Abstract
Purpose
With growing number of obese children, the prevalence of nonalcoholic fatty liver disease (NAFLD) in pediatric population is increasing. Nonalcoholic steatohepatitis (NASH) is a severe form of NAFLD, and can cause morbid complications. It is important to identify patients in order to grade pathologic severities and treat those children who possibly have NASH. This study was performed to evaluate whether the pharmacological therapy is also effective as well as the body weight reduction in pediatric NAFLD.
Methods
Among the 52 children presenting with obesity and hepatopathy, NAFLD was diagnosed through liver biopsy in 29 children, who were 7 to 14 years of age, from January 2006 to December 2011. The patients were advised to reduce their body weight through diverse methods. Medication with Ursodeoxycholic acid (UDCA) and vitamin E was performed in children whose liver functions did not improve or their weight reductions were not successful. The therapeutic effects were monitored and assessed via the biochemical profiles and the physical measurements.
Results
The therapy of vitamin E and UDCA combined with body mass index (BMI) reduction showed significantly higher rate of improvement in clinical profiles, which could be seen in data of aspartate aminotransferase (AST), alanine aminotransferase (ALT), AST/ALT ratio, alkaline phosphatase, total bilirubin and γ-glutamyl transpeptidase. Children whose BMI were successfully reduced showed favorable clinical improvements without any medication, but those without BMI reduction did not show any improvement despite medications.
Conclusion
This study showed that the first line of therapy should be the BMI reduction in NAFLD and drug therapy combined with BMI reduction could have additive therapeutic effect in children with NAFLD.
Keywords: Child, Nonalcoholic fatty liver disease, Vitamin E, Ursodeoxycholic acid, Pharmacotherapy
INTRODUCTION
The prevalence of pediatric nonalcoholic fatty liver disease (NAFLD) differs according to the method of diagnosis, but in the United States, it has been reported to be 3-10% in all children, and 20-53% in overweight children [1]; in Korea, according to the National Health and Nutrition Survey conducted in 1998, an increase in alanine aminotransferase (ALT) of 3.6% in boys and 2.8% in girls, aged 10 through 19, was reported [2]; and in a Japanese study using an ultrasound, the prevalence of pediatric NAFLD was found to be 2.6% [3]. Pediatric NAFLD cases have been reported in patients as young as two, but are most common in 11- to 13-year-olds and increase with age [4]. Nonalcoholic steatohepatitis (NASH) is defined as NAFLD cases that involve damages to hepatocytes, hepatic fibrosis and inflammatory cell infiltration. Pediatric NASH is a risk factor for cirrhosis (2-10%) [4], hepatic insufficiency, and in rare cases, hepatoma [5].
The pathophysiology of NASH has yet to be fully elucidated. However, currently, the most widely accepted hypothesis is the 'two hit' theory. The first hit is an increased movement of free fatty acids (FFA) and fat storage within hepatocytes, due to insulin resistance and hyperinsulinemia. The second hit is inflammation and necrosis of the liver acini, caused by increased oxidative stress to the hepatocytes.
It is known that, at present, no treatment has proven effective for NAFLD. However, weight loss, through diet and exercise, constitutes a basic therapy regime, and treatment of metabolic disorders, such as diabetes and hyperlipidemia, as well as weight loss and drug treatment to improve insulin resistance, and treatment with antioxidants and hepatoprotective agents to control oxidative stress and hepatic fibrosis, are all under investigation.
There has not been a study of pediatric NAFLD patients where vitamin E and ursodeoxycholic acid (UDCA) are jointly administered, but Dufour and colleagues [6] treated 48 adults with vitamin E (800 IU) and UDCA (12-15 mg/kg/day) for two years and found significant improvements in hepatic enzyme levels and NAFLD activity score, relative to the placebo. The present study was conducted to investigate whether or not pharmacological treatment with vitamin E and UDCA, in conjunction with weight loss through diet and exercise, would improve patients' clinical profile more effectively.
