Abstract
Globally, acute malnutrition triggers more than 50% of childhood mortality in children under 5 years old, which implies that about 3.5 million children die of malnutrition each year. Prior to the advent of ready-to-use therapeutic food (RUTF), the management of acute malnutrition was limited to hospitals, resulting in low coverage rates with high mortality, as malnourished cases were indentified at later stages often plagued with complications. However, current availability of RUTF has enabled malnourished children to be treated at communities. Further, because RUTF is dehydrated and sealed, it has the added advantage of a lower risk of bacterial contamination, thereby prolonging its storage life at room temperature. Recent data indicate that Community Management of Acute Malnutrition (CMAM) is as cost effective as other high-impact public health measures such as oral rehydration therapy for acute diarrheal diseases, vitamin A supplementation, and antibiotic treatment for acute respiratory infections. Despite the high efficacy of CMAM programs, CMAM still draws insufficient attention for global implementation, suggesting that CMAM programs should be integrated into local or regional routine health systems. Knowledge gaps requiring further research include: the definition of practical screening criteria for malnourished children at communities, the need for systematic antibiotic therapy during malnutrition treatment, and the dietary management of severe malnutrition in children below 6 months of age.
Keywords: Severe acute malnutrition, Community Management of Acute Malnutrition, Ready-to-use therapeutic food, Developing countries
INTRODUCTION
Although the term malnutrition refers to both under-nutrition and over-nutrition, it generally indicates under-nutrition including protein-energy malnutrition and deficiency of micronutrients.
It is estimated that 52 million children under-five years of age were wasted in 2011. Despite an 11% decrease in wasting cases since 1990 [1], wasting malnutrition still affects 8% of all children under 5 years old. Wasted children are at substantial increased risk of severe acute malnutrition and death, with malnutrition accounting for over 50% of all child deaths worldwide [2]. Although at least one million child deaths are directly caused by malnutrition [3], acute malnutrition may predispose up to 3.5 million children under 5 years old to death [4]. If malnutrition were properly addressed, at least one third of child mortality and morbidity could be averted [5]. It is also speculated about 11% of the total global disability-adjusted life-years are due to childhood malnutrition. Geographically 70-80% of undernourished children of the world live in developing countries [2,6].
In addition to predisposing children to death, malnutrition often affects a child's immune system [7,8]. Malnutrition has seldom detectable effect on antibody responses to parenteral vaccines [9]. On the contrary some researches indicated malnutrition dampens immune response to oral vaccines [10]. Furthermore low efficacy of several oral vaccines in developing countries is speculated to attribute to malnutrition [11-14] though this low performance is not consistent with other reports [15-17].
Malnutrition may be measured in manyways. Clinical grading standard, weight-for-height (WFH) index, height-for-age (HFA) index, weight-for-age (WFA) index, body mass index, and skin fold thickness are among those utilized most frequently in the field.
Generally, there are three different forms of malnutrition resulting from different causes (Table 1). Acute malnutrition results from acute food deprivation and is defined by a decrease of two standard deviations (SD) below the WFH index. Severe acute malnutrition is often complicated by diarrhea, respiratory infection and malaria and is defined by a decrease of three SD below the WHF index. However, chronic malnutrition, described as stunting, is defined by a decrease of two SD below the HFA index. In addition, a composite form of both stunting and wasting is defined by a decrease in the WFA index.
Table 1.
Classification of Malnutrition
*For further information about anthropometric indicators, see reference [1]. †Below the median World Health Organization reference; the standard deviations (SD) score (Z value) is defined as the deviation of the value for an individual from the median value of the reference population, divided by the standard deviation of the reference population. WFH: weight-for-height, HFA: height-for-age, WFA: weight-for-age.
The WFH index is usually quantified using a SD score (Z value, WHZ), which is defined as the deviation of the value for an individual from the median value of the reference population (Z=(x-µ)/σ where x indicates the body weight of a child, µ denotes the median value of the reference population, and σ represents the standard deviation of the population).
For children aged between 6 and 60 months, the criteria for severe acute malnutrition proposed by the World Health Organization (WHO) and the United Nations Children's Fund (UNICEF) include any of the following: 1) WFH index below three SD or Z scores of the median WHO growth reference (2006), 2) visible severe wasting, 3) presence of bipedal edema; and 4) mid upper arm circumference (MUAC) below 115 mm [18].
MANAGEMENT OF SEVERE ACUTE MALNUTRITION
The Ethiopian famine in the mid-70s required efforts to treat large numbers of children with severe acute malnutrition [19]. In 1999, the WHO revised the management manual reflecting advances in the understanding of the pathophysiology of acute malnutrition [20]. These advances include malnutrition associated immunosuppression [21,22], new knowledge on the function of protein [23], and antioxidants in the pathogenesis of malnutrition. This revision with minor adaptations forms the base of current major guidelines [20,24,25], including the WHO/UNICEF initiative for Integrated Management of Childhood Illness [26].
