Abstract
Background and Purpose
The aim of the study was to determine whether KCNQ channels are functionally expressed in bladder smooth muscle cells (SMC) and to investigate their physiological significance in bladder contractility.
Experimental Approach
KCNQ channels were examined at the genetic, protein, cellular and tissue level in guinea pig bladder smooth muscle using RT-PCR, immunofluorescence, patch-clamp electrophysiology, calcium imaging, detrusor strip myography, and a panel of KCNQ activators and inhibitors.
Key Results
KCNQ subtypes 1–5 are expressed in bladder detrusor smooth muscle. Detrusor strips typically displayed TTX-insensitive myogenic spontaneous contractions that were increased in amplitude by the KCNQ channel inhibitors XE991, linopirdine or chromanol 293B. Contractility was inhibited by the KCNQ channel activators flupirtine or meclofenamic acid (MFA). The frequency of Ca2+-oscillations in SMC contained within bladder tissue sheets was increased by XE991. Outward currents in dispersed bladder SMC, recorded under conditions where BK and KATP currents were minimal, were significantly reduced by XE991, linopirdine, or chromanol, and enhanced by flupirtine or MFA. XE991 depolarized the cell membrane and could evoke transient depolarizations in quiescent cells. Flupirtine (20 μM) hyperpolarized the cell membrane with a simultaneous cessation of any spontaneous electrical activity.
Conclusions and Implications
These novel findings reveal the role of KCNQ currents in the regulation of the resting membrane potential of detrusor SMC and their important physiological function in the control of spontaneous contractility in the guinea pig bladder.
Keywords: smooth muscle, potassium channels, contractility, urinary bladder, electrophysiology, immunohistochemistry
Introduction
The ability of the bladder to store and expel urine is achieved through the interaction of the complex arrangement of cells that make up the bladder wall, which include the urothelium, nerves, smooth muscle cells (SMC) and populations of interstitial cells (ICs). Spontaneous contractile activity is a phenomenon common to many smooth muscle preparations and, in the urinary bladder, is considered to underlie the basal tone which enables the bladder to maintain an optimum shape as it expands to accommodate increasing volumes of urine (Turner and Brading, 1997).
Potassium channels have an important role in controlling bladder contractility by regulating the resting membrane potential (r.m.p.) of its SMC and repolarizing the action potential (Petkov, 2011). Several types of potassium currents have been characterized in bladder SMC, including those carried by large-conductance calcium-activated potassium channels (BK) (Imaizumi et al., 1998; Petkov et al., 2001a; Herrera and Nelson, 2002), intermediate-conductance calcium-activated potassium channels (IK) (Ohya et al., 2000), small conductance calcium-activated potassium channels (SK) (Herrera and Nelson, 2002), voltage-gated potassium channels (Kv) (Thorneloe and Nelson, 2003), ATP-sensitive potassium channels (KATP) (Bonev and Nelson, 1993; Petkov et al., 2001b) and two-pore domain K channels (K2P; channel nomenclature follows Alexander et al., 2011). In bladder smooth muscle, BK channels control action potential duration and the r.m.p., whereas SK currents underlie after-hyperpolarizations (Herrera and Nelson, 2002). Kv currents contribute to the regulation of the r.m.p. in addition to repolarizing the action potential (Thorneloe and Nelson, 2003). While there is little convincing evidence that KATP channels are active under normal physiological conditions, activation of these channels generates membrane hyperpolarization, reduction in action potential firing and subsequent bladder relaxation (Petkov, 2011). Recent work on K2P channels shows their involvement in r.m.p. maintenance and sensing or responding to environmental factors, such as pH (Beckett et al., 2008) or stretch (Baker et al., 2008).
KCNQ (Kv7) currents are outwardly rectifying, voltage dependent K+ currents that activate at potentials positive to −60 mV and show little inactivation (Robbins, 2001). KCNQ channels belong to the Kv family, comprise six transmembrane domains and a pore forming loop (6TMD-1P), and have been widely studied in the nervous system, heart and inner ear (Jentsch, 2000; Robbins, 2001). Five genes encoding the KCNQ family of ion channel proteins have been identified, each encoding a different KCNQ α-subunit (1–5). KCNQ1 was initially identified in cardiomyocytes (Wang et al., 1996) and was found to underlie the slow delayed rectifier current (IKs) (Sanguinetti et al., 1996; Romey et al., 1997). Channelopathies result in late repolarization and prolongation of the cardiac action potential, as occurs in long QT syndrome, associated with cardiac arrhythmias and sudden death (Wang et al., 1996; Seebohm et al., 2003). KCNQ2, 3 and 5 generate M-type currents in the nervous system (Wang et al., 1998; Selyanko et al., 2001; Shah et al., 2002; Delmas and Brown, 2005) and KCNQ2 and KCNQ3 gene mutations contribute to benign familial neonatal convulsions or epilepsy (Biervert et al., 1998; Schroeder et al., 1998). In addition to brain and nervous tissue, KCNQ5 is also expressed in skeletal muscle (Schroeder et al., 2000). Genetic mutations of KCNQ4 in hair cells of the cochlea (Rennie et al., 2001; Liang et al., 2006) lead to a form of autosomal dominant progressive deafness (Kubisch et al., 1999). KCNQ4 is also expressed in heart, brain and skeletal muscle (Kharkovets et al., 2000), and shares characteristics of M current (Søgaard et al., 2001).
In vascular smooth muscle, KCNQ channel modulators have been used to demonstrate that KCNQ currents are present in murine portal vein (Ohya et al., 2003; Yeung and Greenwood, 2005; Yeung et al., 2007), rat pulmonary artery (Joshi et al., 2006), rat stomach (Ohya et al., 2002) and rat mesenteric artery (Mackie et al., 2008) in addition to myometrial smooth muscle (McCallum et al., 2009). KCNQ currents are important in the regulation of vascular smooth muscle contractility and vascular tone. Emerging evidence indicates that KCNQ channels might also be present in the bladder as Streng et al. (2004) demonstrated that the KCNQ activator, retigabine, decreased capsaicin-induced bladder overactivity or hypercontractility when delivered intravesically to conscious rats. Furthermore, Rode et al. (2010) demonstrated effects on rat bladder contractility by KCNQ drugs. Gene expression of KCNQ1, 4 and 5 and protein expression of KCNQ4 by Western blotting was recently reported for rat bladder (Svalø et al., 2001). Our previous report of KCNQ currents in ICs of the guinea pig detrusor, which contributed to the r.m.p. (Anderson et al., 2009), showed that KCNQ channels were functional in the bladder.
The aims of the present study were to investigate whether KCNQ currents comprised a component of the total outward current in bladder SMC; to examine whether KCNQ drugs altered the r.m.p. of bladder SMC; and to determine whether KCNQ modulators could affect spontaneous contractile activity or Ca2+-oscillations in bladder tissues. Gene and protein expression of KCNQ channels was investigated using molecular and immunohistochemical techniques. Some preliminary findings of this study have been presented to the Physiological Society (Carson and McCloskey, 2007).
Methods
Ethical approval
All animal care and experimental protocols were in accordance with Schedule 1, Animal Scientific Procedures Act, 1986, UK, and approved by the local ethics committee, Queen's University Belfast. Bladders were removed from male Dunkin-Hartley guinea pigs (Harlan, UK) (200–500 g) that had been killed humanely by cervical dislocation. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2002; McGrath et al., 2001). A total of 121 animals were used in the experiments described here.
Cell preparation
Bladders were opened by longitudinal incision and the mucosa removed to leave the underlying detrusor. Cells were enzymically isolated from the detrusor region as previously described (McCloskey and Gurney, 2002). A heterogeneous yield of cells was typically obtained, including non-contractile IC, which were identified by their branched morphology, and a larger population of typical elongated spindle-shaped SMC which contracted readily upon depolarization. The latter cells were selected for patch clamp experiments.
Electrophysiological recordings
Immediately after dispersal, cells were plated at the bottom of a recording chamber for amphotericin perforated patch-clamp experiments. The bath was constantly perfused with Hanks-PSS (see below for composition) solution at room temperature and the cell of interest was continuously superfused by a drug delivery system consisting of a pipette tip with diameter of 200 μm placed approximately 300 μm away. Borosilicate microelectrodes (2–4 MΩ) were connected via an AD/DA converter (National Instruments Inc., Berkshire, UK) to an Axopatch-1D amplifier (Axon Instruments; Molecular Devices, Sunnyvale, CA, USA) and a personal computer running WinWCP software (Dr Dempster, University of Strathclyde). After gigaseals were obtained, the cell was given a test depolarization (from −60 mV to 40 mV) every 30 s until the outward current developed to its maximal amplitude. Series resistance and the capacitive surge were compensated using circuitry from the Axopatch-1D amplifier. Bladder SMC had mean cell capacitance of 51.5 ± 1.5pF, n = 82. In voltage-clamp experiments, current amplitude (pA) was divided by the cell capacitance (pF) to give current density, pA/pF.