MATERIALS AND METHODS
Methods
1. Target patients
Of the pediatric patients who visited the Department of Pediatrics at Hanyang University Hospital for reasons such as obesity, between July, 2006 and December, 2011, 59 presented with NAFLD, according to the results of their liver ultrasound. We observed their natural progression while supervising their weight loss, then diagnosed 29 patients with NAFLD after a liver biopsy in patients whose aspartate aminotransferase (AST) and ALT levels continued to increase or did not improve for at least six months. We confirmed that these patients did not have hepatitis, metabolic liver disease, parenteral nutrition-associated liver disease, neoplastic liver disease, genetic hemachromatosis or autoimmune hepatitis. For all diagnosed patients, we supervised dietary therapy in consultation with dietitians, and recommended weight loss by guiding their exercise, according to their obesity index, as well as drug treatment. The present study was conducted retrospectively, and vitamin E (800 IU/day) and UDCA (5-10 mg/day) were prescribed to 20 of the 29 total diagnosed pediatric patients. Those with high NAFLD activity levels, according to liver biopsy, have been included; however, drug treatment was not done in cases where the patient and the guardian anticipated a natural improvement by weight loss and behavioral control. The patients visited the pediatric outpatient clinic on a regular basis, while undergoing weight loss and treatment regimes, and their ALT, AST, alkaline phosphatase (ALP), total bilirubin, albumin, total cholesterol, triglyceride (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), total lipid, γ-glutamyl transpeptidase (γ-GT) and glucose levels, among others, were followed up and compared to the levels at the time of diagnosis.
2. Physical measurements
We used the standard values for the physical development of Korean children, provided by the Center for Disease Control and Prevention and the Korean Pediatrics Society in 2007, and calculated body mass index (BMI) percentiles for each sex and age group to be used for the diagnosis of obesity, through physical measurements. The patient's height was measured with a manual measuring apparatus (stadiometer [Harpenden, Holtain, Crymych, UK]) and their weight was measured with a digital scale (Health-o-meter [DS-102, Jenix, Seoul, Korea, 200 kg×0.1 kg]). BMI was calculated as follows: BMI=(weight)/(height)2 (kg/m2)
Relative to the same sex and age group, a BMI in the 95th percentile or greater was classified as obese, and 85-95th percentile as overweight.
3. Liver biopsy
Liver biopsy was performed using an 18-gauge automatic biopsy gun (Autovac [BARD Angiomed, Karlsruhe, Germany]). Immediately before the biopsy, an area in the right intercostal space was selected, avoiding the pleura and blood vessels; the skin was disinfected with povidone-iodine, and local anesthesia was performed on the area of skin puncture. Under ultrasonic guidance, the needle of the biopsy gun was advanced 1-2 cm into the hepatic capsule, and at least 1.5 cm of tissue was biopsied from every patient.
A pathology specialist reviewed the target patient's liver tissue and classified the severity of NAFLD from its microscopic appearance, in accordance with the NAFLD Clinical Research Network (CRN) scoring system, which has been devised by Kleiner et al. [7].
4. Statistical analysis
Windows SAS ver. 9.2 (SAS Institute Inc., Cary, NC, USA) was used for statistical analysis of the data, and all data were expressed as the mean±standard deviation. Fisher's exact test was performed for a comparative analysis of the biopsy results (NAFLD activity score, portal inflammation, fibrosis, etc.) between the case-control groups; Kruskal-Wallis test was performed for a comparative analysis of biochemical profiles. For a comparative analysis of biochemical profiles for judging the efficacy of the treatment (ALT, AST, ALP, total bilirubin, albumin, total cholesterol, TG, HDL, LDL, total lipid, γ-GT, glucose), Wilcoxon signed rank test was performed. A p-value of less than or equal to 0.05 was considered statistically significant.
