Abstract
Cellular stress or injury can result in mitochondrial dysfunction, which has been linked to many chronic neurological disorders including amyotrophic lateral sclerosis (ALS) and Parkinson’s disease (PD). Stressed and dysfunctional mitochondria exhibit an increase in large conductance mitochondrial membrane currents and a decrease in bioenergetic efficiency. Inefficient energy production puts cells, and particularly neurons, at risk of death when energy demands exceed cellular energy production. Here we show that the candidate ALS drug dexpramipexole (DEX; KNS-760704; ((6R)-4,5,6,7-tetrahydro-N6-propyl-2,6-benzothiazole-diamine) and cyclosporine A (CSA) inhibited increases in ion conductance in whole rat brain-derived mitochondria induced by calcium or treatment with a proteasome inhibitor, although only CSA inhibited calcium-induced permeability transition in liver-derived mitochondria. In several cell lines, including cortical neurons in culture, DEX significantly decreased oxygen consumption while maintaining or increasing production of adenosine triphosphate (ATP). DEX also normalized the metabolic profile of injured cells and was protective against the cytotoxic effects of proteasome inhibition. These data indicate that DEX increases the efficiency of oxidative phosphorylation, possibly by inhibition of a CSA-sensitive mitochondrial conductance.
Keywords: leak conductance, mitochondria, bioenergetics, neural metabolism, patch clamp electrophysiology
1. Introduction
Mitochondrial dysfunction has been implicated in ALS and other chronic neurodegenerative disorders, including changes in the structural integrity of mitochondria, disruption of energy metabolism and abnormal calcium (Ca2+) buffering (Beal, 2007; Dodson and Guo, 2007; Jordan et al., 2003; Kawamata and Manfredi, 2010; Pedrini et al., 2010; Perry et al., 2010; Sasaki and Iwata, 1996; Swerdlow et al., 1998). This suggests that when critical proportions of mitochondria in specific neuronal populations become dysfunctional, neurons are put at risk. Necessary functions are compromised and death may result if ATP synthesis cannot match cellular energy requirements (Hinerfeld et al., 2004; Nicholls, 2008). In this study we present evidence of enhancement of the efficiency of oxidative phosphorylation by the candidate ALS drug dexpramipexole (DEX; KNS-760704; (6R)-4,5,6,7-tetrahydro-N6-propyl-2,6-benzothiazole-diamine) (Gribkoff and Bozik, 2008). DEX is a neuroprotective drug previously suggested to slow ALS disease progression in an open label clinical trial (Wang et al., 2008a). It is safe and well-tolerated at doses that should produce pharmacodynamically effective concentrations in the CNS (Bozik et al.), and it demonstrates unprecedented preliminary evidence of drug activity, including reduction of functional decline and decreased mortality in a recent Phase 2 study in subjects with ALS (Bozik, 2009).
DEX is the non-dopaminergic R(+) enantiomer of the dopamine agonist and Parkinson’s disease therapeutic pramipexole (Mirapex®; (6S)-4,5,6,7-tetrahydro-N6-propyl-2,6-benzothiazole-diamine) (Gribkoff and Bozik, 2008). Pramipexole is neuroprotective by a non-dopaminergic mechanism in in vitro and in vivo models of cell death and neurodegenerative diseases (NDDs), but only at higher concentrations (≥10μM) than would be tolerated in humans (Abramova et al., 2002; Ferrari-Toninelli et al., 2010; Iravani et al., 2006; Le et al., 2000). DEX is equally protective and is tolerated at clinical doses which allow its use as a neuroprotective drug (Bozik et al.). Both enantiomers are believed to exert their neuroprotective potential at the level of the mitochondrion (Danzeisen et al., 2006; Ferrari-Toninelli et al., 2010), but their mitochondrial effects have not been well characterized..
Mitochondria produce adenosine triphosphate (ATP) by oxidative phosphorylation. Membrane currents in mitochondria can reduce the efficiency of this process by uncoupling the electron transport system from oxidative phosphorylation. Mitochondrial membrane ion channels participate in the initiation of cell death in conditions such as neuronal trauma and hypoxia/ischemia (Bernardi and Rasola, 2007; Bonanni et al., 2006; Dejean et al., 2005; Jonas, 2009; Miyawaki et al., 2008), and may play a major role in controlling the daily metabolic health of cells by governing the efficiency of oxidative phosphorylation (Lemasters, 2007; Mannella and Kinnally, 2008; Nowikovsky et al., 2009). We have described recently that the anti-apoptotic protein Bcl-xL regulates mitochondrial membrane potential by decreasing an inner membrane leak conductance associated with the ATP synthase (Alavian, 2011; Chen, 2011). We find that these Bcl-xL-associated changes in membrane properties directly increase bioenergetic efficiency of neurons and enhance cell survival (Alavian, 2011). In dysfunctional neuronal mitochondria aberrant membrane currents may contribute to the pathology associated with chronic NDDs such as ALS (Nicholls, 2008). Recently, pramipexole was shown to inhibit membrane currents induced by high calcium (Ca2+) concentrations, a mitochondrial stressor, in rat liver mitoplasts (Sayeed et al., 2006). In the current study we found that DEX and cyclosporine A (CSA) inhibited stress-induced membrane currents in whole brain-derived mitochondria. Profiling the bioenergetics of neurons and other cultured cells, DEX decreased oxygen consumption rates while maintaining or enhancing ATP levels; therefore inhibition of a pathological mitochondrial conductance may produce the observed enhancements of bioenergetic efficiency that underlies the cellular protective effects of DEX.
2. RESULTS
2.1 PSI pre-treatment of rats produced currents in isolated brain mitochondria that were inhibited by DEX and CSA
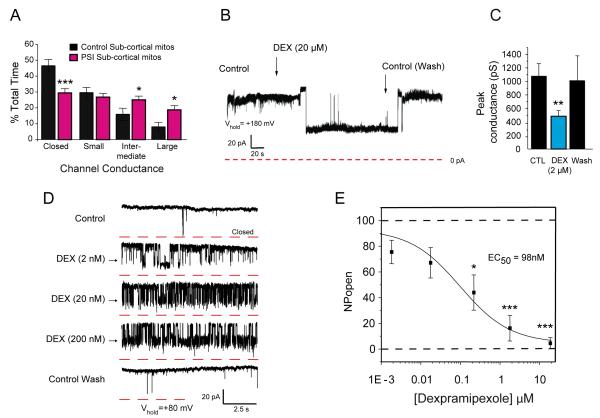
To model changes in aberrant protein accumulation that may lead to chronic neuronal mitochondrial dysfunction we exposed rats in vivo to a proteasome inhibitor (carbobenzoxyl-Ile-Glu(O-t-butyl)-Ala-leucinal; PSI) (McNaught et al., 2004). We previously developed a technique to patch clamp the outer membrane of mitochondria inside living neurons (Jonas et al., 1997). These techniques, used here, were later modified to record from the outer membrane of isolated whole mitochondria (Bonanni et al., 2006; Miyawaki et al., 2008), and are similar to techniques described by others (Kinnally et al., 1987; Kinnally et al., 2006; Lohret et al., 1997; Pavlov et al., 2001). Studies of ischemic injured neurons have shown increased levels of intermediate- (200-750 pS) and large- (>750 pS) conductance channel activity in whole-organelle recordings of isolated mitochondria (Bonanni et al., 2006; Miyawaki et al., 2008). Therefore patch clamp recordings were made from whole mitochondria isolated from a subcortical brain fraction after repeated dosing of rats with the proteasome inhibitor PSI (PSI-mitochondria, see Experimental Procedure). Recordings of PSI-mitochondria incubated in the absence of added Ca2+ exhibited intermediate- and large-conductance channel activity at a significantly higher frequency than recordings from control mitochondria (Fig. 1A).