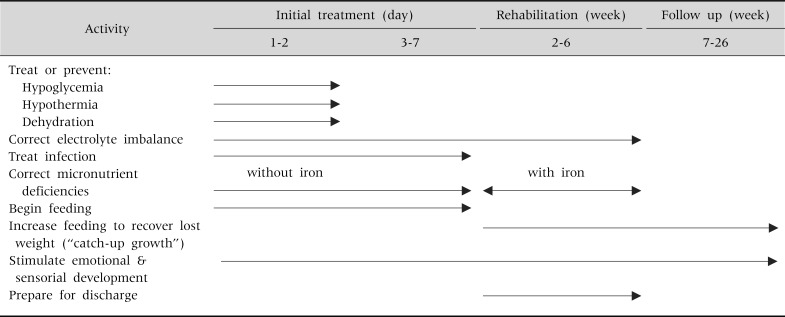
The WHO/UNICEF manual is composed of ten steps in two phases: the stabilization phase and the rehabilitation phase (Table 2). The stabilization phase requires up to seven days and involves restoring homeostasis while treating medical complications. The rehabilitation phase includes rebuilding wasted tissues and may take several weeks.
Table 2.
Time Frame for the Management of a Child with Severe Malnutrition
Cited from 'Management of Severe Malnutrition: a manual for physicians and other senior health workers, World Health Organization, Geneva, 1999 (available online at http://www.who.int/nutrition/publications/en/manage_severe_malnutrition_eng.pdf).
Currently, inpatient care is recommended for children with severe acute malnutrition. When children are admitted to management centers, they are given a mixture of dried skimmed milk, oil, and sugar diluted into clean water that may be supplemented with additional potassium and magnesium [23].
A starter diet for severe malnutrition contains lower protein and sodium. To minimize the risk of inappropriate preparation, ready-made recovery diets are now available and widely used. These are prepared using the severe acute malnutrition basic formula, with an added and improved vitamin and mineral mix.
Inpatient care demands many qualified and trained staff, plus a vast number of inpatients beds. In facilities where sufficient beds and qualified staff are available, compliance with the manual can guarantee a decrease in fatality rates during non-emergency [27,28] and emergency interventions [29].
COMMUNITY MANAGEMENT OF ACUTE MALNUTRITION (CMAM)
During emergency famine situations in developing countries, malnutrition programs are implemented by international organizations with sufficient resources and qualified staff. In contrast, during non-emergency situations, the malnutrition inpatient care model is not sustainable because of poor infrastructure and the paucity of skilled health staff, which is estimated to be fewer than four doctors and 22 nurses per 100,000 people [30], and in addition, hospital based management of malnutrition attains a low coverage rate, which is worsened in situations where dispersed communities live in chronic poverty.
Hospital based management requires care givers to stay with malnourished children during the treatment period, which usually lasts several weeks. This translates into a substantial burden and cost of illness if the care givers are involved in major family activities or if they are the principle source of income involved in time-consuming labor such as agriculture. To circumvent the need for and the burden associated with hospital based management of malnutrition, a CMAM approach can be implemented based on three innovations: 1) ready-to-use therapeutic food (RUTF), 2) community engagement and mobilization, and 3) screening for malnutrition in communities. These innovations are regarded as essential factors to ensure that CMAM can become a popular,successful and effective treatment modality.
RUTF
Because of the described challenges of hospital based management, several trials have been conducted since the 1970s to decentralize the management sites from hospitals to community stabilization centers, primary health-care centers, or homes [31,32]. However, these studies were not effective until RUTF was introduced in the 1990s [33].
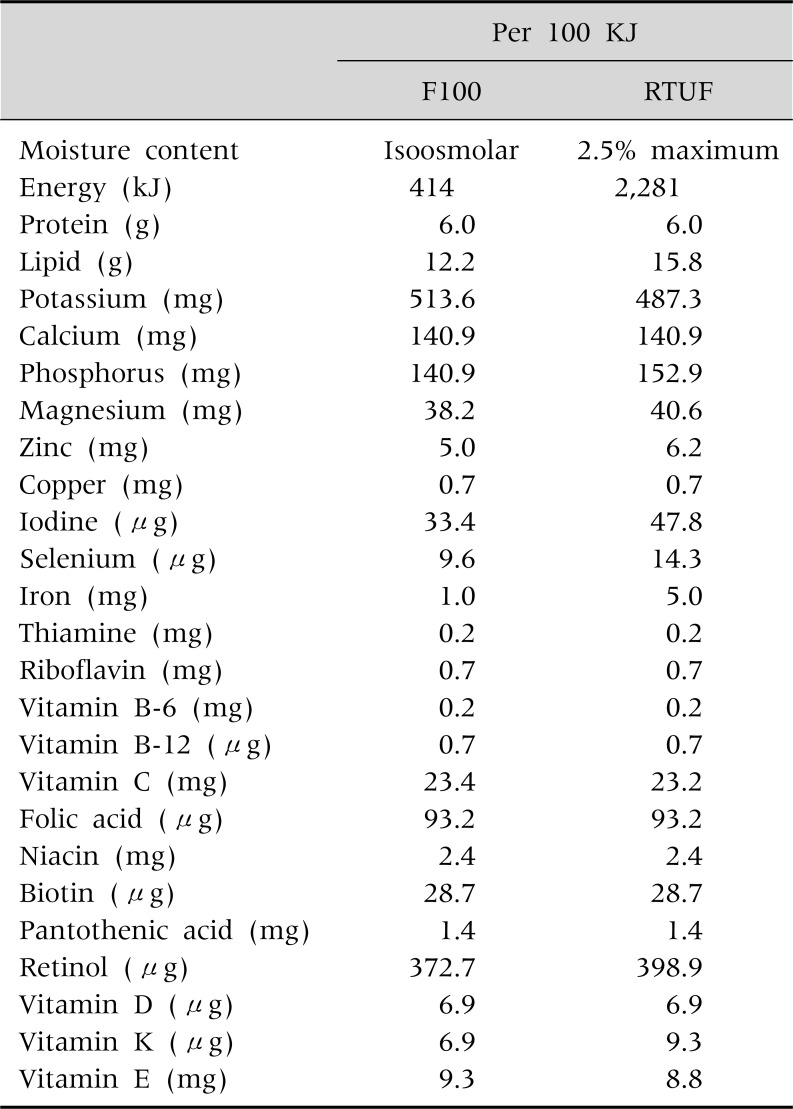
Because RUTF is prepared in the form of energy-dense pastes or biscuits containing no water, it does not easily support bacterial growth. RUTF is made from peanuts, dried milk, oil, sugar and micronutrients [20,34] and it contains more energy density and nutritional equivalent to the milk-based formula, F 100 (Table 3). Thus RUTF can be stored safely at room temperature for several months [35,36]. Furthermore, addition of micronutrients confers greater efficacy to CMAM programs based on the utilization of RUTF [34]. Additionally, children with malnutrition gain weight effectively when they are fed with RUTF [37,38]. Consequently introduction of RUTF has resulted in fundamental changes in the management of severe acute malnutrition, as CMAM can now take place in the community even during non-emergency situations [39].
