (D) GT intestinal tissues show an increase in CYP1A in the epithelial cells and CYP1A-positive vascular endothelial cells (arrow heads) in the lamina propria and submucosa. All tissues sectioned at 4 µm thickness and imaged with a 20× objective. Arrows = vascular endothelial cells, chevrons = gill filaments, asterisks = buccopharyngeal cavity, arrow heads = kidney tubules. Scale bar = 50 µm. All slides were counterstained with hematoxylin (blue). Gill tissues from T1, T2, and T3 were previously used to create images published in Whitehead et al., 20123, although the gill images presented here (T1, T2, and T3) are new images from archived tissues that were processed alongside gills from T4, and head kidney, intestine, and liver tissues depicted in this figure.
The persistence of increased CYP1A protein expression in GT fish more than one year after initial oiling is not surprising since crude oil can remain bound in sediments, facilitating the slow and long-term release of PAHs from sediments and from dietary accumulation from benthic food sources2, 5. By examining sensitive biological responses in resident species with high home-range fidelity (such as the Gulf killifish), it is possible to determine the distribution and persistence of exposures to the toxic components of oil across space and time.
Typically, responses of liver are used for estimating exposure to PAHs due to its capacity for xenobiotic transformation of blood-borne toxicants, although the gill, intestine, and head kidney are also sensitive indicators of exposure to AhR-inducing chemicals, and have become increasingly popular for their utility in determining exposure to PAHs20, 26–28. However, assessing multiple tissues for CYP1A distribution can provide insights into the route of exposure based on the differential expression between organs and their functional roles. The gill and intestine are transport epithelia capable of metabolizing AhR-inducing xenobiotics, including PAHs25, 29, 30. Elevated CYP1A in the gill can indicate water-borne exposure to PAHs, whereas CYP1A increase in the intestine can indicate dietary uptake and metabolism of PAHs as they cross the intestinal epithelium2, 25, 28. Additionally, fish in hyperosmotic environments drink to absorb water across the posterior intestine leading to the accumulation of PAHs via the gastrointestinal tract2. CYP1A expression in the head kidney is indicative of exposure of a central part of the teleost immune system to immunotoxic PAHs and also suggests systemic circulation of PAHs31, 32. The up-regulation and distribution of CYP1A protein throughout the gill, intestine, head kidney, and liver seen in GT fish, in contrast to that seen in fish collected from reference sites, is consistent with exposure to PAHs in contaminating oil, and with persistence of PAHs in sediments enabling long-term exposure to resident biota2, 5.
Developmental impacts of field-collected sediments
Total PAH (tPAH) and alkane content in GT sediments from 2010 and 2011, and WB sediments from 2011 were elevated compared to NBS sediments (Table S3–S4 and Figure S1–S3). SCAT data confirms visible oil at GT and WB throughout December 2012, while no oil was reported at NBS. Based on analytical chemistry data, GT sediments collected during 2010 had almost a 10-fold higher tPAH concentration compared to sediments collected at that site a year later. WB sediments collected in 2011 contained approximately 21% less tPAHs than do GT 2011 sediments collected at the same time, whereas NBS had negligible tPAH content that was less than 1% of the tPAHs found in WB sediments.