FIGURE 1.
DEX inhibited PSI-induced currents in brain-derived mitochondria. A. Histograms show the % total time that patches displayed specified conductance levels in all recorded traces (n=190 recordings from a total of 10 PSI-exposed sub-cortical mitochondria, n=83 recordings from a total of 9 vehicle (DMSO)-exposed sub-cortical mitochondria). Conductance level frequencies for PSI-dosed mitochondria compared with the comparable level in non-PSI-dosed mitochondria, using 2-tailed unpaired t-tests; p=0.0005 for closed PSI-mitochondria compared to closed control, p=0.0426 for intermediate conductance PSI-mitochondria compared to intermediate control, p=0.0192 for large PSI-mitochondrial conductance compared to large control. In all figures, the specific analyses performed and unadjusted or adjusted p values connoting significance are presented in the figure legend. The general level of significance obtained when comparing 2 groups, including pre-planned post hoc comparisons, is indicated on any figure by the number of asterisks above or below the mean value; *=p<0.05, **=p<0.01, ***=p<0.0001.–B. Example of a continuous patch clamp recording from a PSI-mitochondrion before and after bath application of DEX. C. Group data showing peak conductance recorded from mitochondria isolated from subcortex of PSI-injected rats (n=15 mitochondria, except for wash, where n=6 mitochondria). 2-tailed paired t-test, p=0.0352. The wash was not included in the analysis. D. Intermediate-conductance (~500pS) channels recorded from PSI mitochondria before and after DEX and after wash at the indicated concentrations. Holding potential = + 80 mV. Note that in this example many closures reveal sub-conductance states. Sample recordings were obtained at steady-state for each condition. E. Mean inhibitory effect of different concentrations of DEX on NPo, recorded in brain-derived PSI-mitochondria (n=5 mitochondria for all except 20μM, where n=9). One-way ANOVA, p=.000014; pre-planned post hoc comparisons, Bonferroni corrected t-tests, p=0.0455 for 200nM DEX, p=0.00038 for 2μM DEX, p=0.0005 for 20μM DEX; EC50=98nM by logistic fit; Hill slope, nH<1.
DEX inhibited intermediate- and large-conductance channel activity of PSI-mitochondria; the inhibition was concentration-dependent and reversible (Fig. 1B,1C,1D). The decrease in the mean open probability (NPo) of discrete single channel ion currents had an estimated EC50 of 98nM (Fig. 1E). The full effect of the drug could take up to several minutes, and washout followed an approximately equal time course, although in some cases the onset and washout were quite rapid. The concentration-response curve (CRC) was shallow, with a Hill slope <1; full inhibition of PSI-induced aberrant currents (EC100) required DEX concentrations >10μM, which we surmise may have consequences for the potency of the drug in cellular systems (see below).
Similar to pramipexole (Sayeed et al., 2006), DEX may inhibit the pore-forming protein complex known as the mitochondrial permeability transition pore (mPTP) (Bernardi, 1992; Sayeed et al., 2006). The mPTP participates in the initiation of some forms of cell death, although its roles in long term changes in mitochondrial function are likely to be different from its roles in more acute phenomena (Crompton, 1999; Nicholls and Chalmers, 2004). Addition of Ca2+ to isolated mitochondria activates the mPTP (see below); cyclosporine A (CSA) specifically inhibits it (Szabo and Zoratti, 1991; Szabo et al., 1992). CSA effectively blocked channel activity in PSI-mitochondria and significantly decreased the peak membrane conductance (Fig. 2A). Currents sensitive to DEX were equally sensitive to CSA after DEX washout. DEX-sensitive currents in PSI-mitochondria therefore share features with the mPTP, such as inhibition by CSA.
FIGURE 2.
CSA and DEX have similar effects on brain mitochondrial currents, but not on permeability transition in liver mitochondria. A. (Left) Bar graph of mean level of inhibition of peak conductance by 1μM CSA in recordings from PSI-mitochondria (n=7 mitochondria); p=0.0003, paired t-test. (Right) Bar graph of the mean inhibition of peak conductance by 200nM DEX in PSI-mitochondria, wash (>5 min.) and1.0μM CSA (n=3 mitochondria). B. (Left) Bar chart of the mean effect of 100μM Ca2+ on peak membrane conductance (in pS) (n=14 mitochondria); p=0.0092, 2-tailed paired t-test. (Right) Bar chart of the mean inhibition of peak conductance (in pS) by 20μM DEX in the continued presence of Ca2+ (n=7 mitochondria); p=0.0094, 2-tailed paired t-test; n=4 for wash; the wash was not included in the analyses. C. Optical absorbance of fresh, respiring rat liver mitochondria (measured respiratory control ratio >5) before and during Ca2+-induced permeability transition, and its amerlioration by LiCl and CSA, but not DEX. Each point represents the mean±SEM for the group at each time point; n≥12 wells for each condition.
2.2 DEX inhibited outer membrane ion channel conductances in [Ca2+]-treated brain-derived mitochondria
To determine if DEX also modulates mitochondrial ion channel activity during Ca2+-induced mitochondrial stress, an initiator of mPTP activity, isolated control rat brain mitochondria were challenged with high [Ca2+] or with high [Ca2+] followed by DEX. Addition of 100 μM Ca2+resulted in an increase in intermediate- and large-conductance currents recorded under whole-organelle patch clamp. DEX (2 or 20μM, in 100μM Ca2+ (Fig. 2B), effectively decreased these Ca2+-induced mitochondrial membrane currents. These data demonstrate that DEX also potently inhibited Ca2+-induced ion conductance in mitochondria.
2.3 DEX did not inhibit Ca2+-induced mitochondrial transition in liver-derived mitochondria
Unlike a previous report using pramipexole (Sayeed et al., 2006), we found no effect of DEX (or pramipexole) on optically recorded permeability transition in liver mitochondria, following addition of high (50-100μM) external Ca2+. In these experiments, known inhibitors of permeability transition, including CSA and lithium chloride (LiCl) (Azzolin et al.; Shalbuyeva et al., 2007), were effective at blocking Ca2+-induced mitochondrial permeability transition (Fig. 2C). In brain mitochondria, therefore, DEX-sensitive currents may have another role apart from permeability transition in mitochondrial function, such as the regulation of cellular metabolism.
