Abstract
Purpose
Luteolin, a flavone present in many foods and medicinal plants, may have beneficial effects on various human chronic diseases. In the present study, we investigated the hypothesis that luteolin can directly act on vascular endothelial cells (ECs), leading to nitric oxide (NO) production and subsequent vascular relaxation.
Methods
Rat aortic rings were mounted in organ bath. Luteolin was added cumulatively and vessel relaxation of rat aortic rings precontracted with phenylephrine (PE) or potassium was recorded. Endothelial nitric oxide synthase (eNOS) phosphorylation at Ser1177 and NO production from aortic rings and primary human aortic endothelial cells (HAECs) exposed to luteolin were measured by using Western blot and fluorometric assay, respectively.
Results
Luteolin dose-dependently (10-100 μmol/L) elicited relaxation of PE- or potassium-contracted aortic rings. The vasorelaxation effect of luteolin was attenuated by the eNOS inhibitor, N-nitro-L-arginine methyl ester, suggesting that this luteolin action is at least partially mediated by activating eNOS activity. We further found that luteolin dose-dependently (10-100 μmol/L) increased eNOS phosphorylation at Ser1177 (up to 1.9 fold) in isolated rat rings. Consistently, exposure of HAECs to luteolin also increased eNOS phosphorylation and NO production.
Conclusion
Luteolin may be a vascular protective agent by directly acting on vascular ECs to stimulate NO-dependent vascular dilatation.
Keywords: luteolin; vasorelaxation, endothelial cells; eNOS; NO
Introduction
It is known that the classic and specific α (1) adrenergic receptor (α1-AR) agonist, phenylephrine (PE), exerts a positive inotropic effect on vascular smooth muscle which is opposed by products of the vascular endothelium. Specifically, within the cardiovascular system there is a paracrine antagonism through which the product of endothelial nitric oxide synthase (eNOS), nitric oxide (NO), moderates the effects of α1-AR -mediated contraction [1, 2]. That is, the endothelial layer of the arterial vasculature increases NO release by increasing eNOS activity during α1-AR mediated sympathetic agonism, thus moderating arterial blood pressure. Disruption of this concerted mechanism between α1-AR agonism and eNOS stimulation may have clinically relevant ramifications. Impairment of eNOS stimulation and the subsequent decrease in NO release is thought to contribute substantially to molecular mechanisms associated with essential hypertension [3, 4]. Because of this pivotal role for NO in blood pressure homeostasis, modulation of NO bioavailability is considered a major target for treatment of essential hypertension.
Luteolin is a bioflavonoid present in many food products and medicinal plants including Artichoke (Cynara cardunculus), one of the world’s most well-known “medicinal” plants with a long history of pharmacotherapeutic use [5-7]. Recent investigations have showed that luteolin or artichoke extracts have numerous beneficial medicinal properties such as anti-cancer, anti-oxidant, anti-inflammatory, and anti-allergic actions [8-14]. The cardioprotective properties of luteolin or artichoke extracts include lowering plasma lipids [15], inhibiting cholesterol biosynthesis [16], preventing LDL oxidation [17], and increasing eNOS gene expression [18]. There are also studies showing that luteolin improves vascular relaxation and prevents age-associated loss of vasomotor function [19]. How luteolin affects vascular relaxation however, remains controversial. While some studies show that luteolin relaxes smooth muscle independently of NO [20], other research reports roles for luteolin in both NO-coupled vasodilatation and NO-independent modulation of Ca2+ and K+ channels [21, 22]. The current study was undertaken to further clarify the mechanism of luteolin action in vascular relaxation using rat aortic rings and primary human aortic endothelial cells (HAECs).
Methods and Materials
Materials
Primary HAECs and endothelial growth supplements (EGM2) were purchased from Lonza Walkersville (Walkersville, MD, USA); nitrite/nitrate fluorometric assay reagents were purchased from Cayman Chemical (Ann Arbor, MI, USA); antibodies for eNOS and phospho-eNOS were from Cell Signaling Technology (Beverly, MA, USA); nitrocellulose membranes and protein assay kits were from Bio-Rad (Hercules, CA, USA); luteolin, eNOS inhibitor N-nitro-L-arginine methyl ester (L-NAME), PE, and other general chemicals were from Sigma (St. Louis, MO, USA). Stock solutions of luteolin, at 100 mmol/L in dimethylsulfoxide (DMSO), were stored at −80°C before use.