RESULTS
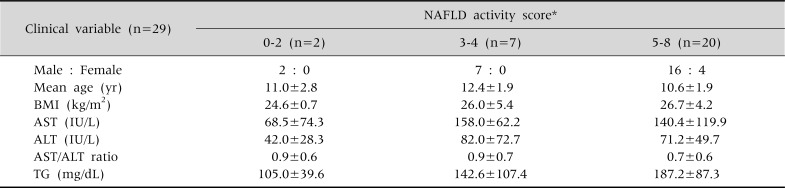
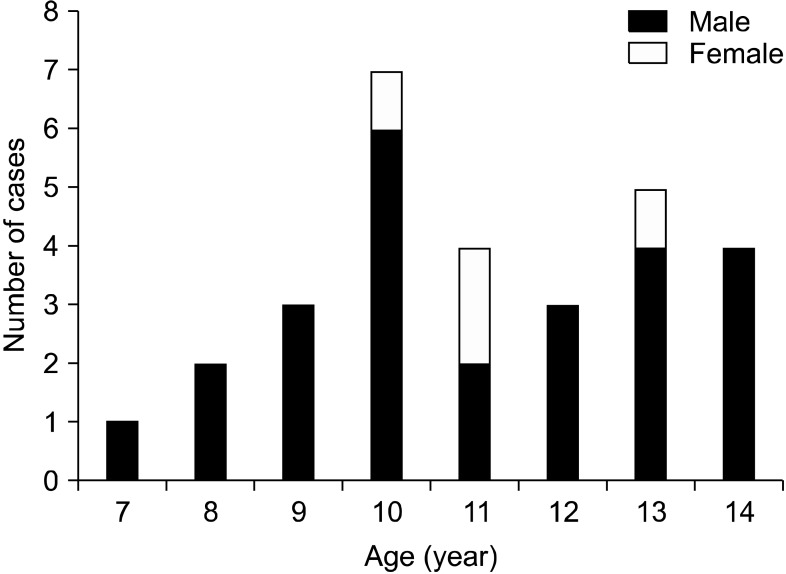
All 29 of the pediatric patients were obese, beyond being overweight. Of them, male patients were the majority, with 25 males and 4 females, the mean age was 11.07±2.00 years (range: 7-14), and their clinical profiles (BMI, TG, ALT, AST) were compared according to the NAFLD activity index, as shown in Table 1 and Fig. 1.
Table 1.
Subject Characteristics in Proportion to the Nonalcoholic Fatty Liver Disease Activity Score
Values are presented as number or mean±standard deviation. *NAFLD activity score: sum of the scores for steatosis (0-3), lobular inflammation (0-3), and ballooning (0-2), ranging from 0 to 8; 0-2 NASH(-), 3-4 probable, 5-8 NASH(+). NAFLD: nonalcoholic fatty liver disease, BMI: body mass index, AST: aspartate aminotransferase, ALT: alanine aminotransferase, TG: triglyceride.
Fig. 1.
Age and sex distributions of the children at the time of diagnosis.
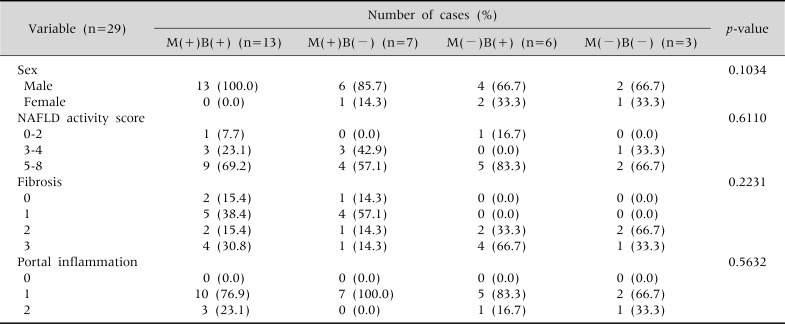
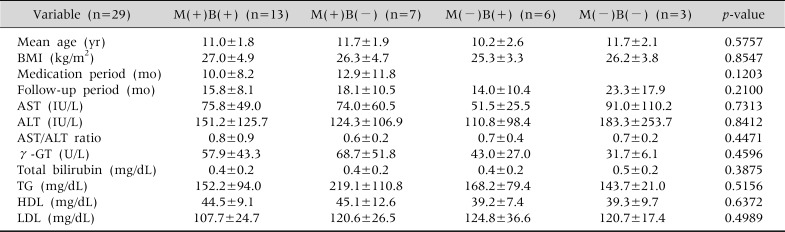
According to whether or not their BMI had decreased, and whether or not vitamin and UDCA had been administered, the 29 patients diagnosed with NAFLD were divided into four groups: a group that had carried out both tasks, two that had performed either one or the other, and one that had simply observed their progress without a reduction in their BMI or drug treatment (Tables 2 and 3).
Table 2.
The Characteristics of Study Groups according to Medication and Body Mass Index Reduction at the Time of the Diagnosis
Fisher's exact test done. M: medication, B: body mass index reduction, NAFLD: nonalcoholic fatty liver disease. p<0.05.
Table 3.