Table 3.
Comparison of the Nutritional Composition of Milk Based Formula, F100 and Ready to Use Therapeutic Food (RUTF)
Cited and modified from 'Community-Based Management of Severe Acute Malnutrition, A Joint Statement by the World Health Organization, the World Food Programme, the United Nations System Standing Committee on Nutrition and the United Nations Children's Fund, May 2007 (available online at http://www.who.int/nutrition/topics/Statement_community_based_man_sev_acute_mal_eng.pdf) and Diop el et al., 2003 [37].
Community engagement and mobilization
The success of a CMAM program relies on early case finding, referral to the community based management program, and effective follow up measures at the community. For this reason, quality engagement of the target communities is pivotal. Community-based programs must be contextualized for each community where the program is implemented. This requires systems to ensure that information gathered during the assessment of the affected community guides program design and planning. Inputs into making the initial assessment more comprehensive are usually rewarded with greater sensitization of the population and more profound mobilization [4].
The identification of severe acute malnutrition in the community
According to several studies, MUAC is a simple, low-cost, and objective method of assessing nutritional status [40-42]. However these studies were conducted in hospitals. In contrast to the hospital setting, where lots of confounding variables are inter-related for mortality, a recent, large-scale community-setting study involving more than 64,000 children in Niger indicated that MUAC has a lower predictive value for malnutrition compared to the WHZ index [43].
In addition, WHZ is frequently not undertaken in practice in sub-Saharan Africa because height is difficult to measure accurately in children and because the measurement of weight depends on the presence of properly-calibrated and functioning scales, which are not often available [44,45].
Since each criteria has an independent predictive value for mortality, the WHO recommends measuring both for screening and allowing children who meet any of these two criteria to be admitted to the CMAM program [1].
METHODS OF COMMUNITY MANAGEMENT [46]
There are four principles that should guide CMAM programs:
1. Maximum coverage and access. Since the community based management program is operated at or near communities, the program should be implemented to achieve the greatest coverage by securing maximum access to the entire population in need.
2. Timeliness. Prior to progress to advanced stage, the severely malnourished cases should be indentified and referred to the management programs in earlier stages before they reach complications. If there are any early warning signs of famine, the case finding activities should be strengthened to prevent escalation of malnutrition cases at communities.
3. Appropriate malnutrition care. Management program should be simple and effective both for severely malnourished children with or without complication, who require outpatient and inpatient care respectively.
4. Care for as long as it is needed. The CMAM program should provide enough care until the malnourished children recover and gain more than 15% of body weight. As long as there are acutely malnourished children in the targeted communities, the program should be sustained.
The CMAM program involves three treatment modalities, namely: inpatients management at the stabilization center established near the target communities, outpatient management, and supplementary feeding. The choice among these three measures is dependent on the severity of the malnutrition and associated complications.