Rat isolated aortic rings
Male Sprague-Dawley rats (250-300 g) were sacrificed using CO2, and the chests were opened immediately to isolate the thoracic aorta. After removal of superficial connective tissues, the aorta was cut into ring segments, 3-4 mm in width, which were then mounted in standard 5- mL organ baths filled with continuously gassed (95% O2:5% CO2) Krebs-Hensleit (KH) buffer (pH =7.4) of the following composition in mmol/L; 118 NaCl, 25 NaHCO3, 3.7 KCl, 1 KH2PO4, 11.1 glucose, 1.2 MgSO4 and 2.0 CaCl2 at 37°C. Aortic rings were equilibrated for 90 min at a resting tension of 2 g. Aortic rings were then pre-contracted with three courses of 80 mmol/L KCl to achieve consistent contraction. After a 30 min washout with KH buffer, all vessels were returned to and stabilize at a resting tension of 2 g. At the conclusion of the experiments, samples were desiccated to dryness at 100°C and results were presented as mg of tension per mg of dry weight. The animal protocol was approved by the Institutional Animal Care and Use Committee at Virginia Polytechnic Institute and State University (Animal Welfare Assurance # A-3208).
Characterization of the vasorelaxant response to luteolin
Aortic rings were constricted by the serial addition of PE to determine the concentration of PE required for 0.5 maximal response (EC50), which was found to be approximately 1 μmol/L. Vessels were then tested for endothelial integrity with a single addition of 10 μmol/L acetylcholine (Ach). Only aortic rings that relaxed with Ach challenge were used in the following experiments. After washout and re-equilibration with KH buffer (until all vessels return to and stabilize at a resting tension of 2 g), the aortic rings were contracted with 1 μmol/L PE . When the contractile response stabilized (steady-state phase for 12-15 min), relaxation was evaluated by cumulative addition of luteolin from 1 μmol/L, 5 μmol/L, 10 μmol/L, 30 μmol/L, and 100 μmol/L. Each concentration was incubated to the steady-state phase for 15 min. Identical concentrations of DMSO alone were added to the control group at the same time interval as the addition of luteolin. In some experiments, cumulative addition of luteolin was made with or without the specific inhibitor of eNOS, L-NAME at 300 μmol/L as described in previous study [23].
Cell culture
Primary HAECs were cultured in M199 medium containing 2% FBS and EGM2 at 37°C in a 5% CO2/95% air environment. The medium was changed every other day until the cells became confluent. HAECs were passaged after 0.05% trypsin treatment and passages 4–6 were used in all experiments.
Western blot analysis
Equal amounts of protein from HAECs or aortic tissue extracts were subjected to Western blot analysis as we described [24, 25]. Membranes were probed with antibody against phospho-eNOS. The immunoreactive proteins were detected by chemiluminescence. Nitrocellulose membranes were then stripped and reprobed with eNOS antibody. The protein bands were digitally imaged for densitometric quantitation with a software program (Gene tools, Synoptics Ltd. Cambridge, UK). The expression of phospho-eNOS was normalized to that of eNOS.
NO measurement
To investigate the effect of luteolin on NO release from ECs, confluent HAECs grown in 12-well plates were serum-starved in Hank’s balanced salts solution (HBSS; 135 mmol/L NaCl, 1.2 mmol/L CaCl2, 1.2 mmol/L MgCl2 1.2, 5 mmol/L KOH, 10 mmol/L HEPES, 10 mmol/L glucose, pH 7.4) supplemented with L-arginine (0.1 mmol/L) for 30 min, followed by stimulation with various concentrations of luteolin (0, 1, 5, 10 μmol/L) for 30 min. This serum-starvation was to prevent the deactivation of the normal enzyme activity by serum proteins, a procedure that is commonly used in the enzyme activity studies [26]. Culture supernatants were then collected for NO assay. NO production was determined by using a fluorometric assay as previously described [27] and normalized to that of protein in the same sample.
Statistical analysis
Data were analyzed with one-way ANOVA using SAS® software and are expressed as mean ± standard error (SE) of three or four independent experiments. Treatment differences were subjected to Tukey’s multiple comparison tests, where P < 0.05 was considered significant.
Results
Luteolin dose-dependently relaxes PE-induced contraction in rat aortic rings
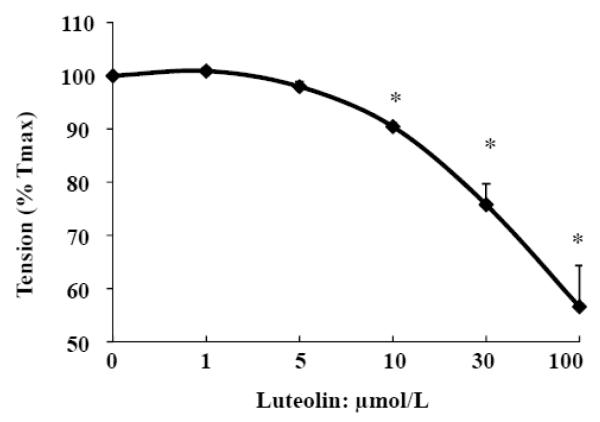
To evaluate the potential effect of luteolin, aortic rings were precontracted by PE (1 μmol/L) and, when the contractile response was stabilized (steady-state phase at 15 min), relaxation was evaluated by the addition of luteolin. Our results show that luteolin (10-100 μmol/L) leads to immediate and dose-dependent vasorelaxation in aortic rings (Fig. 1).