The Characteristics of Study Groups according to Medication and Body Mass Index Reduction at the Time of the Diagnosis
Kruskal-Wallis test done. Values are presented as mean±standard deviation. M: medication, B: body mass index reduction, BMI: body mass index, AST: aspartate aminotransferase, ALT: alanine aminotransferase, γ-GT: γ-glutamyl transpeptidase, TG: triglyceride, HDL: high-density lipoprotein, LDL: low-density lipoprotein. p<0.05.
Patients were observed for 16.76±10.05 months on average, and 20 received drug treatment for an average of 11.00±9.40 months, with some still under treatment and others discontinuing treatment due to poor drug compliance or completed treatment. No unusual side effects were observed during the treatment.
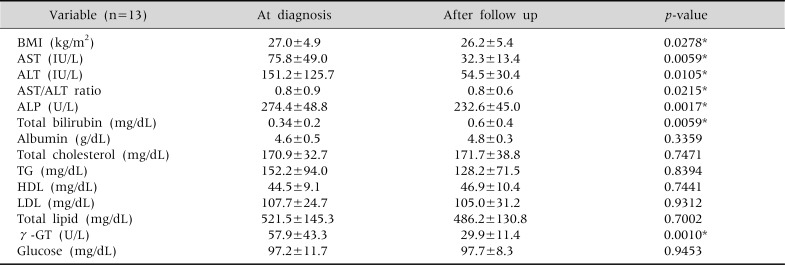
The efficacy of treatment was analyzed by comparing the biochemical profiles of the four patient groups, and in the group that had successfully reduced their BMI and simultaneously carried out drug treatment, statistically significant improvements were observed in AST, ALT, AST/ALT ratio, ALP, total bilirubin and γ-GT levels in the follow-up, compared to the initial levels (Table 4). In particular, an improvement in ALT levels from an average of 151.2 IU/L to 54.5 IU/L, and normalization in AST levels from 75.8 IU/L to 32.3 IU/L were found.
Table 4.
The Changes in Serum Biochemical Tests in the Children with Medication and Body Mass Index Reduction (n=13)
Wilcoxon signed rank test done. Values are presented as mean±standard deviation. BMI: body mass index, AST: aspartate aminotransferase, ALT: alanine aminotransferase, ALP: alkaline phosphatase, TG: triglyceride, HDL: high-density lipoprotein, LDL: low-density lipoprotein, γ-GT: γ-glutamyl transpeptidase. *p<0.05.
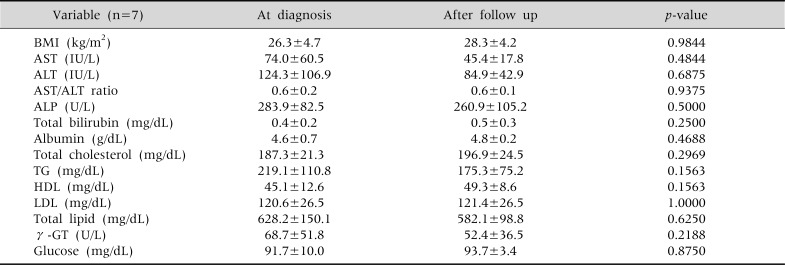
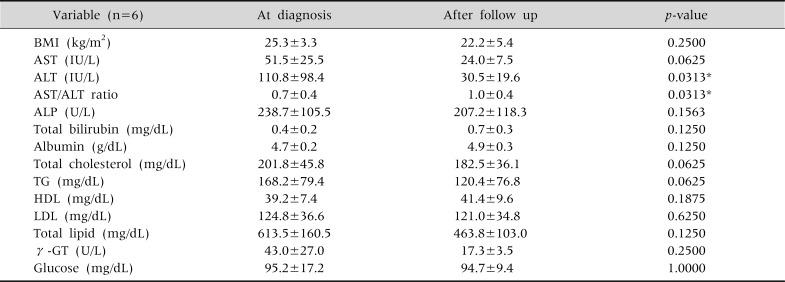
In the group that underwent drug treatment without showing reduction in their BMI, ALT levels tended to improve from an average of 124.3 IU/L to 84.9 IU/L; however, this was not statistically significant, nor were any other differences in their biochemical profile (Table 5). In the group with successful BMI reduction, but without drug treatment, there were statistically significant differences in the levels of ALT and the AST/ALT ratio (Table 6).
Table 5.