RNA extraction and reverse transcription-PCR
Total RNA was extracted from freshly dispersed detrusor cells. Cells were repeatedly washed in PSS by centrifuging, removal of the supernatant, replacing with fresh PSS to minimize the presence of cell debris and to improve the purity of the detrusor cell sample. Guinea pig heart and brain tissue were used as positive controls. The tissue was cut into 5 mg pieces and placed in 150 μL lysis buffer, which also contained 4 ng·μL−1 carrier RNA (Qiagen, Manchester, UK). Tissue was immediately homogenized using a conventional rotor-stator homogenizer for 20–40 s. Proteinase K solution was then added to the homogenate (RNeasy Kit, Qiagen) and incubated at 55°C for 10 min before being centrifuged (2 min, maximum speed) through a QIAshredder (Qiagen). RNA extraction from freshly dispersed bladder cells followed a similar protocol with the exception of homogenization. Total RNA was extracted using RNeasy mini Elute spin columns (Qiagen), which included on-column DNase I treatment. RNA content was quantified using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). The superscript III RT (Invitrogen, Paisley, UK) and a mixture of oligo(dt) primer and random hexamers were used to reverse transcribe the RNA samples. In negative controls, addition of reverse transcriptase was omitted. cDNA was then added to a Hot start Taq polymerase master mix (Qiagen) to which guinea pig KCNQ 1–5 forward and reverse primers (Table 1) were incorporated. KCNQ 1, 2, 3 and 5 primers for RT-PCR used sequences that were demonstrated to work reliably on guinea pig and rat cochlea KCNQ subtypes (Liang et al., 2006), whereas KCNQ 4 primers were designed online using Primer 3: all were purchased from MWG-Biotech. The thermal cycler program used for PCR amplification included a 1 min 95°C denaturation step, a 30 s annealing step, using individual optimized annealing temperatures specific for each primer pair, followed by a 30 s primer extension step at 68°C for 38 cycles. PCR products were separated on 1% agarose (Melford, Ipswich, UK) gels in Tris-borate-EDTA (TBE) buffer (Fisher Scientific, Loughborough, UK), visualized with ethidium bromide staining and documented using a UVIdoc gel system. PCR products were confirmed by sequencing in the University of Manchester Core Facility.
Table 1.
Guinea pig oligonucleotide sequence of primers used for RT-PCR
| Subtypes | Primer sequence (forward and reverse) | Product length (bp) |
|---|---|---|
| KCNQ1 | Forward – 5′ GCT CTG GCC ACC GGG ACC CT-3′ | 434 |
| Reverse – 5′ GAT GCG GCC GGA CTC ATT CA-3′ | ||
| KCNQ2 | Forward – 5′ CAA GTA CCC TCA GAC CTG GAA–3′ | 515 |
| Reverse – 5′ CAG CTC TTG GGC ACC TTG CT–3′ | ||
| KCNQ3 | Forward – 5′ CCA AGG AAT GAA CCA TAT GTA GCC-3′ | 461 |
| Reverse – 5′ CAG AAG AGT CAA GAT GGG CAG GAC-3′ | ||
| KCNQ4 | Forward – 5′ CGC TTC CGG GCC TCT CTA AGA C-3′ | 561 |
| Reverse – 5′ GTC CTC GTG GTC TAC AGG GCT GTG-3′ | ||
| KCNQ5 | Forward – 5′ CCA TTG TTC TCA TCG CTT CA-3′ | 200 |
| Reverse – 5′ TCC AAT GTA CCA GGC TGT GA–3′ |
Immunohistochemistry
Detrusor preparations were fixed in 4% paraformaldehyde, washed in PBS, blocked in 1% BSA and incubated with primary antibodies (in 0.05% Triton-X 100) to KCNQ subtypes 1–5 (KCNQ1 (APC-022), KCNQ2 (APC-050) KCNQ3 (APC-051) from Alomone, 1:200; KCNQ 4 (sc-50417) and KCNQ5 (sc-50416) from Santa Cruz (1:200)) for 24 h. After washing in PBS to remove excess antibody, tissues were incubated in secondary fluorescent antibodies (Alexa 488 anti-rabbit, 1:200, Invitrogen) for 1 h. Immunolabelled tissues were imaged with confocal microscopy (Nikon C1) mounted on a Nikon e90i upright microscope equipped with a 488 nm argon ion laser and the resulting emission fluorescence collected through appropriate filters to a photomultiplier tube. Control experiments were carried out as following: (i) omission of the primary antibody to control for the specificity of the secondary antibody; (ii) no antibodies to control for autofluorescence of the tissue; (iii) absorption of the primary antibody with control antigen (where available) to control for the specificity of the primary antibody. Significant fluorescence was not observed in any of the control samples.
Organ bath contractile studies
After mucosa removal, strips of detrusor (10 mm × 2 mm × 2 mm) were mounted in organ baths to an initial tension of 1 g. Tension was readjusted to 1 g after the strips initially relaxed (1–2 times) until spontaneous contractile activity occurred. This activity was maintained for at least 4 h in the absence of drugs or in the presence of drug vehicles (Figure 2F). Tissues were perfused (1–2 mL per minute) with Krebs solution (see below for composition), 37°C, pH 7.4) and equilibrated for 1 h. Experiments were performed using Intracept Chart Software. The following parameters were measured: phasic contraction amplitude; phasic contraction frequency; area under the phasic contraction curve and tone. Measurements were made over a period of 10 min before and 3 min during exposure to a drug once the maximal effect was achieved. Data were normalized and expressed as % of control.
Figure 2.
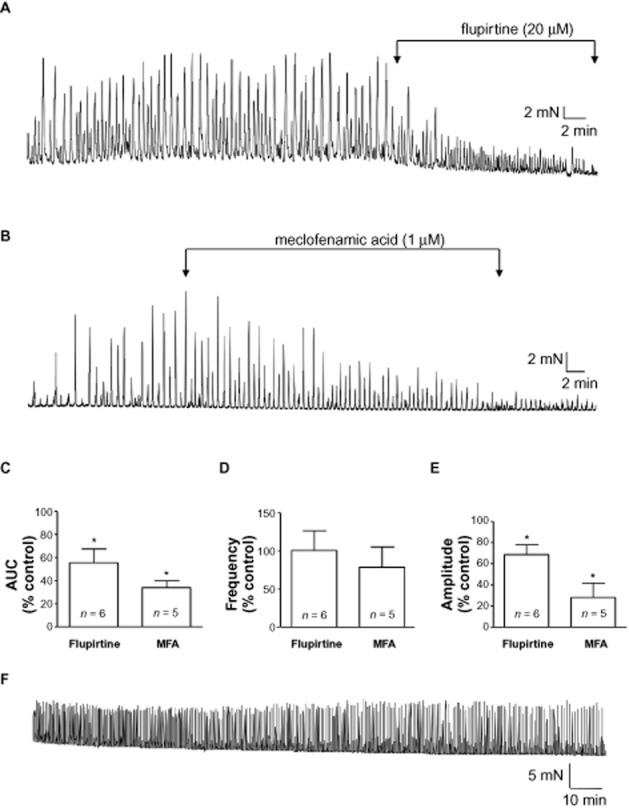
KCNQ channel openers reduce contractility of bladder strips. (A) Example of the reduction in contraction amplitude by the KCNQ opener, flupirtine (20 μM). (B) Meclofenamic acid (MFA; 1 μM) also reduced contraction amplitude. (C, D, E) Summary bar charts for the effects of flupirtine and MFA on contractility measured as area under curve (AUC), frequency and contraction amplitude respectively. All experiments were carried out in the presence of tetrodotoxin (1 μM). *P < 0.05, significantly different from control. (F) Trace from a time-dependent control showing maintenance of spontaneous activity over several hours.
Fluorescent calcium imaging
Preparations of guinea pig bladder containing several smooth muscle bundles were pinned to the Sylgard base of a recording chamber and loaded with Fluo-4 AM (Invitrogen; 1–5 μM in 0.03% Pluronic) for 30 min. Recordings commenced after preparations were perfused (2 mL·min−1) with HEPES-Krebs solution (see below for composition) at 35°C for at least 20 min. Tissues were imaged with a Nikon 80i upright epifluorescent imaging system equipped with an EMCCD camera (DQC-FS, Nikon UK Ltd., Kingston-upon-Thames, UK) via a water dipping objective lens. Data was recorded using WinFluor software (v3.2.25, Dr Dempster, University of Strathclyde) at a frame rate of 20–30 frames per second using 2 × 2 binning from WinFluor, which represented an acceptable compromise between acquisition speed and image resolution.
Offline analysis of Ca2+-oscillations involved drawing a region of interest (ROI) on the SMC and a ROI on part of the image where there were no active cells so that background fluorescence could be subtracted from all measurements. The background-corrected fluorescence (F) at any time point was normalized to baseline fluorescence (F0). F0 was calculated as average fluorescence during 100 frames when there was no activity. The frequency of events was measured in WinFluor and analyzed in Microsoft Excel and Prism software (v4.02, Graphpad, La Jolla, CA, USA).
Data analysis
Results from electrophysiological experiments are summarised as means ± SEM. Statistical comparisons were made using the Student's paired t-test or anova (where more than one intervention occurred) with P < 0.05 considered as significant. Data from the organ bath experiments are summarised as means ± SEM and analysed with Student's t-test, where P < 0.05 was considered as significant. The number of tissues is referred to as ‘n’; experimental series contained tissues from at least four animals. Data from the Ca2+ imaging are expressed as mean ± SEM, and statistical comparisons were made with anova or Student's paired t-test with P < 0.05 considered as significant. The number of observations in an experimental group is denoted as ‘n’. Data were analyzed with Origin (Origin Lab, Northampton, MA, USA), Microsoft Excel and Prism (Graphpad) software.