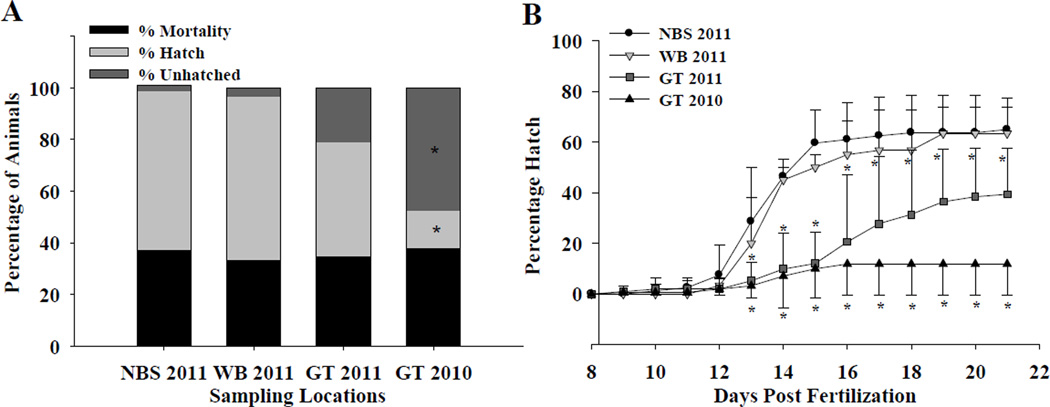
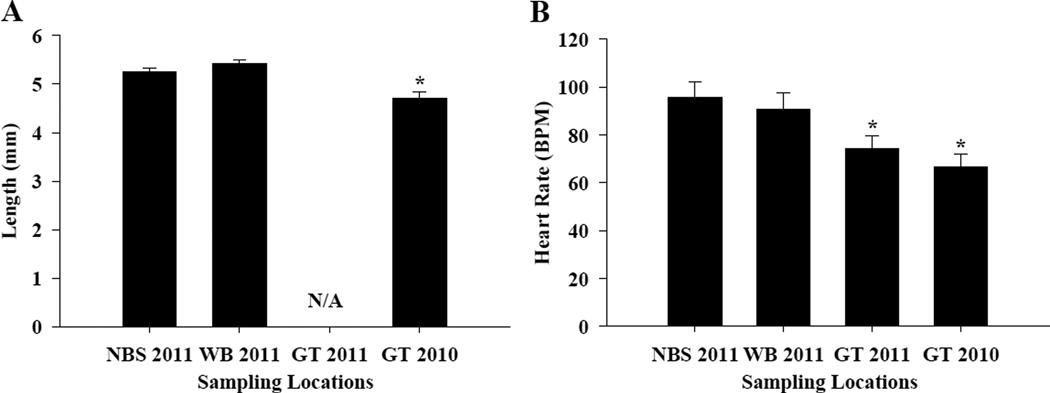
Throughout the 21-day exposure of embryos to these sediments, many of the GT sediment-exposed embryos failed to hatch (Figure 3 A–B). Beginning at day 13, embryos exposed to GT 2010 and GT 2011 sediments had significantly less hatching events compared to embryos exposed to WB or NBS sediments (P<0.05). Accordingly, percent hatch within 21 days post-fertilization for embryos exposed to GT sediments collected in 2010 and 2011 was significantly reduced compared to those exposed to sediments collected in 2011 from NBS or WB (P<0.05). In comparison, there was no significant change in embryonic mortality between treatments. Those embryos that did hatch in the GT sediments were significantly smaller, had pronounced bradycardia during embryonic development (P<0.05) (Figure 4 A–B), and had poor vigor at hatch. Yolk-sac and pericardial edema were observed in fish exposed to GT sediments, but not observed in WB or NBS larvae (data not shown). These data are consistent with the characteristic developmental impairments associated with early life-stage exposures to crude oil and PAHs, and are correlated with an increase in PAHs (and alkanes) in the sediments, coincident with oiling attributed to the DHOS (Table S1–S4 and Figure S1–S3)3, 5, 33, 34. Variation in physiochemical properties of the sediments may influence the toxicity of crude oil to developing embryos, and sex, breeding potential, and other variables are known to influence teleost response to crude oil exposure12, 35. However, these factors are likely minor compared to the influence of oil concentration between sites (Table S1–S4 and Figure S1–S3). The effects seen in these developing fish indicate that resident developing Gulf killifish embryos were exposed to crude oil from these sediments (see below) for at least two breeding seasons, and that this exposure may affect future population demographics at locations where crude oil is present.
Figure 3.
