Abstract
Recent epidemiologic studies suggest that caffeine may be protective against Alzheimer’s Disease (AD). Supportive of this premise, our previous studies have shown that moderate caffeine administration protects/restores cognitive function and suppresses brain β-amyloid (Aβ) production in AD transgenic mice. In the present study, we report that acute caffeine administration to both young adult and aged AD transgenic mice rapidly reduces Aβ levels in both brain interstitial fluid and plasma without affecting Aβ elimination. Long-term oral caffeine treatment to aged AD mice provided not only sustained reductions in plasma Aβ, but also decreases in both soluble and deposited Aβ in hippocampus and cortex. Irrespective of caffeine treatment, plasma Aβ levels did not correlate with brain Aβ levels or with cognitive performance in individual aged AD mice. Although higher plasma caffeine levels were strongly associated with lower plasma Aβ1-40 levels in aged AD mice, plasma caffeine levels were also not linked to cognitive performance. Plasma caffeine and theophylline levels were tightly correlated — both being associated with reduced inflammatory cytokine levels in hippocampus. Our conclusion is two-fold. First, that both plasma and brain Aβ levels are reduced by acute or chronic caffeine administration in several AD transgenic lines and ages, indicating a therapeutic value of caffeine against AD. Second, that plasma Aβ levels are not an accurate index of brain Aβ levels/deposition or cognitive performance in aged AD mice.
Keywords: caffeine, Alzheimer’s Disease, transgenic mice, β-amyloid, plasma, brain interstitial fluid
Introduction
Synthetic anti-Alzheimer’s Disease (AD) drugs currently on the market have mild symptomatic benefits, are not known to be disease-modifying, and can have significant undesirable side-effects. Thus, it would be most desirable to identify an inherently safe and readily available “nutriceutic” compound (e.g. natural and normally in the diet) that can provide therapeutic utility against AD. Caffeine is a methylxanthine, highly concentrated in coffee, and probably the world’s most widely consumed psychoactive substance [1]. Caffeine’s well known ability to increase alertness and arousal primarily involves antagonism of central nervous system adenosine receptors, while additional mechanisms of caffeine action (e.g., phosphodiesterase inhibition, calcium mobilization) have been proposed [2], although they occur only at high, unphysiologic concentrations of caffeine (mM range). Oral caffeine is rapidly and almost completely absorbed via the gastrointestinal tract, with blood caffeine levels quickly equilibrating with brain tissue levels due to caffeine’s unhindered traversal of the blood-brain barrier [3–4]. Caffeine is primarily metabolized in the liver to theophylline and paraxanthine, both of which are at least as physiologically active as caffeine [3]. There are no apparent differences in metabolism of, or physiological responses to, caffeine in elder individuals compared to young individuals; caffeine’s pharmacokinetics are similar after oral or intravenous administration in humans and animals [3].
Recent longitudinal studies spanning 4–10 years suggest that habitual caffeine/coffee intake protects against cognitive impairment in aging humans [5–6]. Moreover, an epidemiologic study evaluated caffeine intake during the 20 years preceding AD diagnosis and found that AD patients consumed markedly less caffeine during that period compared to age-matched individuals without AD [7]. Collectively, these and other observational human studies [8] suggest that habitual caffeine/coffee intake may protect against memory impairment and AD during aging. However, because such studies are not controlled and cannot isolate caffeine’s effects from the myriad of other life-style choices humans make, we performed a highly controlled “protection-based” study in mice [9]. In that study, we found that oral caffeine administration to AD transgenic (APPsw) mice from young adulthood into older age: 1) protected these mice from otherwise certain cognitive impairment in older age, and 2) limited their brain production of the peptide β-amyloid (Aβ). The moderate amount of caffeine intake given to these APPsw mice (human equivalency of 5 cups of coffee per day) suppressed both β-secretase (BACE1) and γ-secretase/PS1 levels in hippocampus, indicating that caffeine can directly impact AD pathogenesis in these AD mice. This study in AD mice is consistent with the human epidemiologic literature in supporting an ability of moderate caffeine intake to reduce risk of AD [7]. Paradoxically, human intake of coffee/caffeine declines appreciably during aging in Western cultures, in part due to caffeine intake restrictions often suggested by health care professions. Such restrictions would appear unwarranted, based on comprehensive literature searches indicating that moderate caffeine intake has no adverse effects on the cardiovascular system, bone status, calcium balance, or the incidence of cancer during aging [4, 10]. Indeed, a recent study involving 18–24 year follow-ups found that coffee intake (4–6 cups per day) was associated with reduced mortality, particularly due to cardiovascular disease [11].
The potential for caffeine to treat established AD has yet to be explored in human studies. As an initial step for elucidating possible efficacy of caffeine to stabilize or reverse established AD, we have recently completed a “treatment-based” study in aged APPsw mice that already contained Aβ pathology (see accompanying paper, this volume). These aged mice were confirmed to be cognitively impaired in working memory prior to receiving several months of oral caffeine treatment. When re-tested, aged APPsw mice receiving caffeine treatment exhibited working memory that was not only substantially better than APPsw mice that did not receive caffeine, but comparable to normal non-transgenic mice [12]. Thus, even with pre-existing and substantial Aβ neuropathology, aged APPsw mice exhibited memory restoration with caffeine treatment, suggesting a therapeutic potential of caffeine in cases of established AD.
Substantial evidence suggests that the brain’s production and aggregation of Aβ peptide represent key events underlying AD pathogenesis. As depicted in Figure 1A, newly produced Aβ enters a dynamic equilibrium between soluble and deposited Aβ in the brain, with continual transport of soluble Aβ out of the brain and into plasma. In view of our findings that caffeine decreases Aβ production in APPsw mice through suppression of both BACE1 and γ-secretase/PS1 [9], we hypothesize that resultant lower brain levels of soluble Aβ will at least acutely result in lower plasma Aβ levels (Figure 1B). This hypothesis is supported by the finding that Aβ is rapidly produced and cleared from the brain [13]. In the present study, we determine the effect of both acute and chronic caffeine administration on plasma and brain Aβ levels in AD transgenic mice. In addition, we explore possible relationships between: 1) plasma and brain pools of Aβ, 2) plasma caffeine/theophylline concentrations and plasma/brain Aβ levels, and 3) plasma Aβ/caffeine levels and cognitive performance. Possible associations between Aβ, caffeine, and cytokines are also considered. Both young adult and aged AD transgenic mice were utilized in these studies to investigate caffeine’s effect at both early (pre-Aβ deposition) and late (robust Aβ deposition) stages of the disease in mice.
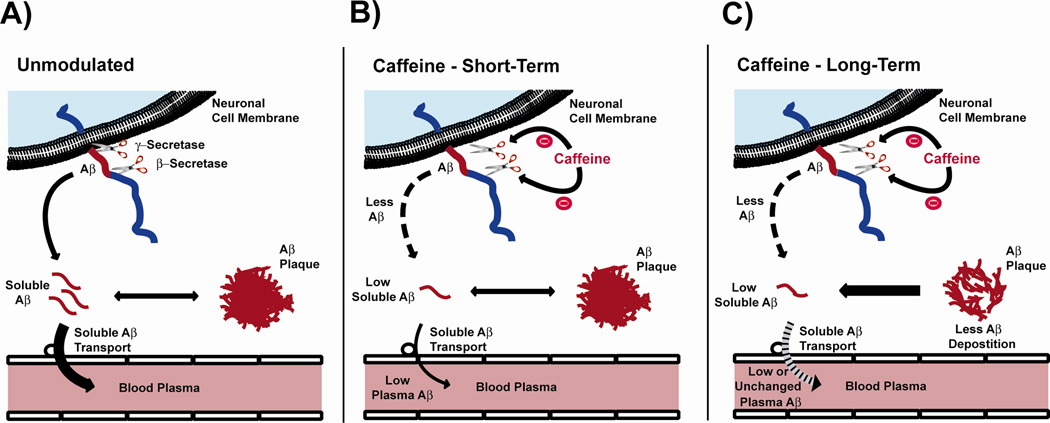
Figure 1. Diagrams depicting brain Aβ production/clearance, the suppressive actions of caffeine on Aβ production, and resultant effects on brain and plasma Aβ levels.
(A) Unmodulated: Aβ is primarily produced in neurons, secreted into the brain extracellular space in soluble form, then enters a dynamic equilibrium between soluble and deposited (insoluble) Aβ. Continual transport of soluble Aβ occurs into plasma. (B) Caffeine: Short-Term: Caffeine suppression of both β- and γ-secretase activities reduces Aβ production, resulting in lower soluble Aβ in brain and plasma. The equilibrium between soluble and deposited Aβ is not impacted by this short-term reduction in brain soluble Aβ levels. (C)Caffeine: Long-Term: Continued caffeine suppression of Aβ production and resultant lower levels of brain soluble Aβ induce a flux of deposited (insoluble) Aβ to the soluble form, which is cleared from brain into plasma via soluble Aβ transport. Plasma Aβ levels may be reduced or not changed, depending on degree of caffeine-induced suppression of Aβ production. In aged APPsw mice given chronic caffeine treatment, their lower brain Aβ levels/deposition results in reversal of cognitive dysfunction.
Based on encouraging results from our completed caffeine administration studies in AD transgenic mice [9, 12], clinical trials in aged individuals are currently being conducted to investigate the effects of caffeine administration on plasma Aβ levels. As such, a major aim of the present study was to attain advance insight into potential results from those clinical trials through similar studies in AD transgenic mice.
