Abstract
This study determined the effects of fucoxanthin on gene expressions related to lipid metabolism in rats with a high-fat diet. Rats were fed with normal fat diet (NF, 7% fat) group, high fat diet group (HF, 20% fat), and high fat with 0.2% fucoxanthin diet group (HF+Fxn) for 4 weeks. Body weight changes and lipid profiles in plasma, liver, and feces were determined. The mRNA expressions of transcriptional factors such as sterol regulatory element binding protein (SREBP)-1c, Carnitine palmitoyltransferase-1 (CPT1), Cholesterol 7α-hydroxylase1 (CYP7A1) as well as mRNA expression of several lipogenic enzymes were determined. Fucoxanthin supplements significantly increased plasma high density lipoprotein (HDL) concentration (P < 0.05). The hepatic total lipids, total cholesterols, and triglycerides were significantly decreased while the fecal excretions of total lipids, cholesterol, and triglycerides were significantly increased in HF+Fxn group (P < 0.05). The mRNA expression of hepatic Acetyl-CoA carboxylase (ACC), Fatty acid synthase (FAS), and Glucose-6-phosphate dehydrogenase (G6PDH) as well as SREBP-1C were significantly lower in HF+Fxn group compared to the HF group (P < 0.05). The hepatic mRNA expression of Hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) and Acyl-CoA cholesterol acyltransferase (ACAT) were significantly low while lecithin-cholesterol acyltransferase (LCAT) was significantly high in the HF+Fxn group (P < 0.05). There was significant increase in mRNA expression of CPT1 and CYP7A1 in the HF+Fxn group, compared to the HF group (P < 0.05). In conclusion, consumption of fucoxanthin is thought to be effective in improving lipid and cholesterol metabolism in rats with a high fat diet.
Keywords: Fucoxanthin, SREBP-1C, CYP7A1, CPT1, lipogenesis
Introduction
Excessive consumption of high-fat diet alters lipid metabolism stimulating β-oxidation of fatty acid, leading to an overall increase in hepatic free fatty acid, causing the development of nonalcoholic fatty liver disease (NAFLD) [1,2]. NAFLD affects approximately 20-30% of the population in developed countries and is a common finding in patients with metabolic syndrome [3].
Fucoxanthin is one of the most abundant carotenoids, and contributes more than 10% of the estimated total production of carotenoids in nature, especially in the marine environment [4]. Fucoxanthin is an orange-colored pigment and has an unusual allenic bonds which gives unique properties [5-7]. Some non-allenic carotenoids, such as lutein, lutein epoxide, and β-carotene 5,6-epoxide, were found to not exhibit the suppressive effect of fat accumulation in adipose tissue or in liver, indicating that the allene bond of fucoxanthin and its metabolites might be important for the inhibition of fat deposit [8,9]. It was found that fucoxanthin significantly reduced plasma and hepatic triglyceride concentrations and cholesterol-regulating enzyme activities, and fecal triglyceride and cholesterol [10-16], as well as fatty acid oxidation enzyme activity in epididymal white adipose tissue of mice [6,11].
The potential lipid lowering effect might be mediated by down-regulating various lipogenic enzyme activities and up-regulating fatty acid β-oxidation activity suggesting that fucoxanthin might act as a regulator of lipid metabolism in fat tissues. Fatty acid biosynthesis is divided into two steps. First step involves the carboxylation of acetyl CoA into malonyl CoA by Acetyl-CoA carboxylase. The second step is the elongation process into long-chain fatty acid catalyzed by fatty acid synthase [17]. In cholestrol metabolism, HMG-CoA is an important enzyme that regulates cholesterol synthesis and decline in this enzymatic activity decreases cholesterol levels both in the plasma and liver. Another important step in cholesterol metabolism is the conversion of cholesterol into bile acid through cytochrome P450-meidiated oxidation. Cytochrome P450 7A1 (CYP7A1) is the rate-limiting enzyme for the dominant pathway of bile acid synthesis from cholesterol [18]. Although several studies focused on lipid lowering functions of fucoxanthin [10-16], the effect of fucoxanthin on mRNA expression of transcriptional factors and enzymes related with lipid or cholesterol-lowering enzymes in liver have been poorly clarified. Hence this study sought to investigate the effects of fucoxanthin on gene expressions related to lipid and cholesterol metabolism in rats with a high fat diet.
Materials and Methods
Animals and diets
Four week old male Sprague-Dawley (Central Lab. Animal Inc., Seoul, Korea) rats were maintained on a conventional diet for 3 days and then divided into two groups using a randomized block design according to weight. Rats were fed with either normal fat diet or high fat diet for 1 week. A normal fat (NF) diet contained 7% fat (soybean oil) and the high fat (HF) diet consisted of 20% fat (13% lard and 7% soybean oil). After 1 week, rats of high fat diet group were divided into two groups: high fat diet only (HF diet) and high fat diet with 0.2% fucoxanthin (HF+Fxn diet). Rats were maintained NF diet, HF diet, and HF+Fxn diet for additional 4 weeks. Fucoxanthin powder extracted from dried seaweed (Undaria pinnatifida) was purchased from Amicogen (Amicogen Inc, Jinju, Korea) and the fucoxanthin powder mainly consisted of glycolipids containing 5% fucoxanthin. The composition (g/kg diet) of the diets is shown in Table 1. Rats were maintained individually in cages with wire-mesh bottoms in conditioned rooms (temperature, 23℃ relative humidity, 55%). All animal studies were conducted in accordance with the Dankook University ethics committee guidelines for the care and use of laboratory animals (approval code: 12-004).
Table 1.