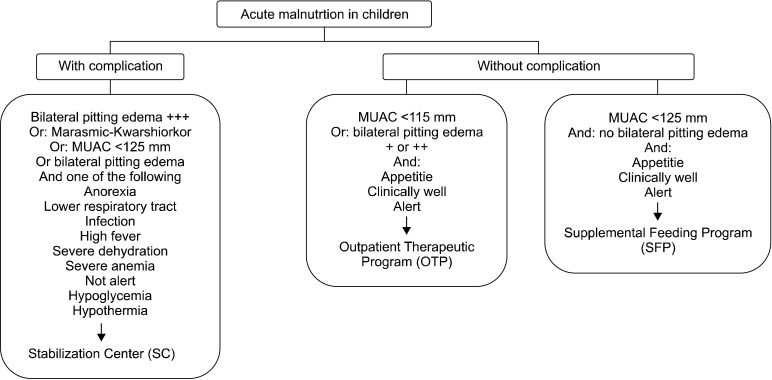
The start point of any CMAM program is community mobilization. Within the target communities, the voluntary health workers identify malnourished children utilizing MUAC and WFH criteria, along with visible evidence of malnutrition and pitting edema. Each identified child is referred to a Stabilization Center (SC), an Outpatient Treatment Program (OTP), or a Supplemental Feeding Program (SFP), according to the severity of her/his malnutrition (Fig. 1).
Fig. 1.
Triage of children with acute malnutrition. Cited and modified from 'Community Based Therapeutic Care, A Field Manual, First Edition,Valid International 2006 (available online at http://www.fantaproject.org/downloads/pdfs/CTC_Manual_v1_Oct06.pdf).
Inpatient Treatment at SC
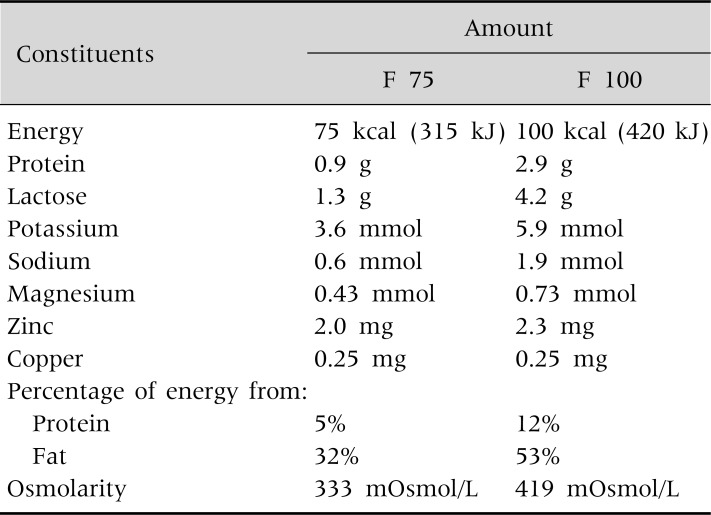
The SC is designed to accommodate severely malnourished children with medical complications and/or anorexia. At the SC, life-threatening complications are identified and treated; specific deficiencies are corrected; metabolic abnormalities are reversed and feeding begins with a formula with less osmolarity, F 75 [20]. This treatment follows the phase I protocols laid out by the WHO, with minor adaptations that replace F 100 milk with RUTF at the end of phase 1 [20] (Table 4).
Table 4.
Composition of F 75 and F 100 Diet
Cited from 'Management of Severe Malnutrition: A manual for physicians and other senior health workers, World Health Organization, Geneva, 1999 (available online at http://www.who.int/nutrition/publications/en/manage_severe_malnutrition_eng.pdf).
OTP
Children with severe acute malnutrition without medical complications are referred to an OTP. They appear at an OTP site weekly or biweekly to receive RUTF, a course of oral broad-spectrum antibiotics, antihelminthic treatment, folic acid, and, if appropriate, vitamin A, measles vaccination, and antimalarial drugs.
OTP involves two ways of admissions:
-
- Admitted from the targeted communities
Children with severe acute malnutrition with no complications are referred directly from the targeted communities by community health workers.
-
- Referred from the SC
The severely malnourished children with complications who are provided appropriate medical care so as to recover from life threatening complications are referred to the OTP.
Supplementary Feeding Program
According to the CMAM manual, those with moderate acute malnutrition without medical complications are to be referred to a SFP. Here, the patients are provided with dry rations. Generally SFPs are often operated in disaster situations, and rarely in developmental settings. At the SFP sites, children are often offered basic health care including immunization and hygiene and sanitation training and so on. It is recommended that all children with severe acute malnutrition be offered at least two months of follow up after they have been discharged from the OTP.
OUTCOME
The mortality rate for severe acute malnutrition cases in health facilities is high in developmental settings, reaching levels of 20% to 30% for wasting only and 50% to 60% for kwashiorkor, a form of malnutrition that occurs when there is not enough protein in the diet [47].
However treatment implemented in specialist units have achieved case fatality rates on the order of 1% to 5% [28,48,49]. Mortality rates achieved by humanitarian agencies treating acute malnutrition during emergencies have also been significantly reduced, often belowthe 10% level [23,29,50,51]. The results are consistent with a recent analysis involving around 87,000 children with severe acute malnutrition in Niger, Malawi, Ethiopia, and North and South Sudan, which revealed mortality rates of 4-5% [33,52,53]. Recovery rates and drop out rate were around 80%, and 11%, respectively [33]. In Niger, a study involving 632 infants aged below 6 months showed a mortality rate of 6% [54].