Materials
Solutions used were of the following compositions (mM):
Hanks' Ca2+-free solution: 141 Na+, 5.8 K+, 130.3 Cl−, 15.5 HCO3−, 0.34 HPO42−, 0.44 H2PO4−, 10 HEPES, 10 glucose and 2.9 sucrose, pH 7.40.
Hanks solution (PSS): 130 Na+, 5.8 K+, 135 Cl−, 4.16 HCO3−, 0.44 H2PO4−, 0.34 HPO42−, 0.4 SO42−, 1.8 Ca2+, 0.9Mg2+, 10 HEPES, 10 glucose and 2.9 sucrose, pH 7.40.
Modified pipette solution to eliminate contribution from Ca2+-activated K+ and KATP: 130 K+, 6.2 Na+, 110 gluconate, 21 Cl−, 0.5 Mg2+, 3 ATP (Na+ salt), 0.1 GTP, 5 HEPES, 5 EGTA, pH 7.2;
Cs+ pipette solution: 130 Cs+, 10.2 Na+, 131 Cl−, 0.5 Mg2+, 2.5 ATP (Na+ salt), 0.1 GTP, 2.5 phosphocreatine, 5 HEPES, 1 EGTA, pH 7.2.
Krebs solution for organ bath tension recordings: 146.2 Na+, 5.9 K+, 133.3 Cl, 25 HCO3−, 1.2 H2PO4−, 11 glucose, 2.5 Ca2+, 1.2 Mg2+ constantly gassed with 95% O2/5% CO2.
HEPES-Krebs for calcium imaging: 125 NaCl, 5.36 KCl, 11 glucose, 10 HEPES, 0.44 KH2PO4, 0.33 NaH2PO4, 1 MgCl2, 1.8 CaCl2.
Stock solutions of XE991, linopirdine (Tocris, Abingdon, UK), meclofenamic acid (Sigma, UK) were made in distilled water; chromanol 293B and flupirtine (Sigma) were dissolved in DMSO. The final concentration of DMSO was 0.1% which does not affect spontaneous activity in this preparation (Hashitani and Brading, 2003).
Results
Functional effect of compounds acting on KCNQ channels in guinea pig bladder strips
During equilibration, detrusor strips typically developed myogenic spontaneous contractions that were maintained for several hours. Experiments were carried out in the presence of 1 μM tetrodotoxin to eliminate any effect of the compounds tested on neuronal channels. Application of the KCNQ channel inhibitor XE991 (10 μM) (Wang et al., 1998; Zaczek et al., 1998) enhanced contractility (Figure 1A). XE991 significantly increased AUC and amplitude (P < 0.05, n = 13), but did not significantly affect frequency (P > 0.05, Figure 1D–F) or baseline tone (P > 0.05). Two other KCNQ channel inhibitors, linopirdine and chromanol 293B, also enhanced spontaneous activity (Figure 1B, C), significantly increasing amplitude and AUC (P < 0.05, n = 5 and n = 9 respectively, Figure 1D,F) but neither drug affected frequency (P > 0.05, Figure 1E) nor baseline tone (P > 0.05).
Figure 1.
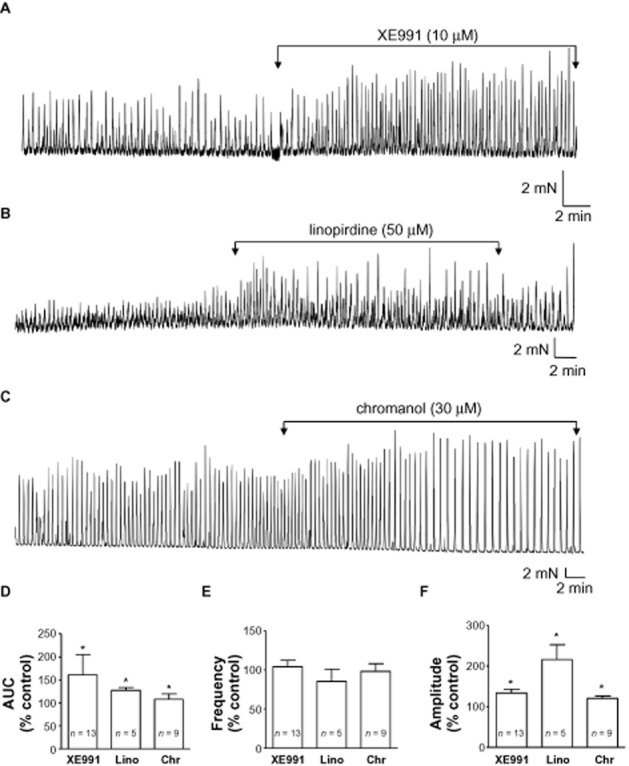
KCNQ channel inhibitors enhance spontaneous contractile activity of bladder strips. (A) An example of the effect of XE991 (10 μM) on myogenic spontaneous activity in a detrusor strip. (B) Linopirdine (Lino; 50 μM) also augmented the amplitude of spontaneous contractions. (C) Example of the increase in contraction amplitude by chromanol 293B (Chr; 30 μM). (D) Summary bar chart of the effect of KCNQ inhibitors on contractility measured as area under curve (AUC) and expressed relative to control (100%). (E) Summary bar chart of the effect of KCNQ inhibitors on frequency. (F) Summary of the effect of KCNQ inhibitors on contraction amplitude. All experiments were carried out in the presence of tetrodotoxin (1 μM). *P < 0.05, significantly different from control.
The anti-convulsant and muscle relaxant flupirtine (Friedel and Fitton, 1993; Kapetanovic et al., 1995), activates KCNQ currents, reducing excitability in neurons (Wladyka and Kunze, 2006). Yeung et al. (2007), demonstrated flupirtine-induced relaxation of pre-contracted blood vessels, similar to the structurally related drug, retigabine (Main et al., 2000; Tatulian et al., 2011; Wuttke et al., 2005). Flupirtine reduced spontaneous contraction amplitude in bladder strips (P < 0.05, n = 6, Figure 2), with little effect on frequency, but reduced baseline tone (n = 6, P < 0.05). Another KCNQ 2/3 activator meclofenamic acid (MFA) was tested (Peretz et al., 2005) and found to significantly reduce contraction amplitude, AUC and baseline tone (n = 5, P < 0.05; Figure 2B–E), without affecting frequency (P > 0.05). A time-dependent control demonstrating maintenance of spontaneous activity under these experimental conditions, in the absence of drugs, is shown in Figure 2F.
Effects of XE991 on calcium oscillations in SMC within tissue sheets
Fluo-4AM loaded bladder tissue preparations containing several smooth muscle bundles, exhibited Ca2+-oscillations and smaller localized events in individual SMC, in addition to coordinated whole bundle flashes (Figure 3). Flashes typically originated at one or both edges of the smooth muscle bundle and propagated to the opposite side. Post hoc x-t analysis of fluorescence intensity demonstrated travel of flashes across the width of the bundle (Figure 3A,C). Application of XE991 (10 μM) significantly enhanced the frequency of whole bundle Ca2+ flashes (Figure 3D, n = 5, P < 0.05). Analysis of Ca2+-oscillations in individual SMC within the bundle showed that large Ca2+-transients occurred along the length of the cell in addition to smaller, localized Ca2+ events (Figure 3B), both of which were increased by XE991.
Figure 3.
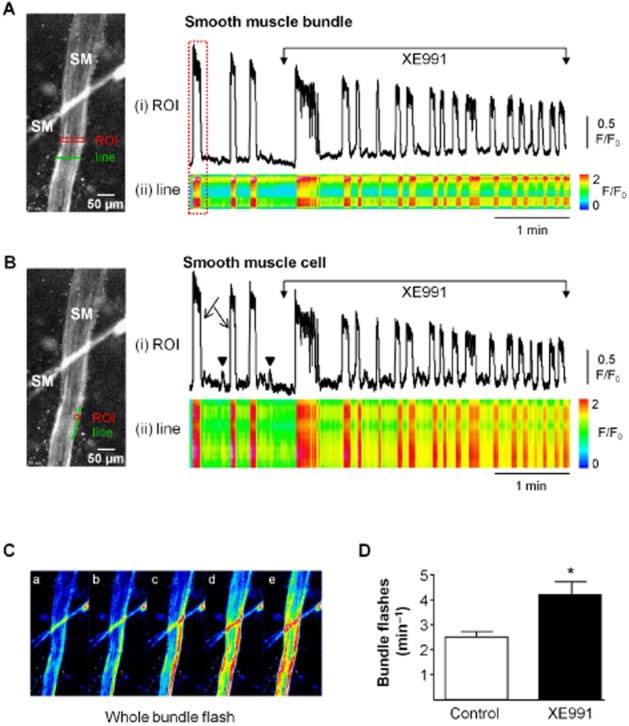
The effect of XE991 on calcium oscillations in SMC within tissue sheets. (A) Bladder tissue preparation loaded with the calcium indicator Fluo-4AM showing 2 smooth muscle bundles (SM). Activity was analysed with a region of interest (ROI) and a line drawn across the bundle. (i) Intensity–time plot of activity in the ROI showing XE991-induced (10 μM) enhancement in the frequency of whole bundle calcium flashes. (ii) Post hoc x-t analysis of fluorescence intensity demonstrates occurrence of the flashes across the width of the bundle. (B) (i) ROI analysis of the smooth muscle cell at the edge of the bundle as indicated on the micrograph showing that activity within a single smooth muscle cell is augmented by XE991. Large Ca2+-transients (arrow) occurred along the length of the cell, as shown in the line analysis below (ii). Smaller, localized Ca2+ events did not propagate along the cell length (arrowhead). Both types of Ca2+ signals were increased by XE991. (C) Consecutive frames from the whole bundle flash highlighted in A show the spread of Ca2+ signal across the bundle from right to left. (D) Summary bar chart of the significant increase in the frequency of smooth muscle bundle flashes by XE991. Control bar refers to pre-drug spontaneous activity. *P < 0.05, significantly different from control: n = 5.