Fig. 1.
Luteolin dose-dependently relaxes phenylephrine (PE)-constricted rat aortic rings. After depolarized with KCL and washed with KH buffer, the rings were precontracted by 1 μmol/L PE for 15 min. Cumulatively increasing doses of luteolin were added to the bath containing the rings, and tension after each addition of luteolin was recorded for 15 min. Data are expressed as mean ± SE from three separate experiments. * P<0.05 vs. vehicle-treated control.
Luteolin induction of vasodilatation is partially inhibited by eNOS inhibitor in rat aortic rings
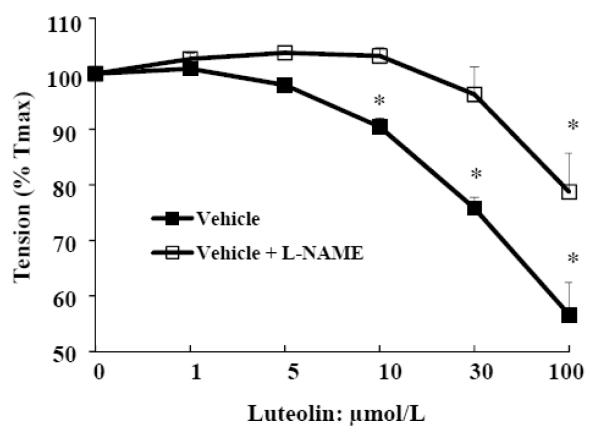
After the PE (1 μmol/L)-induced contraction reached maximum, serial additions of luteolin were made with or without the specific inhibitor of eNOS, L-NAME (300 μmol/L) [23]. As shown in Fig. 2, the effect of luteolin in relaxing PE-induced tension was partly abolished by L-NAME, suggesting that luteolin modulates vascular tension at least partially through the eNOS/NO-dependent pathway.
Fig. 2.
Luteolin-induced vasorelaxation is partially inhibited by the eNOS inhibitor in rat aortic rings. After PE (1 μmol/L)-induced contraction of aortic rings reached the half maximum, incremental doses of luteolin were added to the incubation with or without the specific inhibitor of eNOS, L-NAME (300 μmol/L), and the tension of rings were measured. Data are mean ± SE obtained from three separate experiments. * P<0.05 vs. vehicle-treated control.
Luteolin acutely stimulates eNOS phosphorylation in isolated rat aorta
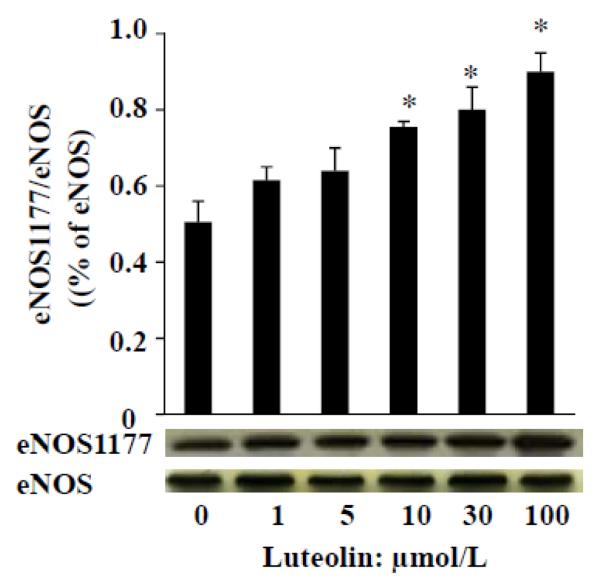
Once PE-induced contraction reached a stable plateau (about 80% contraction), aortic rings were then exposed to different concentrations of luteolin (1, 5, 10, 30 and 100 μmol/L) for 30 min. The rings were immediately processed for measuring eNOS activity as determined by Western blot analysis of phosphorylated eNOS. As shown in Fig. 3, luteolin stimulated phosphorylation of eNOS at Ser 1177, suggesting that the vascular dilatory effect of luteolin in aortic rings is due to the activation of eNOS.
Fig. 3.
Luteolin dose-dependently activates eNOS phosphorylation in rat aorta. Aortic rings were incubated with various concentrations of luteolin for 30 min, followed by determination of the phosphorylated and total eNOS levels as described in “Methods”. The experiment was repeated three times and data are expressed as means ± SE. *P<0.05 vs. vehicle-treated control.