Out of all children with severe acute malnutrition who were referred to CMAM, more than 70% were treated solely in outpatient program [52,55], implying that OTP is the main form of community management of severe acute malnutrition. The overall coverage was predominantly above 70% [39,55] a figure that is higher than in centre-based programs, where coverage is often less than 10% [56,57].
The data of community based programs satisfy the international standards for malnutrition treatment in emergency situations. As for mortality rate, the results far surpass the Sphere project minimum standards demanding the mortality rate to keep below 10% [51].
In addition, CMAM programs are favorable for children living with HIV/AIDS. Recent data of children with HIV infection show a synergistic effect between community-based therapeutic care and antiretroviral-treatment programs against HIV/AIDS. For children living with HIV/AIDS who were referred to decentralized malnutrition management programs, despite their slow weight gain and higher mortality rates compared with HIV negative children, more than half of the children living with HIV/AIDS reached more than 90% Weight/Height [58-60].
Over a six month follow-up period of 124 children after discharge, 17.8% relapsed and another 5 children died, indicating children should be followed up even after "fully" recovering [61]. Collaborating and promoting additional complementary development programs to address the root causes of malnutrition and key health issues would decrease the relapse rate.
Recent data from Bangladesh also indicates that the average cost for a child to recover from severe acute malnutrition in community treatment was one-sixth that of inpatient treatment (US$26 vs. US$1,344 per disability-adjusted life year [62].
Based on data from two programs implemented by Concern Worldwide and designed for emergency responses to famine, the estimated cost per case management ranged between 101 and 197USD, which translates into 12-132 USD for each year of life gained [4].
However, the actual cost is dependent on other variables, such as disease prevalence, the number of cases, accessibility to the management centers, and case-fatality rate in untreated cases [4,55]. The estimated cost for community management of acute malnutrition consistently falls below the 150 USD upper threshold criteria of 'highly cost-effective' interventions suggested by the World Bank. The cost-effectiveness of CMAM programs is therefore analogous to that of other high-impact measures including vitamin A supplementation, oral rehydration therapy for diarrheal disease, and introduction of antibiotics for acute respiratory tract infection [57].
CONCLUSION AND SUGGESTION
RUTF is an innovation that, together with community mobilization and effective screening, has made it possible to treat malnourished children at communities under highly cost-effective CMAM programs.
However there is still some room for improvement. WHO and UNICEF recommend to four critera for admission into CMAM, including: weight for height, visible severe wasting, presence of edema, and MUAC. As stated earlier, the weight for height criteria is difficult to measure accurately in children, while the measurement of weight depends on the presence of properly-calibrated and functioning scales, which are not readily available in some settings. Thus, new simple and practival WHO inclusion/admission criteria should be introduced. Furthermore, there is a need for more knowledge on the potential benefits of administering systematic antibiotic therapy and on feasible methods for the dietary management of severe malnutrition in children below 6 months of age.
More importantly, despite the existing data documenting the efficacy of CMAM programs, the programs often catch inadequate attention. Thus the program should be integrated into routine health systems. Simultaneous development projects related to basic infrastructure for clean water and sanitation facilities, roads and transportation systems, and sustainable household incomes to secure food sources could facilitate the success of future CMAM programs and reduce the rate of malnutrition relapse among children.
ACKNOWLEDGEMENTS
The authors acknoweledge editorial assistance from Dr. Raúl Gómez Román and the IVI Communications & Advocacy Unit.
References
- 1.UNICEF-WHO-The World Bank Joint Child Malnutrition, Levels & Trends in Child Malnutrition. 2012. [Accessed on Nov 10, 2012]. Available at http://www.who.int/nutgrowthdb/jme_unicef_who_wb.pdf.
- 2.Caulfield LE, de Onis M, Blössner M, Black RE. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr. 2004;80:193–198. doi: 10.1093/ajcn/80.1.193. [DOI] [PubMed] [Google Scholar]
- 3.Collins S, Sadler K, Dent N, Khara T, Guerrero S, Myatt M, et al. Key issues in the success of community-based management of severe malnutrition. Food Nutr Bull. 2006;27(3 Suppl):S49–S82. doi: 10.1177/15648265060273S304. [DOI] [PubMed] [Google Scholar]
- 4.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, et al. Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 5.Mason JB, Musgrove P, Habicht JP. At least one-third of poor countries' disease burden is due to malnutrition. Disease Control Priorities Project (DCPP) Working Paper No. 1. Fogarty International Center of the National Institutes of Health; 2003. Mar, [Accessed on Nov 10, 2012]. Available at: http://www.dcp2.org/file/17/wp1.pdf. [Google Scholar]
- 6.Bryce J, Coitinho D, Darnton-Hill I, Pelletier D, Pinstrup-Andersen P Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: effective action at national level. Lancet. 2008;371:510–526. doi: 10.1016/S0140-6736(07)61694-8. [DOI] [PubMed] [Google Scholar]