Compositions of the diet (g/kg diet)
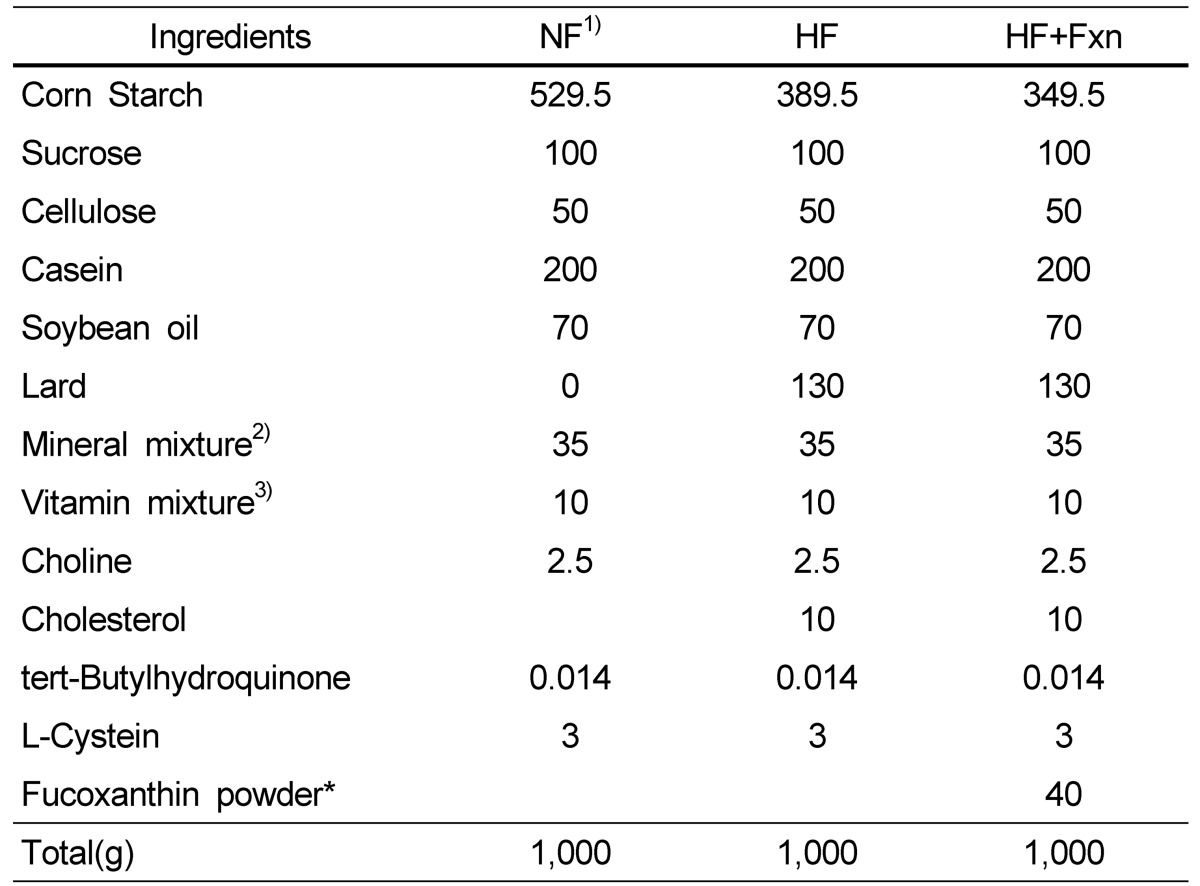
1)NF; normal fat diet, HF; high fat diet, HF + Fxn; high fat diet + 0.2% fucoxanthin (*Fucoxanthin powder contained 5% fucoxanthin extracted from seaweeds)
2)Mineral mixture (per kg): Calcium carbonate anhydrous, 357 g; Potassium phosphate monobasic, 196 g; Potassium citrate tripotassium monohydrate, 70.78 g; Potassium sulfate Sodium chloride, 74 g: Magnesium oxide, 24 g; Ferric citrate, 6.06 g; Zinc carbonate, 1.65 g; Sodium meta-silicate, 1.45 g; Manganous carbonate, 0.63 g; Cupric carbonate, 0.30 g; Chromium potassium sulfate, 0.275 g; Boric acid, 81.5 mg; Sodium fluoride, 63.5 mg; Nickel carbonate, 31.8 mg; Lithium chloride, 17.4 mg; Sodium selenate anhydrous, 10.25 mg; Potassium iodate, 10.0 mg; Ammonium paramolybdate, 6.66 mg; Powdered sucrose, 221.026 g
3)Vitamin mixture (per kg): Nicotinic acid, 3.0 g; Ca Pantothenate, 1.6 g; Pyridoxine HCl, 0.7 g; Thiamin HCl, 0.6 g; Riboflavin, 0.6 g; Folic acid, 0.2 g; Biotin, 0.02 g; Vitamin B12, 2.5 g; Vitamin E, 15.0 g;Vitamin A, 0.8 g;Vitamin D3, 0.25 g;Vitamin K-1, 0.075 g; Powdered sucrose, 974.655 g
Sample preparations of plasma, liver and feces
The rats were anesthetized with ethyl ether and dissected. Blood was collected from the heart with heparinized syringe. The plasma from the blood was collected by centrifugation at 4,000 × g for 30 min after allowing the blood to settle for 30 min at 4℃. The plasma was then stored at -70℃. The liver was removed and the wet weights were measured [19]. The livers were stored at -70℃ for lipid analysis. Stool samples were individually collected twice over 24 hr and completely dried by heating at 105℃.
Lipid measurements in plasma, liver and feces
Plasma total lipids were determined as described by Frings and Dunn [20], with slight modifications. Plasma total cholesterol and triacylglycerol were measured enzymatically using a commercial kit (Asan Pharmaceutical Co., Seoul, Korea). Total lipids in the liver and feces were extracted with chroloform: methanol (2:1,v/v) using a modified method described by Folch et al. [21]. After extraction, total lipid, triaclyglycerols, and cholesterol were measured with the same methods used for plasma lipid determination.
Total RNA isolation
Total liver RNA was isolated using TRI-reagent (Sigma aldrich, MO, USA) according to the manufacturer's protocol [19]. Liver samples (0.2 g) were homogenized in 1 mL TRI Reagent, and then stored the homogenate for 5 min at room temperature to permit the complete dissociation of nucleoprotein complexes. The homogenate was mixed with 200 µL chloroform (Sigma aldrich, MO, USA), shacked vigorously, stored for 10 min, and centrifuged at 12,000 g for 10 min. After transferring the supernatant, 500 µL of isopropanol were added, stored at room temperature for 5 min, and then centrifuged at 12,000 g for 10 min at 4℃.