2.4 DEX enhanced metabolic efficiency of cultured cells
We had recently reported that the anti-apoptotic protein Bcl-xL regulates inner membrane properties and cellular bioenergetics (Alavian, 2011; Chen, 2011). We therefore undertook to determine if DEX altered metabolic function in neurons and other cell types. Unlike CSA, DEX has no known cellular effects other than its mitochondrial modulation, and is nontoxic even at high concentrations in vitro. Consistent with a role in metabolism, DEX (10μM) increased ATP levels in cultured hippocampal neurons (DIV 14) by 11% compared to vehicle-treated cultures (Fig. 3A). Under basal conditions, mitochondrial oxygen uptake contributes both to the production of ATP by oxidative phosphorylation and to counteracting inner membrane ‘leak’ which decreases coupling between the electron transport system and ATP production (Andrews et al., 2005; Brand, 2005). To determine if oxygen uptake was altered by DEX, oxygen flux was measured from single hippocampal neurons using a self-referencing oxygen-sensitive electrode (Alavian, 2011; Gleichmann et al., 2009; Land et al., 1999) (Fig.3B,3C,3D). Single neuron measurements target the cell of interest, and self-referencing electrodes improve the signal:noise ratio compared to fluorescence-based techniques (Gerencser et al., 2009).The average oxygen flux of control neurons was stable over 5 min. Acute application of 10μM DEX lowered average oxygen flux by ~16% to a new stable level after 1-5 min (Fig. 3C,3D). These data, in conjunction with the increased ATP levels in identically treated neurons, suggest that DEX-treated neurons couple ATP production more efficiently to oxygen uptake.
FIGURE 3.
DEX modulation of cellular bioenergetics. A. Effect of DEX (10μM, 12 hr. incubation prior to measurements) on ATP levels, measured in a luciferase assay in cultured hippocampal neurons (n=21 wells each condition, 2 independent cultures, p=0.0024, unpaired t-test). B. Image of an oxygen-sensitive electrode placed in position to record oxygen uptake by a single cultured hippocampal neuron. Shown are the recording and self-referencing positions of the electrode. Scale bar with arrow represents 10μm; measurement of oxygen level in proximity of neurons occurred ~1-2μM from cell surface and the reference point was 10-12μM away from cell surface. C. Single self-referencing oxygen electrode recording from a cultured hippocampal neuron after addition of DEX (10μM) or an equivalent volume of water to the bath. D. Histogram of group data (mean ± SEM) for recordings as in C before and after addition of DEX (10μM; n=14 neurons) or an equivalent volume of water (n=6 neurons). Unpaired t-test, p≤0.0001. E. Effect of DEX on OCR on rat cortical cultures exposed to digitonin(10μg/ml) and rotenone (100nM) (45 min.) followed by succinate and ADP injection. Oxygen consumption rate (OCR) was measured by a Seahorse® flux analyzer. OCR at baseline (i.), 30μM DEX (n=10 wells) or vehicle injection (n=10 wells) (ii.), 10mM succinate (iii.), 1mM ADP (iv.) and 10μM antimycin A (v.). 10 wells/treatment. F. Group histograms illustrating the effect of DEX on OCR under the indicated conditions relative to control values ± SEM. For succinate, p=0.1211 (2-tailed t-test). For ADP injection, p=0.0001 (2-tailed t-test). Primary rat cortical cultures, from several separate isolations and multiple independent plates, were pretreated with DEX (30μM; n=95 wells) or control medium (n=94 wells) for 1 hr., then incubated with digitonin (10μg/ml), rotenone (100nM), and DEX (30μM) prior to OCR measurements. G. Initial slope of ATP production following ADP addition in isolated cortical neurons in absence and presence of 30μM DEX. Third bar indicates ATP level after antimycin A (AMA) addition at end of experiment. H. Maximal level of ATP production for experiment shown in G. I. Citrate synthase levels in absence and presence of 30μM DEX after the flux experiments in F (n=17 wells/treatment arm).
To further study the effects of DEX on neurons, oxygen consumption rate (OCR) was studied in digitonin-permeabilized primary cortical neurons (9 DIV) using a Seahorse® multi-well flux analyzer. When permeabilized neurons were incubated in control medium containing the complex I inhibitor rotenone (Fig. 3Ei), acute application of DEX (30μM) did not affect the already low levels of basal respiration (Fig. 3Eii). In control cells, addition of the complex II substrate succinate produced a significant increase in OCR (Fig. 3Eiii); following a pulse of ADP (see Methods), OCR was further increased (Fig. 3Eiv). In contrast, however, in DEX-treated cells, the increase in OCR produced by succinate and following the ADP pulse was less than in control cells (Fig. 3Eiii,3Eiv), consistent with the single neuron oxygen recordings. In other experiments, when DEX was applied to digitonin-treated cortical neurons 1 hr. prior to the onset of recording, cells likewise had lower OCR during succinate incubation, and significantly lower OCR following an ADP pulse, relative to controls (Fig. 3F). To control for possible changes in cell number, mitochondrial biomass, or mitochondrial integrity after DEX treatment, measurements of the initial slope of ATP synthesis following an ADP pulse, maximal ATP levels, and citrate synthase activity were studied and were found to be unchanged (Fig. 3G,3H,3I).
Incubation of SH-SY5Y neuroblastoma cells in DEX (1-100μM; 24 hr), also significantly increased ATP levels relative to control values, with maximal group increases of ~18% (Fig. 4). DEX (30μM) also modestly decreased basal OCR in these cells. To determine whether the changes in ATP or oxygen consumption were related to an increase in glycolysis, SH-SY5Y cells were incubated in galactose, a sugar that exclusively requires mitochondrial (rather than glycolytic) metabolism to produce ATP. Cells treated with DEX in galactose-containing medium had similar DEX-induced increases in ATP levels, suggesting that the effects of DEX on cellular ATP levels were mediated via effects on mitochondrial metabolism (Fig. 4).
FIGURE 4.
Dexpramipexole enhances mitochondrial metabolism in cells grown in a medium where glucose is replaced with galactose. SH-SY5Y neuroblastoma cells were exposed to the indicated concentrations of dexpramipexole for 24 hr. before measurement of ATP levels by a luciferin-luciferase assay. Data shown represent mean±SEM. Statistical analyses: 1-Way-ANOVA, p=0.0001 for both normal and galactose medium; pre-planned post hoc comparisons (Bonferroni corrected t-tests) for normal medium and galactose medium, respectively, p=0.0068 and p=0.0018 for 1 μM, p=0.0013 and p=0.0039 for 3 μM, p=0.0001 (galactose) for 10 μM, p=0.0009 and p=0.0001 for 30 μM, p=0.0129 (galactose) for 100 μM dexpramipexole; n=14-17 wells/concentration for each substrate).