Material and Methods
Animals
In these various studies, all mice (with two exceptions) had a mixed background of 56.25% C57, 12.5% B6, 18.75% SJL, and 12.5% Swiss-Webster and were derived from a cross between heterozygous mice carrying the mutant APPK670N, M671L gene (APPsw) with heterozygous PS1 (Tg line 6.2) mice. This resulted in offspring consisting of mutant APPsw, PS1, APPsw+PS1, and non-transgenic (NT) genotypes. The two exceptions were: 1) APPsw+PS1 mice used in the 7-day gavage treatment study, which had a B6C3 background and were obtained from Jackson Labs (Bar Harbor, MA), and 2) Tg2576 mice used in the in vivo microdialysis study, which had a C57/BL6/SJL background and were a gift from Karen Hsiao Ashe (University of Minnesota; Hsaio et al. 1996). All mice were maintained on a 12 hours dark and 12 hours light cycle with ad libitum access to rodent chow and water/caffeinated water. All animal procedures were performed in AAALAC-certified facilities under protocols approved by Institutional Animal Care and Use Committees at USF and the JA Haley VA Hospital.
General Protocol
A spectrum of studies involving both acute and chronic caffeine administration was performed in APPsw/Tg2576 and APPsw+PS1 transgenic lines, with plasma, neurochemical, and/or behavioral measures collected. It should be underscored that these transgenic lines have measurable levels of soluble Aβ in both brain and plasma in young adulthood, with their brain levels of Aβ increasing appreciably during aging. This results in Aβ plaque formation beginning around 10–11 months of age for APPsw/Tg2576 mice and by 6 months of age for APPsw+PS1 mice. Thus, the 3–4 month old APPsw/Tg2576 mice used in these studies had no Aβ deposition, while the 14 month and older APPsw mice exhibited age-dependent Aβ deposition. All APPsw+PS1 mice utilized in these studies were at least 15 months of age and therefore had robust Aβ deposition. The general protocol for each study is indicated below:
Acute caffeine cffects on plasma Aβ levels
Acute (single treatment) administration of caffeine or saline vehicle was given by i.p. injection or gavage to the following groups of mice: 3–4 month old APPsw mice (i.p.), 14 month old APPsw mice (i.p. or gavage), and 14 month old APPsw+PS1 mice (gavage). A pre-treatment blood sample (0.15 ml) was taken by sub-mandibular vein puncture 3–4 days before treatment. At 3–4 hours following caffeine (1.5 mg/0.2 ml; Sigma, St. Louis, MO) or vehicle administration, another blood sample was taken. In these acute studies, each group of caffeine- or vehicle-treated animals consisted of 5–7 Tg mice. For all acute caffeine treatment studies, an equivalent volume of 0.9% saline (0.2 ml) was given immediately following any blood sample taken.
Long-term caffeine effects on plasma Aβ levels
Longer term caffeine administration was given by gavage to aged APPsw+PS1 mice. At 3–4 days following a pre-treatment blood sample, 15–20M old APPsw+PS1 mice (n=6) were started on twice-daily caffeine treatment (1.5 mg/0.2 ml each) via gavage for 7 consecutive days. A blood sample was taken on the final day of caffeine treatment, as well as 9 days thereafter. A second group of four 20 month old APPsw+PS1 mice were pre-treatment bled, then given two caffeine treatments (1.5 mg/0.2 ml each) via gavage every 4th day for up to two months. In this second long-term study, blood samples were taken every 8th day during treatment, always on the day following a treatment. As was the case for acute studies, volume replacement with 0.9% saline occurred immediately following each blood sample.
In vivo microdialysis: Acute caffeine effects on interstitial fluid (ISF) levels of Aβ in hippocampus
In vivo microdialysis was used to assess brain ISF Aβx-40 in the hippocampus of awake, freely moving Tg2576 mice. Microdialysis was performed similar to previously described methods [14]. This technique samples soluble molecules within the extracellular fluid that are smaller than 38-kilodaltons, the molecular weight cut off of the probe membrane. Aβ capable of entering the probe has been dubbed “exchangeable Aβ or eAβ” [14]. The pool of eAβ is in dynamic equilibrium with the total pool of ISF Aβ. During microdialysis, mice were housed in a constant light condition and remained awake with freedom of movement and ad lib food and water during microdialysis. Microdialysis perfusion buffer was artificial CSF containing 0.15% bovine serum albumin that was filtered through a 0.1 µm membrane. Flow rate was a constant 1.0 µl/minute, which recovers 23.4 ± 1.7% (mean ± SEM) of exchangeable Aβ within the brain ISF of Tg2576 mice. Samples were collected every 30–60 minutes with a refrigerated fraction collector into polypropylene tubes and assessed for Aβ by sandwich ELISA at the completion of each experiment, as described by Cirrito et al. [15]. Briefly, Aβx-40 was assessed using an Aβ40-specific mouse monoclonal antibody, mHJ2, as a coating antibody and a biotinylated central domain antibody, mHJ5.1, as the detecting antibody, followed by streptavidin-poly-HRP-40 (Fitzgerald Industries). All ELISA assays were developed using Super Slow ELISA TMB (Sigma, St. Louis, MO) and absorbance read on a Bio-Tek FL-600 plate reader (Winooski, Vermont) at 650 nm. Basal levels of ISF Aβ were defined as the mean concentration of Aβ over 5–6 hours preceding drug treatment. The mean in vivo concentration of basal ISF eAβ was 3.9 ± 0.78 ng/ml (n=6). For each animal, all Aβ levels were normalized to the basal Aβ concentration. Once basal ISF Aβ levels were established, Tg2576 mice were administered caffeine i.p. and ISF Aβ levels were sampled every 30 minutes until the end of the study. Mice were studied at 3 months of age, which is prior to Aβ deposition in this mouse model.
ISF Aβ half-life was determined similar to Cirrito et al. [14]. Microdialysis probes were inserted as described previously. Basal levels of ISF Aβ were established for five hours, followed by i.p. administration of 30 mg/kg caffeine or vehicle (PBS). Three hours after treatment, the γ-secretase inhibitor Compound E (Alexis Biochemicals, San Diego, CA; 100 nM) was added to the microdialysis perfusion buffer to rapidly inhibit Aβ production near the probe. The IC50 for this compound to inhibit γ-secretase activity in vitro is 0.3 nM. Microdialysis samples were collected every 30 minutes for an additional four hours and then assessed for Aβx-40 by sandwich ELISA. The half-life of Aβ was calculated based on the slope of decline in ISF Aβ levels [14] beginning one hour after the onset of Compound E administration.
Long-term caffeine effects in behaviorally-tested mice
At 18–19 months of age, APPsw and non-transgenic (NT) littermate controls were pre-tested in the Radial arm water maze (RAWM) task of working memory according to our established protocol [9, 16]. Following confirmation that APPsw mice were cognitively-impaired, they were divided into two groups, with half of Tg mice started on caffeine administered in their drinking water (0.3 mg/ml, providing a daily dose of ≈ 1.5 mg caffeine/mouse) and the other half remaining on standard tap water, as detailed previously [9]. At 4–5 weeks into caffeine treatment, all mice were re-tested in the RAWM, with both last block and overall errors being analyzed for working memory Trials 4 and 5. Following completion of behavioral testing at 20–21 months of age, mice were deeply anesthetized with sodium pentobarbital, a blood sample was then taken for neurochemical analysis, followed by transcardial perfusion with 100 ml of 0.9% saline. Postmortem brains were immediately removed and bisected sagitally. The hippocampus and cerebral cortex was dissected from the right hemisphere and processed for soluble Aβ1–40 and Aβ1–42 determinations by ELISA, as well as for cytokine levels. Briefly, 30 mg brain samples were homogenized in 400 µl RIPA buffer (100 mM Tris [pH8.0 ], 150 mM NaCl, 0.5% DOC, 1% NP-40, 0.2% SDS, and 1 tablet proteinase inhibitor per 100 ml (S8820, Sigma, St. Louis, MO), and sonicated for 20 seconds on ice. Samples were then centrifuged for 30 min at 27,000 g at 4°C, and supernatants were transferred into new screw cap tube. The supernatants obtained from this protocol were then stored at −80°C for determination of soluble Aβ levels using ELISA kits (KHB3482 for 40, KHB3442 for 42, Invitrogen, CA). Standard and samples were mixed with detection antibody and loaded on the antibody pre-coated plate as the designated wells. HRP-conjugated antibody was added after wash, and substrates were added for colorimetric reaction, and then stopped with sulfuric acid. Optical density was obtained and concentrations were calculated according a standard curve. Plasma Aβ1–40 and 42 levels were determined with the same protocol and using the same ELISA kits. Homogenates from the right hippocampus and cerebral cortex were also analyzed for cytokine levels (see next section).
The left hemisphere was histologically processed for analysis of total Aβ deposition, as previously described [17]. Briefly, equally-spaced 5-µm sections at the hippocampal level were immunostained for total Aβ deposition using a biothinylated human Aβ monoclonal antibody (clone 4G8; Covance Res. Products, Everyville, CA). Quantitative image analysis was then performed according to Mori et al. [18] and Aβ burden was determined as a percentage of immunolabeled area (positive pixels) relative to the full area captured (total pixels). Quantitative image analysis was done based on previous methods with modifications [18–19]. Images were acquired using an Olympus BX60 microscope with an attached digital camera system (DP-70, Olympus, Tokyo, Japan). The digital image was then routed into a Windows PC for quantitative analysis using SimplePCI software (Compix Inc.,Imaging Systems, Cranberry Township, PA). Images of five 5-µm sections (150 µm apart) through the hippocampus were captured from each animal, and a threshold optical density was obtained that discriminated staining from background. Each region of interest was manually edited to eliminate artifacts. For Aβ burden analysis, data are reported as percentage of immunolabeled area captured (positive pixels) relative to the full area captured (total pixels). Each analysis was done by a single examiner blinded to sample identities.