The Changes in Serum Biochemical Tests and Body Mass Index in the Children with only Medication (n=7)
Wilcoxon signed rank test done. Values are presented as mean±standard deviation. BMI: body mass index, AST: aspartate aminotransferase, ALT: alanine aminotransferase, ALP: alkaline phosphatase, TG: triglyceride, HDL: high-density lipoprotein, LDL: low-density lipoprotein, γ-GT: γ-glutamyl transpeptidase. p<0.05.
Table 6.
The Changes in Serum Biochemical Tests and Body Mass Index in the Children with only Body Mass Index Reduction (n=6)
Wilcoxon signed rank test done. Values are presented as mean±standard deviation. BMI: body mass index, AST: aspartate aminotransferase, ALT: alanine aminotransferase, ALP: alkaline phosphatase, TG: triglyceride, HDL: high-density lipoprotein, LDL: low-density lipoprotein, γ-GT: γ-glutamyl transpeptidase. *p<0.05.
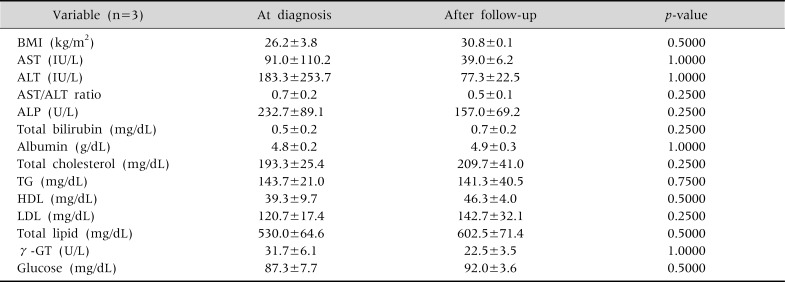
Finally, in the group whose progress was simply monitored without BMI reduction or drug treatment, no notable improvements were found (Table 7).
Table 7.
The Changes in Serum Biochemical Tests and Body Mass Index in the Children with Neither Medication Nor Body Mass Index Reduction (n=3)
Wilcoxon signed rank test done. Values are presented as mean±standard deviation. BMI: body mass index, AST: aspartate aminotransferase, ALT: alanine aminotransferase, ALP: alkaline phosphatase, TG: triglyceride, HDL: high-density lipoprotein, LDL: low-density lipoprotein, γ-GT: γ-glutamyl transpeptidase. p<0.05.
DISCUSSION
In children, liver biopsy is again considered the gold standard for diagnosing NAFLD. The grading system for NAFLD was first established in 1999 by Brunt and colleagues, who, adapting the grading system for chronic hepatitis, described the disease stages on a scale according to the degree of adiposis, ballooning degeneration, and inflammation of liver acini and portal vein, as well as the degree of fibrosis and structural remodeling. Since then, NAFLD CRN has devised a more detailed grading system for lesions pertaining to NAFLD on the basis of the grading and stage criteria, suggested by Brunt and others [7].
Hepatic enzyme levels, such as ALT, AST, γ-GT, TG, and LDL, are known to be elevated in NAFLD patients [8], and ALT is used as a screening test especially in the diagnosis of pediatric NAFLD. However, in obese children, medical imaging tests can diagnose NAFLD even with normal hepatic enzyme levels [9]. In a study of 176 pediatric NAFLD patients, whose diagnoses were confirmed by liver biopsy, the severity of NAFLD was correlated to an increase in AST and γ-GT levels and the severity of hepatic fibrosis was related to increased levels of AST among others [10], and a prospective study using indicators of NAFLD fibrosis (age, waist size, neutral fat) reported that the presence or absence of fibrosis could be predicted in children [11]. There have also been reports that the degree of NAFLD activity and fibrosis in children is related to the ratios of TG/HDL, cholesterol/HDL, and LDL/HDL [12]. Currently, there are no serum or biomarker tests that can replace a biopsy [4]. However, there has been a study in which needless biopsy was avoided in approximately half of the cases by using serum biomarkers in a single or in combination [4]. In a study by Jee et al. [13], the severity of liver pathology and the results of liver ultrasound tests were highly correlated, and in pediatric patients, who are less amenable to invasive tests, the severity of NAFLD could even be estimated when tested by a professional medical imaging specialist.