After removing the supernatant, the RNA pellet was washed with 75% ethanol following by subsequent centrifugation at 7,500 g for 5 min at 4℃. After removing the ethanol wash, samples were dried thoroughly and then dissolved RNA in 50 µL RNase-free dH2O/0.1 mmol/L EDTA through a pipette tip. The purity of each RNA preparation was evaluated by the ratio of the absorbance at 260 nm to that at 280 nm. The final preparation of total RNA is essentially free of DNA and proteins and has a 260/280 ratio 1.7-2.0.
Reverse Transcription (RT)
cDNA was synthesized using 3 µg of total RNA with Super Script II reverse transcriptase (Invitrogen, CA, USA). In 1.5 mL tubes, 3 µg total RNA from sample and 1 µL of oligo DT (0.5 µg/µL, Invitrogen, CA, USA) were added to bring the final volume to 12 µL with Depc water, fllowed by incubating 70℃ for 10 min [22]. A reaction buffer (First strand Buffer 4 µL, 0.1 mol/L DTT 2 µL, 10 mmol/L NTP 1 µL) was added into the mixture and incubated at 42℃ for 5 min. After incubating, 0.5 µL superscript II reverse transcriptase (Invitrogen, CA, USA) was added and incubated at 42℃ for 100 min. and 70℃ for 15 min. Rnase Depc water, 80 µL, was finally added into mixture and storing at -20℃ until subsequent analysis.
Realtime PCR
Real-time detection PCR was performed by the modified method recently described in detail [22,23]. The prepared cDNA sample, 2 µL, were mixed with SYBR green Master mix (Applied biosystems, CA, USA), 10 µL, sense/antisense primer (Table 2), 1 µL each, and DEPC water 6 µL. The initial activation of the primer at 95℃ for 10 min was followed by 40 cycles of 95℃ for 15 min and 60℃ for 1 min. Expression of mRNA was carried out with an Applied Biosystems StepOne Plus RT-PCR system (Applied biosystems, CA, USA). Fold difference for gene expression was calculated using 2-ΔΔ. CT using the endogenous control gene.
Table 2.
Primer sequences used for realtime PCR
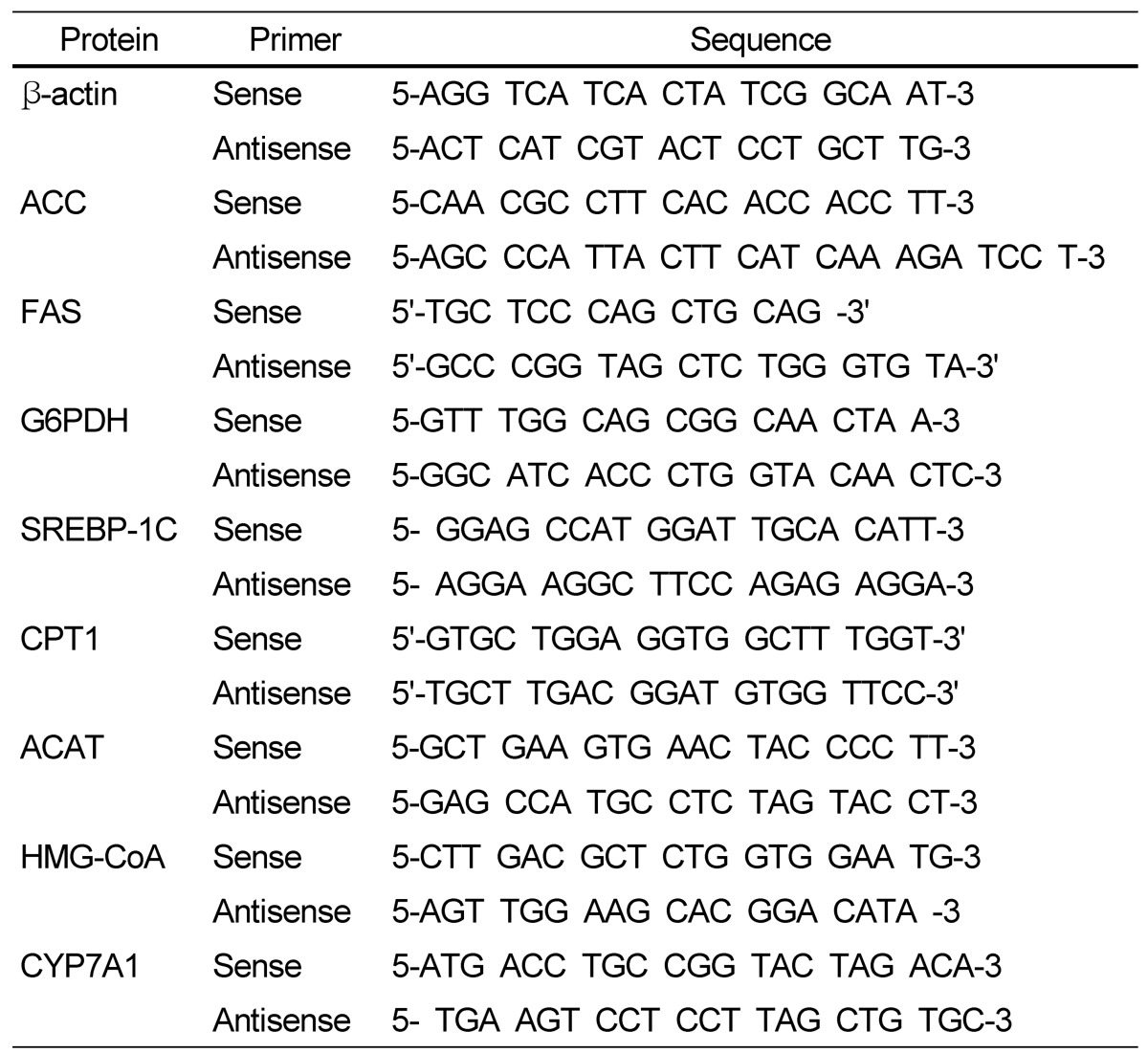
T, Thymine; A, Adenin; C, Cytosine; G, Guanine
Statistical analysis
The statistical analysis was performed using Statistical Analysis System (SAS Institute, Cary, NC, USA). Data are expressed as mean with standard deviation, and statistically significant differences among groups were evaluated using one way-ANOVA (analysis of variance). Statistically significant differences among the means of groups were tested at α = 0.05 using Duncan's multiple range tests.