Plasma Neurochemical Analysis
Plasma from blood samples was analyzed for levels of Aβ1–40, caffeine, theophylline (a caffeine metabolite), and cytokines. Aβ1–40 levels were determined according to the aforementioned methodology involving brain tissues. Plasma caffeine and theophylline concentration were measured with ELISA Kits from Neogen (WI, USA) by following the manufacture protocol. In brief, the enzyme conjugate solution was prepared by diluting the 180X enzyme conjugate stock 1 to 180 in the EIA buffer provided. Caffeine (or theophylline) standard was then diluted with EIA buffer at two fold dilutions from 200ng/ml to 0.39ng/ml. Then 20 ul standard of each dilution was added into the coated plate. Plasma samples were then diluted with EIA buffer, with 20 ul of this dilution added into the coated plate. Both standard and samples were run in duplicate in the plate. Positive and negative controls of 20 ul were loaded to each plate. Then 180 ul of diluted drug-enzyme conjugate was added into each well and mixed by gently shaking the plate. Plates were covered with plastic film and incubated at room temperature for 45 minutes. During the incubation, a 10x wash buffer was diluted to 1X with DI water and mixed thoroughly. Once incubation was completed, the liquid was dumped from the wells. Plates were then taped on a clean lint-free towel to remove any remaining liquid in the wells. Then each well was washed with 300ul of diluted wash buffer 3 times. After completing the last wash step, the bottom of the wells was wiped with a lint-free towel to remove any liquid on the outside of the wells. Then 150 ul of the K-Blue Substrate was added to each well with a multi-channel pipette. The plate was then mixed by gently shaking, followed by incubation at room temperature for 5 to 20 minutes. To stop the enzyme reaction, 50 ul of red stop solution was added to each well and gently mixed. The absorbance was then measured with plate reader (Synergy HT, Biotek, VT, USA) at a wavelength of 650 nm. The absorbance was converted into concentration using Gen5 software.
Cytokine expression profiles were detected using the Bio-Rad Bio-Plex kits (Bio-Rad, catalogue # 171F11181). Samples and standards were prepared using company protocols with the initial concentration of standards ranging from 32 ng/ml to 1.95 pg/ml. Plasma samples were prepared for analysis by diluting 1 volume of the serum sample with 3 volumes of the Bio-Plex mouse sample diluents. Details of this procedure were performed by followed the protocol provided by the manufacture. Finally, the plates were read. Each cytokine level was calculated based on its own standard curve. Brain cytokine and chemokine levels were detected with the same method.
Statistical Analyses
Group comparisons involving levels of soluble/deposited Aβ, caffeine, theophylline, and cytokines were performed using ANOVA. For determination of pre-treatment versus post-treatment differences in plasma Aβ level, paired t-tests were employed. All other statistical analyses are as indicated in the text. In order to test if relationships were present between plasma, neurochemical, and behavioral measures, correlation analysis was performed using the Systat analytical software package.
Results
Acute caffeine administration reduces plasma Aβ level
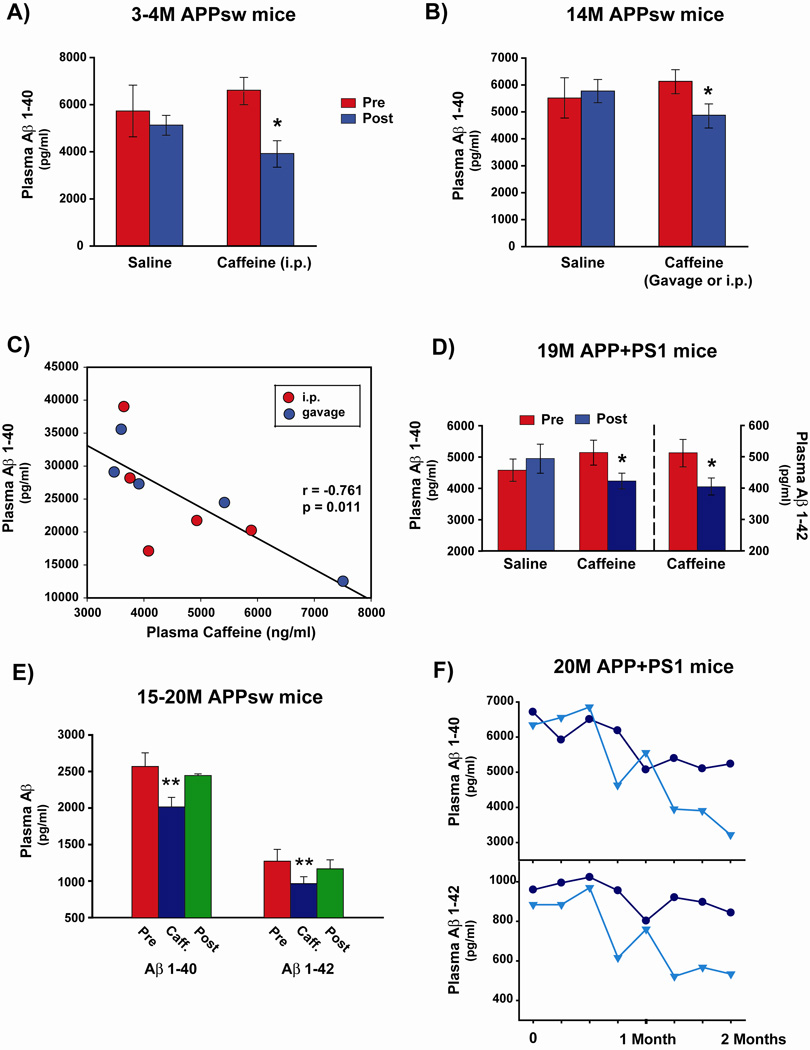
We have previously found that Aβ1–40 and Aβ1–42 generation is decreased in N2a neuronal cell cultures following 6 hours of caffeine treatment [9]. Moreover, these reductions in generation of both Aβ1–40 and Aβ1–42 peptides were dose-dependent, occurring at caffeine concentrations (≤10 µM), which are typically present in plasma following coffee consumption in humans. Given caffeine’s rapid delivery to all body organs including the brain, we therefore hypothesized that acute caffeine administration would quickly suppress brain Aβ production in AD transgenic mice, resulting in a significant reduction in plasma Aβ levels within hours. In an initial study in which 3–4 month old APPsw mice (pre-plaque) were given a single intraperitoneal (i.p.) injection of caffeine (1.5 mg), plasma Aβ1–40 levels were significantly reduced by 41% at 3 hours post-treatment compared to pre-treatment levels (Figure 2A); vehicle injection failed to affect plasma Aβ levels in other littermate APPsw mice. Caffeine-induced reductions in plasma Aβ1–40 levels were also observed in aged 14-month old APPsw mice (plaque-bearing) at 3 hours following either a single i.p. or gavage treatment with the same dose of caffeine (Figure 2B). Moreover, plasma caffeine concentrations in these same 14-month old APPsw mice were negatively correlated with plasma Aβ1–40 levels — higher plasma caffeine levels were associated with lower plasma Aβ levels in individual animals (Figure 2C). Even aged 19-month APPsw+PS1 mice (plaque-bearing) exhibited significant reductions in plasma Aβ1–40 and Aβ1–42 levels at 3 hours following gavage treatment with caffeine (Figure 2D).
Figure 2. Caffeine treatment induces a rapid and sustained decrease in plasma Aβ levels.
(A,B,D) A single i.p. or gavage treatment with caffeine significantly reduces plasma Aβ levels in 3–4 month old (A) and 14 month old (B) APPsw mice; as well as in 19 month old APP+PS1 mice (D). In all three studies, saline vehicle treatment had no effect. (C) Plasma caffeine levels in 14M old APPsw mice are inversely correlated with plasma Aβ1-40 levels. (E) Oral caffeine treatment for one week to 15–20 month old APPsw mice results in decreased plasma levels of both Aβ1-40 and Aβ1-42 immediately following treatment, with a rebound in plasma Aβ levels occurring by 9 days after cessation of caffeine treatment. Each group in A, B, D, and E consisted of 4 – 7 mice. (F) As exemplified by two aged 20 month old APP+PS1 mice, oral caffeine administration every 4th day over a 2-month period induces a sustained and continual decrease in plasma levels of both Aβ1-40 and Aβ1-42 over the treatment period. Post-treatment versus pre- or delayed post-treatment Aβ levels were evaluated by paired t-tests. *p<0.05–0.01 versus pre-treatment levels; **p<0.05 versus both pre-treatment and 9-day post-treatment levels.
Long-term oral caffeine treatment provides a sustained reduction in plasma Aβ levels
Our prior work has demonstrated that hippocampal levels of both soluble and insoluble Aβ are reduced by greater than 30% following 5½ months of oral caffeine treatment (≈1.5 mg/day) to young adult APPsw mice [9]. In the same study, we also found this same treatment to be effective in reducing insoluble hippocampal Aβ levels in aged 17-month old APPsw mice following 18 days of treatment. We therefore hypothesized that long-term oral caffeine treatment would result in a sustained suppression of plasma Aβ levels. In fact, aged 15–20 month old APPsw mice (plaque-bearing) that were given oral treatment with 1.5 mg caffeine twice daily for one week did exhibit significant reductions in plasma levels of both Aβ1–40 and Aβ1–42 compared to pre-treatment values (Figure 2E). At 9 days following cessation of this caffeine treatment, however, plasma levels of both Aβ1–40 and Aβ1–42 had returned to their pre-treatment levels. In a second long-term treatment study, 20-month old APPsw+PS1 mice (plaque-bearing) were given oral caffeine treatment (1.5 mg) twice daily every 4th day over a 2 month period. Periodic analysis of plasma Aβ1–40 and Aβ1–42 levels during this treatment period revealed not only a sustained reduction in both Aβ peptides, but also a continuing decrease in their levels throughout the 2-month treatment. Given that the half-life of caffeine in rodents is only 0.7 to 1.2 hours [3], these later observations suggest that the effects of caffeine on Aβ suppression greatly exceed its own half-life.