Luteolin also induces vasodilation of KCl-contracted aorta
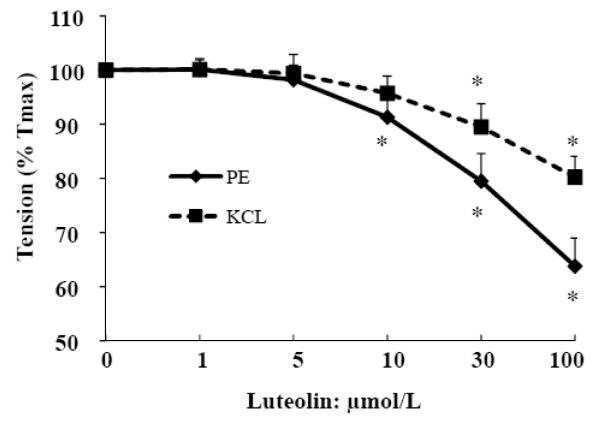
In addition to PE [28], vascular tension can be stimulated by other factors, such as KCl [29] and angiotensin II [30]. We therefore further determined if luteolin is able to reduce vascular tension caused by KCl. In this regard, aortic rings were constricted with either KCl or PE and then relaxed with increasing concentrations of luteolin. As shown in Fig. 4, luteolin can relax KCl-contracted vessel rings, but the magnitude of this effect is less than that from PE-contracted aortic rings. This observation suggests that luteolin may also suppress contraction by an ionotropic mechanism, in addition to increasing NO production.
Fig. 4.
Luteolin-induced vasoralaxation capacity depends on preconstrictors. After depolarized with KCL and washed with KH buffer, Aortic rings were contracted by PE or KCL at the half-log concentration for 15 min. The relaxation responses of vessel rings to incremental concentrations of luteolin were then evaluated. Data are expressed as mean ± SE derived from three separate experiments. * P<0.05 vs. vehicle-treated control.
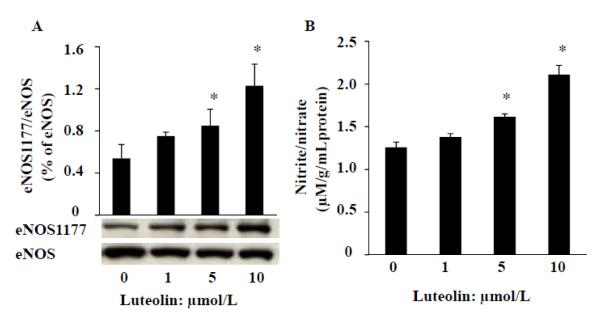
Luteolin stimulates eNOS phosphorylation and NO production in HAECs
To determine whether luteolin can also stimulate eNOS in human ECs, we incubated HAECs with various concentrations of luteolin for 30 min. As shown in Fig. 5, luteolin dose-dependently activated eNOS phosphorylation at Ser 1177 (A) and increased NO release (B) in HAECs. These findings suggest that luteolin activation of eNOS is not species-specific.
Fig. 5.
Luteolin stimulates eNOS phosphorylation and NO production in HAECs. Serum-starved HAECs were incubated with various concentration of luteolin in HBSS buffer for 30 min. A, HBSS buffer washed cells were collected for eNOS measurement by Western blot; B, NO production was evaluated by measurement of nitrite and nitrate (NOx) released to the culture media and normalized to the protein content in the same sample. Data are expressed as mean ± SE from three separate experiments.*P<0.05 vs. vehicle-treated control.
Discussion
Our current study demonstrates that luteolin induces rapid activation of eNOS both in rat aortic rings and HAECs, leading to NO production in HAECs and relaxation of PE- or KCI-induced contraction in rat aortic vessels. Our observation supports a possible role for the inclusion of luteolin in regimens to combat essential hypertension.
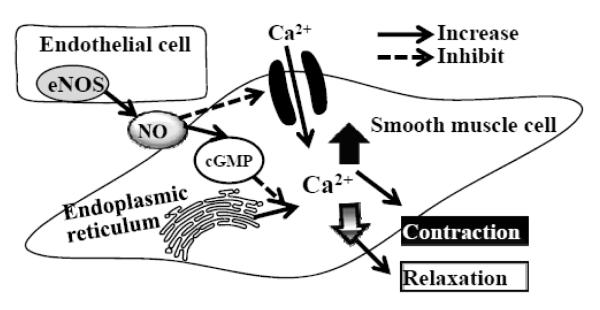
Blood pressure is controlled by the tonic constriction and dilation of blood vessels, vasomotion, the degree of which depends on the flux of cytosolic free Ca2+ levels ([Ca2+]i) in smooth muscle cells (SMCs) [31-33]. Essential hypertension results from mechanical changes in vascular elasticity and in response to vasoactive agonists that increase [Ca2+]i within SMCs. This increased SMC [Ca2+]i comes from either extracellular Ca2+ influx through Ca2+-permeable ion channels and/or intercellular Ca2+ release from endoplasmic reticulum. In either case the increase in cytosolic Ca2+ combines with calmodulin which subsequently leads to myosin phosphorylation and SMC contraction. Conversely, to lower blood pressure, the cytosolic [Ca2+]i and/or the sensitivity of the contractile apparatus for [Ca2+]i must decrease. A prominent physiological mechanism for such a reduction in vasomotor tone is initiated within the endothelium by increasing eNOS synthesis and/or increasing its activity, either of which increases NO release. The increased NO in turn down-regulates SMC cytosolic [Ca2+] and/or the sensitivity of the contractile apparatus for Ca2+ via activation of gunalyate cylase and production of cGMP (cyclic guanosine monophosphate) [34, 35], as illustrated in Fig. 6.