Results
Body weight changes after fucoxanthin supplementation
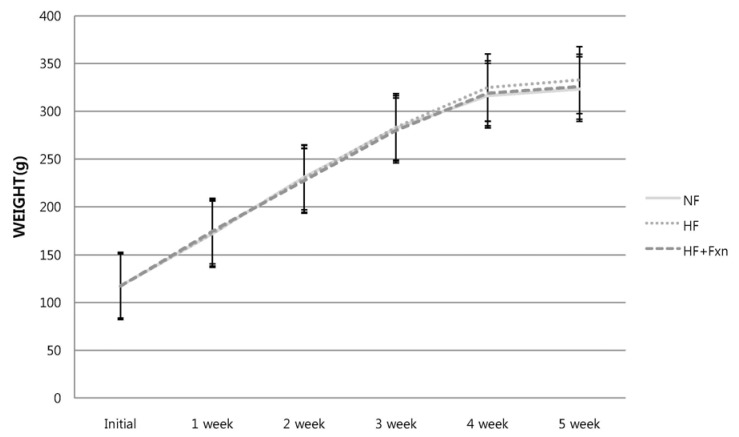
Although the mean weight gain of high fat diet with fucoxanthin group (209.9 ± 8.8 g) was lower than that of the high fat diet only group (215.2 ± 9.7 g), no significant differences in weight gain were observed between two groups (Fig. 1). There is a trend showing rats feeding fucoxanthin maintained their weight just like rats with normal fat diet and lower weight than weights of rats with high fat diet.
Fig. 1.
Changes of body weight in the rats
Lipid concentrations in plasma, liver and feces
The effects of fucoxanthin on lipid concentrations in plasma, liver, and feces are shown in Table 3. Fucoxanthin supplements significantly increased plasma HDL concentration when it compared to that of high fat only diet (12.9 ± 4.1 mg/100 mL in HF group, 20.4 ± 6.7 mg/100 mL in HF+Fxn group, P < 0.05). Although plasma triglyceride concentration with fucoxanthin supplements (86.2 ± 5.1 mg/100 mL) was decreased, compared with the HF group (98.3 ± 9.3 mg/100 mL), no statistical significance was shown. No significant changes in total lipids or cholesterol concentrations were observed between HF group and HF+Fxn group.
Table 3.
Lipid concentrations of plasma, liver, and feces in rats fed with experimental diets
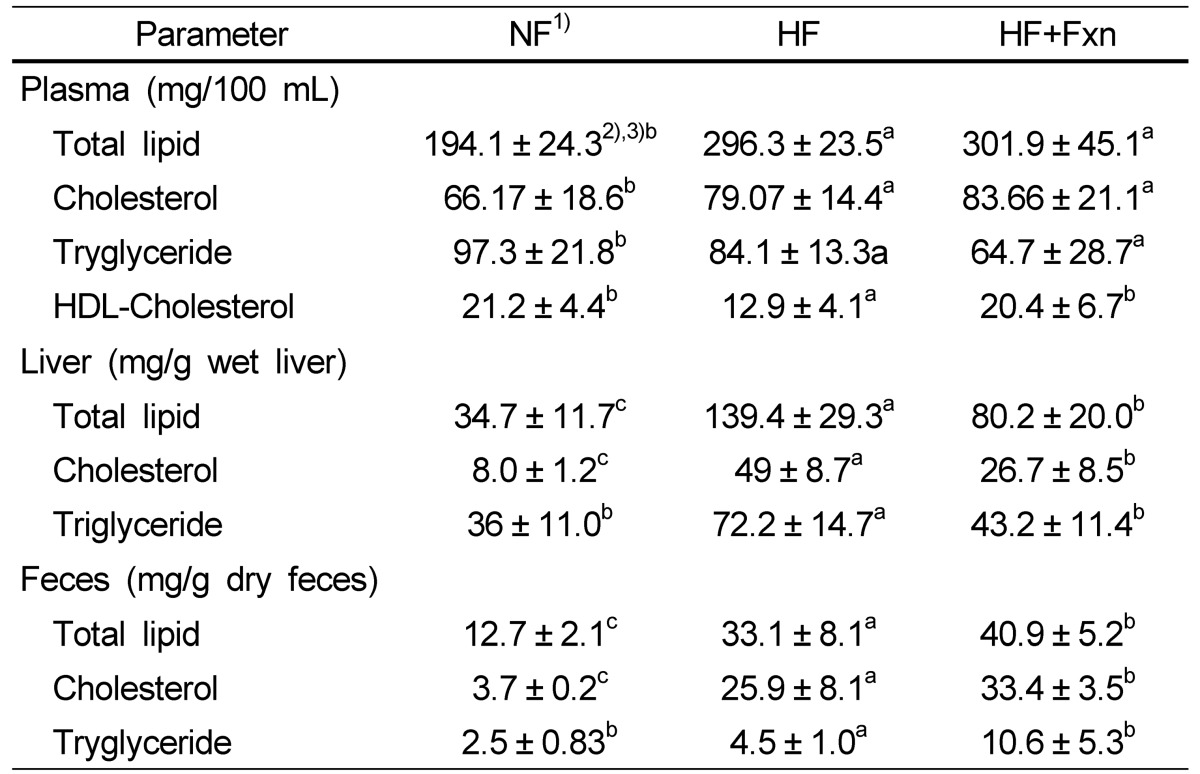
1)NF, normal fat; HF, high fat; HF+Fxn, high fat with 0.2% fucoxanthin
2)Mean ± SD
3)The significant was determined by Duncan's multiple range test at α = 0.05.