Caffeine administration lowers brain interstitial fluid levels of Aβ in vivo
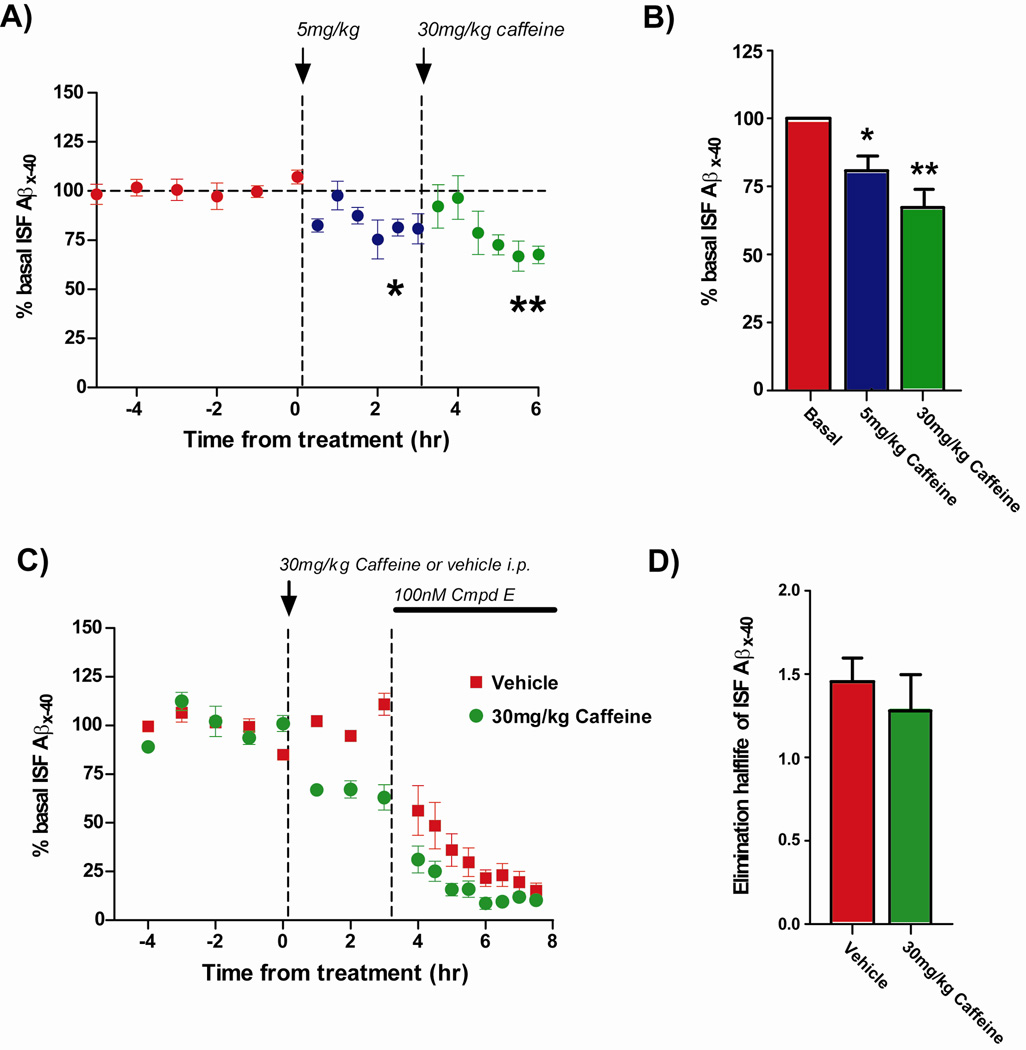
Aβ is primarily produced in neurons and secreted into the brain extracellular space where it is normally soluble within the interstitial fluid (ISF). To determine if caffeine administration acutely affects brain ISF Aβ levels, 3-month old Tg2576 mice (pre-Aβ plaque) were treated with several doses of caffeine while performing in vivo microdialysis to measure ISF Aβ levels. Microdialysis probes were implanted into the hippocampus, permitting us to sample ISF Aβ levels every 30 minutes for up to 24 hours in awake, behaving animals [14–15]. Basal ISF Aβ levels were determined over 6 hours in each mouse followed by i.p. administration of caffeine at 5 mg/kg (~0.15 mg/mouse), then at 30 mg/kg (~1 mg/mouse) three hours later (Figure 3A). Caffeine significantly lowered ISF Aβ levels by 19% and 32% at the low and high doses, respectively, as compared to the basal ISF Aβ levels in each mouse (Figure 3B). There was also a trend that Aβ levels were reduced to a greater extent by the 30 mg/kg dose of caffeine than by the 5 mg/kg dose (p=0.1).
Figure 3. Caffeine lowers ISF Aβ levels.
(A) Brain ISF Aβx-40 levels were measured by in vivo microdialysis. Prior to treatment with caffeine, ISF Aβ levels fluctuated very little. A low dose (5 mg/kg) and a higher dose (30 mg/kg) of caffeine caused ISF Aβ levels to decrease significantly compared to basal levels. (B) Following a 5 mg/kg dose of caffeine i.p., ISF Aβx-40 levels decreased by 19% compared to basal levels (*p<0.05), while a 30 mg/kg administration decreased Aβ levels by 33% (**p<0.01, n=6 per group). The mean concentration of ISF Aβ represents an average of hours 2–3 after each dose of caffeine when Aβ levels had stabilized. (C) Tg2576 were pre-treated with vehicle or 30 mg/kg caffeine for 3 hours (n=6 per group), followed by administration of 100 nM Compound E, a γ-secretase inhibitor, via reverse microdialysis. In both groups, ISF Aβ levels decreased rapidly when APP cleavage was blocked. (D) The elimination half-life of ISF Aβx-40 was comparable in vehicle-treated and caffeine-treated mice, suggesting that caffeine does not affect ISF Aβ elimination, but likely impacts Aβ production instead.
Caffeine can have many affects in a living animal which could potentially alter Aβ production or Aβ elimination, thus lowering ISF Aβ levels [3, 20]. As such, we determined the elimination half-life of ISF Aβ in mice treated with vehicle or 30 mg/kg caffeine. Basal ISF Aβ levels were measured in Tg2576 mice, followed by i.p. administration of 30 mg/kg caffeine or vehicle (Figure 3C). As expected, caffeine reduced ISF Aβ levels by 35% compared to no change in vehicle-treated mice. Three hours later, the microdialysis perfusion buffer was switched to contain a potent γ-secretase inhibitor, Compound E, to rapidly block Aβ production. In vehicle-treated mice, the elimination half-life of Aβ was 1.5 hours (Figure 3D), which is similar to previous reports of the ISF Aβ half-life in this mouse model [21]. Importantly, the half-life of ISF Aβ in caffeine-treated mice (1.3 hours) was not significantly different from vehicle-treated mice. This suggests that caffeine does not alter ISF Aβ elimination, but likely impacts some aspect of Aβ production instead.
Long-term oral caffeine treatment to aged, cognitively-impaired APPsw mice reduces brain soluble and deposited Aβ
To determine the effects of chronic caffeine treatment in cognitively-impaired Alzheimer’s mice, caffeine (~1.5 mg/day) was orally administered in drinking water to 18–19 month old APPsw mice (plaque-bearing) that were confirmed to be impaired in the radial arm water maze (RAWM) task of working memory prior to treatment. At 4–5 weeks into caffeine treatment, impaired APPsw mice that had been given caffeine (Tg/Caff) exhibited substantially better RAWM working memory performance compared to the continuing impairment of control APP mice [12]. After euthanizia at 20–21 months of age (2 months into caffeine treatment), soluble Aβ1–42 levels in both cortex and hippocampus of Tg/Caff mice were significantly reduced by 51% and 59%, respectively, compared to Tg controls (Figure 4A). Cortical and hippocampal Aβ1–40 levels were also reduced by chronic caffeine treatment, although the decrease in hippocampal Aβ1–40 did not reach statistical significance (p=0.09). Compared to Tg controls, plasma Aβ1–40 levels were not significantly decreased in Tg/Caff mice. However, when Tg/Caff mice were divided into two sub-groups based on higher vs. lower plasma caffeine levels, a significant decrease in plasma Aβ1–40 was evident in higher plasma caffeine mice (See up-coming section involving plasma caffeine). Finally, and in the same behaviorally-tested aged Tg mice, chronic caffeine treatment resulted in a remarkable 40% reduction in hippocampal Aβ deposition compared to Tg controls (Figure 4B). Brain levels of insoluble Aβ (as measured by ELISA) were also reduced in caffeine-treated Tg mice, as exemplified by the 29% and 33% decreases in Aβ1–40 and Aβ1–42, respectively, seen in posterior cortex (data not shown).
Figure 4. Long-term caffeine administration to aged, cognitively-impaired APP mice reduces soluble Aβ levels in both cortex and hippocampus, while also decreasing deposited (insoluble) Aβ in hippocampus.
Caffeine was administered to 18–19 month old APPsw mice for two months in their drinking water. Both brain Aβ1-40 and Aβ1-42 were decreased in caffeine-treated Tg mice. Although plasma Aβ levels were unaffected for all Tg/Caff mice inclusively, see Figure 6B. Immunohistochemical Aβ deposition in the hippocampus was reduced by 40% in caffeine treatment mice. *p<0.05; **p<0.001. Each group consisted of 5–8 mice.