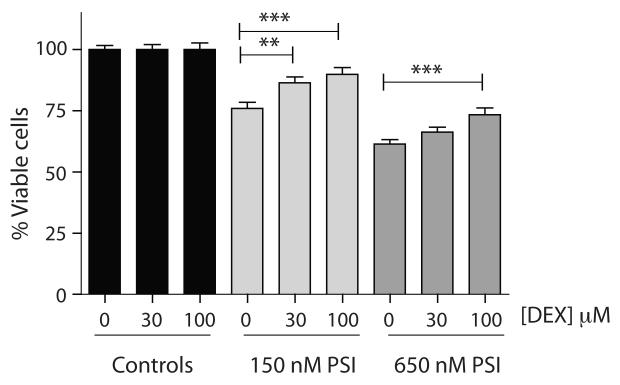
To determine if the protective properties of DEX on PSI-induced mitochondrial membrane currents were linked to protective effects on metabolism, we used two cell lines to examine the effects of PSI and DEX on bioenergetics and cell survival. To measure effects on bioenergetics in the absence of cell death, a CRC for cell death by PSI was established in C2C12 cells (Fig. 5A), and a PSI concentration that was near the inflection point of the killing curve (30nM) was used to stress but not kill C2C12 myoblasts. Under these conditions, neither ATP levels nor cell viability (Fig. 5B) were significantly affected in any group (PSI, DEX or the combination, relative to control). In PSI, however, although C2C12 cells did not display a significant change in OCR, they did display a very significant increase in ECAR (Fig. 5C), indicating a higher relative level of glycolytic activity to mitochondrial metabolism following low-level PSI treatment. In contrast, cells incubated only in DEX displayed a significant decrease in OCR, which was not accompanied by a change in ECAR (Fig. 5C), indicative of an increase in basal mitochondrial efficiency of DEX-treated cells. Co-incubation with DEX and PSI resulted in a profile identical to DEX alone (Fig. 5C), suggesting that DEX blocked the PSI-induced bioenergetic changes. This normalization of respiratory profiles may be protective under conditions where cell survival is at risk. When SH-SY5Y cells were exposed to lethal levels of PSI, pretreatment with DEX significantly reduced cell death (Fig. 6).
FIGURE 5.
DEX altered bioenergetic parameters and ATP production in the C2C12 myoblast cell line. A. Cellular viability of C2C12 cells after 18 hr. exposure to PSI at indicated concentrations. Data are presented as a percentage of the vehicle-treated control ±SEM (n=10 wells at each concentration). A sub-lethal concentration of PSI, 30nM, was used in subsequent experiments to stress C2C12 cells. B. (Left) ATP levels (%control) after18 hr exposure to indicated agents. (2-way-ANOVA; p=0.1763; n=18 wells for each group). (Right) cell viability after18 hr exposure to indicated agents (2-way-ANOVA, p=0.2824). Data are presented as a percentage of the vehicle-treated control±SEM (n = 14-17 wells for each group; for all conditions multiple plates and cell platings contributed to all data obtained). C. (Left) Oxygen consumption rate (OCR) and (Right) extracellular acidification rate (ECAR) of cells exposed to DEX (30μM) and/or PSI (30nM), or no drug (control) for 18 hr. (control, black bars, n=27; DEX, n=24; PSI, n=12, from 5 independent multiwell plates, from multiple cell platings). (*p=0.0477; **p=0.0002, blue bars). ***p=0.00015, gray bar; (n=9, OCAR ***p=0.0009, red bars). Data are expressed as a percentage of the corresponding control OCR or ECAR value, and p values represent results of pre-planned post hoc comparisons (Tukey HSD) following one-way ANOVA (p=0.001). Data shown represent mean±SEM.
FIGURE 6.
Exposure of SH-SY5Y cells to high concentrations of PSI compromises cell viability. Cells were pre-treated with DEX for 24 hr. prior to 24 hr. exposure to PSI at indicated concentrations. Pre-exposure to dexpramipexole (DEX) significantly reduced the PSI-mediated cell death (2-factor MANOVA, DEX+150 nM PSI vs. 150 nM PSI, p=0.001, DEX+650 nM PSI vs. 650 nM PSI, p=0.001; pre-planned post hoc Bonferroni-corrected t-tests, 150 nM PSI vs. 150 nM PSI + 30 μM DEX, p=0.031; 150 nM PSI + 100 μM DEX, p=0.001; 650 nM PSI + 100 μM DEX, p=0.001; all other comparisons were not statistically significant). Bar graphs represent normalized mean±SEM; n=41-98 wells/condition.
3. DISCUSSION
In the current study we found that DEX and CSA potently and effectively inhibited ion channel activity evoked by Ca2+ or PSI pre-treatment in brain-derived mitochondria. We have previously shown that intermediate- and large-conductance channel activity is present at higher frequency in mitochondria isolated from affected brain regions of rodents exposed in vivo to global ischemic injury (Bonanni et al., 2006; Miyawaki et al., 2008). These previous works suggested that the ischemia-induced channel is comprised of a complex of proteins including an inner membrane-localized divalent-sensitive component (possibly the mPTP) and outer membrane proteins including a pro-apoptotic version of Bcl-xL, and the voltage-dependent anion channel (VDAC). Therefore the activity inhibited by DEX in our studies may reflect an ion conductance associated with both the inner and outer mitochondrial membranes. The current work does not localize the site(s) of action of DEX to any particular membrane or component, although effects of DEX on bioenergetic properties of the cells, as in our studies with Bcl-xL (Alavian, 2011; Chen, 2011), suggest at least a partial inner membrane localization of the DEX effects.
Increases or unchanged ATP levels were observed in neurons and other cells after exposure to DEX, while basal OCR levels were significantly reduced. In addition, DEX normalized ECAR levels and lowered OCR in PSI-treated C2C12 cells, suggesting that it ameliorated injury-induced mitochondrial stress. The results of experiments in which DEX was administered in the setting of PSI exposure suggest that DEX restored a ‘normal’ bioenergetic phenotype in compromised cells. The timeframe of most chronic NDDs is long, and small changes in mitochondrial bioenergetic modulation may have meaningful effects over the course of the years during which neurons are at risk.
While both CSA and DEX similarly inhibited conductance in these preparations from brain-derived mitochondria at submaximal concentrations, only CSA was able to block transition measured optically in liver mitochondria. This suggests either that CSA and DEX have differential effects, at least in liver mitochondria, or it may simply mean that there is a difference in the degree to which these agents can maximally inhibit the ion channel complex (not examined here for CSA). Nevertheless, we did not observe any consistent effect of either DEX or pramipexole on mitochondrial transition in liver mitochondria.
DEX inhibited PSI-induced cell death in a concentration-dependent manner when tested in SH-SY5Y neuroblastoma cells. In our study, as in previous studies (Abramova et al., 2002; Cassarino et al., 1998; Danzeisen et al., 2006), effective cytoprotective concentrations of DEX (or pramipexole in most previous studies), and concentrations required for significant effects on metabolic efficiency in cells, were significantly higher than the EC50 for mitochondrial current inhibition. Because of the shallow slopes of this CRC, concentrations of the drug producing complete elimination of these aberrant currents (an EC100), correspond to 10-30μM or even higher concentrations, and this range may be required for enhanced bioenergetic efficiency and significant cytoprotection.