A–B. Hatching success and mortality of embryos exposed to sediments collected from an unoiled reference location in North Bay Sansbois in 2011 (NBS 2011), from oiled locations in Wilkinson Bay in 2011 (WB 2011) and Grande Terre Island (GT 2011 and 2010). Embryos were fertilized and observed daily for 21 days for mortality and hatching. (A) Percentage of hatched and unhatched larvae and percentage of mortalities. Unhatched embryos are defined as those that did not hatch by day 21 post fertilization. Mortalities are animals that died within the 21 day period. Asterisks indicate significant difference compared to the NBS reference sediment exposure (P≤0.001). (B) After day 13, embryos exposed to GT 2010 and GT 2011 sediments hatched significantly less compared to embryos exposed to WB or NBS sediments. Error bars indicate standard error and are one sided to prevent overlapping and crowding of symbols. Asterisks indicate significant difference compared to the NBS reference sediment exposure (P≤0.05).
Figure 4.
A–B. Early life-stage phenotypic effects of embryos exposed to sediments collected from an unoiled reference location in North Bay Sansbois in 2011 (NBS 2011), from oiled locations in Wilkinson Bay in 2011 (WB 2011) and Grande Terre Island (GT 2011 and 2010). (A) Larval length at ≤24 hours post hatch was significantly lower for GT 2010 sediment exposed fish. GT 2011 exposed fish were unavailable for length measurements. (B) Heart rate of embryos at 8 DPF. Error bars indicate standard error. Asterisks in both graphs indicate a significant difference compared to the NBS reference sediment exposure (P≤0.001).
Immunohistochemistry of sediment-exposed larvae
Larvae collected at <24 hours post hatch had elevated CYP1A protein when exposed to oiled sediments (WB 2011, GT 2010, and GT 2011) compared to larvae exposed to unoiled (NBS) sediment (Figure 5 A–B and Figure S4). Elevated CYP1A protein in oiled sediment-exposed larvae was found in vascular endothelial cells throughout the body, and in the gill, buccopharyngeal epithelium, liver, head kidneys, and heart (Figure 5B). NBS sediment-exposed fish showed slightly elevated CYP1A in kidney tubules, heart, and liver tissue, although elevated expression of CYP1A was not observed in other tissues. Larvae exposed to GT sediments that had substantially more CYP1A protein than the other sediment-exposed fish, including stronger staining of the external epithelial surfaces. Staining in the external epithelia was present in WB larvae, albeit to a much lesser extent than that of GT sediment-exposed larvae, probably because PAH and alkane concentrations were elevated in WB sediments at levels just below that of GT 2011 sediments (Table S1–S2 and Figure S1–S3). Despite the increased CYP1A expression in WB sediment-exposed larvae, no bradycardial or developmental effects were noted in these animals (Figure 4 A–B). It is likely that the dosage of toxicants needed to elicit observable phenotypic effects is higher than that found in WB sediments, but lower than that found in GT sediments collected at the same time in 2011(Figure S3–S4 and Figure S1–S2), or that other physical or chemical characteristics of these sediments contribute to the effects seen in these animals.