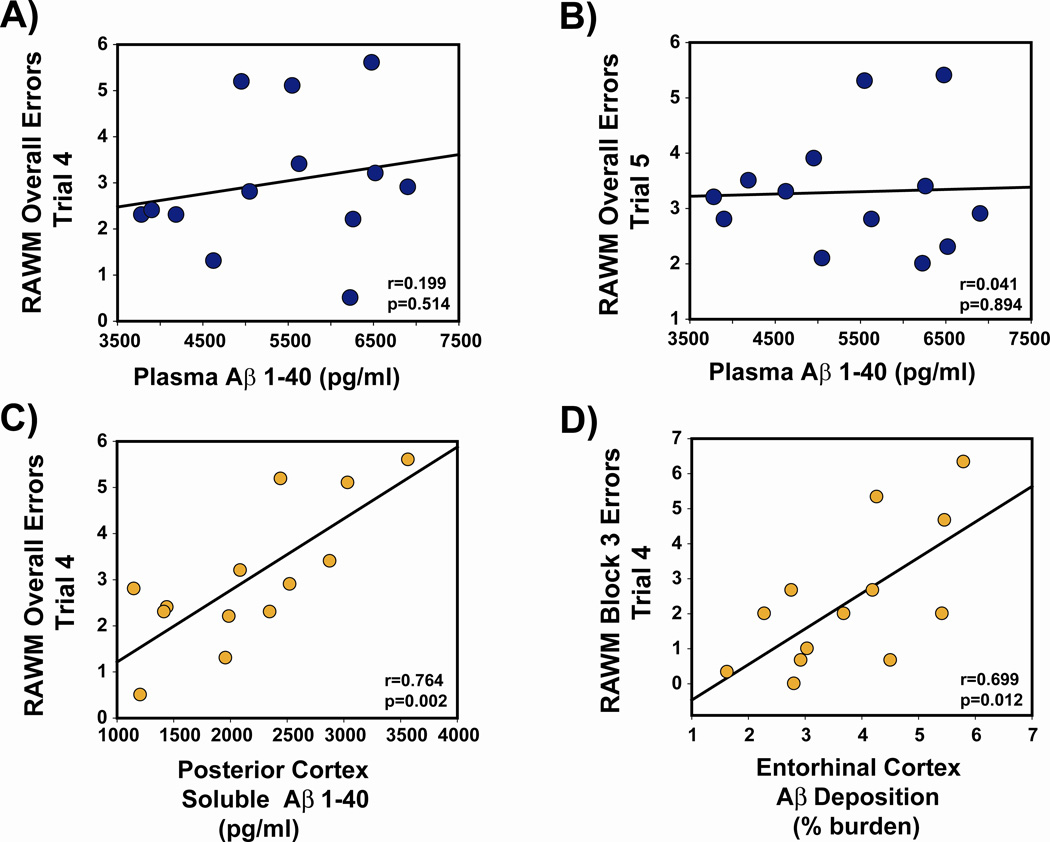
Plasma Aβ levels do not correlate with brain Aβ levels or cognitive performance in aged APPsw mice
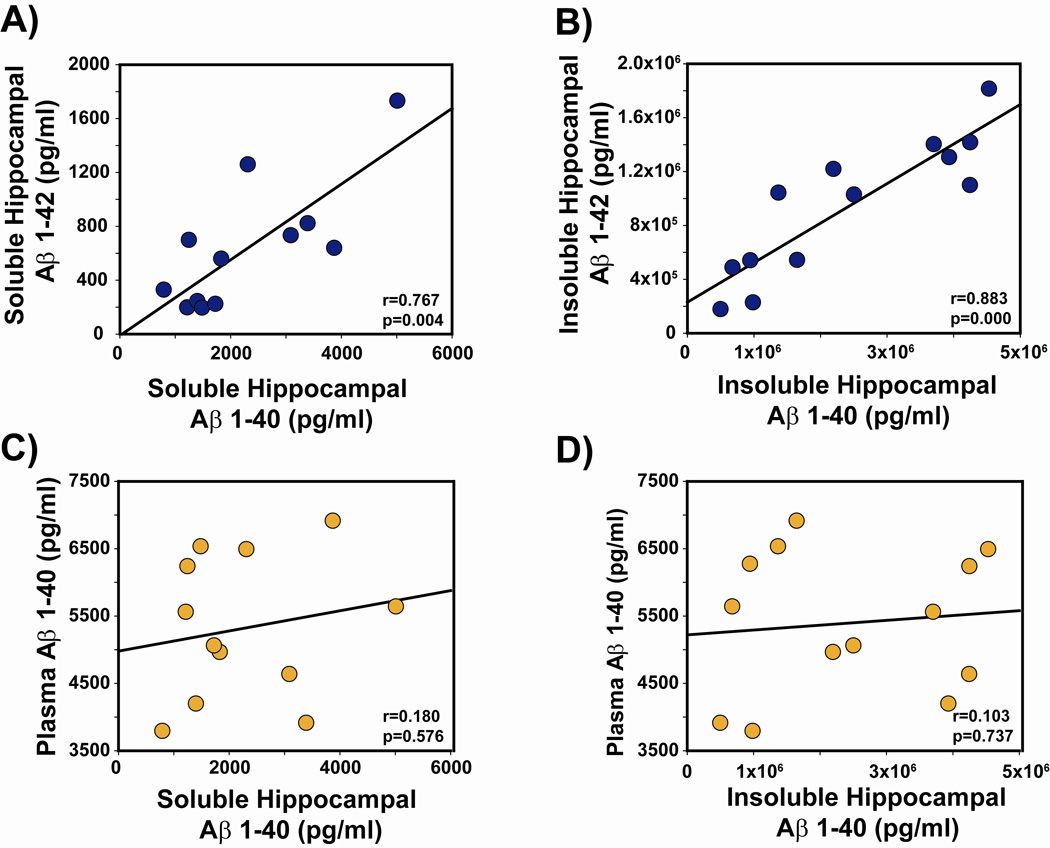
To explore the relationship between brain and plasma pool of Aβ, as well as the association between various Aβ pools and cognitive performance, we next performed correlation analysis between these measures in the same aged APPsw mice whose brain and plasma Aβ measures were presented in Figure 4. These 20–21 month old APPsw mice exhibited strong correlations between Aβ1–40 and Aβ1–42 levels in hippocampus and cortex, and for both soluble and insoluble Aβ pools (Figure 5, A and B). These correlations were present irrespective of whether all Tg mice, or only Tg controls were included. In sharp contrast, plasma Aβ1–40 was not correlated with any of the 8 brain Aβ measures evaluated (e.g., hippocampus or cortex, soluble or insoluble, Aβ1–40 or Aβ1–42). Figure 5, C and D shows two representative plots involving hippocampal soluble/insoluble Aβ1–40 versus plasma Aβ1–40. The lack of correlations between brain and plasma Aβ measures was present irrespective of whether all Tg mice, or only Tg controls were included. Finally, there were no correlations between plasma Aβ1–40 levels and four measures of cognitive performance in the RAWM task of working memory; two of these correlations are shown in Figure 6 (A and B). However, as we have consistently shown in prior studies [22–25], Aβ levels in cerebral cortex, entorhinal cortex, and hippocampus were closely linked to RAWM performance — higher brain Aβ levels were strongly correlated with poorer working memory performance (Figure 6C and D). Thus, plasma levels of Aβ are not an accurate index of: 1) soluble or insoluble brain Aβ, or 2) cognitive performance.
Figure 5. Plasma Aβ levels do not correlate with brain Aβ levels in aged APPsw mice.
(A,B) Strong correlations are present between levels of Aβ1-40 and Aβ1-42 for both soluble and insoluble Aβ in hippocampus, irrespective of caffeine treatment. By contrast, no correlations existed between hippocampus (or cortex) and plasma for soluble Aβ levels (C) or insoluble Aβ levels (D) irrespective of caffeine treatment.
Figure 6. Plasma Aβ levels do not correlate with cognitive performance in aged APPsw mice.
(A,B) No correlations were present between plasma Aβ levels and RAWM working memory. (C,D). By contrast, strong correlations were evident between brain Aβ levels/deposition and cognitive impairment, as exemplified by correlations involving soluble Aβ levels in posterior cortex (C) and Aβ deposition in entorhinal cortex (D). Each symbol represents an individual Tg mouse. All correlations involve all Tg mice collectively (Tg controls and Tg/Caff), although identical results were observed when only Tg controls were included in the analysis.
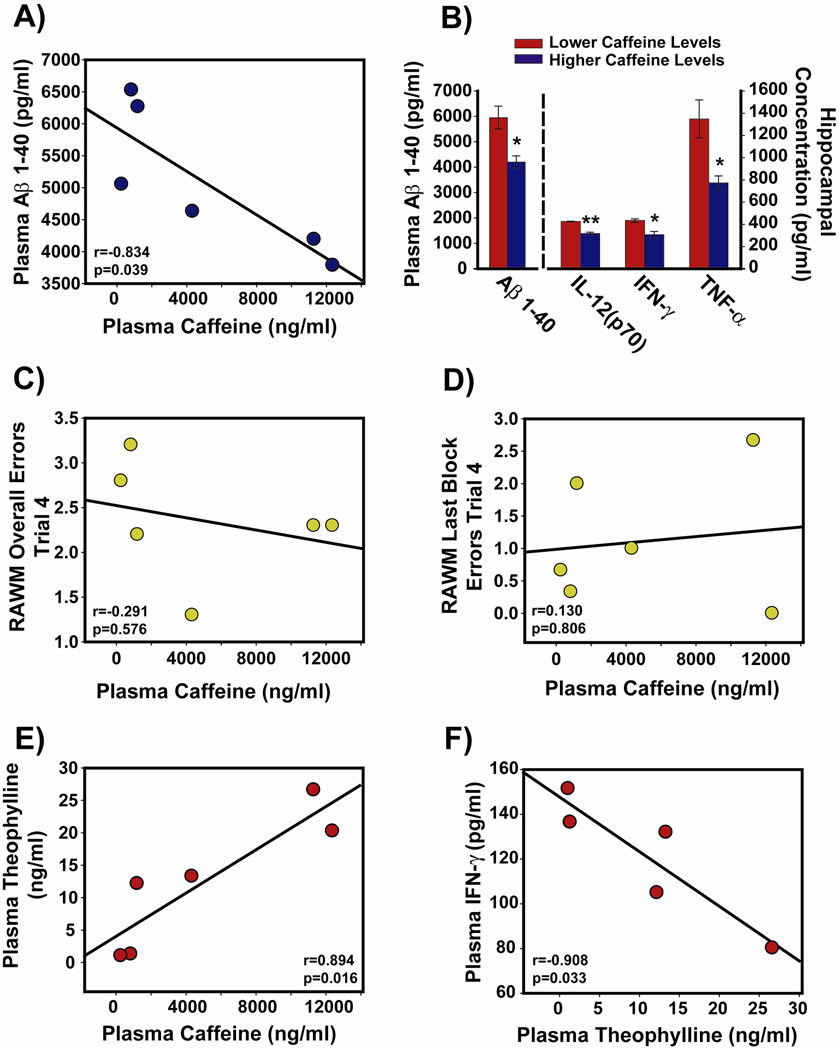
The relationship between plasma caffeine levels in aged APPsw mice and their plasma/brain Aβ levels and cognitive performance
Plasma taken at euthanasia from the 6 behaviorally-tested 20–21 month old APPsw mice that have been given oral caffeine treatment for 2 months was analyzed for concentrations of caffeine and theophylline (a major biologically-active metabolite of caffeine). A strong inverse correlation was evident between plasma caffeine concentration in those mice and their plasma levels of Aβ1–40 (Figure 7A), with higher plasma caffeine levels associated with lower plasma Aβ levels. Because there was a significant range in plasma caffeine concentrations among these animals, the three APPsw mice with the highest plasma caffeine levels [mean = 9331 ng/ml] were compared to the three mice with the lowest levels for plasma Aβ levels [mean = 769 ng/ml]. Mice with higher plasma caffeine levels had significantly reduced plasma Aβ1–40 levels compared to those with lower plasma caffeine levels (Figure 7B). However, plasma caffeine levels in these APPsw mice were not correlated with their Aβ1–40 or Aβ1–42 levels in either hippocampus or cerebral cortex (data not shown). Moreover, as was the case for plasma Aβ levels (Figure 6, A and B), there were no correlations between plasma caffeine levels and RAWM working memory performance in aged APPsw mice (Figure 7, C and D). Plasma caffeine levels were, however, strongly correlated with plasma levels of theophylline (Figure 7E). Importantly for human relevance, the 6 aged APPsw mice chronically treated with caffeine had a mean plasma caffeine concentration of 26±11.5 µM, which is comparable to plasma caffeine levels in humans following several cups of coffee [3].