Mitochondrial dysfunction has long been implicated in the pathogenesis of neurodegenerative disease (Baron et al., 2007; Beal, 2007; Dupuis et al., 2004). Proteasomal dysfunction is a potential mechanism for accumulation of undegraded proteins in mitochondria (Naiki and Nagai, 2009), and this has been suggested as one of many potentially stressful events coupled to the onset of neurodegeneration in diseases with diverse clinical presentation including ALS, Alzheimer’s disease, and PD (Bandopadhyay and de Belleroche, 2010; Hanger et al., 2009; Shaw and Valentine, 2007). We have demonstrated that mitochondrial stress induced by either PSI or high Ca2+ resulted in increased membrane current in mitochondrial membranes, and produced a shift in the bioenergetic profile of cultured cells. This membrane current likely constitutes a leak conductance of the inner mitochondrial membrane which results in a reduction in bioenergetic efficiency. If this inefficiency cannot be countered by sufficient compensatory increases in metabolism of available substrate and OCR, or by shifting to glycolysis, it may stress energy supplies, resulting in increased risk of cellular and particularly neuronal death when energy demand exceeds supply. These data suggest that DEX reduces this risk by inhibiting aberrant mitochondrial leak conductance, resulting in neuroprotection and, quite possibly, therapeutic benefit in NDDs. Recently, in an initial carefully controlled Phase 2 study in subjects with ALS, DEX-treated subjects exhibited a lower rate of decline in measures of motor function, and suffered a lower level of mortality over the course of the study (Cudkowicz et al., 2011). These initial clinical results suggest that the cellular effects of DEX have important consequences in ALS. The results from the current study represent a first step in understanding the specific mechanism by which DEX exerts its neuroprotective actions.
4. EXPERIMENTAL PROCEDURES
4.1 Isolation of Brain-derived Mitochondria
Standard techniques were adapted for isolating brain-derived mitochondria (Brown et al., 2004). Mitochondria were stored in isolation buffer (IB: 250mM sucrose, 20mM HEPES, pH 7.2, 1mM EDTA, and 0.5% BSA) at −80 °C. For mitochondria treated with a proteasome inhibitor, male Sprague-Dawley rats (age 6 weeks, ~200 gm, 3-6 rats/group/condition, at least 2 different preparations for each experimental paradigm) were injected with the ubiquitin proteasome inhibitor Z-lle-Glu(OtBu)-Ala-Leu-al (PSI; Peptides International Inc, Kentucky,USA; s.c. every other day for 1 or 2 weeks; 6.0mg/kg PSI in DMSO, or with DMSO, 200 μL/rat) (McNaught et al., 2004), and mitochondria isolated and stored as above.
4.2 Dexpramipexole
DEX ((6R)-4,5,6,7-tetrahydro-N6-propyl-2,6-benzothiazole-diamine) was prepared by contract with Albany Molecular Research Inc. (AMRI), and was determined to have chemical purity >99.99%, and enantiomeric (R+) purity relative to pramipexole of >99.95%.
4.3 Electrophysiological recording from rat brain-derived mitochondria
Patch-clamp recordings were made from frozen de-energized mitochondria in intracellular solution (120mM potassium chloride, 8mM NaCl, 0.5mM EGTA, 10mM HEPES, pH adjusted to 7.3) at room temperature (22–25°C). Pipettes (80-100MΩ) were filled with the same solution; recordings were made using a Heka 8 amplifier with Vm held at positive voltages up to + 180 mV. Data were recorded at 20 kHz and filtered at 500-1000 Hz. DEX (Knopp Neurosciences, Pittsburgh, PA; purity established to be >99.95%) was prepared as a 10mM aqueous stock and diluted in intracellular recording solution; cyclosporine A (CSA; Sigma, St. Louis, MO) was prepared as an 8mM stock solution in EtOH and diluted in buffer. Reagents were rapidly perfused into the recording chamber. Peak membrane conductance was measured as the peak amount of current (pA) from zero, converted to pS by assuming a linear I-V relationship. Discrete channel conductances were sorted by activity (% occurrence of a conductance level per unit time measured using pCLAMP 10 software, Molecular Devices, Sunnyvale, CA); levels defined as closed (no current), small (<200pS), intermediate (>200pS and <750pS) and large (>750pS) (Bonanni et al., 2006). Mean open time (NPo; open time of discrete single channels times the apparent number of single channels in the patch) was used to assess drug effect.
4.4 Preparation and Maintenance of Cultured Cells
Primary cultures of cortical or hippocampal neurons were prepared as described previously (Li et al., 2008; Wang et al., 2008b), plated at 8×105 cells/well for Seahorse XF24 experiments and grown in Neurobasal A supplemented with B27, 1mM glutamine and penicillin/streptomycin for 4 days in vitro (DIV). On DIV 7, media without antibiotics was substituted. The cultures were used on DIV 9-10.
Undifferentiated SH-SY5Y human neuroblastoma cells (ATCC, Manasses, VA) were maintained in humidified 5% CO in 1:1 Ham’s F12 nutrient mixture with Glutamax® 2 and Minimal Essential Medium with L-glutamine (MEM-Alpha), 10% FBS, and 1% penicillin/streptomycin (Gibco® Invitrogen Corp., Carlsbad, CA).
The C2C12 mouse myoblast cell line (ATCC, Manasses, VA) was cultured in Dulbecco’s Modified Eagle Medium (DMEM; Gibco®; Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS), 2mM GlutaMax® and penicillin/streptomycin. Cells were maintained in 10% CO2 humidified at 37°C.
4.5 Cell Viability and Cellular ATP quantitation
Cellular viability was measured using CellTiter-Blue® (Promega Corp., Madison, WI) in black-walled optical imaging multiwell plates, and cellular ATP was measured using the CellTiter-Glo® (Promega Corp., Madison, WI) luminescence assay in opaque-walled multiwell plates according to the manufacturer’s protocol.
4.6 Cell culture oxygen consumption experiments
Oxygen uptake was measured from single hippocampal neurons grown in culture (DIV 14-17) (Li et al., 2008). A 2-4 μm (tip diameter) oxygen-sensing electrode oscillated 10 μm close-to and away-from the cell every 3 sec. The difference in current detected at the two positions was translated into oxygen flux (Gleichmann et al., 2009; Land et al., 1999). DEX (10μM) or control solution was added after a 5 min. baseline measurement, and flux was measured for >5 min. post-treatment. In these cells ATP was measured by a luciferase assay (details?).
In other experiments OCR and ECAR were measured with a Seahorse® XF24 Flux Analyzer (Seahorse Bioscience, Billerica, MA). For PSI experiments on C2C12 cells, basal values were assessed in non-buffered DMEM Assay Medium without glucose, glutamine or pyruvate, and supplemented with 25mM glucose, 6mM Glutamax®, and 1mM sodium pyruvate.
For primary cortical neurons the assay had 2 modifications: 1) MAS1 buffer (70mM sucrose, 220mM mannitol, 5mM KH2PO4, 5mMmgCl2, 2mM HEPES, 1mM EGTA, 0.2% FA-free BSA; pH 7.2; Sigma-Aldrich, St. Louis, MO) was used instead of Assay Media, and 2) 10x concentrated digitonin (10μg/mL), rotenone (100nM) and DEX (30μM) or control (equivalent volume of water) were added to the cells 45 minutes prior to assay. Succinate and ADP stocks were diluted to 10x concentrations in pre-warmed XF MAS buffer, pH 7.2. Using a Plate Prep Station (Seahorse Bioscience, Billerica, MA), cells were washed with 1 mL buffer and were brought to 607μL with buffer. DEX or water (68μL; 10x concentrated) was added to the wells, and cells incubated at 37°C in a non-CO2 incubator for 45 minutes to 1 hour.