Fig. 6.
Regulation of vascular smooth muscle tone. Blood pressure is controlled by the contraction and relaxation of smooth muscle cells (SMC) in blood vessels, the degree of which depends on the flux of SMC cytosolic Ca2+ regulated by either extracellular Ca2+ influx through Ca2+-permeable ion channels and/or intercellular Ca2+ release from endoplasmic reticulum. Endothelial released nitric oxide, produced by the enzyme endothelial nitric oxide synthase (eNOS), down-regulates SMC cytosolic [Ca2+] by either inhibiting extracellular Ca2+ influx and/or intercellular Ca2+ release from endoplasmic reticulum through activation of gunalyate cylase and production of cGMP (cyclic guanosine monophosphate) .
In the present study, we found that luteolin-induced relaxation cannot be abolished completely by inhibition of eNOS activity, indicating that luteolin may suppress SMC contraction by multiple mechanisms. A comparison of the vasorelaxant effect of luteolin on PE-and KCl-contracted aorta found that the capacity of luteolin to reverse PE-induced contraction was greater than its capacity to block KCl-induced tension. This disparity may reflect the difference by which these two agents elicit vasoconstriction.
PE-induced vasoconstriction is initiated by binding to its specific receptor (α1ARs) which then activates phospholipase C, leading to inositol 1,4,5-trisphosphate and diacylglycerol production [36, 37] and subsequent mobilization of intracellular Ca2+ stores. On the other hand, KCl-induced contractions are produced via the opening of voltage-dependent Ca2+ channels [29]. Therefore, the greater efficacy of luteolin to reverse PE- compared with KCl-constricted aortic rings may be attributed to the different mechanism of contraction. Interestingly, similar disparate results have been observed with other NO producing agents including acetylcholine chloride, diethylanine NONOate and glyceryltrinitrate [38].
Because of the role of NO in preventing and treating essential hypertension, increasing endogenous NO bioavailability is a priority strategy that may help maintain normal vascular tone. In this regard, natural phytochemicals, like flavonoids, are particularly attractive and more than dozen of flavonoids have been tested. An earlier study [39] showed that the order of common flavonoid potency for vascular relaxation have been established as follows: flavones (epigenin and luteolin) > flavonols (kaempferol and quercetin) > isoflavones (genistein and daizein) > flavanes (catechin and epicatechin). While some of flavonoids, such as genistein [24, 27, 40] and epicatechin [41, 42], have been shown to activate eNOS and NO production, no study, to our knowledge, has investigated if luteolin can directly stimulate eNOS activity in ECs. Li et al reported that luteolin and whole extract from Artichoke up-regulates eNOS expression and mRNA transcription in human ECs [18]. The current study demonstrates that luteolin rapidly activates eNOS phosphorylation both in rat aortic rings and HAECs (Fig. 3 & 5), and most importantly, this eNOS activation and vasorelaxant effect of luteolin (10-100 μM) may be physiologically relevant because plasma concentrations of luteolin after oral intake can reach up to 19.3 μmol/L [43], suggesting that dietary supplementation of luteolin could be useful both clinically to reduce and practically to prevent hypertension. Although endothelium and eNOS were already damaged in most pathophysiological conditions, the damaged endothelium and eNOS expression/activity can be reversed or partially restored by either naturally occurring or pharmacological vasoactive agents such as genistein [40] and •-lapachone, a well-known substrate of NAD(P)H:quinone oxidoreductase (NQO1), in hypertensive rats [44], indicating that luteolin could be a potentially useful and low-cost agent for preventing and treating hypertension.
The activation of eNOS can be regulated by several intracellular kinase-mediated signaling pathways such as PI3K/Akt, PKA, or AMP-activated kinase (26). It is still unclear how luteolin induces rapid eNOS activation in ECs. An earlier study showed that luteolin dose-dependently (10-300 μmol/L) inhibits cAMP-specific phosphodiesterase [20], thereby allowing cAMP accumulation. We recently demonstrated that activation of the cAMP/PKA cascade leads to increased eNOS phosphorylation and NO production in ECs [27]. Therefore, it is speculated that luteolin may activate eNOS through a cAMP/PKA-mediated mechanism. We are currently investigating this hypothesis.