Figure 7. Effects of caffeine and theophylline on plasma, brain, and cognitive measures.
In aged 20–21 month old APPsw mice that had been treated for 2 months with caffeine and cognitively evaluated (n=6), blood and brain tissues taken at euthanasia were analyzed. (A) High plasma caffeine levels correlated with lower plasma Aβ levels. (B) Mice with higher plasma caffeine levels had lower plasma Aβ1-40 levels and lower hippocampal cytokine levels than those mice with lower caffeine levels. *p<0.05; **p<0.01. (C,D) As exemplified by these 2 correlation graphs, plasma caffeine levels in individual mice were not correlated with their radial arm water maze (RAWM) working memory performance. (E) Plasma caffeine and plasma theophylline levels were strongly correlated. (F) High plasma theophylline levels correlated with lower plasma IFN-γ levels.
The association of Aβ and caffeine levels in plasma with cytokines in plasma/brain
Aβ and caffeine have been reported to have pro- and anti-inflammatory actions, respectively [20, 26]. Therefore, we investigated whether plasma levels of these two compounds impacted plasma or brain cytokine levels in aged APPsw mice (plaque-bearing) following 2 months of oral caffeine treatment. Plasma Aβ1–40 levels were not correlated with plasma cytokine levels for all Tg mice collectively, irrespectively of caffeine treatment (data not shown). As well, plasma caffeine levels in caffeine-treated Tg mice were not correlated with plasma cytokine levels (data not shown). Indeed, there were largely no differences in “plasma” cytokine levels between non-transgenic, Tg, and Tg/Caff groups in this study (data not shown). Although this was also the case for “brain” cytokine levels, closer inspection revealed potential effects of caffeine on levels of key inflammatory cytokines. Specifically among caffeine-treated Tg mice, those mice with higher caffeine levels had significantly reduced levels of hippocampal cytokines compared to mice with lower caffeine levels (Figure 7B). These results suggest an anti-inflammatory action of caffeine in a key brain area for cognitive function. The caffeine metabolite “theophylline” may also be contributory to caffeine’s anti-inflammatory effect, as exemplified by the lower levels of plasma interferon (IFN)-γ in APPsw mice having higher plasma theophylline levels (Figure 7F). Indeed, plasma levels of theophylline in caffeine-treated APPsw mice: 1) were correlated with lower cytokine levels in both hippocampus and cortex, and 2) showed the same anti-inflammatory profile as caffeine (Figure 7B) when analyzed in terms of high vs. low theophylline levels (data not shown).
Discussion
In this report, we show that caffeine treatment to AD transgenic mice lowers Aβ levels in plasma and brain ISF within a few hours and can provide continued reductions in plasma Aβ through oral treatment periods of 1–8 weeks. Even in aged, cognitively-impaired APPsw mice bearing pre-existing and substantial Aβ burdens, oral caffeine administration over several months reduced both soluble and deposited brain Aβ. Relatedly, this same caffeine treatment reverses the poor memory performance of aged APPsw mice back to the level of non-transgenic (normal) mice [12]. However, because there were no correlations between hippocampal and plasma Aβ levels in individual mice, we conclude that plasma Aβ levels are not an accurate index of brain Aβ levels in aged AD mice. As well, because there were no correlations between plasma Aβ levels and cognitive performance in the same aged AD mice, we conclude that blood Aβ levels are not an accurate index of cognitive performance. From an overall perspective, then, plasma Aβ levels are not a viable biomarker for the cognitive and neurochemical characteristics of aged AD mice. The inability of plasma Aβ1–40 levels to acutely reflect decreased brain Aβ1–40 levels may involve a consequent, acute decrease in Aβ1–40 transporter activity (below saturability levels) at the BBB [27]. Secondly, our collective results underscore that caffeine, its metabolites, and/or analogs should be considered for prevention and treatment trials in AD because caffeine can suppress Aβ production, but not its clearance from the brain.
The concentration of Aβ in brain tissue and cerebrospinal fluid (CSF) is some 50–100 times higher than in plasma [28]. It is thus likely that a sizable amount of plasma Aβ1–40 originates from production in, and out of, the brain. Indeed, Aβ is rapidly produced and cleared from the brain, with similar ISF clearance rates in Alzheimer’s Tg mice and humans [13, 14]. Given the relatively short (<2 hour) half-life of ISF Aβ in Tg mice [14], it is apparent that newly produced Aβ enters a dynamic equilibrium between soluble and insoluble/deposited Aβ in the brain, with continual transport of soluble Aβ out of the brain and into plasma (Figure 1, Unmodulated). In the present study, a single treatment with caffeine rapidly reduced both brain ISF and plasma levels of Aβ1–40 within several hours, indicating a direct and immediate effect of caffeine on brain Aβ levels. Alternatively, because blood platelets appear to be the primary source of circulating APP and Aβ, it is possible that caffeine was independently lowering plasma Aβ by suppressing Aβ production from blood platelets. The peripheral production of Aβ could certainly be one explanation for the lack of correlations between plasma and brain Aβ — or between plasma Aβ and any behavioral/neurochemical markers evaluated in this study.
A single treatment with caffeine did not affect the half-life of ISF Aβ, demonstrating that caffeine had affected brain Aβ production rather than its elimination. Underscoring this premise, our prior studies [9, 12] show that caffeine affects Aβ production through suppression of both β-secretase (BACE1) and γ-secretase/PS1 expression. Whether or not this caffeine-induced suppression of Aβ production is direct or involves adenosine receptor blockade/mediation is currently unknown and a subject of current research in our laboratories. Acutely, such decreased Aβ production would result in lower soluble Aβ in brain ISF and consequently lower plasma Aβ levels (Figure 1, Caffeine- Short-Term). This is exactly what we observed in AD transgenic mice that were young adults (no brain Aβ deposition), as well as aged Tg mice (robust Aβ deposition). It is noteworthy that even caffeine treatment every fourth day was sufficient to provide a sustained reduction in plasma Aβ levels to aged Tg mice over several months. Thus, the Aβ-reducing effects of caffeine exceed its own half-life, which does not preclude adenosine receptor-mediated effects of caffeine — indeed adenosine receptor activation has been shown to impact gene expression/signal transduction [3].
We recently reported that caffeine, when administered in drinking water from young adulthood through older age, protected APPsw mice from otherwise certain impairment and reduced their brain Aβ levels [9]. These results, suggestive that moderate daily intake of caffeine (the human equivalent of 5 cups of coffee daily) could delay or reduce the risk of AD, underscore epidemiologic studies reporting that caffeine is protective against both AD [7]and cognitive impairment associated with normal aging [5, 6, 8]. In a new study [12], we show that this same caffeine treatment paradigm, when given for two months to cognitively-impaired APPsw mice, reverses their working memory impairment to normal levels. We further report that this 2-month caffeine administration regime, given to aged APPsw mice with pre-existing and substantial Aβ burdens, reduces both soluble and deposited brain Aβ levels — in essence, reversing their brain Aβ neuropathology. Although the mechanism(s) for caffeine-induced reversal of Aβ deposition requires further investigation, we hypothesize that “chronic” caffeine suppresses Aβ production long-term, resulting in consistently lower brain levels of soluble ISF Aβ (Figure 1, Caffeine – Long-Term). This would then induce a flux of insoluble Aβ out of the deposited form and into the soluble form due to dynamic equilibrium. Newly-solubilized Aβ would then be cleared into the plasma via both blood-brain-barrier transport and bulk fluid flow. The dynamic equilibrium between soluble (ISF) and insoluble/deposited Aβ in the brain is highlighted by our earlier work showing that young adult and aged APPsw mice had similar steady-state levels of ISF Aβ, but rapid inhibition of Aβ production resulted in a two-fold longer Aβ half-life in the aged, Aβ deposit-bearing mice [14]. We believe this longer Aβ half-life is caused by solubilization of a portion of the deposited Aβ pool due to the dynamic ISF Aβ ↔ deposited Aβ equilibirum.