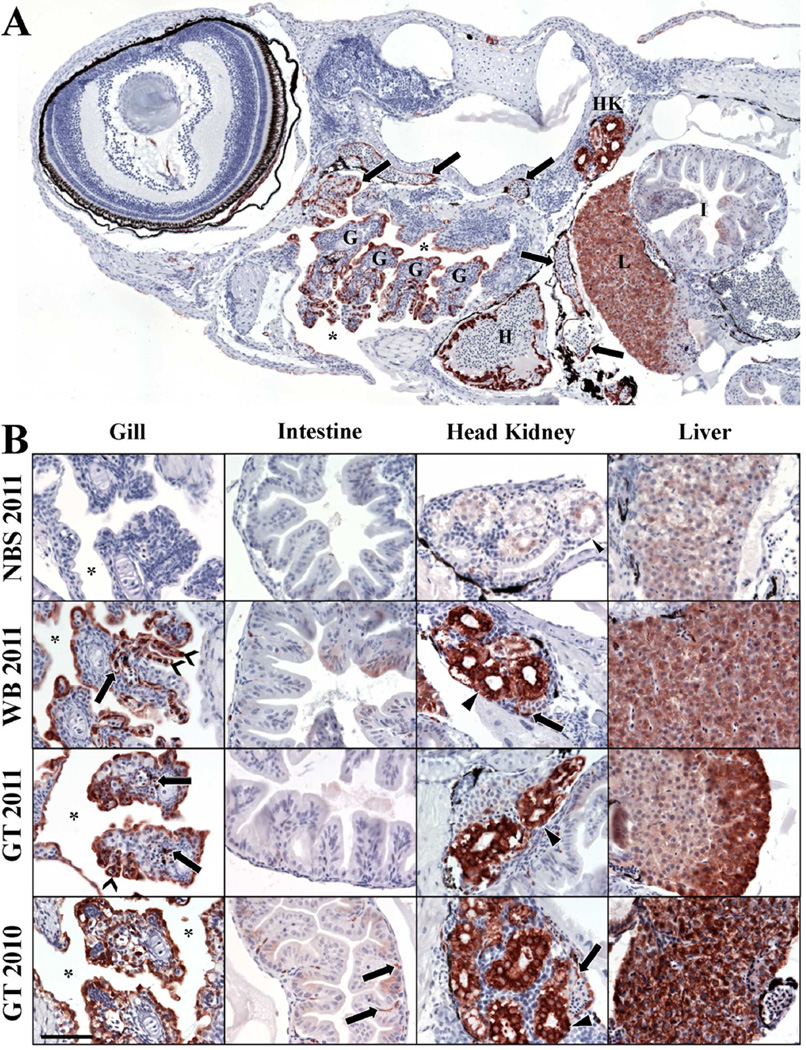
Figure 5.
A–B. Distribution of CYP1A protein (burgundy staining) in larval tissues at ≤ 24 h post hatch exposed throughout development to sediments collected from an unoiled reference location in North Bay Sansbois in 2011 (NBS 2011), from a mildly oiled location in Wilkinson Bay in 2011 (WB 2011), and a heavily oiled location at Grande Terre Island in 2010 and 2011 (GT 2011 and 2010). (A) Representative image of larvae at <24 hours post hatch that were exposed to WB sediment throughout embryonic development. CYP1A was mostly found localized in gill (G), intestine (I), head kidney (HK), liver (L), heart (H) endothelium, vascular endothelial cells (arrows), and in epithelial cells lining the buccopharyngeal cavity (asterisks) in fish exposed to oiled sediments (i.e. WB or GT sediments). Fish exposed to unoiled sediment from NBS showed staining for CYP1A in endothelia within the heart and kidney tubules (See supplementary data, S2). Larvae were sectioned at 4 µm. Figure depicts a montage of 12 images captured using a 20× objective. (B) Tissue sections from larvae at <24 hours post hatch. Gill tissues from fish exposed to WB 2011, GT 2011, and GT 2010 sediments had CYP1A-positive vascular endothelial cells and increased CYP1A in epithelial and pillar cells in the gill filaments and lamellae. Epithelial cells lining the buccopharyngeal cavity also showed increased CYP1A in these fish. Intestine showed light staining for CYP1A protein in the epithelial cells and endothelial cells in the submucosa in these fish. Head kidneys tubules and vascular endothelial cells in fish exposed to oiled sediment had higher CYP1A staining compared to fish exposed to unoiled NBS 2011 sediment. Hepatocytes of liver tissue of fish exposed to oiled sediments showed increased CYP1A expression compared to those of fish exposed to unoiled NBS sediment. Images captured through a 40× objective. Arrows = vascular endothelial cells, chevrons = gill lamellae, asterisks = bucopharyngeal cavity, arrow heads = kidney tubules. All tissues from A and B were sectioned at 4µm. Scale bar = 50µm. All slides were counterstained with hematoxylin (blue).