Raw basal OCR and ECAR values were normalized as a percent of the mean control value for each individual experiment to minimize the between-plate variability. Normalized data from experiments were expressed as mean ±SEM. The number of measurements (n) is equal to the number of wells at each condition, but in every case all conditions were averaged across multiple plates and multiple cell preparations.
4.7 Statistical analyses and Curve Fitting
For comparisons involving 2 groups, paired or unpaired Student’s t-tests (2-tailed) were used. In all figures, *=p<0.05, **=p<0.01, and ***=p<0.001 to denote significance level, and exact p values are provided in the figure legends. For more than 2 groups, one-way or 2-way analyses of variance (ANOVA), or 2-factor MANOVA, were performed; in the case of a significant F-test, the p value is provided in the figure legend, and pre-planned post hoc comparisons (Bonferroni-corrected t-tests or Tukey’s HSD) were performed and significance levels displayed in figures and exact p values provided as described above. Where possible, p values for tests are presented to 2 significant digits. All statistical analyses were performed using GraphPad Prism 5, InStat (GraphPad Software, La Jolla, CA) or SPSS (IBM Corporation, Somers, NY).
ACKNOWLEDGEMENTS
This work was supported by a grant from Knopp Biosciences LLC to Yale Medical School, and NIH grant numbers NS045876 and NS064967 to EAJ, and Young Researchers Grant 2007, Dementia with Lewy bodies: new diagnostic markers and therapeutic implications, from the Italian Ministry of Health to LB.
Abbreviations
- CSA
cyclosporine A
- DEX
dexpramipexole, (6R)-4,5,6,7-tetrahydro-N6-propyl-2,6-benzothiazole-diamine
- ECAR
extracellular acidification rate
- OCR
oxygen consumption rate
- PSI
Z-lle-Glu(OtBu)-Ala-Leu-al
Footnotes
CONFLICT OF INTEREST STATEMENT Steven I. Dworetzky, Jamie E. Mangold, Armando P. Signore, and Ulrike DeMarco are employees of Knopp Biosciences, LLC, and all own equity in the company. Valentin K. Gribkoff and Damon R. Demady are former employees of Knopp Neurosciences; Damon R. Demady has a continuing equity interest in the company. Elizabeth A. Jonas is the recipient of a research grant from Knopp Neurosciences, but otherwise has no financial interest in the sponsoring company or in the drug dexpramipexole. Laura Bonnani, Marco Onofrj, Astrid Thomas, Silvio Sacchetti, Kambiz N. Alavian, Ping Zhang, Hongmei Li, Panah Nabili, Emma Lazrove and Peter J.S. Smith have no financial interest in Knopp Neuroscience or in the drug dexpramipexole.
REFERENCES
- Abramova NA, Cassarino DS, Khan SM, Painter TW, Bennett JP., Jr. Inhibition by R(+) or S(−) pramipexole of caspase activation and cell death induced by methylpyridinium ion or beta amyloid peptide in SH-SY5Y neuroblastoma. Journal of Neuroscience Research. 2002;67:494–500. doi: 10.1002/jnr.10127. [DOI] [PubMed] [Google Scholar]
- Alavian L, Collis, Bonanni, Zeng, Sacchetti, Lazrove, Nabili, Flaherty, Graham, Chen, Messerli, Mariggio, Rahner, McNay, Shore, Smith, Hardwick, Jonas Bcl-x(L) regulates metabolic efficiency of neurons through interaction with the mitochondrial F(1)F(O) ATP synthase. Nat Cell Biol. 2011;13:1224–33. doi: 10.1038/ncb2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews ZB, Diano S, Horvath TL. Mitochondrial uncoupling proteins in the CNS: in support of function and survival. Nature Reviews Neuroscience. 2005;6:829–40. doi: 10.1038/nrn1767. [DOI] [PubMed] [Google Scholar]
- Azzolin L, von Stockum S, Basso E, Petronilli V, Forte MA, Bernardi P. The mitochondrial permeability transition from yeast to mammals. FEBS Letters. 584:2504–9. doi: 10.1016/j.febslet.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandopadhyay R, de Belleroche J. Pathogenesis of Parkinson’s disease: emerging role of molecular chaperones. Trends in Molecular Medicine. 2010;16:27–36. doi: 10.1016/j.molmed.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Baron M, Kudin AP, Kunz WS. Mitochondrial dysfunction in neurodegenerative disorders. Biochemical Society Transactions. 2007;35:1228–31. doi: 10.1042/BST0351228. [DOI] [PubMed] [Google Scholar]
- Beal MF. Mitochondria and neurodegeneration. Novartis Foundation Symposium. 2007;287:183–92. doi: 10.1002/9780470725207.ch13. discussion 192-6. [DOI] [PubMed] [Google Scholar]
- Bernardi P. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore by the proton electrochemical gradient. Evidence that the pore can be opened by membrane depolarization. Journal of Biological Chemistry. 1992;267:8834–9. [PubMed] [Google Scholar]
- Bernardi P, Rasola A. Calcium and cell death: the mitochondrial connection. Sub-Cellular Biochemistry. 2007;45:481–506. doi: 10.1007/978-1-4020-6191-2_18. [DOI] [PubMed] [Google Scholar]
- Bonanni L, Chachar M, Jover-Mengual T, Li H, Jones A, Yokota H, Ofengeim D, Flannery RJ, Miyawaki T, Cho CH, Polster BM, Pypaert M, Hardwick JM, Sensi SL, Zukin RS, Jonas EA. Zinc-dependent multi-conductance channel activity in mitochondria isolated from ischemic brain. Journal of Neuroscience. 2006;26:6851–62. doi: 10.1523/JNEUROSCI.5444-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozik ME, Mather JL, Kramer WG, Gribkoff VK, Ingersoll EW. Safety, Tolerability, and Pharmacokinetics of KNS-760704 (Dexpramipexole) in Healthy Adult Subjects. J Clin Pharmacol. 51:1177–85. doi: 10.1177/0091270010379412. [DOI] [PubMed] [Google Scholar]
- Bozik MEI, E.W., Volles L, Mather JM, Amburgey CA, Moritz JM, Archibald DG, Sullivan M, Gribkoff VK, Miller R, Mitsumoto H, Moore D, Schoenfeld D, Shefner J, Cudkowicz M. KNS-760704-CL201, Part 1: A 12-Week Phase 2 Study of the Safety, Tolerability, and Clinical Effects of KNS-760704 in ALS Subjects Abstract. Amyotrophic Lateral Sclerosis. 2009;10:28–29. [Google Scholar]
- Brand MD. The efficiency and plasticity of mitochondrial energy transduction. Biochemical Society Transactions. 2005;33:897–904. doi: 10.1042/BST0330897. [DOI] [PubMed] [Google Scholar]