Effects of XE991 on SMC outward currents and the r.m.p
Bladder SMC were voltage clamped using K+-filled pipettes containing 5 mM EGTA and 3 mM ATP to inhibit Ca2+-activated K+ and KATP channels and depolarized to evoke outward potassium currents. This approach was successfully used by Evans et al. (1996) and Yeung and Greenwood (2005), and avoids the use of channel blocking drugs, which may interfere with the conductance of interest. Typical outward currents evoked on stepping to +40 mV from a holding potential of −60 mV, followed by repolarization to −60 mV are shown in Figure 4A. To test the idea that a component of the outward current was mediated by KCNQ channels, XE991 (3, 10 and 30 μM) was applied to the cell. XE991 reduced the control current in a concentration-dependent manner with mean current reduced from 620pA ± 104pA to 507 ± 94pA by 10 μM XE991 (n = 7, P < 0.01), and further reduced to 402pA ± 34pA by 30 μM XE991 (P < 0.01). The XE991-sensitive current was obtained by subtracting the trace in 30 μM XE991 from the control trace (inset) showing the typical non-inactivating nature of the current. Current amplitude evoked at +40 mV in the presence of drug was normalized to control and mean current (± SEM) was plotted as % inhibition against concentration in Figure 4B. Data was fitted with a Hill equation (E/Emax = Emin + (Emax − Emin)[xn/(kn + xn)] where E/Emax was the relative response to XE991, kn was the IC50 and n was the Hill coefficient). The XE991 IC50 was 9.9 ± 0.003 μM.
Figure 4.
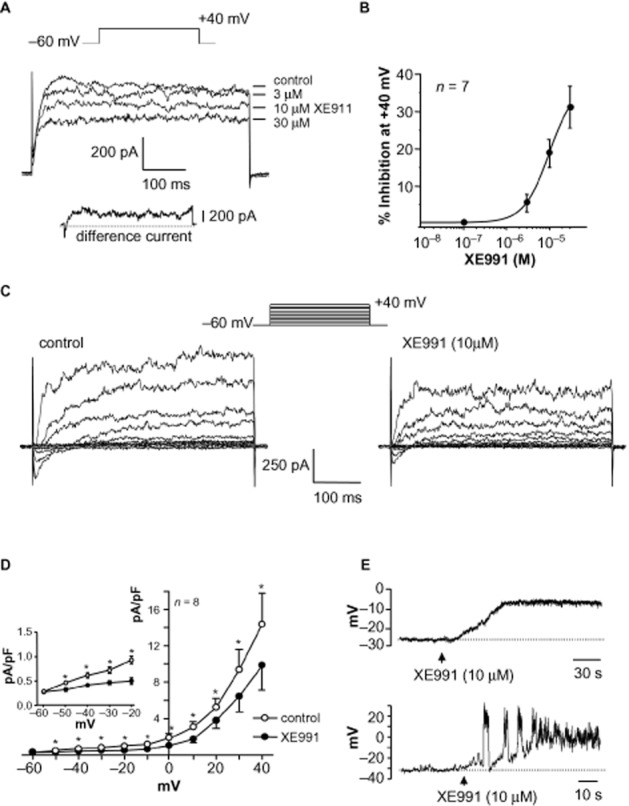
Effect of XE991 on outward current and resting membrane potential in SMC. (A) Currents were evoked by stepping from −60 mV to +40 mV using a pipette solution containing EGTA (5 mM) and ATP (3 mM) to eliminate BK and KATP currents. XE991 reduced the total outward current in a concentration-dependent fashion. The inset trace shows the non-inactivating XE991-sensitive current, obtained by subtracting the trace in 30 μM XE991 from the control trace. (B) Concentration–response curve for the effect of XE991 on peak outward current. Current amplitude in presence of drug was normalized to control and plotted as % inhibition. Mean data for seven cells was fitted with a Hill equation, which calculated the IC50 to be 9.9 ± 0.003 μM. (C) Family of currents evoked by stepping from −60 mV to a range of increasingly positive potentials as denoted in the voltage protocol. XE991 (10 μM) reduced the amplitude of outward currents across the voltage range. (D) Summary current density–voltage graph illustrating the effect of XE991 in eight cells. Inset shows the current density data for −50 mV to −20 mV on an expanded scale. *P < 0.05, significant effect of XE991. (E) An example of the depolarization caused by XE991 (10 μM) in a quiescent SMC (upper trace). The XE991-evoked depolarization was sometimes sufficient to evoke spontaneous transient depolarizations (lower trace).
XE991 (10 μM) reduced the amplitude of currents across the voltage range tested in Figure 4C. The current–voltage relationship of mean current density in the absence and presence of drug (n = 8) is shown in Figure 4D, with the inset showing current density at potentials between −50 and −20 mV. Although inhibition of outward current by XE991 occurred within 3 min, previous studies have shown variable time courses for maximal effect (Gu et al., 2005; Yeung and Greenwood, 2005; Joshi et al., 2006; Vervaeke et al., 2006). In the present study, XE991 was applied for up to 10 min, and its reduction of outward current was not reversed on washout.
As KCNQ currents have different sensitivities to tetraethylammonium (TEA) depending on their composition of specific KCNQ α-subunits (see Discussion), XE991 was tested in the presence of TEA. After TEA exposure (30 mM), XE991 (30 μM) significantly reduced the residual outward current from 60pA ± 10pA to 47pA ± 10pA (n = 5, P < 0.05). As the electrode solution contained 5 mM EGTA, any blocking effect of TEA would be expected to be on Kv and not BK, indicating that KCNQ subtypes were present, some of which were TEA-insensitive.
The action of KCNQ modulators in the contractile experiments could be explained by an effect on bladder SMC r.m.p. Bladder SMC studied in current clamp-mode had mean r.m.p. of −32 mV ± 2 (n = 14, range from −23 mV to −45 mV), and although the majority of cells were quiescent, spontaneous oscillations were sometimes observed (5/14 cells). XE991 (10 μM) produced membrane depolarization (mean 20 ± 3 mV; n = 6, P < 0.05, Figure 4E) that was maintained during drug application (up to 10 min, Figure 4E) and was irreversible. In 2/6 cells, this depolarization was sufficient to trigger transient depolarizations (figure 4E, lower panel).
Effect of other KCNQ channel inhibitors on SMC currents
The KCNQ inhibitor linopirdine (30 μM) produced effects similar to those of XE991 (Figure 5A) by reducing the outward current at +40 mV (n = 5, P < 0.05). Chromanol 293B, an open channel inhibitor that inhibits IKs in cardiac myocytes (Busch et al., 1996; Suessbrich et al., 1996) is specific for KCNQ1 channels (Bleich et al., 1997). Chromanol (30 μM) significantly decreased bladder SMC currents (n = 7, P < 0.05), as shown in the example and summary bar chart in Figures 5B & D. In current clamp, chromanol induced a mean depolarization of −6.4 ± 0.9 mV (P < 0.05, n = 5).
Figure 5.
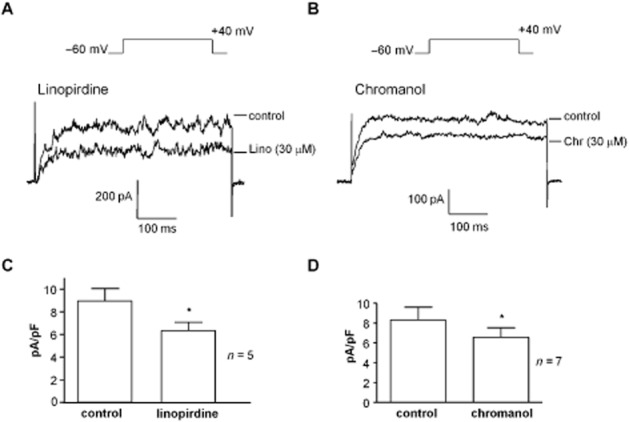
Effect of linopirdine and chromanol on SMC outward current. (A) Currents were evoked by stepping to +40 mV and were reduced by linopirdine (Lino; 30 μM). (B) Outward currents were reduced by chromanol 293B (Chr; 30 μM). (C, D) Summary bar charts of the significant reduction of SMC outward current by linopirdine (n = 5) and chromanol 293B (n = 7). *P < 0.05, significantly different from control.