Most NAFLD patients have elevated levels of blood glucose, insulin and FFA due to inappropriate nutrition or overnutrition. Such conditions cause resistance to glucose uptake in insulin-responsive adipose tissue and skeletal muscle, as well as resistance to the inhibition of neutral fat hydrolysis [1,4]. Numerous studies have reported that weight loss can reduce hepatic steatosis and hepatic enzyme levels in the serum, and in 85 biopsy-confirmed pediatric NAFLD patients, controlling life habits for one year resulted in improved ultrasound contrast and hepatic enzyme levels in 57 patients [14]. However, there has not been a large-scale randomized controlled trial, and moreover, it is difficult to standardize the appropriate level of weight reduction and the period of sustained weight loss, or to quantify dietary and exercise regimen [15]. However, an improved insulin sensitivity is known to be correlated to the length of exercise [16].
UDCA, a hydrophilic bile acid, tends to reduce liver damage by competitively opposing the activity of endogenous hydrophobic bile acids that promote apoptosis in hepatocytes. Many studies have reported on diseases in which UDCA is effective, in particular, cholestatic disorder [17]. In a year-long open-label trial in adult NASH patients, UCDA improved the degree of fatty degeneration among hepatic enzyme levels and histological results [18]. However, in a subsequent large-scale controlled study, low doses of UDCA (13-15 mg/kg/day) were prescribed to adult patients for two years and no improvements in hepatic enzyme levels or histological profile were observed, relative to the placebo [19]. Leuschner et al. [20] conducted a randomized, double-blind clinical trial of high-dose UDCA in adult NASH patients, treating them with high doses of UDCA (23-28 mg/kg/day) and comparing their Brunt scores and NAFLD activity scores histologically, but did not find significant differences overall in the histological scores and fibrosis compared to the placebo. However, in the treatment group, inflammation of hepatic lobules and γ-GT levels were improved. In another report, adults treated with UDCA (30 mg/kg/day) for 12 months showed improved ALT, AST and γ-GT levels, albeit without a histological report [21].
Oxidative stress plays a central role in the pathophysiology of NASH. Lipid peroxidation stimulates cytokine production, leading to hepatic stellate cell activation and promotion of fibrosis. In a study of patients in the NASH risk group, decreased serum vitamin E concentrations were found [22]. In addition, a study has demonstrated improved serum ALT and histological profiles in an open-label trial with adults treated with vitamin E (300 IU every day) for one year [23], and a placebo-controlled study administered a larger dose of vitamin E (1,000 IU every day) with vitamin C to adults for six months and saw a marked improvement in the degree of fibrosis, although a similar degree of improvement was seen in the placebo group [24]. Lavine et al. [25] conducted a randomized, double-blind, placebo-controlled multi-institutional clinical trial in children, in which patients were given 800 IU of vitamin E (58 patients) or 1,000 mg metformin (57 patients) or placebo (58 patients) for 96 weeks (two years). The results indicated that ALT was inadequate for predicting inflammation or fibrosis of hepatocytes in children, but was significantly correlated to NAFLD activity score and the degree of fibrosis. Moreover, a significant effect was observed histologically in the ballooning degeneration score, NAFLD activity score and the improvement rate of NASH in vitamin E-treated group relative to placebo.
The most accurate indicator of the efficacy of NAFLD treatment would be a second liver biopsy, but this could not be performed as a follow-up test since most guardians did not consent due to the invasive nature of the test; instead, a comparative analysis of the biochemical profiles was conducted.
Rather than using weight loss as one of the indicators for assigning groups to compare the treatment efficacy, we looked at BMI reduction, achieved through exercise, behavioral control and dietary therapy. A problem arises if treatment efficacy is to be demonstrated by weight loss, since children are growing individuals unlike adults; and as such, weight loss cannot simply be interpreted as fat reduction, and one must take into account of the changes in body composition according to the age, sex and level of maturity. Additionally, this study is a retrospective one, where the lengths of therapy and drug treatment are not uniform in each group; therefore, BMI is more appropriate than weight loss as an indicator of the changes in patients over a time period that cannot be considered short.
In this study, we confirmed that for pediatric NAFLD patients, BMI reduction through exercise and dietary therapy must be the basic and primary therapeutic approach. It was observed that in conjunction with BMI reduction, vitamin E and UDCA treatment has a therapeutic effect, but no marked improvements in biochemical profiles, typified by the levels of ALT and others, were seen if pharmacological treatment was given without BMI reduction. However, drug treatment may be expected to improve patients' clinical profile more effectively, since combining drug treatment with BMI reduction, rather than BMI reduction alone, led to a clear improvement, not only in ALT levels and AST/ALT ratio but also in a greater number of indicators, such as BMI, AST, ALP, total bilirubin, and γ-GT levels.