In contrast to caffeine’s clear ability to reduce brain Aβ levels through chronic two-month treatment to aged Tg mice, these same mice did not have significantly reduced Aβ levels in their plasma. However, correlation analysis revealed not only a strong inverse correlation between plasma caffeine levels and plasma Aβ levels in these aged Tg mice, but also a differential effect of high vs. low plasma caffeine levels — aged Tg mice with high plasma caffeine levels had significantly reduced plasma Aβ levels compared to those with low plasma caffeine levels. Thus, whether or not a chronic reduction in plasma Aβ levels occurs in aged Tg mice would appear to be dependent on plasma caffeine levels. Our elucidation of the same inverse correlation between plasma caffeine and plasma Aβ in young adult Tg mice given a single caffeine treatment indicates that this relationship transcends: 1) length of caffeine treatment, 2) age of Tg mouse recipient, and 3) whether or not Aβ plaques are present. Indeed, given the fact that plasma caffeine concentrations in both age groups were well within physiologic range, it would not be surprising for a similar inverse relationship between plasma caffeine and plasma Aβ to be present in humans. In the context that caffeine suppresses γ-secretase [9, 12], the reduced plasma Aβ1–40 levels seen in our aged APPsw mice with higher plasma caffeine levels is consistent with the reported reduction of plasma Aβ levels in AD patients following a similar 6 week treatment with a γ-secretase inhibitor [29].
For aged APPsw mice, strong correlations were evident between Aβ1–40 and Aβ1–42 levels (both soluble and insoluble) in hippocampus and cortex irrespective of caffeine treatment. Moreover, we have found brain Aβ levels from aged APPsw mice to be strongly correlated with cognitive impairment [12, 23–25]. Thus, our prior work has established an intimate and presumably causative association between “brain” Aβ levels and cognitive impairment. In sharp contrast, results from the present study’s aged APPsw mice clearly show that no such relationship exists between “plasma” Aβ levels and cognitive performance. Furthermore, plasma Aβ levels in these mice were not correlated with any of eight brain Aβ measures. We conclude that, at least in aged AD transgenic mice, plasma Aβ levels are not an accurate index of soluble or insoluble brain Aβ levels, nor are they an accurate index of cognitive performance. This conclusion is consistent with a previous study in old APP transgenic mice [30], as well as a recent report showing that plasma Aβ levels in AD patients did not correlate with Aβ levels measured in neocortex following death [31] nor with brain Aβ burden as measured in vivo with amyloid imaging [32]. As with plasma Aβ, we also found no correlations between plasma caffeine concentrations and brain Aβ levels or cognitive performance. Thus, higher plasma caffeine levels were associated with lower plasma Aβ levels, but were not reflective of brain Aβ levels or cognitive performance.
From this report’s studies involving several AD transgenic lines, it is apparent that baseline plasma levels of Aβ remain relatively stable during aging and irrespective of APPsw vs. APPsw+PS1 transgenicity (Figures 2 and 4). This finding suggests either close regulation of plasma Aβ in AD transgenic mice or limited/saturable Aβ transport into the blood. Regarding the latter, we propose that during aging in AD Tg mice, there is a limit to the amount of Aβ that can be transported out of the brain and into plasma. After this limited transport capacity is reached, any additional Aβ would remain in the brain and “stored” in Aβ deposits through the dynamic Aβ equilibrium present with ISF Aβ (Figure 1).
The ability of plasma Aβ levels to be predictive of impending AD cognitive impairment in humans is currently unresolved, with some studies reporting that high plasma Aβ1–40 levels are indicative of increased AD risk [33] and others showing no predictive ability of plasma Aβ for progression to AD [34]. Results from the present study are consistent with the later of these assertions in that baseline plasma Aβ levels remained essentially unchanged during aging in our inbred AD transgenic mice (compare control Aβ levels in Figure 2A,B, D, and F) , yet these mice develop robust brain Aβ pathology and become cognitively impaired as they age. The question nonetheless remains: Are studies involving the predictive value of plasma Aβ equivocal because the assays being utilized are inadequate, or is plasma Aβ simply just not a good biomarker for AD? Indeed, some laboratories are focusing on signaling proteins and intercellular communication factors, rather than plasma Aβ, as potential biomarkers for AD. A notable recent study employing this strategy elucidated a set of 18 signaling proteins in plasma that collectively were highly accurate in classifying subjects as aged normal or AD [35].
Our measurement of plasma caffeine and theophylline levels from aged Tg mice given 2 months of caffeine treatment underscore the human relevance and physiologic significance of the caffeine treatment in this long-term study. At the caffeine concentration in drinking water that was employed, plasma caffeine levels averaged 26 µM — equivalent to the plasma caffeine concentration expected in humans following intake of several cups of coffee [3]. This caffeine concentration is very close to the 20 µM concentration that we found to be optimal in our N2a cell culture studies for suppression of Aβ1–40 and Aβ1–42 production, although caffeine concentrations in the much lower nM range were also effective [9]. Predictably, plasma theophylline levels were tightly correlated with plasma caffeine levels. As with plasma caffeine levels, however, no correlations where evident between plasma theophylline and plasma Aβ or cognitive performance. As one of several active metabolites of caffeine, theophylline may be responsible for a significant portion of the cognitive, neuropathologic, and neurochemical benefits of caffeine. This premise is underscored by the fact that caffeine metabolites such as theophylline have a considerably longer half-life than caffeine. Indeed, brain concentrations of theophylline in mice following long-term caffeine ingestion are usually higher than those of caffeine [36]. We are currently exploring the contribution of such caffeine metabolites to the benefits currently being attributed to caffeine in our AD transgenic mice.
No correlations were found between plasma Aβ levels and plasma/brain cytokine levels, suggesting their independence of one another. Although plasma caffeine levels were also not correlated with plasma cytokines, a clear reduction in hippocampal inflammatory cytokines was evident for caffeine-treated mice with higher plasma caffeine levels compared to those with lower caffeine levels. This was not only also true for plasma theophylline levels, but higher plasma theophylline levels were strongly and consistently correlated with lower inflammatory cytokine levels in both hippocampus and cortex — these theophylline findings suggest a more profound interaction between this caffeine metabolite and brain cytokines than caffeine itself, perhaps due to the aforementioned higher brain concentrations of theophylline [36]. The ability of caffeine and theophylline to provide beneficial anti-inflammatory effects in the brain of AD transgenic mice are consistent with a large body of data supportive of their anti-inflammatory capacities [20] and, as such, could represent a potent “non-amyloidogenic” mechanism of caffeine action that may contribute to its ability to protect against/reverse cognitive impairment and synaptic dysfunction. Other such beneficial mechanisms of caffeine action in AD mice (and perhaps in human AD) include caffeine’s antioxidant actions [37], its ability to block disruptions of the blood-brain barrier [38], and its well-established antagonism of brain A1 and/or A2a adenosine receptors [1–2, 39,40] — indeed, adenosine receptor antagonism may be central to most or all of caffeine’s beneficial actions against AD and any combination of “caffeinergic” actions may collectively provide the cognitive benefits we have documented in AD transgenic mice, with.
Anti-Alzheimer’s drugs currently on the market (cholinesterase inhibitors and NMDA receptor antagonists) have minimal benefits and do not appear to address AD pathogenesis. Although caffeine is the most widely consumed psychoactive agent in the world, its intake in Western countries decreases substantially during aging. Given that: 1) blood caffeine concentrations equilibrate almost instantaneously with brain caffeine levels [3], 2) caffeine appears to suppress AD pathogenesis [9, 12], 3) caffeine has few, if any, deleterious side effects for most individuals [10], and 4) caffeine is readily available and inexpensive, therapeutic actions of this psychoactive agent against AD could provide immediate benefits. Based on the robust protective [9] and treatment effects [12] of caffeine that we have observed in AD transgenic mice, Phase II clinical trials involving acute caffeine administration are currently in progress. To our knowledge, no prior studies have investigated the effect of acute or chronic caffeine administration on plasma biomarkers related to AD. It is important to note that this report’s acute caffeine administration studies involved AD transgenic mice “naïve” to caffeine, whereas clinical trials involve many occasional or habitual caffeine users. Therefore, it is difficult to predict the impact of acute caffeine administration in these ongoing human trials.
To summarize, the present report demonstrates that caffeine can acutely decrease brain and plasma Aβ levels, as well as reduce brain Aβ deposition and improve cognitive function following chronic administration. As such, caffeine appears to have excellent potential as a safe and effective therapeutic against AD. We also provide evidence from multiple studies and methodologies indicating that plasma Aβ is not an accurate index of brain Aβ levels/deposition or cognitive performance in aged AD mice.
Acknowledgements
This research was supported by grants to G.A. and H.P. within the NIA-designated Florida Alzheimer’s Disease Research Center (P50AG025711), AG029524 (JRC), AG13956 (DMH), Cure Alzheimer’s Fund (DMH and JRC), and funds from the Byrd Alzheimer’s Center & Research Institute.