KCNQ channel activators on SMC outward current and r.m.p
The KCNQ activator flupirtine (20 μM) increased current amplitude across the voltage range (Figure 6A). Summary data is presented in the current–voltage plot in Figure 6 (n = 6, P < 0.05). Flupirtine was also observed to reduce inward currents (Figure 6C) at the beginning of the current step, but enhanced the outward current (at 0 mV). The effect on inward current was examined using Cs+-filled pipettes, to eliminate K+ currents, and stepping to 0 mV, where the inward current was maximal. Figure 6C demonstrates the reduction (but not abolition) of inward Ca2+ current by flupirtine (20 μM), typical of six experiments. Flupirtine (20 μM) produced a reversible hyperpolarization (mean 5 ± 1 mV, n = 7, P < 0.0001) when applied for 30–40 s to quiescent cells (Figure 6D) and abolished activity in cells that had exhibited spontaneous transient depolarizations (Figure 6D right panel). MFA (20 μM) significantly increased current amplitude at +40 mV (n = 11, P < 0.05; Figure 6E, F). Unlike flupirtine, MFA had no apparent effect on inward currents.
Figure 6.
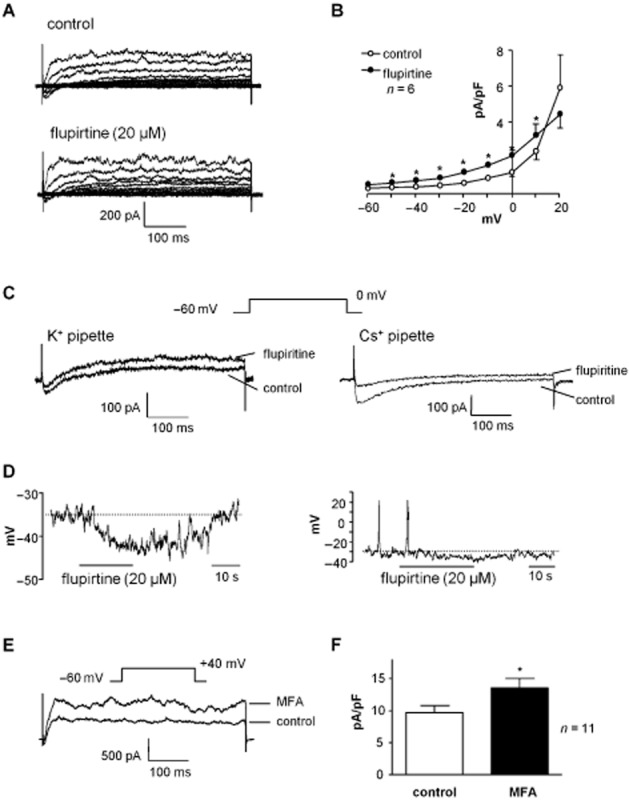
Effect of KCNQ activators on SMC currents and resting membrane potential. (A) Example of a family of outward currents in SMC enhanced by flupirtine (20 μM). (B) Summary of six similar experiments in which flupirtine significantly reduced the outward current at potentials between −50 and +10 mV. (C) The current evoked by stepping to 0 mV had both inward and outward components. Flupirtine reduced the inward component at the beginning of the trace and increased the outward current over the remainder of the sweep. Right panel shows an inward Ca2+ current (at 0 mV), which was recorded using Cs+-filled electrodes in order to eliminate outward K+ currents. Flupirtine reduced the inward current amplitude. (D) Example of a current-clamp recording where flupirtine (20 μM) caused a reversible membrane hyperpolarization in quiescent cells (left panel). Cells which exhibited spontaneous electrical activity in the absence of drugs (right panel) ceased firing on hyperpolarization induced by flupirtine. (E) Meclofenamic acid (MFA, 20 μM) increased amplitude of currents at +40 mV. (F) Summary bar chart showing significant enhancement of current amplitude by MFA in 11 cells. *P < 0.05, significantly different from control.
Molecular identification of KCNQ subtypes expressed in bladder cells
The molecular identification of KCNQ1-5 genes in dispersed guinea pig detrusor cells using end-point RT-PCR is shown in Figure 7. All five KCNQ genes were expressed in guinea pig detrusor cells. Bands of the expected sizes (KCNQ1, 434 bp; KCNQ2, 515 bp; KCNQ3, 461 bp; KCNQ4, 561 bp; KCNQ5, 200 bp) were amplified, as observed with ethidium bromide staining under UV light, and confirmed by sequencing. Amplification of KCNQ2 in guinea pig bladder resulted in two bands at 520 and 486 bp, which did not disappear upon optimization of primer annealing temperature, in agreement with Liang et al. (2006), who demonstrated alternative spliced forms of KCNQ2 with similar PCR band sizes. There is a small possibility that nerve fragments were present in the cell suspension and that neuronal or other cellular KCNQ channels contributed to the bands; however, this would be minimal, and the results are more likely to represent KCNQ transcripts in the concentrated SMC cell suspension.
Figure 7.
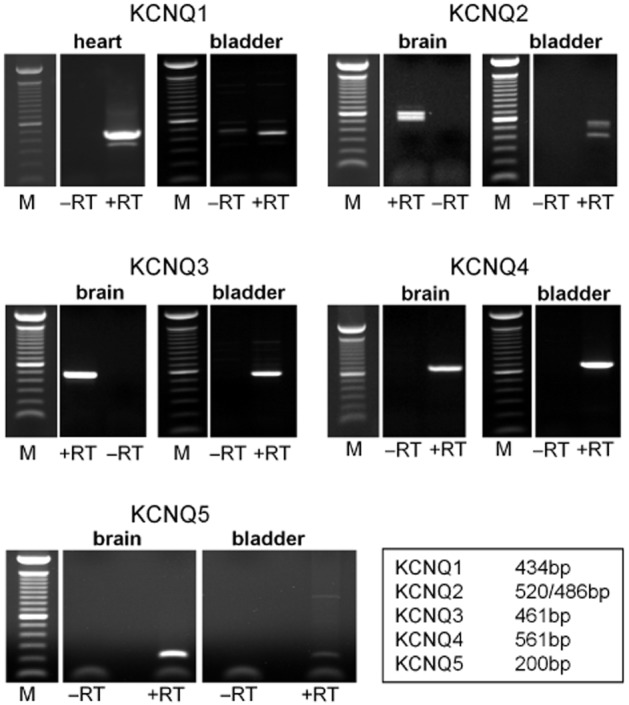
Identification of KCNQ genes in guinea pig bladder cells. RT-PCR analysis of KCNQ1-5 gene family members in RNA extracted from dispersed guinea pig detrusor cells. Guinea pig heart and brain tissues were used as positive controls. ‘+RT’ and ‘−RT’ represent the inclusion or omission of reverse transcriptase respectively in the reverse transcription of mRNA to cDNA process. Bands corresponding to KCNQ1-5, as indicated by the base pair table, were detected in guinea pig detrusor cells.
KCNQ protein subtypes expressed in bladder tissue
Immunohistochemical labelling of guinea pig bladder tissues with antibodies to the five KCNQ subtypes confirmed the expression of all five family members (Figure 8). The micrographs demonstrate the presence of KCNQ channels in SMC and IC, based on morphology and location from previous published work (McCloskey and Gurney, 2002). Successful double-labelling experiments with IC markers and KCNQ antibodies were not feasible due to differences in the fixatives required, but the images suggest that KCNQ1-5 are present in both SMC and IC, which are present on the edge of, and in the spaces between, smooth muscle bundles. KCNQ4 appears to be predominantly expressed in the SMC, as has been shown in vascular smooth muscle (Joshi et al., 2009). Significant fluorescence was not present in negative control tissues, where the primary antibody had been omitted (Figure 8F), or where no antibodies were present to control for tissue autofluorescence (Figure 8G). Control antigens were not available for the KCNQ4 and KCNQ5 antibodies, but pre-absorption with the antigen peptides used to generate the KCNQ1-3 antibodies also prevented staining. Positive controls were not carried out in the present study; a parallel study by some of the authors on pulmonary artery (Joshi et al., 2009) showed staining with a panel of KCNQ antibodies.
Figure 8.
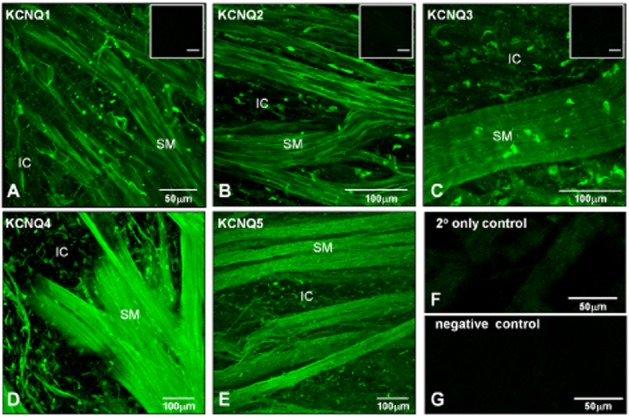
Detection of KCNQ gene expression by immunohistochemistry. (A–E) Whole-mount preparations of guinea pig detrusor were incubated with antibodies to KCNQ1-5 subtypes. Immunoreactivity was detected in both smooth muscle bundles (SM) and in IC adjacent to and between the smooth muscle bundles for the five KCNQ subtypes tested. Inset micrographs in (A–C) show minimal immunofluorescence in pre-absorption controls for KCNQ1-3; the scale bars indicate 50 μm. (F) Micrograph of secondary antibody only control slide, showing minimal immunofluorescence. (G) Micrograph of negative control (no antibodies) demonstrating absence of autofluorescence.