In summary, this study demonstrates for the first time, to our knowledge, that luteolin can directly act on vascular ECs, leading to rapid eNOS activation and NO production, thereby causing relaxation of vascular tension in rat aortic rings. Consistently, luteolin also induces eNOS activity and subsequent NO production in primary HAECs. These findings provide a basic mechanism underlying potentially laudatory physiological effects of luteolin in the vasculature.
Acknowledgements
We would like to thank the financial supports from the National Center for Complementary and Alternative Medicine of National Institute of Health (1R21AT004694 and 1R21AT002739 to D. L) and American Diabetes Association (1-08-JF-30 to D. Liu).
Abbreviations
- Ach
acetylcholine
- α1-AR
α (1) adrenergic receptor
- [Ca2+]i
cytosolic Ca2+ levels
- cGMP
cyclic guanosine monophosphate
- DMSO
dimethylsulfoxide
- EGM2
endothelial growth supplements
- eNOS
endothelial nitric oxide synthase
- FBS
fetal bovine serum
- HAECs
human aortic endothelial cells
- HBSS
Hank’s balanced salt solution
- KH
Krebs-Hensleit
- L-NAME
N-nitro-L-arginine methyl ester
- NO
nitric oxide
- PE
phenylephrine
- PKA
protein kinase A
- SMCs
smooth muscle cells
Footnotes
Conflict of interest The authors declare that they have no conflicts of interest.
Contributor Information
Hongwei Si, Department of Human Nutrition, Foods and Exercise, College of Agriculture and Life Sciences, Virginia Tech, Blacksburg, VA 24061, USA; Department of Family and Consumer Sciences , College of Agriculture, Human and Natural Sciences, Tennessee State University, Nashville, TN 37209, USA.
Richard P. Wyeth, Edward Via College of Osteopathic Medicine, Virginia campus, Blacksburg, VA 24060, USA
Dongmin Liu, Department of Human Nutrition, Foods and Exercise, College of Agriculture and Life Sciences, Virginia Tech, Blacksburg, VA 24061, USA.
References
- 1.Wang YG, Dedkova EN, Ji X, Blatter LA, Lipsius SL. Phenylephrine acts via IP3-dependent intracellular NO release to stimulate L-type Ca2+ current in cat atrial myocytes. J Physiol. 2005;567:143–157. doi: 10.1113/jphysiol.2005.090035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin W, Furchgott RF, Villani GM, Jothianandan D. Depression of contractile responses in rat aorta by spontaneously released endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1986;237:529–538. [PubMed] [Google Scholar]
- 3.Schulz E, Jansen T, Wenzel P, Daiber A, Munzel T. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxidants & redox signaling. 2008;10:1115–1126. doi: 10.1089/ars.2007.1989. [DOI] [PubMed] [Google Scholar]
- 4.Ribera-Casado JM. Ageing and the cardiovascular system. Z Gerontol Geriatr. 1999;32:412–419. doi: 10.1007/s003910050138. [DOI] [PubMed] [Google Scholar]
- 5.Miean KH, Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agricult Food Chem. 2001;49:3106–3112. doi: 10.1021/jf000892m. [DOI] [PubMed] [Google Scholar]
- 6.Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 7.Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 8.Chowdhury AR, Sharma S, Mandal S, Goswami A, Mukhopadhyay S, Majumder HK. Luteolin, an emerging anti-cancer flavonoid, poisons eukaryotic DNA topoisomerase I. Biochem J. 2002;366:653–661. doi: 10.1042/BJ20020098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yee SB, Lee JH, Chung HY, Im KS, Bae SJ, Choi JS, Kim ND. Inhibitory effects of luteolin isolated from Ixeris sonchifolia Hance on the proliferation of HepG2 human hepatocellular carcinoma cells. Arch Pharmacal Res. 2003;26:151–156. doi: 10.1007/BF02976662. [DOI] [PubMed] [Google Scholar]
- 10.Lv PC, Li HQ, Xue JY, Shi L, Zhu HL. Synthesis and biological evaluation of novel luteolin derivatives as antibacterial agents. Eur J Med Chem. 2008;44:908–914. doi: 10.1016/j.ejmech.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Shimoi K, Saka N, Kaji K, Nozawa R, Kinae N. Metabolic fate of luteolin and its functional activity at focal site. BioFactors (Oxford, England) 2000;12:181–186. doi: 10.1002/biof.5520120129. [DOI] [PubMed] [Google Scholar]
- 12.Xagorari A, Papapetropoulos A, Mauromatis A, Economou M, Fotsis T, Roussos C. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J Pharmacol Exp Ther. 2001;296:181–187. [PubMed] [Google Scholar]