- Brown MR, Sullivan PG, Dorenbos KA, Modafferi EA, Geddes JW, Steward O. Nitrogen disruption of synaptoneurosomes: an alternative method to isolate brain mitochondria. Journal of Neuroscience Methods. 2004;137:299–303. doi: 10.1016/j.jneumeth.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Cassarino DS, Fall CP, Smith TS, Bennett JP., Jr. Pramipexole reduces reactive oxygen species production in vivo and in vitro and inhibits the mitochondrial permeability transition produced by the parkinsonian neurotoxin methylpyridinium ion. Journal of Neurochemistry. 1998;71:295–301. doi: 10.1046/j.1471-4159.1998.71010295.x. [DOI] [PubMed] [Google Scholar]
- Chen YB, Aon MA, Hsu YT, Soane L, Teng X, McCaffery JM, Cheng WC, Qi B, Li H, Alavian KN, Dayhoff-Brannigan M, Zou S, Pineda FJ, O’Rourke B, Ko YH, Pedersen PL, Kaczmarek LK, Jonas EA, Hardwick JM. Bcl-xL regulates mitochondrial energetics by stabilizing the inner membrane potential. J Cell Biol. 2011;195:263–76. doi: 10.1083/jcb.201108059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochemical Journal. 1999;341:233–49. [PMC free article] [PubMed] [Google Scholar]
- Danzeisen R, Schwalenstoecker B, Gillardon F, Buerger E, Krzykalla V, Klinder K, Schild L, Hengerer B, Ludolph AC, Dorner-Ciossek C, Kussmaul L. Targeted antioxidative and neuroprotective properties of the dopamine agonist pramipexole and its nondopaminergic enantiomer SND919CL2x [(+)2-amino-4,5,6,7-tetrahydro-6-Lpropylamino-benzathiazole dihydrochloride] Journal of Pharmacology & Experimental Therapeutics. 2006;316:189–99. doi: 10.1124/jpet.105.092312. [DOI] [PubMed] [Google Scholar]
- Dejean LM, Martinez-Caballero S, Guo L, Hughes C, Teijido O, Ducret T, Ichas F, Korsmeyer SJ, Antonsson B, Jonas EA, Kinnally KW. Oligomeric Bax is a component of the putative cytochrome c release channel MAC, mitochondrial apoptosis-induced channel. Molecular Biology of the Cell. 2005;16:2424–32. doi: 10.1091/mbc.E04-12-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson MW, Guo M. Pink1, Parkin, DJ-1 and mitochondrial dysfunction in Parkinson’s disease. Current Opinion in Neurobiology. 2007;17:331–7. doi: 10.1016/j.conb.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Dupuis L, Gonzalez de Aguilar JL, Oudart H, de Tapia M, Barbeito L, Loeffler JP. Mitochondria in amyotrophic lateral sclerosis: a trigger and a target. Neurodegenerative Diseases. 2004;1:245–54. doi: 10.1159/000085063. [DOI] [PubMed] [Google Scholar]
- Ferrari-Toninelli G, Maccarinelli G, Uberti D, Buerger E, Memo M. Mitochondria-targeted antioxidant effects of S(−) and R(+) pramipexole. BMC Pharmacology. 2010;10:2. doi: 10.1186/1471-2210-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerencser AA, Neilson A, Choi SW, Edman U, Yadava N, Oh RJ, Ferrick DA, Nicholls DG, Brand MD. Quantitative microplate-based respirometry with correction for oxygen diffusion. Anal Chem. 2009;81:6868–78. doi: 10.1021/ac900881z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleichmann M, Collis LP, Smith PJ, Mattson MP. Simultaneous single neuron recording of O2 consumption, [Ca2+]i and mitochondrial membrane potential in glutamate toxicity. Journal of Neurochemistry. 2009;109:644–55. doi: 10.1111/j.1471-4159.2009.05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribkoff VK, Bozik ME. KNS-760704 [(6R)-4,5,6,7-tetrahydro-N6-propyl-2, 6-benzothiazole-diamine dihydrochloride monohydrate] for the treatment of amyotrophic lateral sclerosis. CNS Neuroscience & Therapeutics. 2008;14:215–26. doi: 10.1111/j.1755-5949.2008.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanger DP, Seereeram A, Noble W. Mediators of tau phosphorylation in the pathogenesis of Alzheimer’s disease. Expert Review of Neurotherapeutics. 2009;9:1647–66. doi: 10.1586/ern.09.104. [DOI] [PubMed] [Google Scholar]
- Hinerfeld D, Traini MD, Weinberger RP, Cochran B, Doctrow SR, Harry J, Melov S. Endogenous mitochondrial oxidative stress: neurodegeneration, proteomic analysis, specific respiratory chain defects, and efficacious antioxidant therapy in superoxide dismutase 2 null mice. Journal of Neurochemistry. 2004;88:657–67. doi: 10.1046/j.1471-4159.2003.02195.x. [DOI] [PubMed] [Google Scholar]
- Iravani MM, Haddon CO, Cooper JM, Jenner P, Schapira AH. Pramipexole protects against MPTP toxicity in non-human primates. Journal of Neurochemistry. 2006;96:1315–21. doi: 10.1111/j.1471-4159.2005.03625.x. [DOI] [PubMed] [Google Scholar]
- Jonas EA, Knox RJ, Kaczmarek LK. Giga-ohm seals on intracellular membranes: a technique for studying intracellular ion channels in intact cells. Neuron. 1997;19:7–13. doi: 10.1016/s0896-6273(00)80343-8. [DOI] [PubMed] [Google Scholar]
- Jonas EA. Molecular participants in mitochondrial cell death channel formation during neuronal ischemia. Experimental Neurology. 2009;218:203–12. doi: 10.1016/j.expneurol.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan J, Cena V, Prehn JH. Mitochondrial control of neuron death and its role in neurodegenerative disorders. Journal of Physiology & Biochemistry. 2003;59:129–41. doi: 10.1007/BF03179878. [DOI] [PubMed] [Google Scholar]
- Kawamata H, Manfredi G. Mitochondrial dysfunction and intracellular calcium dysregulation in ALS. Mechanisms of Ageing & Development. 2010;131:517–26. doi: 10.1016/j.mad.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnally KW, Tedeschi H, Mannella CA. Evidence for a novel voltage-activated channel in the outer mitochondrial membrane. FEBS Letters. 1987;226:83–7. doi: 10.1016/0014-5793(87)80555-0. [DOI] [PubMed] [Google Scholar]
- Kinnally KW, Martinez-Caballero S, Dejean LM. Detection of the mitochondrial apoptosis-induced channel (MAC) and its regulation by Bcl-2 family proteins. Current Protocols in Toxicology. 2006:12. doi: 10.1002/0471140856.tx0212s30. Chapter 2, Unit2. [DOI] [PubMed] [Google Scholar]
- Land SC, Porterfield DM, Sanger RH, Smith PJ. The self-referencing oxygen-selective microelectrode: detection of transmembrane oxygen flux from single cells. Journal of Experimental Biology. 1999;202:211–8. doi: 10.1242/jeb.202.2.211. [DOI] [PubMed] [Google Scholar]
- Le WD, Jankovic J, Xie W, Appel SH. Antioxidant property of pramipexole independent of dopamine receptor activation in neuroprotection. Journal of Neural Transmission. 2000;107:1165–73. doi: 10.1007/s007020070030. [DOI] [PubMed] [Google Scholar]