Discussion
The present study has provided molecular, cellular and tissue evidence that KCNQ channels are functionally expressed in guinea pig bladder. Patch-clamp experiments demonstrated that a component of outward current in bladder SMC was carried by KCNQ channels and that the r.m.p. was partly mediated by KCNQ currents. RT-PCR demonstrated gene expression of KCNQ1, 2, 3, 4 and 5 in SMC, and the corresponding proteins were immunolabelled in detrusor tissues.
Effect of KCNQ inhibitors
KCNQ currents from bladder SMC exhibited typical properties, including activation positive to −60 mV, voltage dependence, outward rectification, lack of inactivation and inhibition by XE991, linopirdine and chromanol 293B. XE991 and its less potent analogue linopirdine are open-channel blockers that directly interact with KCNQ channel protein rather than acting via G-protein or Ca2+-mediated messenger pathways (Costa and Brown, 1997; Lamas et al., 1997). XE991 and linopirdine are effective inhibitors of KCNQ/M currents, although they are not subtype specific (Aiken et al., 1995; Lamas et al., 1997; Wang et al., 1998). In bladder SMC, XE991 was more potent against outward current than linopirdine, consistent with the findings of others; XE991 inhibits KCNQ/M currents with an IC50 of 1 μM (Wang et al., 1998) compared with linopirdine, IC50 3.4–7.4 μM (Costa and Brown, 1997; Lamas et al., 1997). The findings with chromanol 293B indicate that KCNQ1 is also functionally active.
Effect of KCNQ activators
Flupirtine, and its more potent analogue, retigabine, increase KCNQ/M-like currents and reduce excitability in neurons (Tatulian et al., 2011; Martire et al., 2004; Peretz et al., 2005; Wladyka and Kunze, 2006), and act on all KCNQ subtypes except KCNQ1. MFA augments KCNQ2/3 channel activity by shifting the voltage activation curve leftward and slowing the deactivation kinetics, leading to membrane hyperpolarization (without affecting KCNQ1, Peretz et al., 2005). Flupirtine and MFA enhanced outward currents in bladder SMC, confirming the presence of some, or all of the KCNQ 2–5 subunits. The loss of effect of flupirtine at 20 mV and above is consistent with the voltage-dependent effect of its related analogue, retigabine, on neuronal KCNQ currents (Tatulian et al., 2011).
KCNQ and the r.m.p
The functional significance of KCNQ channels in guinea pig bladder was demonstrated in current-clamp studies. The experimental conditions where KATP channels and repolarizing Ca2+-activated K+ currents were minimized, resulted in spontaneous transient depolarizations with prolonged durations compared with action potentials. The inhibitors of KCNQ channels (XE991 and chromanol) reduced the SMC r.m.p and induced transient depolarizations. Consistent with this, flupirtine induced a hyperpolarization and simultaneous cessation of any firing. These findings suggest that in bladder SMC, KCNQ channels regulate excitability by acting as a brake on the repetitive firing of electrical activity.
KCNQ subtypes
Five gene members of the KCNQ family of ion channel proteins have been identified, each encoding a different KCNQ α-subunit (1–5). Considerable diversity in the expression of KCNQ has been noted between tissues, with channels expressed as heteromultimers (e.g. KCNQ2/KCNQ3) or homomultimers with accessory subunits, such as the KCNE or minimal K+ channel proteins (minK). Variation in the sensitivity of KCNQ1-5 subunits to TEA is a useful tool for elucidating the composition of KCNQ subunits in a given cell (Wang et al., 1998; Hadley et al., 2000; Shah et al., 2002). Hadley et al. (2000) demonstrated in transfected CHO cells that the TEA sensitivity of KCNQ2 was high (IC50 0.3 mM), whereas KCNQ3 (IC50 > 30 mM) had a low sensitivity, and intermediate sensitivities were found in KCNQ1 and KCNQ4 (IC50 5 mM and 3 mM respectively). KCNQ5 channels expressed in human brain had an IC50 value of 71 mM for TEA (Schroeder et al., 2000). In the present study, the reduction of the TEA-resistant (30 mM) current in bladder SMC by KCNQ blockers could be explained by an effect on KCNQ3 or KCNQ5 channel subunits, as this concentration blocks KCNQ 1, 2 and 4 but not 3 and 5 (Hadley et al., 2000). Taken together with our findings from the use of KCNQ activators and inhibitors, our results suggest that KCNQ1, 2, 3, 4 and 5 subunits may all be functionally expressed in SMC from the guinea pig bladder, consistent with the PCR and immunohistochemistry data. While we cannot be absolutely certain that residual BK currents were not present and therefore accounting for some of the TEA effect, the experimental conditions were set to minimize BK activity. This could be further explored in the future using bladder cells and tissue from BK knockout mice. To understand the relative contributions of the different subunits, it would be helpful to know which KCNQ subunits predominate in the SMC and which KCNE β subunits are present to modulate their activity.
Functional significance of KCNQ channels in bladder
KCNQ channels are important in setting vascular tone; Yeung et al. (2007) demonstrated relaxation of pre-contracted murine aorta with flupirtine and MFA. In contrast, KCNQ inhibitors constricted rat mesenteric (Mackie et al., 2008) and rat or mouse intrapulmonary arteries (Joshi et al., 2006). Here, the putative functional role of KCNQ channels in bladder was demonstrated in bladder strips, where myogenic spontaneous contractions were affected by KCNQ drugs, indicating that KCNQ channels are active under these conditions. These findings are consistent with the current-clamp studies of isolated cells, in which KCNQ inhibitors reduced the membrane potential and often elicited transient depolarizations, whereas KCNQ activators induced hyperpolarization and simultaneous cessation of any spontaneous transient depolarizations. We interpret the reduction in contractility induced by flupirtine with a degree of caution, however, as this could be partly explained by its effect on L-type Ca2+ currents.
The effects of KCNQ channel inhibitors and activators on bladder strip contractility are reasonably explained by a direct action on SMC and not arising from depolarization of intramural nerves leading to neurotransmitter release, as experiments were performed in the presence of tetrodotoxin. Interestingly, the compounds tested did affect contraction amplitude and AUC analysis but had no overall significant effect on frequency. Moreover, KCNQ channel inhibitors did not significantly enhance baseline tone, typical of other phasic smooth muscle tissues, such as uterus (McCallum et al., 2009) and colon (Jepps et al., 2009). Ca2+ experiments demonstrated that at the single cell level and within smooth muscle bundles, XE991 enhanced the frequency of Ca2+-oscillations consistent with the depolarization found in current clamp experiments. Therefore, in normal bladder, KCNQ channels seem to provide a fine-tuning control mechanism over smooth muscle excitability, where inhibition of KCNQ channels can augment spontaneous contractions but is not sufficient to cause sustained contraction, which would be undesirable during bladder filling. The arrangement of smooth muscle in the bladder, comprising interlocking bundles of SMC rather than a sheet arrangement, as is found in the vasculature or the gut, prevents spread of excitation across the wall and subsequent coordinated contractions.
We previously reported KCNQ currents in detrusor IC and found that they contributed to the r.m.p. (Anderson et al., 2009). Although the functional roles of detrusor IC are not yet fully understood, if they do act to modulate smooth muscle activity, KCNQ channels would provide one ionic mechanism of controlling their excitability. Streng et al. (2004) provided evidence for the functional role of KCNQ channels in the bladder in in vivo experiments on rats, where intravesical administration of the KCNQ channel opener, retigabine, decreased baseline bladder pressures in rat detrusor, increased micturition volume and decreased capsaicin-induced detrusor overactivity. Recent studies of KCNQ inhibitors and activators on rat bladder demonstrate effects on contractility (Rode et al., 2010; Svalø et al., 2001). Our study supports these findings and provides the first direct electrophysiological evidence for the existence of KCNQ channels in bladder SMC. It seems feasible that the development of bladder-specific KCNQ openers could provide treatment options for the overactive bladder. Moreover, gene mutations of KCNQ subunits may well underlie bladder disorders, as with some cardiovascular, nervous and auditory pathologies.
Commercially available compounds were used here, and we interpret the results within the normal understanding of pharmacological specificity. These compounds are widely used in research into cardiovascular or neuronal KCNQ channels and are established as reliable inhibitors or activators of KCNQ channels (see Miceli et al., 2008; Brown and Passmore, 2009). We could not be certain that the KCNQ inhibitors and activators would not affect other ion channels in bladder SMC and therefore designed our experimental conditions to eliminate other K+ currents where possible. The use of a panel of KCNQ channel inhibitors and activators strengthens the evidence that the effects observed were due to KCNQ channel modulation and are less likely to be non-selective actions on other voltage-dependent currents.
The current findings advance our knowledge of the diversity of K+ channel expression in bladder smooth muscle. The roles of BK, SK, delayed rectifier and KATP channels in bladder electrical activity are well established, yet there are more K+ channels present, including novel TASK (Beckett et al., 2008) and TREK channels (Baker et al., 2008), in addition to the KCNQ channels. Clearly, bladder SMC contain a complement of K+ channels that can be finely tuned to work together in response to the physiological requirements of the organ during filling and micturition. Modulation of KCNQ by muscarinic neurotransmitters in the bladder presents an intriguing area of future work; this mechanism is well established for the neuronal KCNQ M current, where muscarinic stimulation inhibits KCNQ channel activity.