The hepatic total lipid was significantly decreased in the HF+Fxn group (139.4 ± 9.3 mg/g), when it compared with HF group (80.2 ± 7.1 mg/g) (P < 0.05). Hepatic cholesterols and triglycerides were also significantly lower in the HF+Fxn group than in the HF group (P < 0.05). The fecal triglyceride contents (10.6 ± 5.8 mg/g) were significantly higher in the HF+Fxn group than in HF group (4.5 ± 1.0 mg/g) (P < 0.05). The concentration of fecal cholesterol and total lipids were also significantly increased when fucoxanthin was added in high fat diet (P < 0.05).
mRNA expression of transcription factors and enzymes related to lipid metabolism
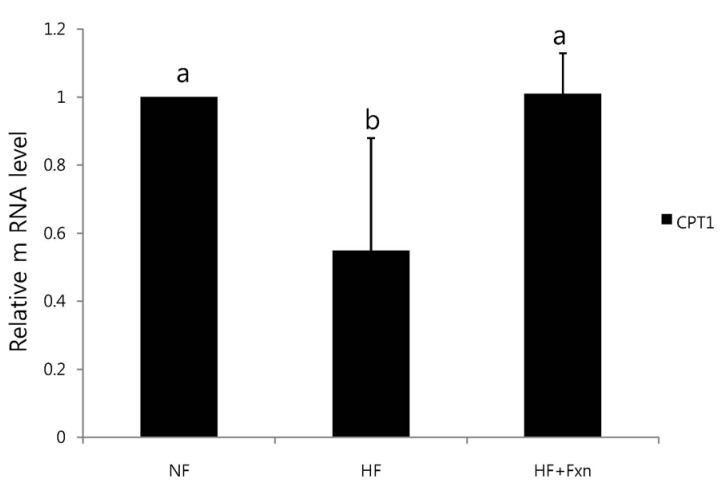
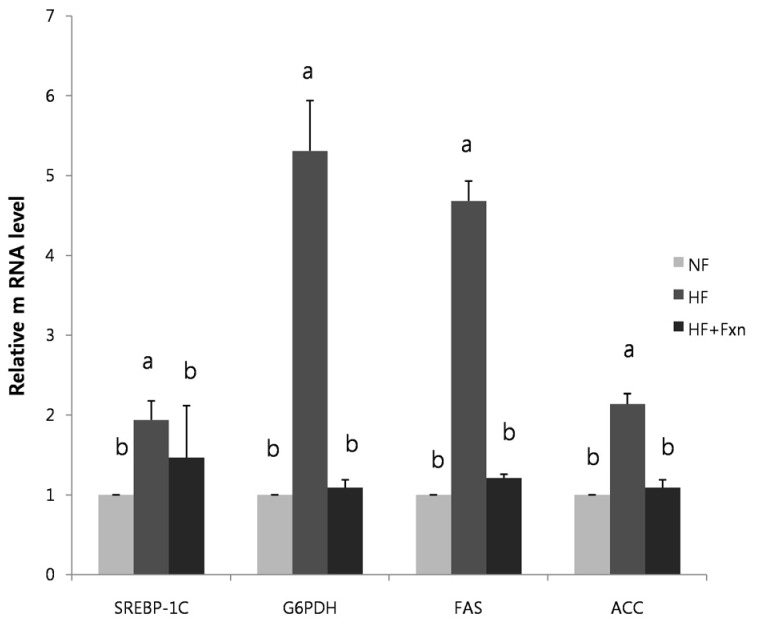
When the rats were fed with high fat diet, the mRNA expression of hepatic SREBP-1c, ACC, FAS, and G6PDH were significantly increased, compared to NF group (P < 0.05). The mRNA expression of hepatic SREBP-1c, ACC, FAS, and G6PDH were significantly lower in the HF+Fxn group compared to the HF group (P < 0.05) (Fig. 2). In the livers of rats with a high fat diet, the mRNA expressions of CPT1 were significantly decreased, compared to the NF group (P < 0.05) (Fig. 3). The relative mRNA expression of hepatic CPT1 in HF+Fxn group was significantly higher than that in the HF group (P < 0.05).
Fig. 2.
Effect of fucoxanthin on mRNA expression of transcription factor and enzymes related to lipid metabolism in the liver of rats. Total RNA was isolated using TRI-reagenet and cDNA was synthesized using 3 µg of total RNA with SuperScript II reverse transcriptase. Realtime PCR was performed using SYBR green and standard procedures to assess the mRNA expression of primer in liver samples obtained from each group. An Applied Biosystem StepOne softwere v2.1 was used. Each bar represents the mean ± SD of three independent experiments. Different letters above each bar indicate significant differences among groups at α = 0.05 as determined by Duncan's multiple range test. SREBP-1C, Sterol regulatory element binding protein; G6PDH, Glucose-6-phosphate dehydrogenase; FAS, Fatty acid synthase; ACC, Acetyl-CoA carboxylase.
Fig. 3.
Effect of fucoxanthin on mRNA expression of CPT1 in the liver of rats. Total RNA was isolated using TRI-reagenet and cDNA was synthesized using 3 µg of total RNA with SuperScript II reverse transcriptase. Realtime PCR was performed using SYBR green and standard procedures to assess the mRNA expression of primer in liver samples obtained from each group. An Applied Biosystem StepOne softwere v2.1 was used. Each bar represents the mean ± SD of three independent experiments. Different letters above each bar indicate significant differences among groups at α = 0.05 as determined by Duncan's multiple range test. CPT1, Carnitine palmitoyltransferase-1.