- Lemasters JJ. Modulation of mitochondrial membrane permeability in pathogenesis, autophagy and control of metabolism. Journal of Gastroenterology & Hepatology. 2007;22(Suppl 1):S31–7. doi: 10.1111/j.1440-1746.2006.04643.x. [DOI] [PubMed] [Google Scholar]
- Li H, Chen Y, Jones AF, Sanger RH, Collis LP, Flannery R, McNay EC, Yu T, Schwarzenbacher R, Bossy B, Bossy-Wetzel E, Bennett MV, Pypaert M, Hickman JA, Smith PJ, Hardwick JM, Jonas EA. Bcl-xL induces Drp1-dependent synapse formation in cultured hippocampal neurons. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2169–74. doi: 10.1073/pnas.0711647105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohret TA, Jensen RE, Kinnally KW. Tim23, a protein import component of the mitochondrial inner membrane, is required for normal activity of the multiple conductance channel, MCC. Journal of Cell Biology. 1997;137:377–86. doi: 10.1083/jcb.137.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella CA, Kinnally KW. Reflections on VDAC as a voltage-gated channel and a mitochondrial regulator. Journal of Bioenergetics & Biomembranes. 2008;40:149–55. doi: 10.1007/s10863-008-9143-0. [DOI] [PubMed] [Google Scholar]
- McNaught KS, Perl DP, Brownell AL, Olanow CW. Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson’s disease.[see comment] Annals of Neurology. 2004;56:149–62. doi: 10.1002/ana.20186. [DOI] [PubMed] [Google Scholar]
- Miyawaki T, Mashiko T, Ofengeim D, Flannery RJ, Noh KM, Fujisawa S, Bonanni L, Bennett MV, Zukin RS, Jonas EA. Ischemic preconditioning blocks BAD translocation, Bcl-xL cleavage, and large channel activity in mitochondria of postischemic hippocampal neurons. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4892–7. doi: 10.1073/pnas.0800628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naiki H, Nagai Y. Molecular pathogenesis of protein misfolding diseases: pathological molecular environments versus quality control systems against misfolded proteins. Journal of Biochemistry. 2009;146:751–6. doi: 10.1093/jb/mvp119. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Chalmers S. The integration of mitochondrial calcium transport and storage. Journal of Bioenergetics & Biomembranes. 2004;36:277–81. doi: 10.1023/B:JOBB.0000041753.52832.f3. [DOI] [PubMed] [Google Scholar]
- Nicholls DG. Oxidative stress and energy crises in neuronal dysfunction. Annals of the New York Academy of Sciences. 2008;1147:53–60. doi: 10.1196/annals.1427.002. [DOI] [PubMed] [Google Scholar]
- Nowikovsky K, Schweyen RJ, Bernardi P. Pathophysiology of mitochondrial volume homeostasis: potassium transport and permeability transition. Biochimica et Biophysica Acta. 2009;1787:345–50. doi: 10.1016/j.bbabio.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Pavlov EV, Priault M, Pietkiewicz D, Cheng EH, Antonsson B, Manon S, Korsmeyer SJ, Mannella CA, Kinnally KW. A novel, high conductance channel of mitochondria linked to apoptosis in mammalian cells and Bax expression in yeast. Journal of Cell Biology. 2001;155:725–31. doi: 10.1083/jcb.200107057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrini S, Sau D, Guareschi S, Bogush M, Brown RH, Jr., Naniche N, Kia A, Trotti D, Pasinelli P. ALS-linked mutant SOD1 damages mitochondria by promoting conformational changes in Bcl-2. Human Molecular Genetics. 2010;19:2974–86. doi: 10.1093/hmg/ddq202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JJ, Shin DS, Tainer JA. Amyotrophic lateral sclerosis. Advances in Experimental Medicine & Biology. 2010;685:9–20. doi: 10.1007/978-1-4419-6448-9_2. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Iwata M. Ultrastructural study of the synapses of central chromatolytic anterior horn cells in motor neuron disease. Journal of Neuropathology & Experimental Neurology. 1996;55:932–9. doi: 10.1097/00005072-199608000-00009. [DOI] [PubMed] [Google Scholar]
- Sayeed I, Parvez S, Winkler-Stuck K, Seitz G, Trieu I, Wallesch CW, Schonfeld P, Siemen D. Patch clamp reveals powerful blockade of the mitochondrial permeability transition pore by the D2-receptor agonist pramipexole. FASEB Journal. 2006;20:556–8. doi: 10.1096/fj.05-4748fje. [DOI] [PubMed] [Google Scholar]
- Shalbuyeva N, Brustovetsky T, Brustovetsky N. Lithium desensitizes brain mitochondria to calcium, antagonizes permeability transition, and diminishes cytochrome C release. Journal of Biological Chemistry. 2007;282:18057–68. doi: 10.1074/jbc.M702134200. [DOI] [PubMed] [Google Scholar]
- Shaw BF, Valentine JS. How do ALS-associated mutations in superoxide dismutase 1 promote aggregation of the protein? Trends in Biochemical Sciences. 2007;32:78–85. doi: 10.1016/j.tibs.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Parks JK, Cassarino DS, Trimmer PA, Miller SW, Maguire DJ, Sheehan JP, Maguire RS, Pattee G, Juel VC, Phillips LH, Tuttle JB, Bennett JP, Jr., Davis RE, Parker WD., Jr. Mitochondria in sporadic amyotrophic lateral sclerosis. Experimental Neurology. 1998;153:135–42. doi: 10.1006/exnr.1998.6866. [DOI] [PubMed] [Google Scholar]
- Szabo I, Zoratti M. The giant channel of the inner mitochondrial membrane is inhibited by cyclosporin A. Journal of Biological Chemistry. 1991;266:3376–9. [PubMed] [Google Scholar]
- Szabo I, Bernardi P, Zoratti M. Modulation of the mitochondrial megachannel by divalent cations and protons. Journal of Biological Chemistry. 1992;267:2940–6. [PubMed] [Google Scholar]
- Wang H, Larriviere KS, Keller KE, Ware KA, Burns TM, Conaway MA, Lacomis D, Pattee GL, Phillips LH, 2nd, Solenski NJ, Zivkovic SA, Bennett JP., Jr. R+ pramipexole as a mitochondrially focused neuroprotectant: initial early phase studies in ALS. Amyotrophic Lateral Sclerosis. 2008a;9:50–8. doi: 10.1080/17482960701791234. [DOI] [PubMed] [Google Scholar]
- Wang S, Xing Z, Vosler PS, Yin H, Li W, Zhang F, Signore AP, Stetler RA, Gao Y, Chen J. Cellular NAD replenishment confers marked neuroprotection against ischemic cell death: role of enhanced DNA repair. Stroke. 2008b;39:2587–95. doi: 10.1161/STROKEAHA.107.509158. [DOI] [PMC free article] [PubMed] [Google Scholar]