In conclusion, we have presented here the first electrophysiological evidence for KCNQ currents in bladder SMC and their role in control of the r.m.p. Evidence for gene and cellular expression of five members of the KCNQ family in the guinea pig bladder detrusor is also presented. The activity of KCNQ channel inhibitors and activators on bladder strips suggest that KCNQ currents have a novel role in the regulation of bladder contractility.
Acknowledgments
Financial support from The Wellcome Trust (grant 074591/Z/04Z) is gratefully acknowledged. C Carson was supported by a DEL studentship from Queen's University Belfast.
Experiments were performed by U. A. A., C. C., L. J. and S. J. Study concept and design by K. D. M. and A. M. G. Data analysis and interpretation, all authors. Manuscript was drafted by K. D. M., U. A. A. and C. C. and was critically evaluated by all authors.
Glossary
- BK
large conductance calcium activated potassium channel
- IC
interstitial cell
- IK
intermediate conductance calcium activated potassium channel
- K2P
two-pore domain potassium channels
- KATP
ATP-sensitive potassium channel
- Kv
voltage-gated potassium channels
- MFA
meclofenamic acid
- SK
small conductance calcium activated potassium channel
- SMC
smooth muscle cell
- TTX
tetrodotoxin
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- Aiken SP, Lampe BJ, Murphy PA, Brown BS. Reduction of spike frequency adaptation and blockade of M-current in rat CA1 pyramidal neurones by linopirdine (DuP 996), a neurotransmitter release enhancer. Br J Pharmacol. 1995;115:1163–1168. doi: 10.1111/j.1476-5381.1995.tb15019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson UA, Carson C, McCloskey KD. KCNQ currents and their contribution to resting membrane potential and the excitability of interstitial cells of Cajal from the guinea pig bladder. J Urol. 2009;182:330–336. doi: 10.1016/j.juro.2009.02.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SA, Hennig GW, Han J, Britton FC, Smith TK, Koh SD. Methionine and its derivatives increase bladder excitability by inhibiting stretch-dependent K+ channels. Br J Pharmacol. 2008;153:1259–1271. doi: 10.1038/sj.bjp.0707690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett EA, Han I, Baker SA, Han J, Britton FC, Koh SD. Functional and molecular identification of pH-sensitive K+ channels in murine urinary bladder smooth muscle. BJU Int. 2008;102:113–124. doi: 10.1111/j.1464-410X.2008.07541.x. [DOI] [PubMed] [Google Scholar]
- Biervert C, Schroeder BC, Kubisch C, Berkovic SF, Propping P, Jentsch TJ, et al. A potassium channel mutation in neonatal human epilepsy. Science. 1998;279:403–406. doi: 10.1126/science.279.5349.403. [DOI] [PubMed] [Google Scholar]
- Bleich M, Briel M, Busch AE, Lang HJ, Gerlach U, Gögelein H, et al. KvLQT channels are inhibited by the K+ channel blocker 293B. Pflugers Arch. 1997;434:499–501. doi: 10.1007/s004240050427. [DOI] [PubMed] [Google Scholar]
- Bonev AD, Nelson MT. ATP-sensitive potassium channels in smooth muscle cells from guinea pig urinary bladder. Am J Physiol. 1993;264:C1190–C2000. doi: 10.1152/ajpcell.1993.264.5.C1190. [DOI] [PubMed] [Google Scholar]
- Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. Br J Pharmacol. 2009;156:1185–1195. doi: 10.1111/j.1476-5381.2009.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch AE, Suessbrich H, Waldegger S, Sailer E, Greger R, Lang H, et al. Inhibition of IKs in guinea pig cardiac myocytes and guinea pig IsK channels by the chromanol 293B. Pflugers Arch. 1996;432:1094–1096. doi: 10.1007/s004240050240. [DOI] [PubMed] [Google Scholar]
- Carson C, McCloskey KD. Potassium channels and spontaneous activity in guinea-pig bladder. 2007. Proc Life Sciences PC572.
- Costa AM, Brown BS. Inhibition of M-current in cultured rat superior cervical ganglia by linopirdine: mechanism of action studies. Neuropharmacology. 1997;36:1747–1753. doi: 10.1016/s0028-3908(97)00155-x. [DOI] [PubMed] [Google Scholar]
- Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci. 2005;6:850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- Evans AM, Osipenko ON, Gurney AM. Properties of a novel K+ current that is active at resting potential in rabbit pulmonary artery smooth muscle cells. J Physiol. 1996;496:407–420. doi: 10.1113/jphysiol.1996.sp021694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedel HA, Fitton A. Flupirtine. A review of its pharmacological properties, and therapeutic efficacy in pain states. Drugs. 1993;45:548–569. doi: 10.2165/00003495-199345040-00007. [DOI] [PubMed] [Google Scholar]
- Gu N, Vervaeke K, Hu H, Storm JF. Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells. J Physiol. 2005;566:689–715. doi: 10.1113/jphysiol.2005.086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley JK, Noda M, Selyanko AA, Wood IC, Abogadie FC, Brown DA. Differential tetraethylammonium sensitivity of KCNQ1-4 potassium channels. Br J Pharmacol. 2000;129:413–415. doi: 10.1038/sj.bjp.0703086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Brading AF. Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. Br J Pharmacol. 2003;140:159–169. doi: 10.1038/sj.bjp.0705320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera GM, Nelson MT. Differential regulation of SK and BK channels by Ca2+ signals from Ca2+ channels and ryanodine receptors in guinea-pig urinary bladder myocytes. J Physiol. 2002;541:483–492. doi: 10.1113/jphysiol.2002.017707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi Y, Torii Y, Ohi Y, Nagano N, Atsuki K, Yamamura H, et al. Ca2+ images and K+ current during depolarization in smooth muscle cells of the guinea-pig vas deferens and urinary bladder. J Physiol. 1998;510:705–719. doi: 10.1111/j.1469-7793.1998.705bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci. 2000;1:1–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- Jepps TA, Greenwood IA, Moffatt JD, Sanders KM, Ohya S. Molecular and functional characterization of Kv7 K+ channel in murine gastrointestinal smooth muscles. Am J Physiol Gastrointest Liver Physiol. 2009;297:G107–G115. doi: 10.1152/ajpgi.00057.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Balan P, Gurney AM. Pulmonary vasoconstrictor action of KCNQ potassium channel blockers. Respir Res. 2006;7:1–10. doi: 10.1186/1465-9921-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Sedivy V, Hodyc D, Herget J, Gurney AM. KCNQ modulators reveal a key role for KCNQ potassium channels in regulating the tone of rat pulmonary artery smooth muscle. J Pharmacol Exp Ther. 2009;329:368–376. doi: 10.1124/jpet.108.147785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapetanovic IM, Yonekawa WD, Kupferberg HJ. The effects of D-23129, a new experimental anticonvulsant drug, on neurotransmitter amino acids in the rat hippocampus in vitro. Epilepsy Res. 1995;22:67–73. doi: 10.1016/0920-1211(95)00050-x. [DOI] [PubMed] [Google Scholar]
- Kharkovets T, Hardelin JP, Safieddine S, Schweizer M, El-Amraoui A, Petit C, et al. KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proc Natl Acad Sci U S A. 2000;97:4333–4338. doi: 10.1073/pnas.97.8.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubisch C, Schroeder BC, Friedrich T, Lütjohann B, El-Amraoui A, Marlin S, et al. and Jentsch TJ KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell. 1999;96:437–446. doi: 10.1016/s0092-8674(00)80556-5. [DOI] [PubMed] [Google Scholar]
- Lamas JA, Selyanko AA, Brown DA. Effects of a cognition-enhancer, linopirdine (DuP 996), on M-type potassium currents (IK(M)) and some other voltage- and ligand-gated membrane currents in rat sympathetic neurons. Eur J Neurosci. 1997;9:605–616. doi: 10.1111/j.1460-9568.1997.tb01637.x. [DOI] [PubMed] [Google Scholar]
- Liang GH, Jin Z, Ulfendahl M, Järlebark L. Molecular analyses of KCNQ1-5 potassium channel mRNAs in rat and guinea pig inner ears: expression, cloning, and alternative splicing. Acta Otolaryngol. 2006;126:6–52. doi: 10.1080/00016480500416777. [DOI] [PubMed] [Google Scholar]
- McCallum LA, Greenwood IA, Tribe RM. Expression and function of K(v)7 channels in murine myometrium throughout oestrous cycle. Pflugers Arch. 2009;457:1111–1120. doi: 10.1007/s00424-008-0567-5. [DOI] [PubMed] [Google Scholar]
- McCloskey KD, Gurney AM. Kit positive cells in the guinea pig bladder. J Urol. 2002;168:832–836. [PubMed] [Google Scholar]