- 7.Nassar MF, Younis NT, Tohamy AG, Dalam DM, El Badawy MA. T-lymphocyte subsets and thymic size in malnourished infants in Egypt: a hospital-based study. East Mediterr Health J. 2007;13:1031–1042. doi: 10.26719/2007.13.5.1031. [DOI] [PubMed] [Google Scholar]
- 8.Salimonu LS, Johnson AO, Williams AI, Adeleye GI, Osunkoya BO. Lymphocyte subpopulations and antibody levels in immunized malnourished children. Br J Nutr. 1982;48:7–14. doi: 10.1079/bjn19820082. [DOI] [PubMed] [Google Scholar]
- 9.Savy M, Edmond K, Fine PE, Hall A, Hennig BJ, Moore SE, et al. Landscape analysis of interactions between nutrition and vaccine responses in children. J Nutr. 2009;139:2154S–2218S. doi: 10.3945/jn.109.105312. [DOI] [PubMed] [Google Scholar]
- 10.Hafez M, Aref GH, Mehareb SW, Kassem AS, El-Tahhan H, Rizk Z, et al. Antibody production and complement system in protein energy malnutrition. J Trop Med Hyg. 1977;80:36–39. [PubMed] [Google Scholar]
- 11.Vesikari T, Itzler R, Matson DO, Santosham M, Christie CD, Coia M, et al. Efficacy of a pentavalent rotavirus vaccine in reducing rotavirus-associated health care utilization across three regions (11 countries) Int J Infect Dis. 2007;11(Suppl 2):S29–S35. doi: 10.1016/S1201-9712(07)60019-8. [DOI] [PubMed] [Google Scholar]
- 12.Greenwood BM, Bradley-Moore AM, Bradley AK, Kirkwood BR, Gilles HM. The immune response to vaccination in undernourished and well-nourished Nigerian children. Ann Trop Med Parasitol. 1986;80:537–544. doi: 10.1080/00034983.1986.11812062. [DOI] [PubMed] [Google Scholar]
- 13.Chopra K, Kundu S, Chowdhury DS. Antibody response of infants in tropics to five doses of oral polio vaccine. J Trop Pediatr. 1989;35:19–23. doi: 10.1093/tropej/35.1.19. [DOI] [PubMed] [Google Scholar]
- 14.Chandra RK. Reduced secretory antibody response to live attenuated measles and poliovirus vaccines in malnourished children. Br Med J. 1975;2:583–585. doi: 10.1136/bmj.2.5971.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, et al. Rotavirus Efficacy and Safety Trial (REST) Study Team. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 16.Ciarlet M, Schödel F. Development of a rotavirus vaccine: clinical safety, immunogenicity, and efficacy of the pentavalent rotavirus vaccine, RotaTeq. Vaccine. 2009;27(Suppl 6):G72–G81. doi: 10.1016/j.vaccine.2009.09.107. [DOI] [PubMed] [Google Scholar]
- 17.Block SL, Vesikari T, Goveia MG, Rivers SB, Adeyi BA, Dallas MJ, et al. Pentavalent Rotavirus Vaccine Dose Confirmation Efficacy Study Group. Efficacy, immunogenicity, and safety of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine at the end of shelf life. Pediatrics. 2007;119:11–18. doi: 10.1542/peds.2006-2058. [DOI] [PubMed] [Google Scholar]
- 18.WHO Child Growth Standards and the Identification of Severe Acute Malnutrition in Infants and Children. A Joint Statement by the World Health Organization and the United Nations Children's Fund. 2009. [Accessed on Nov 5, 2012]. Available at: http://www.who.int/nutrition/publications/severemalnutrition/9789241598163_eng.pdf. [PubMed]
- 19.Mason JB, Hay RW, Leresche J, Peel S, Darley S. Treatment of severe malnutrition in relief. Lancet. 1974;1:332–335. doi: 10.1016/s0140-6736(74)93077-3. [DOI] [PubMed] [Google Scholar]
- 20.WHO. Management of severe malnutrition: a manual for physicians and other senior health workers. Geneva: World Health Organization; 1999. [Accessed on Nov 18, 2012]. Available at: http://whqlibdoc.who.int/hq/1999/a57361.pdf. [Google Scholar]
- 21.Tomkins A, Watson F. Malnutrition and infection. A review. Geneva: WHO. Advisor committee on coordination/Subcommittee on nutrition; 1989. [Accessed on Dec 4, 2012]. Available at: http://www.unscn.org/layout/modules/resources/files/Policy_paper_No_5.pdf. [Google Scholar]
- 22.Cunningham-Rundles S. Effects of nutritional status on immunological function. Am J Clin Nutr. 1982;35(5 Suppl):1202–1210. doi: 10.1093/ajcn/35.5.1202. [DOI] [PubMed] [Google Scholar]
- 23.Golden MH, Briend A. Treatment of malnutrition in refugee camps. Lancet. 1993;342:360. doi: 10.1016/0140-6736(93)91491-4. [DOI] [PubMed] [Google Scholar]
- 24.WHO; UNHCR; WFP; IFRC. The management of nutrition in major emergencies. Geneva: WHO; 2000. [Accessed on Dec 4, 2012]. Availabe at: http://whqlibdoc.who.int/publications/2000/9241545208.pdf. [Google Scholar]
- 25.Boelaert M, Davis A, Le Lin B, Michelet M, Ritmeijer K, Van Der Kam S, et al. Nutrition guidelines. 1st. Paris: Medecins sans Frontieres; 1995. [Google Scholar]
- 26.WHO. Improving child health. IMCI: the integrated approach. Geneva: World Health Organization; 1997. [Accessed on Dec 4, 2012]. Available at: http://whqlibdoc.who.int/hq/1997/WHO_CHD_97.12_Rev.2.pdf. [Google Scholar]
- 27.Ahmed T, Ali M, Ullah MM, Choudhury IA, Haque ME, Salam MA, et al. Mortality in severely malnourished children with diarrhoea and use of a standardised management protocol. Lancet. 1999;353:1919–1922. doi: 10.1016/S0140-6736(98)07499-6. [DOI] [PubMed] [Google Scholar]
- 28.Deen JL, Funk M, Guevara VC, Saloojee H, Doe JY, Palmer A, et al. Implementation of WHO guidelines on management of severe malnutrition in hospitals in Africa. Bull World Health Organ. 2003;81:237–243. [PMC free article] [PubMed] [Google Scholar]
- 29.Prudhon C, Briend A, Laurier D, Golden MH, Mary JY. Comparison of weight- and height-based indices for assessing the risk of death in severely malnourished children. Am J Epidemiol. 1996;144:116–123. doi: 10.1093/oxfordjournals.aje.a008898. [DOI] [PubMed] [Google Scholar]
- 30.WHO. WHO global health atlas-human resources for health. [Accessed on Nov 10, 2012]. http://apps.who.int/globalatlas/docs/HRH/HTML/SASA_Aug08.htm.