References
- 1.Fisone G, Borgkvist A, Usiello A. Caffeine as a psychomotor stimulant: mechanism of action. Cell Mol Life Sci. 2004;61:857–872. doi: 10.1007/s00018-003-3269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daly J. Caffeine analogs: biomedical impact. Cell Mol Life Sci. 2007;64:2153–2169. doi: 10.1007/s00018-007-7051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fredholm B, Bättig K, Holmén J, Nehlig A, Zvartau E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 4.Higdon J, Frei B. Coffee and health: a review of recent human research. Crit Rev Food Sci Nutr. 2006;46:101–123. doi: 10.1080/10408390500400009. [DOI] [PubMed] [Google Scholar]
- 5.Ritchie K, Carrière I, de Mendonca A, Portet F, Dartigues J, Rouaud O, Barberger-Gateau P, Ancelin M. The neuroprotective effects of caffeine: a prospective population study (the Three City Study) Neurology. 2007;69:536–545. doi: 10.1212/01.wnl.0000266670.35219.0c. [DOI] [PubMed] [Google Scholar]
- 6.van Gelder B, Buijsse B, Tijhuis M, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Coffee consumption is inversely associated with cognitive decline in elderly European men: the FINE Study. Eur J Clin Nutr. 2007;61:226–232. doi: 10.1038/sj.ejcn.1602495. [DOI] [PubMed] [Google Scholar]
- 7.Maia L, de Mendonça A. Does caffeine intake protect from Alzheimer’s disease? Eur J Neurol. 2002;9:377–382. doi: 10.1046/j.1468-1331.2002.00421.x. [DOI] [PubMed] [Google Scholar]
- 8.Rosso A, Lippa C, Mossey J. Caffeine: Neuroprotective Functions in Cognition and Alzheimer""s Disease. Am J Alzheimers Dis Other Demen. 2008 doi: 10.1177/1533317508320083. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arendash G, Schleif W, Rezai-Zadeh K, Jackson E, Zacharia L, Cracchiolo J, Shippy D, Tan J. Caffeine protects Alzheimer's mice against cognitive impairment and reduces brain beta-amyloid production. Neuroscience. 2006;142:941–952. doi: 10.1016/j.neuroscience.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Nawrot P, Jordan S, Eastwood J, Rotstein J, Hugenholtz A, Feeley M. Effects of caffeine on human health. Food Addit Contam. 2003;20:1–30. doi: 10.1080/0265203021000007840. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Garcia E, van Dam R, Li T, Rodriguez-Artalejo F, Hu F. The relationship of coffee consumption with mortality. Ann Intern Med. 2008;148:904–914. doi: 10.7326/0003-4819-148-12-200806170-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arendash G, Mori T, Cao C, Mamcarz M, Runfeldt M, Dickson A, Rezai-Zadeh K, Tan J, Citron B, Lin X, Echeverria V, Potter H. Caffeine reverses cognitive impairment and decreases brain Aβ levels in aged Alzheimer’s mice. 2008. Submitted for publication. [DOI] [PubMed] [Google Scholar]
- 13.Bateman R, Munsell L, Morris J, Swarm R, Yarasheski K, Holtzman D. Human amyloid-beta synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat Med. 2006;12:856–861. doi: 10.1038/nm1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cirrito J, May P, O'Dell M, Taylor J, Parsadanian M, Cramer J, Audia J, Nissen J, Bales K, Paul S, DeMattos R, Holtzman D. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J Neurosci. 2003;23:8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cirrito J, Kang J, Lee J, Stewart F, Verges D, Silverio L, Bu G, Mennerick S, Holtzman D. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ethell D, Shippy D, Cao C, Cracchiolo J, Runfeldt M, Blake B, Arendash G. Abeta-specific T-cells reverse cognitive decline and synaptic loss in Alzheimer's mice. Neurobiol Dis. 2006;23:351–361. doi: 10.1016/j.nbd.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Cracchiolo J, Mori T, Nazian S, Tan J, Potter H, Arendash G. Enhanced cognitive activity--over and above social or physical activity--is required to protect Alzheimer's mice against cognitive impairment, reduce Abeta deposition, and increase synaptic immunoreactivity. Neurobiol Learn Mem. 2007;88:277–294. doi: 10.1016/j.nlm.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mori T, Town T, Tan J, Yada N, Horikoshi Y, Yamamoto J, Shimoda T, Kamanaka Y, Tateishi N, Asano T. Arundic Acid ameliorates cerebral amyloidosis and gliosis in Alzheimer transgenic mice. J Pharmacol Exp Ther. 2006;318:571–578. doi: 10.1124/jpet.106.105171. [DOI] [PubMed] [Google Scholar]
- 19.Tan J, Town T, Crawford F, Mori T, DelleDonne A, Crescentini R, Obregon D, Flavell R, Mullan M. Role of CD40 ligand in amyloidosis in transgenic Alzheimer's mice. Nat Neurosci. 2002;5:1288–1293. doi: 10.1038/nn968. [DOI] [PubMed] [Google Scholar]
- 20.Horrigan L, Kelly J, Connor T. Caffeine suppresses TNF-alpha production via activation of the cyclic AMP/protein kinase A pathway. Int Immunopharmacol. 2004;4:1409–1417. doi: 10.1016/j.intimp.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Cirrito J, Yamada K, Finn M, Sloviter S, Bales K, May P, Schoepp D, Paul S, Mennerick S, Holtzman D. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 22.Arendash G, Gordon M, Diamond D, Austin L, Hatcher J, Jantzen P, DiCarlo G, Wilcock D, Morgan D. Behavioral assessment of Alzheimer's transgenic mice following long-term Abeta vaccination: task specificity and correlations between Abeta deposition and spatial memory. DNA Cell Biol. 2001;20:737–744. doi: 10.1089/10445490152717604. [DOI] [PubMed] [Google Scholar]
- 23.Arendash G, King D, Gordon M, Morgan D, Hatcher J, Hope C, Diamond D. Progressive, age-related behavioral impairments in transgenic mice carrying both mutant amyloid precursor protein and presenilin-1 transgenes. Brain Res. 2001;891:42–53. doi: 10.1016/s0006-8993(00)03186-3. [DOI] [PubMed] [Google Scholar]
- 24.Leighty R, Nilsson L, Potter H, Costa D, Low M, Bales K, Paul S, Arendash G. Use of multimetric statistical analysis to characterize and discriminate between the performance of four Alzheimer's transgenic mouse lines differing in Abeta deposition. Behav Brain Res. 2004;153:107–121. doi: 10.1016/j.bbr.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Arendash G, Jensen M, Salem N, Hussein N, Cracchiolo J, Dickson A, Leighty R, Potter H. A diet high in omega-3 fatty acids does not improve or protect cognitive performance in Alzheimer's transgenic mice. Neuroscience. 2007;149:286–302. doi: 10.1016/j.neuroscience.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 26.Tuppo E, Arias H. The role of inflammation in Alzheimer's disease. Int J Biochem Cell Biol. 2005;37:289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Kandimalla K, Curran G, Holasek S, Gilles E, Wengenack T, Poduslo J. Pharmacokinetic analysis of the blood-brain barrier transport of 125I–amyloid beta protein 40 in wild-type and Alzheimer's disease transgenic mice (APP,PS1) and its implications for amyloid plaque formation. J Pharmacol Exp Ther. 2005;313:1370–1378. doi: 10.1124/jpet.104.081901. [DOI] [PubMed] [Google Scholar]
- 28.Saido T. Aβ Metabolism and Alzheimer’s Disease (Landes Bioscience) 2003 [Google Scholar]
- 29.Siemers E, Quinn J, Kaye J, Farlow M, Porsteinsson A, Tariot P, Zoulnouni P, Galvin J, Holtzman D, Knopman D, Satterwhite J, Gonzales C, Dean R, May P. Effects of a gamma-secretase inhibitor in a randomized study of patients with Alzheimer disease. Neurology. 2006;66:602–604. doi: 10.1212/01.WNL.0000198762.41312.E1. [DOI] [PubMed] [Google Scholar]
- 30.DeMattos R, Bales K, Cummins D, Paul S, Holtzman D. Brain to plasma amyloid-beta efflux: a measure of brain amyloid burden in a mouse model of Alzheimer's disease. Science. 2002;295:2264–2267. doi: 10.1126/science.1067568. [DOI] [PubMed] [Google Scholar]
- 31.Freeman S, Raju S, Hyman B, Frosch M, Irizarry M. Plasma Abeta levels do not reflect brain Abeta levels. J Neuropathol Exp Neurol. 2007;66:264–271. doi: 10.1097/NEN.0b013e31803d3ae4. [DOI] [PubMed] [Google Scholar]
- 32.Fagan A, Mintun M, Mach R, Lee S, Dence C, Shah A, LaRossa G, Spinner M, Klunk W, Mathis C, DeKosky S, Morris J, Holtzman D. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 33.van Oijen M, Hofman A, Soares H, Koudstaal P, Breteler M. Plasma Abeta(1–40) and Abeta(1–42) and the risk of dementia: a prospective case-cohort study. Lancet Neurol. 2006;5:655–660. doi: 10.1016/S1474-4422(06)70501-4. [DOI] [PubMed] [Google Scholar]
- 34.Hansson O, Zetterberg H, Blennow K. Evaluation of plasma Abeta40 and Abeta42 as predictors of conversion to Alzheimer's disease in patients with mild cognitive impairment. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.03.027. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 35.Ray S, Britschgi M, Herbert C, Takeda-Uchimura Y, Boxer A, Blennow K, Friedman L, Galasko D, Jutel M, Karydas A, Kaye J, Leszek J, Miller B, Minthon L, Quinn J, Rabinovici G, Robinson W, Sabbagh M, So Y, Sparks D, Tabaton M, Tinklenberg J, Yesavage J, Tibshirani R, Wyss-Coray T. Classification and prediction of clinical Alzheimer's diagnosis based on plasma signaling proteins. Nat Med. 2007;13:1359–1362. doi: 10.1038/nm1653. [DOI] [PubMed] [Google Scholar]
- 36.Johansson B, Georgiev V, Kuosmanen T, Fredholm B. Long-term treatment with some methylxanthines decreases the susceptibility to bicuculline- and pentylenetetrazol-induced seizures in mice. Relationship to c-fos expression and receptor binding. Eur J Neurosci. 1996;8:2447–2458. doi: 10.1111/j.1460-9568.1996.tb01539.x. [DOI] [PubMed] [Google Scholar]
- 37.Azam S, Hadi N, Khan N, Hadi S. Antioxidant and prooxidant properties of caffeine, theobromine and xanthine. Med Sci Monit. 2003;9:BR325–BR330. [PubMed] [Google Scholar]
- 38.Chen X, Gawryluk J, Wagener J, Ghribi O, Geiger J. Caffeine blocks disruption of blood brain barrier in a rabbit model of Alzheimer's disease. J Neuroinflammation. 2008;5:12. doi: 10.1186/1742-2094-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dall’Igna O, Fett P, Gomes M, Souza D, Cunha R, Lara D. Caffeine and adenosine A(2a) receptor antagonists prevent beta-amyloid (25–35)-induced cognitive deficits in mice. Exp Neurol. 2007;203:241–245. doi: 10.1016/j.expneurol.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Cunha G, Canas P, Melo C, Hockemeyer J, Müller C, Oliveira C, Cunha R. Adenosine A2A receptor blockade prevents memory dysfunction caused by beta-amyloid peptides but not by scopolamine or MK-801. Exp Neurol. 2008;210:776–781. doi: 10.1016/j.expneurol.2007.11.013. [DOI] [PubMed] [Google Scholar]