- 13.Horvathova K, Novotny L, Tothova D, Vachalkova A. Determination of free radical scavenging activity of quercetin, rutin, luteolin and apigenin in H2O2-treated human ML cells K562. Neoplasma. 2004;51:395–399. [PubMed] [Google Scholar]
- 14.Seelinger G, Merfort I, Schempp CM. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med. 2008;74:1667–1677. doi: 10.1055/s-0028-1088314. [DOI] [PubMed] [Google Scholar]
- 15.Englisch W, Beckers C, Unkauf M, Ruepp M, Zinserling V. Efficacy of Artichoke dry extract in patients with hyperlipoproteinemia. Arzneimittelforschung. 2000;50:260–265. doi: 10.1055/s-0031-1300196. [DOI] [PubMed] [Google Scholar]
- 16.Gebhardt R. Prevention of taurolithocholate-induced hepatic bile canalicular distortions by HPLC-characterized extracts of artichoke (Cynara scolymus) leaves. Planta Med. 2002;68:776–779. doi: 10.1055/s-2002-34417. [DOI] [PubMed] [Google Scholar]
- 17.Zapolska-Downar D, Zapolski-Downar A, Naruszewicz M, Siennicka A, Krasnodebska B, Koldziej B. Protective properties of artichoke (Cynara scolymus) against oxidative stress induced in cultured endothelial cells and monocytes. Life Sci. 2002;71:2897–2808. doi: 10.1016/s0024-3205(02)02136-7. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Xia N, Brausch I, Yao Y, Forstermann U. Flavonoids from artichoke (Cynara scolymus L.) up-regulate endothelial-type nitric-oxide synthase gene expression in human endothelial cells. J Pharmacol Exp Ther. 2004;310:926–932. doi: 10.1124/jpet.104.066639. [DOI] [PubMed] [Google Scholar]
- 19.Rossoni G, Grande S, Galli C, Visioli F. Wild artichoke prevents the age-associated loss of vasomotor function. J Agricult Food Chem. 2005;53:10291–10296. doi: 10.1021/jf052499s. [DOI] [PubMed] [Google Scholar]
- 20.Ko WC, Shih CM, Leu IJ, Chen TT, Chang JP. Mechanisms of relaxant action of luteolin in isolated guinea pig trachea. Planta Med. 2005;71:406–411. doi: 10.1055/s-2005-864133. [DOI] [PubMed] [Google Scholar]
- 21.Uydes-Dogan BS, Takir S, Ozdemir O, Kolak U, Topcu G, Ulubelen A. The comparison of the relaxant effects of two methoxylated flavones in rat aortic rings. Vascul Pharmacol. 2005;43:220–226. doi: 10.1016/j.vph.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Calderone V, Chericoni S, Martinelli C, Testai L, Nardi A, Morelli I, Breschi MC, Martinotti E. Vasorelaxing effects of flavonoids: investigation on the possible involvement of potassium channels. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:290–298. doi: 10.1007/s00210-004-0964-z. [DOI] [PubMed] [Google Scholar]
- 23.Mundy AL, Haas E, Bhattacharya I, Widmer CC, Kretz M, Hofmann-Lehmann R, Minotti R, Barton M. Fat intake modifies vascular responsiveness and receptor expression of vasoconstrictors: implications for diet-induced obesity. Cardiovasc Res. 2007;73:368–375. doi: 10.1016/j.cardiores.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Liu D, Jiang H, Grange RW. Genistein activates the 3′,5′-cyclic adenosine monophosphate signaling pathway in vascular endothelial cells and protects endothelial barrier function. Endocrinology. 2005;146:1312–1320. doi: 10.1210/en.2004-1221. [DOI] [PubMed] [Google Scholar]
- 25.Liu D, Si H, Reynolds KA, Zhen W, Jia Z, Dillon JS. Dehydroepiandrosterone protects vascular endothelial cells against apoptosis through a Galphai protein-dependent activation of phosphatidylinositol 3-kinase/Akt and regulation of antiapoptotic Bcl-2 expression. Endocrinology. 2007;148:3068–3076. doi: 10.1210/en.2006-1378. [DOI] [PubMed] [Google Scholar]
- 26.Kitamura T, Kimura K, Makondo K, Furuya DT, Suzuki M, Yoshida T, Saito M. Proinsulin C-peptide increases nitric oxide production by enhancing mitogen-activated protein-kinase-dependent transcription of endothelial nitric oxide synthase in aortic endothelial cells of Wistar rats. Diabetologia. 2003;46:1698–1705. doi: 10.1007/s00125-003-1232-3. [DOI] [PubMed] [Google Scholar]
- 27.Liu D, Homan LL, Dillon JS. Genistein acutely stimulates nitric oxide synthesis in vascular endothelial cells by a cyclic adenosine 5′-monophosphate-dependent mechanism. Endocrinology. 2004;145:5532–5539. doi: 10.1210/en.2004-0102. [DOI] [PubMed] [Google Scholar]