- 31.Cook R. Is hospital the place for the treatment of malnourished children? J Trop Pediatr Environ Child Health. 1971;17:15–25. doi: 10.1093/tropej/17.1.15. [DOI] [PubMed] [Google Scholar]
- 32.Bengoa JM. Nutrition rehabilitation centres. J Trop Pediatr. 1967;13:169–176. [PubMed] [Google Scholar]
- 33.Ashworth A. Efficacy and effectiveness of community-based treatment of severe malnutrition. Food Nutr Bull. 2006;27(3 Suppl):S24–S48. doi: 10.1177/15648265060273S303. [DOI] [PubMed] [Google Scholar]
- 34.Briend A. Treatment of severe malnutrition with a therapeutic spread. ENN Field Exchange. 1997;3:15. Available at: http://fex.ennonline.net/2/treatment. (Accessed on Dec 4, 2012) [Google Scholar]
- 35.Briend A, Golden MH. Treatment of severe child malnutrition in refugee camps. Eur J Clin Nutr. 1993;47:750–754. [PubMed] [Google Scholar]
- 36.Briend A, Lacsala R, Prudhon C, Mounier B, Grellety Y, Golden MH. Ready-to-use therapeutic food for treatment of marasmus. Lancet. 1999;353:1767–1768. doi: 10.1016/S0140-6736(99)01078-8. [DOI] [PubMed] [Google Scholar]
- 37.Diop el HI, Dossou NI, Ndour MM, Briend A, Wade S. Comparison of the efficacy of a solid ready-to-use food and a liquid, milk-based diet for the rehabilitation of severely malnourished children: a randomized trial. Am J Clin Nutr. 2003;78:302–307. doi: 10.1093/ajcn/78.2.302. [DOI] [PubMed] [Google Scholar]
- 38.Navarro-Colorado C, Laquière S. Clinical trial of BP100 vs F100 milk for rehabilitation of severe malnutrition. ENN Field Exchange. 2005;24:22–24. Available at: http://fex.ennonline.net/24/clinical. (Accessed on Nov 11 2012) [Google Scholar]
- 39.Khara T, Collins S Community-based Therapeutic Care (CTC) Emergency Nutrition Network (ENN) Special Supplement Series No. 2. 2004. Nov, [Accessed on Nov 11, 2012]. Available at http://www.ennonline.net/pool/files/ife/supplement23.pdf.
- 40.Myatt M, Khara T, Collins S. A review of methods to detect cases of severely malnourished children in the community for their admission into community-based therapeutic care programs. Food Nutr Bull. 2006;27(3 Suppl):S7–S23. doi: 10.1177/15648265060273S302. [DOI] [PubMed] [Google Scholar]
- 41.Pelletier DL. The relationship between child anthropometry and mortality in developing countries: implications for policy, programs and future research. J Nutr. 1994;124(10 Suppl):2047S–2081S. doi: 10.1093/jn/124.suppl_10.2047S. [DOI] [PubMed] [Google Scholar]
- 42.Briend A, Dykewicz C, Graven K, Mazumder RN, Wojtyniak B, Bennish M. Usefulness of nutritional indices and classifications in predicting death of malnourished children. Br Med J (Clin Res Ed) 1986;293:373–375. doi: 10.1136/bmj.293.6543.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lapidus N, Luquero FJ, Gaboulaud V, Shepherd S, Grais RF. Prognostic accuracy of WHO growth standards to predict mortality in a large-scale nutritional program in Niger. PLoS Med. 2009;6:e39. doi: 10.1371/journal.pmed.1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.English M, Esamai F, Wasunna A, Were F, Ogutu B, Wamae A, et al. Assessment of inpatient paediatric care in first referral level hospitals in 13 districts in Kenya. Lancet. 2004;363:1948–1953. doi: 10.1016/S0140-6736(04)16408-8. [DOI] [PubMed] [Google Scholar]
- 45.Berkley J, Mwangi I, Griffiths K, Ahmed I, Mithwani S, English M, et al. Assessment of severe malnutrition among hospitalized children in rural Kenya: comparison of weight for height and mid upper arm circumference. JAMA. 2005;294:591–597. doi: 10.1001/jama.294.5.591. [DOI] [PubMed] [Google Scholar]
- 46.Valid International, Community Based Therapeutic Care, first edition 2006. [Accessed on Nov 20, 2012]. Available at: http://www.fantaproject.org/downloads/pdfs/CTC_Manual_v1_Oct06.pdf.