In Whitehead et al., 20123, adult Gulf killifish collected at GT in 2010 exhibited molecular and protein-level responses that are diagnostic of exposure to crude oil. In that study, although PAH concentrations were similarly low in tissues and water samples between all sites, PAHs were elevated in GT sediments relative to reference sites3. The methods used in the experiments reported here were designed to assess the potential for field-collected sediments from oiled sites to elicit lethal or sub-lethal effects. By utilizing field-collected sediments, we attempted to characterize the developmental potential of Gulf killifish at locations that received oil from the DHOS. The results suggest that sediments were a persistent source of biologically-available AhR-activating toxicants at oiled sites for over one year following the landfall of oil. Furthermore, since PAHs in field-collected water and tissues of resident animals have tended to be below the detection limits of analytical chemistry in areas that received contaminating oil, biological responses appear to be more sensitive indicators of contamination3, 36.
IMPLICATIONS
Integration of diverse endpoints spanning multiple levels of biological organization in both laboratory and field studies with indigenous organisms as site-specific indications of ecosystem health enriches the understanding of the effects of environmental change. In seeking to characterize the effects of the DHOS on at-risk fish, a field study examining the health of populations of Gulf killifish was initiated prior to the landfall of oil, and is ongoing. Here we presented evidence of exposure to PAHs in adult fish coincident with the oil contamination from the DHOS. Genome expression profiling indicates significant divergence in genomic responses of fish from an oiled location compared to reference sites. These responses are diagnostic of exposure to the toxic components of oil, and are highly correlated with CYP1A protein expression responses in the gills, liver, head kidney, and intestine of adult and larval fish. Exposure to sediments from oiled locations caused cardiovascular defects in embryonic fish, delayed hatching, and reduced overall hatching success. Those larvae that do hatch are smaller and have yolk-sac and pericardial edema. These data include results that encompass two breeding seasons and indicate that contaminating oil from the DHOS impacts organismal fitness, which may translate into longer-term effects at the population level for Gulf killifish and other biota that live or spawn in similar habitats.
Supplementary Material
ACKNOWLEDGMENTS
We thank the College of Science and the Office of Research and Economic Development at Louisiana State University for generously helping to fund initial field work and critical bridge funding support. Thanks to David Roberts and Eve McCulloch for field assistance. Special thanks to Captain Les Barios for transportation and shelter in Barataria Bay, Dr. E. William Wischusen and the students enrolled in Biology 1208 laboratories sections 13 and 14 in the spring of 2011 for help in monitoring embryo mortality, hatch, and phenotypic characteristics, and M. Scott Miles for analytical chemistry of sediment samples.
Funding Sources
This research was funded in part by the Gulf of Mexico Research Initiative (to F. G. and A.W.), the National Science Foundation (DEB-1048206 and DEB-1120512 to A. W.), and the National Institutes of Health (R15-ES016905-01 to C. D. R.)