- 28.Collins EM, Walsh MP, Morgan KG. Contraction of single vascular smooth muscle cells by phenylephrine at constant [Ca2+]i. Am J Physiol. 1992;262:H754–762. doi: 10.1152/ajpheart.1992.262.3.H754. [DOI] [PubMed] [Google Scholar]
- 29.Karaki H, Urakawa N, Kutsky P. Potassium-induced contraction in smooth muscle. Nihon Heikatsukin Gakkai Zasshi. 1984;20:427–444. doi: 10.1540/jsmr1965.20.427. [DOI] [PubMed] [Google Scholar]
- 30.Peach MJ. Renin-angiotensin system: biochemistry and mechanisms of action. Physiol Rev. 1977;57:313–370. doi: 10.1152/physrev.1977.57.2.313. [DOI] [PubMed] [Google Scholar]
- 31.Sharma RV, Bhalla RC. Calcium and abnormal reactivity of vascular smooth muscle in hypertension. Cell Calcium. 1988;9:267–274. doi: 10.1016/0143-4160(88)90007-3. [DOI] [PubMed] [Google Scholar]
- 32.Furspan PB, Lamb FS, Ross PV, Webb RC, Bohr DF. Calcium and vascular smooth muscle membrane in hypertension. Prog Clin Biol Res. 1986;219:225–243. [PubMed] [Google Scholar]
- 33.Puscas L, Gilau L, Coltau M, Pasca R, Domuta G, Baican M, Hecht A. Calcium channel blockers reduce blood pressure in part by inhibiting vascular smooth muscle carbonic anhydrase I. Cardiovasc Drugs Ther. 2000;14:523–528. doi: 10.1023/a:1007893207279. [DOI] [PubMed] [Google Scholar]
- 34.Carvajal JA, Germain AM, Huidobro-Toro JP, Weiner CP. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J Cell Physiol. 2000;184:409–420. doi: 10.1002/1097-4652(200009)184:3<409::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 35.Lehen’kyi VV, Zelensky SN, Stefanov AV. Ca2+-sensitivity and cGMP-independent effects of NO in vascular smooth muscle. Nitric Oxide. 2005;12:105–113. doi: 10.1016/j.niox.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Wu DQ, Lee CH, Rhee SG, Simon MI. Activation of phospholipase C by the alpha subunits of the Gq and G11 proteins in transfected Cos-7 cells. J Biol Chem. 1992;267:1811–1817. [PubMed] [Google Scholar]
- 37.Wang L, Rolfe M, Proud CG. Ca(2+)-independent protein kinase C activity is required for alpha1-adrenergic-receptor-mediated regulation of ribosomal protein S6 kinases in adult cardiomyocytes. Biochem J. 2003;373:603–611. doi: 10.1042/BJ20030454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Hove CE, Van der Donckt C, Herman AG, Bult H, Fransen P. Vasodilator efficacy of nitric oxide depends on mechanisms of intracellular calcium mobilization in mouse aortic smooth muscle cells. Br J Pharmacol. 2009;158:920–930. doi: 10.1111/j.1476-5381.2009.00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu YC, Leung SW, Yeung DK, Hu LH, Chen GH, Che CM, Man RY. Structure-activity relationships of flavonoids for vascular relaxation in porcine coronary artery. Phytochemistry. 2007;68:1179–1188. doi: 10.1016/j.phytochem.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Si H, Liu D. Genistein, a soy phytoestrogen, upregulates the expression of human endothelial nitric oxide synthase and lowers blood pressure in spontaneously hypertensive rats. J Nutr. 2008;138:297–304. doi: 10.1093/jn/138.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brossette T, Hundsdorfer C, Kroncke KD, Sies H, Stahl W. Direct evidence that (−)-epicatechin increases nitric oxide levels in human endothelial cells. Eur J Nutr. 2011;50:595–599. doi: 10.1007/s00394-011-0172-9. [DOI] [PubMed] [Google Scholar]
- 42.Ramirez-Sanchez I, Maya L, Ceballos G, Villarreal F. (−)-epicatechin activation of endothelial cell endothelial nitric oxide synthase, nitric oxide, and related signaling pathways. Hypertension. 2010;55:1398–1405. doi: 10.1161/HYPERTENSIONAHA.109.147892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimoi K, Okada H, Furugori M, Goda T, Takase S, Suzuki M, Hara Y, Yamamoto H, Kinae N. Intestinal absorption of luteolin and luteolin 7-O-beta-glucoside in rats and humans. FEBS Lett. 1998;438:220–224. doi: 10.1016/s0014-5793(98)01304-0. [DOI] [PubMed] [Google Scholar]
- 44.Kim YH, Hwang JH, Noh JR, Gang GT, Kim do H, Son HY, Kwak TH, Shong M, Lee IK, Lee CH. Activation of NAD(P)H:quinone oxidoreductase ameliorates spontaneous hypertension in an animal model via modulation of eNOS activity. Cardiovasc Res. 2010;91:519–527. doi: 10.1093/cvr/cvr110. [DOI] [PubMed] [Google Scholar]