- Mackie AR, Brueggemann LI, Henderson KK, Shiels AJ, Cribbs LL, Scrogin KE, et al. Vascular KCNQ potassium channels as novel targets for the control of mesenteric artery constriction by vasopressin. J Pharmacol Exp Ther. 2008;325:475–483. doi: 10.1124/jpet.107.135764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main MJ, Cryan JE, Dupere JR, Cox B, Clare JJ, Burbidge SA. Modulation of KCNQ2/3 potassium channels by the novel anticonvulsant retigabine. Mol Pharmacol. 2000;58:53–62. doi: 10.1124/mol.58.2.253. [DOI] [PubMed] [Google Scholar]
- Martire M, Castaldo P, D'Amico M, Preziosi P, Annunziato L, Taglialatela M. M channels containing KCNQ2 subunits modulate norepinephrine, aspartate, and GABA release from hippocampal nerve terminals. J Neurosci. 2004;24:592–597. doi: 10.1523/JNEUROSCI.3143-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli F, Soldovieri MV, Martire M, Taglialatela M. Molecular pharmacology and therapeutic potential of neuronal Kv7-modulating drugs. Curr Opin Pharmacol. 2008;8:65–74. doi: 10.1016/j.coph.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Ohya S, Kimura S, Kitsukawa M, Muraki K, Watanabe M, Imaizumi Y. SK4 encodes intermediate conductance Ca2+-activated K+ channels in mouse urinary bladder smooth muscle cells. Jpn J Pharmacol. 2000;84:97–100. doi: 10.1254/jjp.84.97. [DOI] [PubMed] [Google Scholar]
- Ohya S, Asakura K, Muraki K, Watanabe M, Imaizumi Y. Molecular and functional characterization of ERG, KCNQ, and KCNE subtypes in rat stomach smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2002;282:G277–G287. doi: 10.1152/ajpgi.00200.2001. [DOI] [PubMed] [Google Scholar]
- Ohya S, Sergeant GP, Greenwood IA, Horowitz B. Molecular variants of KCNQ channels expressed in murine portal vein myocytes. Circ Res. 2003;92:1016–1023. doi: 10.1161/01.RES.0000070880.20955.F4. [DOI] [PubMed] [Google Scholar]
- Peretz A, Nachman R, Uziyel Y, Gibor G, Shabat D, Attali B. Meclofenamic acid and diclofenac, novel templates of KCNQ2/Q3 potassium channel openers, depress cortical neuron activity and exhibit anticonvulsant properties. Mol Pharmacol. 2005;67:1053–1066. doi: 10.1124/mol.104.007112. [DOI] [PubMed] [Google Scholar]
- Petkov GV. Role of potassium ion channels in detrusor smooth muscle function and dysfunction. Nat Rev Urol. 2011;9:30–40. doi: 10.1038/nrurol.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. Beta1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol. 2001a;537:443–452. doi: 10.1111/j.1469-7793.2001.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov GV, Heppner TJ, Bonev AD, Herrera GM, Nelson MT. Low levels of K(ATP) channel activation decrease excitability and contractility of urinary bladder. Am J Physiol Regul Integr Comp Physiol. 2001b;280:R1427–R1433. doi: 10.1152/ajpregu.2001.280.5.R1427. [DOI] [PubMed] [Google Scholar]
- Rennie KJ, Weng T, Correia MJ. Effects of KCNQ channel blockers on K+ currents in vestibular hair cells. Am J Physiol Cell Physiol. 2001;280:C473–C480. doi: 10.1152/ajpcell.2001.280.3.C473. [DOI] [PubMed] [Google Scholar]
- Robbins J. KCNQ potassium channels: physiology, pathophysiology, and pharmacology. Pharmacol Ther. 2001;90:1–19. doi: 10.1016/s0163-7258(01)00116-4. [DOI] [PubMed] [Google Scholar]
- Rode F, Svalø J, Sheykhzade M, Rønn LC. Functional effects of the KCNQ modulators retigabine and XE991 in the rat urinary bladder. Eur J Pharmacol. 2010;638:121–127. doi: 10.1016/j.ejphar.2010.03.050. [DOI] [PubMed] [Google Scholar]
- Romey G, Attali B, Chouabe C, Abitbol I, Guillemare E, Barhanin J, et al. Molecular mechanism and functional significance of the MinK control of the KvLQT1 channel activity. J Biol Chem. 1997;272:16713–16716. doi: 10.1074/jbc.272.27.16713. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, et al. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- Schroeder BC, Kubisch C, Stein V, Jentsch TJ. Moderate loss of function of cyclic-AMP-modulated KCNQ2/KCNQ3 K+ channels causes epilepsy. Nature. 1998;396:687–690. doi: 10.1038/25367. [DOI] [PubMed] [Google Scholar]
- Schroeder BC, Hechenberger M, Weinreich F, Kubisch C, Jentsch TJ. KCNQ5, a novel potassium channel broadly expressed in brain, mediates M-currents. J Biol Chem. 2000;275:24089–24095. doi: 10.1074/jbc.M003245200. [DOI] [PubMed] [Google Scholar]
- Seebohm G, Pusch M, Chen J, Sanguinetti MC. Pharmacological activation of normal and arrhythmia-associated mutant KCNQ1 potassium channels. Circ Res. 2003;93:941–947. doi: 10.1161/01.RES.0000102866.67863.2B. [DOI] [PubMed] [Google Scholar]
- Selyanko AA, Hadley JK, Brown DA. Properties of single M-type KCNQ2/KCNQ3 potassium channels expressed in mammalian cells. J Physiol. 2001;534:15–24. doi: 10.1111/j.1469-7793.2001.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MM, Mistry M, Marsh SJ, Brown DA, Delmas P. Molecular correlates of the M-current in cultured rat hippocampal neurons. J Physiol. 2002;544:29–37. doi: 10.1113/jphysiol.2002.028571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søgaard R, Ljungstrøm T, Pedersen KA, Olesen SP, Jensen BS. KCNQ4 channels expressed in mammalian cells: functional characteristics and pharmacology. Am J Physiol Cell Physiol. 2001;280:C859–C866. doi: 10.1152/ajpcell.2001.280.4.C859. [DOI] [PubMed] [Google Scholar]
- Streng T, Christoph T, Andersson KE. Urodynamic effects of the K+ channel (KCNQ) opener retigabine in freely moving, conscious rats. J Urol. 2004;172:2054–2058. doi: 10.1097/01.ju.0000138155.33749.f4. [DOI] [PubMed] [Google Scholar]
- Suessbrich H, Bleich M, Ecke D, Rizzo M, Waldegger S, Lang F, et al. Specific blockade of slowly activating I(sK) channels by chromanols – impact on the role of I(sK) channels in epithelia. FEBS Lett. 1996;396:271–275. doi: 10.1016/0014-5793(96)01113-1. [DOI] [PubMed] [Google Scholar]
- Svalø J, Hansen HH, Rønn LC, Sheykhzade M, Munro G, Rode F. Kv7 positive modulators reduce detrusor overactivity and increase bladder capacity in rats. Basic Clin Pharmacol Toxicol. 2001;110:145–153. doi: 10.1111/j.1742-7843.2011.00765.x. [DOI] [PubMed] [Google Scholar]
- Tatulian L, Delmas P, Abogadie FC, Brown DA. Activation of expressed KCNQ potassium currents and native neuronal M-type potassium currents by the anti-convulsant drug retigabine. J Neurosci. 2011;21:5535–5545. doi: 10.1523/JNEUROSCI.21-15-05535.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneloe KS, Nelson MT. Properties and molecular basis of the mouse urinary bladder voltage-gated K+ current. J Physiol. 2003;549:65–74. doi: 10.1113/jphysiol.2003.039859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner WH, Brading AF. Smooth muscle of the bladder in the normal and the diseased state: pathophysiology, diagnosis and treatment. Pharmacol Ther. 1997;75:77–110. doi: 10.1016/s0163-7258(97)00038-7. [DOI] [PubMed] [Google Scholar]
- Vervaeke K, Gu N, Agdestein C, Hu H, Storm JF. Kv7/KCNQ/M-channels in rat glutamatergic hippocampal axons and their role in regulation of excitability and transmitter release. J Physiol. 2006;576:235–256. doi: 10.1113/jphysiol.2006.111336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, et al. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, VanRaay TJ, et al. Positional cloning of a novel potassium channel gene: KvLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- Wladyka CL, Kunze DL. KCNQ/M-currents contribute to the resting membrane potential in rat visceral sensory neurons. J Physiol. 2006;575:175–189. doi: 10.1113/jphysiol.2006.113308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuttke TV, Seebohm G, Bail S, Maljevic S, Lerche H. The new Anticonvulsant retigabine favors voltage-dependent opening of the Kv7.2 (KCNQ2) channel by binding to its activation gate. Mol Pharmacol. 2005;67:1009–1017. doi: 10.1124/mol.104.010793. [DOI] [PubMed] [Google Scholar]
- Yeung SY, Pucovský V, Moffatt JD, Saldanha L, Schwake M, Ohya S, et al. Molecular expression and pharmacological identification of a role for K(v)7 channels in murine vascular reactivity. Br J Pharmacol. 2007;151:758–770. doi: 10.1038/sj.bjp.0707284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung SYM, Greenwood IA. Electrophysiological and functional effects of the KCNQ channel blocker XE991 on murine portal vein smooth muscle cells. Br J Pharmacol. 2005;146:585–595. doi: 10.1038/sj.bjp.0706342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaczek R, Chorvat RJ, Saye JA, Pierdomenico ME, Maciag CM, Logue AR, et al. Two new potent neurotransmitter release enhancers, 10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone and 10,10-bis(2-fluoro-4-pyridinylmethyl)-9(10H)-anthracenone: comparison to linopirdine. J Pharmacol Exp Ther. 1998;285:724–730. [PubMed] [Google Scholar]