- 47.Schofield C, Ashworth A. Why have mortality rates for severe malnutrition remained so high? Bull World Health Organ. 1996;74:223–229. [PMC free article] [PubMed] [Google Scholar]
- 48.Golden M. The effects of malnutrition in the metabolism of children. Trans R Soc Trop Med Hyg. 1988;82:3–6. doi: 10.1016/0035-9203(88)90245-3. [DOI] [PubMed] [Google Scholar]
- 49.Golden MHN. The Oxford Textbook of Medicine. Oxford: Oxford University Press; 1996. Severe Malnutrition; pp. 1278–1296. [Google Scholar]
- 50.Collins S, Sadler K. Outpatient care for severely malnourished children in emergency relief programmes: a retrospective cohort study. Lancet. 2002;360:1824–1830. doi: 10.1016/S0140-6736(02)11770-3. [DOI] [PubMed] [Google Scholar]
- 51.Sphere Project Team. The Sphere Project: Humanitarian charter and minimum standards in disaster response. Geneva: The Sphere Project; 2011. [Accessed on Nov 20, 2012]. Available at http://www.spherehandbook.org/en/management-of-acute-malnutrition-and-micronutrient-deficiencies-standard-2-severe-acute-malnutrition/ [DOI] [PubMed] [Google Scholar]
- 52.Tectonidis M. Crisis in Niger--outpatient care for severe acute malnutrition. N Engl J Med. 2006;354:224–227. doi: 10.1056/NEJMp058240. [DOI] [PubMed] [Google Scholar]
- 53.Collins S, Dent N, Binns P, Bahwere P, Sadler K, Hallam A. Management of severe acute malnutrition in children. Lancet. 2006;368:1992–2000. doi: 10.1016/S0140-6736(06)69443-9. [DOI] [PubMed] [Google Scholar]
- 54.Vygen SB, Roberfroid D, Captier V, Kolsteren P. Treatment of severe acute malnutrition in infants aged <6 months in niger. J Pediatr. 2012 doi: 10.1016/j.jpeds.2012.09.008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 55.WHO. Report of an informal consultation on the community-based management of severe malnutrition in children. [Accessed on Nov 11, 2012]. Accessible at: http://www.who.int/nutrition/topics/Meeting_report.pdf.
- 56.Van Damme W. Medical assistance to self-settled refugees. Guinea 1990-1996. Antwerp: ITG Press; 1998. [Assessed on Dec 4, 2012]. Available at http://dspace.itg.be/bitstream/10390/4986/1/1998shso0011.pdf. [Google Scholar]
- 57.Jha P, Bangoura O, Ranson K. The cost-effectiveness of forty health interventions in Guinea. Health Policy Plan. 1998;13:249–262. doi: 10.1093/heapol/13.3.249. [DOI] [PubMed] [Google Scholar]
- 58.Sandige H, Ndekha MJ, Briend A, Ashorn P, Manary MJ. Home-based treatment of malnourished Malawian children with locally produced or imported ready-to-use food. J Pediatr Gastroenterol Nutr. 2004;39:141–146. doi: 10.1097/00005176-200408000-00003. [DOI] [PubMed] [Google Scholar]
- 59.Manary MJ, Ndkeha MJ, Ashorn P, Maleta K, Briend A. Home based therapy for severe malnutrition with ready-to-use food. Arch Dis Child. 2004;89:557–561. doi: 10.1136/adc.2003.034306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ndekha MJ, Manary MJ, Ashorn P, Briend A. Home-based therapy with ready-to-use therapeutic food is of benefit to malnourished, HIV-infected Malawian children. Acta Paediatr. 2005;94:222–225. doi: 10.1111/j.1651-2227.2005.tb01895.x. [DOI] [PubMed] [Google Scholar]
- 61.Ashraf H, Alam NH, Chisti MJ, Mahmud SR, Hossain MI, Ahmed T, et al. A follow-up experience of 6 months after treatment of children with severe acute malnutrition in Dhaka, Bangladesh. J Trop Pediatr. 2012;58:253–257. doi: 10.1093/tropej/fmr083. [DOI] [PubMed] [Google Scholar]
- 62.Puett C, Sadler K, Alderman H, Coates J, Fiedler JL, Myatt M. Cost-effectiveness of the community-based management of severe acute malnutrition by community health workers in southern Bangladesh. Health Policy Plan. 2012 doi: 10.1093/heapol/czs070. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]