The limitations of this study are as follows: first, statistical analyses were difficult given that a small number of patients, 29, were divided into four groups to judge the treatment efficacy; second, the lengths of treatment and follow-up were not uniform for all patients, as this was a retrospective test; and third, hepatic enzyme levels at the time of the biopsy were set as the reference, such that pediatric NAFLD patients with normal hepatic enzyme levels might have been excluded.
Moreover, in order to prove the efficacy of drug administration in treating NAFLD, one must draw a comparison between drug treatment and non-treatment groups, regardless of BMI reduction, and verify whether or not it has statistical significance; in the present study, it was shown not to be statistically significant. However, it was difficult to compare the therapeutic efficacy due to the small number of patients in each group, and as mentioned above, it may be meaningful that the biochemical indicators that were improved in the drug treatment group with BMI reduction were clearer and more numerous than in the untreated group with BMI reduction only; a comparative study with a larger group of patients would demonstrate more clearly, whether or not the drug treatment is effective. Unfortunately, the difficulty of obtaining patient and parental consent for invasive tests in pediatric patients poses a problem for clinical studies.
In this study of NAFLD pediatric patients, whose diagnoses were confirmed by liver biopsy, we saw that combining vitamin E and UDCA treatment with BMI reduction through exercise and dietary therapy improve the patients' clinical symptoms and biochemical profiles, and that drug treatment alone, without BMI reduction, does not improve NAFLD. More accurate clinical information may be obtained through liver biopsy, which is an invasive procedure, but a randomized comparative study with a larger number of patients in the future may yield better information. A range of approaches are required in treating obesity-related NAFLD patients, but in patients that do not readily improve upon BMI reduction through exercise and dietary therapy alone, supplementary pharmacological treatment may be expected to enhance the therapeutic results.
References
- 1.Loomba R, Sirlin CB, Schwimmer JB, Lavine JE. Advances in pediatric nonalcoholic fatty liver disease. Hepatology. 2009;50:1282–1293. doi: 10.1002/hep.23119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park HS, Han JH, Choi KM, Kim SM. Relation between elevated serum alanine aminotransferase and metabolic syndrome in Korean adolescents. Am J Clin Nutr. 2005;82:1046–1051. doi: 10.1093/ajcn/82.5.1046. [DOI] [PubMed] [Google Scholar]
- 3.Tominaga K, Kurata JH, Chen YK, Fujimoto E, Miyagawa S, Abe I, et al. Prevalence of fatty liver in Japanese children and relationship to obesity. An epidemiological ultrasonographic survey. Dig Dis Sci. 1995;40:2002–2009. doi: 10.1007/BF02208670. [DOI] [PubMed] [Google Scholar]
- 4.Widhalm K, Ghods E. Nonalcoholic fatty liver disease: a challenge for pediatricians. Int J Obes (Lond) 2010;34:1451–1467. doi: 10.1038/ijo.2010.185. [DOI] [PubMed] [Google Scholar]
- 5.Mencin AA, Lavine JE. Nonalcoholic fatty liver disease in children. Curr Opin Clin Nutr Metab Care. 2011;14:151–157. doi: 10.1097/MCO.0b013e328342baec. [DOI] [PubMed] [Google Scholar]
- 6.Dufour JF, Oneta CM, Gonvers JJ, Bihl F, Cerny A, Cereda JM, et al. Swiss Association for the Study of the Liver. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin e in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2006;4:1537–1543. doi: 10.1016/j.cgh.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 7.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 8.Seo JW. Nonalcoholic fatty liver disease in children. Korean J Pediatr Gastroenterol Nutr. 2011;14:209–221. [Google Scholar]