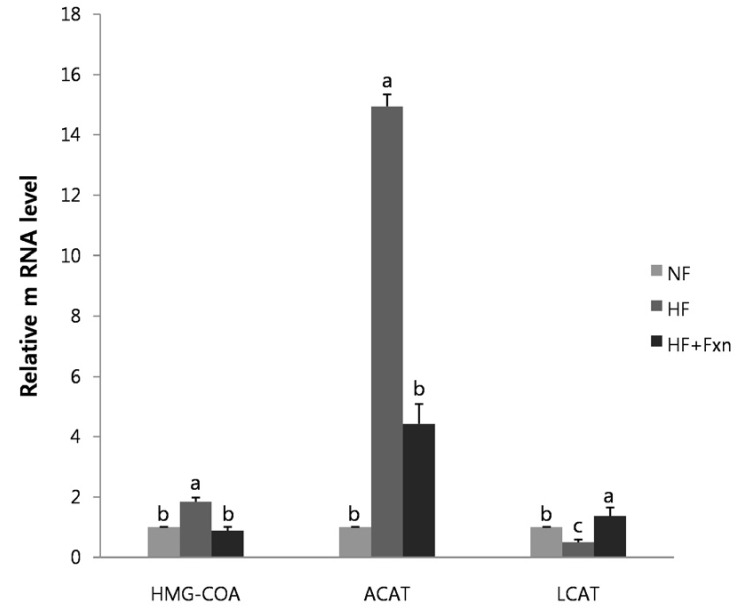
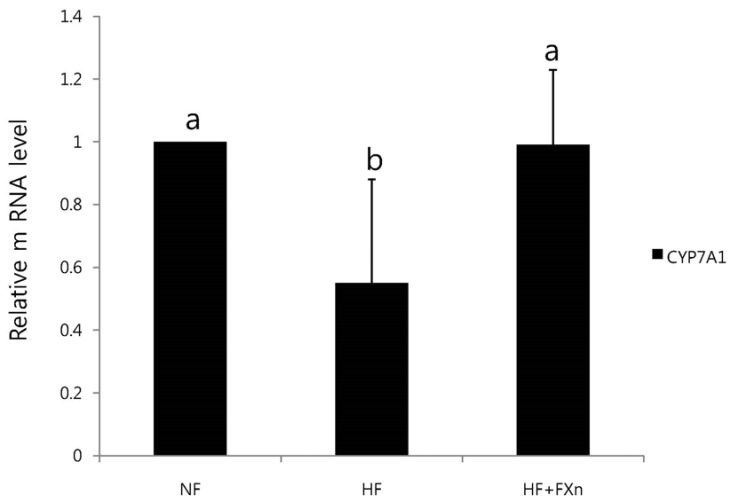
The mRNA expression of hepatic HMG-CoA and ACAT in HF group was significantly increased (P < 0.05) while LCAT was significantly decreased (P < 0.05), compared to NF group (Fig. 4). The hepatic mRNA expression of HMG-CoA and ACAT in HF+Fxn group was significantly lower than those in HF group (P < 0.05). The mRNA expression of LCAT was the highest in HF+Fxn group (P < 0.05). The mRNA expression of CYP7A1 in high fat fed rats was significantly decreased, compared to NF group (P < 0.05) (Fig. 5). There was significant increase in mRNA expression of CYP7A1 in HF+Fxn group compared to HF group (P < 0.05).
Fig. 4.
Effect of fucoxanthin on mRNA expression of enzymes related to cholesterol synthesis in the liver of rats. Total RNA was isolated using TRI-reagenet and cDNA was synthesized using 3 µg of total RNA with SuperScript II reverse transcriptase. Realtime PCR was performed using SYBR green and standard procedures to assess the mRNA expression of primer in liver samples obtained from each group. An Applied Biosystem StepOne softwere v2.1 was used. Each bar represents the mean ± SD of three independent experiments. Different letters above each bar indicate significant differences among groups at α = 0.05 as determined by Duncan's multiple range test. HMG-CoA, Hydroxy-3-methylglutaryl coenzyme A; ACAT, Acyl-CoA cholesterol acyltransferase; LCAT, lecithin-cholesterol acyltransferase.
Fig. 5.
Effect of fucoxanthin on the mRNA expression of CYP7A1 in the liver of rats. Total RNA was isolated using TRI-reagenet and cDNA was synthesized using 3 µg of total RNA with SuperScript II reverse transcriptase. Realtime PCR was performed using SYBR green and standard procedures to assess the mRNA expression of primer in liver samples obtained from each group. An Applied Biosystem StepOne softwere v2.1 was used. Each bar represents the mean ± SD of three independent experiments. Different letters above each bar indicate significant differences among groups at α = 0.05 as determined by Duncan's multiple range test. CYP7A1, Cholesterol 7α-hydroxylase1.
Discussion
Recently, lipid-lowering functions of fucoxanthin in the body have been paid much attention. However, the mechanisms responsible for lipid-lowering actions of fucoxanthin on liver are poorly understood. This study used 0.2% fucoxantin as dietary supplements in high fat fed rats, and that fucoxanthin was extracted from Undaria pinnatifida, brown seaweed, which is a commonly used food in Asia and it is well known as major fucoxanthin sources [4,5].
In this study, fucoxanthin supplementation (0.2%) in rats fed high fat diet was effective in decreasing liver triglyceride and total cholesterol, and in increasing fecal excretions of those lipids. This study observed a decline in the mRNA expressions of lipogenic enzymes such as ACC, FAS, and G6PDH in high fat with fucoxanthin group. A significant decrease in the transcriptional factor of SREBP-1c expression with fucoxanthin supplement was also found in this study. Therefore, lowered hepatic triglyceride and cholesterol levels after fucoxanthin supplementation in high fat fed rats seemed to be related with decreased transcriptional factor, SREBP1-c, which inhibits, in turn, gene expressions of lipogenic enzymes such as ACC, FAS, and G6PDH, thus resulting in inhibiting accumulation of triglycerides or cholesterol in liver. Lipid biosynthesis usually occurs in hepatic tissue and typically involves the following lipogenic enzymes: ACC, FAS, G6PDH, and ME [22]. In this process, SREBP-1c functions as an important transcriptional factor that mediates insulin function and activates fatty acid and cholesterol biosynthesis pathways [23,24]. It is believed that SREBP-1c greatly increase the expression of genes related with lipogenic pathway in liver [25,26]. Jeon's study [13] showed that supplementation with both doses of fucoxanthin extracts (0.05% or 0.2%) in high fat fed rats significantly reduced body weight and plasma and hepatic triglyceride, and/or cholesterol concentrations. Other studies reported that fucoxanthin lowered serum or hepatic lipids concentrations or lowered lipogenic enzyme activities [10,12,13,17], but this is the first study to see the effect of fucoxanthin on mRNA expression of transcriptional factor, SREBP-1c and lipogenic enzymes such as ACC, FAS, and G6PDH in the liver of rats fed high fat diets.