ABBREVIATIONS
- DHOS
Deepwater Horizon oil spill
- EVOS
Exxon Valdez oil spill
- AhR
Aryl hydrocarbon receptor
- PAH
polycyclic aromatic hydrocarbon
- GT
Grande Terre Island
- BSL
Bay St. Louis, MS
- BLB
Bayou La Batre, LA
- T1
Trip 1
- T2
Trip 2
- T3
Trip 3, T3
- CYP1A
cytochrome P4501A
- mAb
monoclonal antibody
- WB
Wilkinson Bay
- NOAA
National Oceanographic and Atmospheric Administration
- SCAT
Shoreline Cleanup and Assessment Technique
- NBS
North Bay Sansbois
- PTFE
polytetrafluoroethylene
- AFS
artificially-formulated seawater
- DPF
days post fertilization
- BPM
beats per minute
- tPAHs
total PAHs
Footnotes
ASSOCIATED CONTENT
Supporting Information. Tables of field sampling sites, analytical chemistry data from sediments, and comparison of representative sediment-exposed larva are included as supplementary data. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
REFERENCES
- 1.Crone TJ, Tolstoy M. Magnitude of the 2010 Gulf of Mexico Oil Leak. Science. 2010;330(6004):634–634. doi: 10.1126/science.1195840. [DOI] [PubMed] [Google Scholar]
- 2.Woodin BR, Smolowitz RM, Stegeman JJ. Induction of cytochrome P4501A in the intertidal fish Anoplarchus purpurescens by prudhoe bay crude oil and environmental induction in fish from Prince William Sound. Environmental Science & Technology. 1997;31(4):1198–1205. [Google Scholar]
- 3.Whitehead A, Dubansky B, Bodinier C, Garcia TI, Miles S, Pilley C, Raghunathan V, Roach JL, Walker N, Walter RB, Rice CD, Galvez F. Genomic and physiological footprint of the Deepwater Horizon oil spill on resident marsh fishes. Proceedings of the National Academy of Sciences. 2012;109(50):20298–20302. doi: 10.1073/pnas.1109545108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peterson CH, Rice SD, Short JW, Esler D, Bodkin JL, Ballachey BE, Irons DB. Long-term ecosystem response to the Exxon Valdez oil spill. Science. 2003;302(5653):2082–2086. doi: 10.1126/science.1084282. [DOI] [PubMed] [Google Scholar]
- 5.Short JW, Rice SD, Heintz RA, Carls MG, Moles A. Long-term effects of crude oil on developing fish: Lessons from the Exxon Valdez oil spill. Energy Sources. 2003;25(6):509–517. [Google Scholar]
- 6.Claireaux G, Desaunay Y, Akcha F, Auperin B, Bocquene G, Budzinski FN, Cravedi JP, Davoodi F, Galois R, Gilliers C, Goanvec C, Guerault D, Imbert N, Mazeas O, Nonnotte G, Nonnotte L, Prunet P, Sebert P, Vettier A. Influence of oil exposure on the physiology and ecology of the common sole (Solea solea): Experimental and field approaches. Aquat. Living Resour. 2004;17(3):335–351. [Google Scholar]
- 7.Davoodi F, Claireaux G. Effects of exposure to petroleum hydrocarbons upon the metabolism of the common sole (Solea solea) Mar. Pollut. Bull. 2007;54(7):928–934. doi: 10.1016/j.marpolbul.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Claireaux G, Davoodi F. Effect of exposure to petroleum hydrocarbons upon cardio-respiratory function in the common sole (Solea solea) Aquat. Toxicol. 2010;98(2):113–119. doi: 10.1016/j.aquatox.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Hontela A. Interrenal dysfunction in fish from contaminated sites: In vivo and in vitro assessment. Environ. Toxicol. Chem. 1998;17(1):44–48. [Google Scholar]
- 10.Frederick LA, Van Veld PA, Rice CD. Bioindicators of immune function in creosote-adapted estuarine killifish, Fundulus heteroclitus. J. Toxicol. Env. Health Pt A. 2007;70(17):1433–1442. doi: 10.1080/15287390701382910. [DOI] [PubMed] [Google Scholar]
- 11.Rice CD, Kergosien DH, Adams SM. Innate Immune Function as a Bioindicator of Pollution Stress in Fish. Ecotoxicology and Environmental Safety. 1996;33(2):186–192. doi: 10.1006/eesa.1996.0024. [DOI] [PubMed] [Google Scholar]