- 9.Manco M, Marcellini M, Devito R, Comparcola D, Sartorelli MR, Nobili V. Metabolic syndrome and liver histology in paediatric non-alcoholic steatohepatitis. Int J Obes (Lond) 2008;32:381–387. doi: 10.1038/sj.ijo.0803711. [DOI] [PubMed] [Google Scholar]
- 10.Nobili V, Alkhouri N, Alisi A, Ottino S, Lopez R, Manco M, et al. Retinol-binding protein 4: a promising circulating marker of liver damage in pediatric nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:575–579. doi: 10.1016/j.cgh.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 11.Alkhouri N, Carter-Kent C, Lopez R, Rosenberg WM, Pinzani M, Bedogni G, et al. A combination of the pediatric NAFLD fibrosis index and enhanced liver fibrosis test identifies children with fibrosis. Clin Gastroenterol Hepatol. 2011;9:150–155. doi: 10.1016/j.cgh.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Nobili V, Alkhouri N, Bartuli A, Manco M, Lopez R, Alisi A, et al. Severity of liver injury and atherogenic lipid profile in children with nonalcoholic fatty liver disease. Pediatr Res. 2010;67:665–670. doi: 10.1203/PDR.0b013e3181da4798. [DOI] [PubMed] [Google Scholar]
- 13.Jee SJ, Kim YJ, Song SY, Paik SS. Association among histopathology, clinical manifestation, and ultrasonographic grades in pediatric non-alcoholic fatty liver disease. Korean J Gastroenterol. 2011;57:158–165. doi: 10.4166/2011.57.3.158. [DOI] [PubMed] [Google Scholar]
- 14.Nobili V, Marcellini M, Devito R, Ciampalini P, Piemonte F, Comparcola D, et al. NAFLD in children: a prospective clinical-pathological study and effect of lifestyle advice. Hepatology. 2006;44:458–465. doi: 10.1002/hep.21262. [DOI] [PubMed] [Google Scholar]
- 15.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 16.Houmard JA, Tanner CJ, Slentz CA, Duscha BD, McCartney JS, Kraus WE. Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol. 2004;96:101–106. doi: 10.1152/japplphysiol.00707.2003. [DOI] [PubMed] [Google Scholar]
- 17.Paumgartner G, Beuers U. Mechanisms of action and therapeutic efficacy of ursodeoxycholic acid in cholestatic liver disease. Clin Liver Dis. 2004;8:67–81. doi: 10.1016/S1089-3261(03)00135-1. [DOI] [PubMed] [Google Scholar]
- 18.Laurin J, Lindor KD, Crippin JS, Gossard A, Gores GJ, Ludwig J, et al. Ursodeoxycholic acid or clofibrate in the treatment of non-alcohol-induced steatohepatitis: a pilot study. Hepatology. 1996;23:1464–1467. doi: 10.1002/hep.510230624. [DOI] [PubMed] [Google Scholar]
- 19.Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770–778. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 20.Leuschner UF, Lindenthal B, Herrmann G, Arnold JC, Rössle M, Cordes HJ, et al. NASH Study Group. High-dose ursodeoxycholic acid therapy for nonalcoholic steatohepatitis: a double-blind, randomized, placebo-controlled trial. Hepatology. 2010;52:472–479. doi: 10.1002/hep.23727. [DOI] [PubMed] [Google Scholar]
- 21.Ratziu V, de Ledinghen V, Oberti F, Mathurin P, Wartelle-Bladou C, Renou C, et al. FRESGUN. A randomized controlled trial of high-dose ursodesoxycholic acid for nonalcoholic steatohepatitis. J Hepatol. 2011;54:1011–1019. doi: 10.1016/j.jhep.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 22.Strauss RS National Health and Nutrition Examination Survey. Comparison of serum concentrations of alpha-tocopherol and beta-carotene in a cross-sectional sample of obese and nonobese children (NHANES III) J Pediatr. 1999;134:160–165. doi: 10.1016/s0022-3476(99)70409-9. [DOI] [PubMed] [Google Scholar]
- 23.Hasegawa T, Yoneda M, Nakamura K, Makino I, Terano A. Plasma transforming growth factor-beta1 level and efficacy of alpha-tocopherol in patients with non-alcoholic steatohepatitis: a pilot study. Aliment Pharmacol Ther. 2001;15:1667–1672. doi: 10.1046/j.1365-2036.2001.01083.x. [DOI] [PubMed] [Google Scholar]
- 24.Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98:2485–2490. doi: 10.1111/j.1572-0241.2003.08699.x. [DOI] [PubMed] [Google Scholar]
- 25.Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, et al. Nonalcoholic Steatohepatitis Clinical Research Network. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]