This study also showed that supplementation of fucoxanthin with high fat diet significantly increased fecal excretions of triglyceride and cholestrol. This result is consistent to Woo et al.'s findings [16] that fucoxanthin increased fecal weights and fats in C57BL/6N mice, and other's findings suggesting carotenoids increases fecal excretion fat and cholesterol [27,28]. Those findings suggested that the hypolipidemic effect of carotenoids was resulted from inhibiting pancreatic lipase's activity, leading to the malabsorption of fat and cholesterol. Thus, this study suggests that lowered hepatic lipids levels after feeding fucoxanthin in high fat diet is related with the increased fecal excretion of triglycerides or cholesterol, in addition to deceased de novo lipogenesis in liver by the action of SREBP-1c.
In this study, fucoxanthin supplement (0.2%) led to increase CPT1 expression in the fucoxanthin supplemented group than the high-fat diet group. Since CPT1 mediates the transport of acyl-CoA across the membrane and stimulates the oxidation of long chain fatty acids [12], it can be suggested that fucoxanthin exhibited hepatic lipid -lowering property which may be also partly mediated via increase of hepatic CPT1 expression. Hu et al. [15] also reported that fucoxanthin with conjugated linoleic acid was significantly increased mRNA expression of CPT1A in peripheral white adipose tissue of rat. In Park's study [10], supplementation of the 0.69% Undaria pinnatifida ethanol extract (which is equal amount of 0.02% pure fucoxanthin (w/w)) in rats stimulated the fatty acid oxidation activity resulting in a decrease in the hepatic lipid droplet accumulation.
To understand how hepatic cholesterol content decreased by fucoxanthin, the genetic expression of cholesterol metabolism regulating enzymes, such as HMG-CoA reductase ACAT, and LCAT as well as transcription factor, CYP7A1, were determined in this study. In this study, the mRNA expression of enzymes involved in cholesterol synthesis, such as HMG-CoA reductase, ACAT, and LCAT, tended to be higher in the high fat with fucoxanthin-fed rats. The HMG-CoA reductase is an important enzyme that regulates cholesterol synthesis and ACAT and LCAT catalyzes esterification of free cholesterol into cholesterol ester (CE), involving in cholesterol absorption in the intestines and lipoprotein production in the liver [29]. Therefore, the demonstrated effect of fucoxanthin in this study is ultimately thought to reduce hepatic cholesterol levels. Woo et al. [16] also reported that fucoxanthin (0.2% in diet) inhibit several enzyme's activity such as hepatic HMG-CoA reductase or ACAT activity in C57BL/6N mice fed a high-fat diet.
Human CYP7A1 gene defect can cause cholesterol accumulation in the liver, which has been associated with hypercholesterolemia [30]. This study observed a significant increase in CYP7A1 expression in the fucoxanthin group than the high-fat diet group. This result indicates that fucoxanthin consumption increases CYP7A1 expression in liver which in turn increased fecal excretion of cholesterol or bile and thus reducing cholesterol accumulation in liver.
In conclusion, data from this study indicate that fucoxanthin consumption decreases lipid accumulation in the liver of rats with a high fat diet. Its consumption also proved to suppress mRNA expression of transcription factors and enzymes that participate in hepatic lipogenesis. Furthermore, data showed that the consumption increased expression of enzymes that stimulate fatty acid oxidation and decreased expression of cholesterol synthesizing enzyme. Therefore, consumption of fucoxanthin is thought to be effective in improving lipid metabolism in rats with a high fat diet.
Footnotes
The present research was conducted by the research fund of Dankook University in 2011.
References
- 1.Barres R, Zierath JR. DNA methylation in metabolic disorders. Am J Clin Nutr. 2011;93:897S–900S. doi: 10.3945/ajcn.110.001933. [DOI] [PubMed] [Google Scholar]
- 2.Marchesini G, Marzocchi R, Agostini F, Bugianesi E. Nonalcoholic fatty liver disease and the metabolic syndrome. Curr Opin Lipidol. 2005;16:421–427. doi: 10.1097/01.mol.0000174153.53683.f2. [DOI] [PubMed] [Google Scholar]
- 3.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 4.MacArtain P, Gill CI, Brooks M, Campbell R, Rowland IR. Nutritional value of edible seaweeds. Nutr Rev. 2007;65:535–543. doi: 10.1301/nr.2007.dec.535-543. [DOI] [PubMed] [Google Scholar]
- 5.Chandini SK, Ganesan P, Suresh PV, Bhaskar N. Seaweeds as a source of nutritionally beneficial compounds - a review. J Food Sci Technol. 2008;45:1–3. [Google Scholar]
- 6.Maeda H, Hosokawa M, Sashima T, Funayama K, Miyashita K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem Biophys Res Commun. 2005;332:392–397. doi: 10.1016/j.bbrc.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Miyashita K, Maeda H, Okada T, Abe M, Hosokawa M. Anti-obesity and anti-diabetic effects of allenic carotenoid, fucoxanthin. Agro Food Ind Hi Tech. 2010;21:24–27. [Google Scholar]
- 8.Okada T, Nakai M, Maeda H, Hosokawa M, Sashima T, Miyashita K. Suppressive effect of neoxanthin on the differentiation of 3T3-L1 adipose cells. J Oleo Sci. 2008;57:345–351. doi: 10.5650/jos.57.345. [DOI] [PubMed] [Google Scholar]