- 12.Spies RB, Stegeman JJ, Hinton DE, Woodin B, Smolowitz R, Okihiro M, Shea D. Biomarkers of hydrocarbon exposure and sublethal effects in embiotocid fishes from a natural petroleum seep in the Santa Barbara Channel. Aquat. Toxicol. 1996;34(3):195–219. [Google Scholar]
- 13.Marty GD, Hinton DE, Short JW, Heintz RA, Rice SD, Dambach DM, Willits NH, Stegeman JJ. Ascites, premature emergence, increased gonadal cell apoptosis, and cytochrome P4501A induction in pink salmon larvae continuously exposed to oil-contaminated gravel during development. Canadian Journal of Fisheries and Aquatic Sciences. 1997;75(6):989–1007. [Google Scholar]
- 14.Heintz RA, Rice SD, Wertheimer AC, Bradshaw RF, Thrower FP, Joyce JE, Short JW. Delayed effects on growth and marine survival of pink salmon (Oncorhynchus gorbuscha) after exposure to crude oil during embryonic development. Mar. Ecol.-Prog. Ser. 2000;208:205–216. [Google Scholar]
- 15.Incardona JP, Collier TK, Scholz NL. Oil spills and fish health: exposing the heart of the matter. J. Expo. Sci. Environ. Epidemiol. 2011;21(1):3–4. doi: 10.1038/jes.2010.51. [DOI] [PubMed] [Google Scholar]
- 16.Hicken CE, Linbo TL, Baldwin DH, Willis ML, Myers MS, Holland L, Larsen M, Stekoll MS, Rice SD, Collier TK, Scholz NL, Incardona JP. Sublethal exposure to crude oil during embryonic development alters cardiac morphology and reduces aerobic capacity in adult fish. Proceedings of the National Academy of Sciences. 2011;108(17):7086–7090. doi: 10.1073/pnas.1019031108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Soysa TY, Ulrich A, Friedrich T, Pite D, Compton S, Ok D, Bernardos R, Downes G, Hsieh S, Stein R, Lagdameo MC, Halvorsen K, Kesich L-R, Barresi M. Macondo crude oil from the Deepwater Horizon oil spill disrupts specific developmental processes during zebrafish embryogenesis. BMC Biology. 2012;10(1):40. doi: 10.1186/1741-7007-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitehead A, Pilcher W, Champlin D, Nacci D. Common mechanism underlies repeated evolution of extreme pollution tolerance. Proceedings of the Royal Society B: Biological Sciences. 2012;279(1728):427–433. doi: 10.1098/rspb.2011.0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 20.Sarasquete C, Segner H. Cytochrome P4501A (CYP1A) in teleostean fishes. A review of immunohistochemical studies. Science of the Total Environment. 2000;247(2–3):313–332. doi: 10.1016/s0048-9697(99)00500-8. [DOI] [PubMed] [Google Scholar]
- 21.Rice CD, Schlenk D, Ainsworth J, Goksøyr A. Cross-reactivity of monoclonal antibodies against peptide 277–294 of rainbow trout CYP1A1 with hepatic CYP1A among fish. Mar. Environ. Res. 1998;46(1–5):87–91. [Google Scholar]
- 22.Clark BW, Matson CW, Jung D, Di Giulio RT. AHR2 mediates cardiac teratogenesis of polycyclic aromatic hydrocarbons and PCB-126 in Atlantic killifish (Fundulus heteroclitus) Aquat. Toxicol. 2010;99(2):232–240. doi: 10.1016/j.aquatox.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumura F. The significance of the nongenomic pathway in mediating inflammatory signaling of the dioxin-activated Ah receptor to cause toxic effects. Biochem. Pharmacol. 2009;77(4):608–626. doi: 10.1016/j.bcp.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Stevens EA, Mezrich JD, Bradfield CA. The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology. 2009;127(3):299–311. doi: 10.1111/j.1365-2567.2009.03054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.VanVeld PA, Vogelbein WK, Cochran MK, Goksoyr A, Stegeman JJ. Route-specific cellular expression of cytochrome P4501A (CYP1A) in fish (Fundulus heteroclitus) following exposure to aqueous and dietary benzo[a] pyrene. Toxicol. Appl. Pharmacol. 1997;142(2):348–359. doi: 10.1006/taap.1996.8037. [DOI] [PubMed] [Google Scholar]
- 26.Ortiz-Delgado JB, Segner H, Sarasquete C. Cellular distribution and induction of CYP1A following exposure of gilthead seabream, Sparus aurata, to waterborne and dietary benzo[a]pyrene and 2,3,7,8-tetrachlorodibenzo-p-dioxin: An immunohistochemical approach. Aquat. Toxicol. 2005;75(2):144–161. doi: 10.1016/j.aquatox.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Jonsson EM, Brandt I, Brunstrom B. Gill filament-based EROD assay for monitoring waterborne dioxin-like pollutants in fish. Environmental Science & Technology. 2002;36(15):3340–3344. doi: 10.1021/es015859a. [DOI] [PubMed] [Google Scholar]
- 28.Jonsson EM, Abrahamson A, Brunstrom B, Brandt I. Cytochrome P4501A induction in rainbow trout gills and liver following exposure to waterborne indigo, benzo[a]pyrene and 3,3 ',4,4 ',5-pentachlorobiphenyl. Aquat. Toxicol. 2006;79(3):226–232. doi: 10.1016/j.aquatox.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Andersson T, Pärt P. Benzo[a]pyrene metabolism in isolated perfused rainbow trout gills. Mar. Environ. Res. 1989;28(1–4):3–7. [Google Scholar]
- 30.Kleinow KM, James MO, Tong Z, Venugopalan CS. Bioavailability and biotransformation of benzo[a]pyrene in an isolated perfused in situ catfish intestinal preparation. Environ. Health Perspect. 1998;106(3):155–166. doi: 10.1289/ehp.98106155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakayama A, Riesen I, Kollner B, Eppler E, Segner H. Surface marker-defined head kidney granulocytes and B lymphocytes of rainbow trout express benzo a pyrene-inducible cytochrome P4501A protein. Toxicol. Sci. 2008;103(1):86–96. doi: 10.1093/toxsci/kfn024. [DOI] [PubMed] [Google Scholar]
- 32.Brand DG, Fink R, Bengeyfield W, Birtwell IK, McAllister CD. Salt water-acclimated pink salmon fry (Oncorhynchus gorbuscha) develop stress-related visceral lesions after 10-day exposure to sublethal concentrations of the water-soluble fraction of north slope crude oil. Toxicol. Pathol. 2001;29(5):574–584. doi: 10.1080/019262301317226384. [DOI] [PubMed] [Google Scholar]
- 33.Incardona JP, Collier TK, Scholz NL. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol. Appl. Pharmacol. 2004;196(2):191–205. doi: 10.1016/j.taap.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 34.Incardona JP, Carls MG, Day HL, Sloan CA, Bolton JL, Collier TK, Scholz NL. Cardiac Arrhythmia Is the Primary Response of Embryonic Pacific Herring (Clupea pallasi) Exposed to Crude Oil during Weathering. Environmental Science & Technology. 2009;43(1):201–207. doi: 10.1021/es802270t. [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Gomez C, Vethaak AD, Hylland K, Burgeot T, Kohler A, Lyons BP, Thain J, Gubbins MJ, Davies IM. A guide to toxicity assessment and monitoring effects at lower levels of biological organization following marine oil spills in European waters. Ices Journal of Marine Science. 2010;67(6):1105–1118. [Google Scholar]
- 36.Ylitalo GM, Krahn MM, Dickhoff WW, Stein JE, Walker CC, Lassitter CL, Garrett ES, Desfosse LL, Mitchell KM, Noble BT, Wilson S, Beck NB, Benner RA, Koufopoulos PN, Dickey RW. Federal seafood safety response to the Deepwater Horizon oil spill. Proceedings of the National Academy of Sciences. 2012 doi: 10.1073/pnas.1108886109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.