- 9.Yim MJ, Hosokawa M, Mizushina Y, Yoshida H, Saito Y, Miyashita K. Suppressive effects of amarouciaxanthin A on 3T3-L1 adipocyte differentiation through down-regulation of PPARγ and C/EBPα mRNA expression. J Agric Food Chem. 2011;59:1646–1652. doi: 10.1021/jf103290f. [DOI] [PubMed] [Google Scholar]
- 10.Park HJ, Lee MK, Park YB, Shin YC, Choi MS. Beneficial effects of Undaria pinnatifida ethanol extract on diet-induced-insulin resistance in C57BL/6J mice. Food Chem Toxicol. 2011;49:727–733. doi: 10.1016/j.fct.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 11.Maeda H, Hosokawa M, Sashima T, Miyashita K. Dietary combination of fucoxanthin and fish oil attenuates the weight gain of white adipose tissue and decreases blood glucose in obese/diabetic KK-Ay mice. J Agric Food Chem. 2007;55:7701–7706. doi: 10.1021/jf071569n. [DOI] [PubMed] [Google Scholar]
- 12.Woo MN, Jeon SM, Shin YC, Lee MK, Kang MA, Choi MS. Anti-obese property of fucoxanthin is partly mediated by altering lipid-regulating enzymes and uncoupling proteins of visceral adipose tissue in mice. Mol Nutr Food Res. 2009;53:1603–1611. doi: 10.1002/mnfr.200900079. [DOI] [PubMed] [Google Scholar]
- 13.Jeon SM, Kim HJ, Woo MN, Lee MK, Shin YC, Park YB, Choi MS. Fucoxanthin-rich seaweed extract suppresses body weight gain and improves lipid metabolism in high-fat-fed C57BL/6J mice. Biotechnol J. 2010;5:961–969. doi: 10.1002/biot.201000215. [DOI] [PubMed] [Google Scholar]
- 14.Maeda H, Hosokawa M, Sashima T, Murakami-Funayama K, Miyashita K. Anti-obesity and anti-diabetic effects of fucoxanthin on diet-induced obesity conditions in a murine model. Mol Med Rep. 2009;2:897–902. doi: 10.3892/mmr_00000189. [DOI] [PubMed] [Google Scholar]
- 15.Hu X, Li Y, Li C, Fu Y, Cai F, Chen Q, Li D. Combination of fucoxanthin and conjugated linoleic acid attenuates body weight gain and improves lipid metabolism in high-fat diet-induced obese rats. Arch Biochem Biophys. 2012;519:59–65. doi: 10.1016/j.abb.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Woo MN, Jeon SM, Kim HJ, Lee MK, Shin SK, Shin YC, Park YB, Choi MS. Fucoxanthin supplementation improves plasma and hepatic lipid metabolism and blood glucose concentration in high-fat fed C57BL/6N mice. Chem Biol Interact. 2010;186:316–322. doi: 10.1016/j.cbi.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Mayes PA. Biosynthesis of fatty acids. In: Murray RK, Harper HA, editors. Harper's Biochemistry. 25th ed. Stamford (CT): Appleton and Lange; 2000. pp. 230–257. [Google Scholar]
- 18.Chen J, Zhao H, Ma X, Han X, Luo L, Wang L, Han J, Liu B, Wang W. The effects of jiang-zhi-ning and its main components on cholesterol metabolism. Evid Based Complement Alternat Med. 2012;2012:928234. doi: 10.1155/2012/928234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Frings CS, Dunn RT. A colorimetric method for determination of total serum lipids based on the sulfo-phospho-vanillin reaction. Am J Clin Pathol. 1970;53:89–91. doi: 10.1093/ajcp/53.1.89. [DOI] [PubMed] [Google Scholar]
- 21.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 22.Bettzieche A, Brandsch C, Hirche F, Eder K, Stangl GI. L-cysteine down-regulates SREBP-1c-regulated lipogenic enzymes expression via glutathione in HepG2 cells. Ann Nutr Metab. 2008;52:196–203. doi: 10.1159/000138123. [DOI] [PubMed] [Google Scholar]
- 23.Foretz M, Guichard C, Ferré P, Foufelle F. Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc Natl Acad Sci U S A. 1999;96:12737–12742. doi: 10.1073/pnas.96.22.12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 25.Shimomura I, Hammer RE, Richardson JA, Ikemoto S, Bashmakov Y, Goldstein JL, Brown MS. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev. 1998;12:3182–3194. doi: 10.1101/gad.12.20.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diraison F, Dusserre E, Vidal H, Sothier M, Beylot M. Increased hepatic lipogenesis but decreased expression of lipogenic gene in adipose tissue in human obesity. Am J Physiol Endocrinol Metab. 2002;282:E46–E51. doi: 10.1152/ajpendo.2002.282.1.E46. [DOI] [PubMed] [Google Scholar]
- 27.Nicolle C, Cardinault N, Aprikian O, Busserolles J, Grolier P, Rock E, Demigné C, Mazur A, Scalbert A, Amouroux P, Rémésy C. Effect of carrot intake on cholesterol metabolism and on antioxidant status in cholesterol-fed rat. Eur J Nutr. 2003;42:254–261. doi: 10.1007/s00394-003-0419-1. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto M, Hosokawa M, Matsukawa N, Hagio M, Shinoki A, Nishimukai M, Miyashita K, Yajima T, Hara H. Suppressive effects of the marine carotenoids, fucoxanthin and fucoxanthinol on triglyceride absorption in lymph duct-cannulated rats. Eur J Nutr. 2010;49:243–249. doi: 10.1007/s00394-009-0078-y. [DOI] [PubMed] [Google Scholar]
- 29.Siperstein MD. Regulation of cholesterol biosynthesis in normal and malignant tissues. Curr Top Cell Regul. 1970;2:65–100. [Google Scholar]
- 30.Pullinger CR, Eng C, Salen G, Shefer S, Batta AK, Erickson SK, Verhagen A, Rivera CR, Mulvihill SJ, Malloy MJ, Kane JP. Human cholesterol 7alpha-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J Clin Invest. 2002;110:109–117. doi: 10.1172/JCI15387. [DOI] [PMC free article] [PubMed] [Google Scholar]