Abstract
The emerging role of endothelial inflammation in diabetes has stimulated research interest in the effects of nutrition on related indices. In the current study we investigated whether the nutrient composition of dietary formula as reflected in glycemic index (GI) may be predictive of postprandial endothelial inflammation in non-diabetic subjects. A double-blinded, randomized, crossover study was conducted in non-diabetic subjects (n = 8/group). Each subject consumed three types of diabetes-specific dietary formulas (high-fiber formula [FF], high-monounsaturated fatty acid (MUFA) formula [MF] and control formula [CF]) standardized to 50 g of available carbohydrates with a 1-week interval between each. The mean glycemic index (GI) was calculated and 3-hour postprandial responses of insulin, soluble intercellular adhesion molecule-1 (sICAM-1), nitrotyrosine (NT) and free fatty acids (FFA) were measured. The MF showed the lowest mean GI and significantly low area under the curve (AUC) for insulin (P = 0.038), but significantly high AUCs for sICAM-1 (P < 0.001) and FFA (P < 0.001) as compared to the CF and FF. The FF showed intermediate mean GI, but significantly low AUC for NT (P < 0.001) as compared to the CF and MF. The mean GI was not positively correlated to any of the inflammatory markers evaluated, and in fact negatively correlated to changes in FFA (r = -0.473, P = 0.006). While the MF with the lowest GI showed the highest values in most of the inflammatory markers measured, the FF with intermediate GI had a modest beneficial effect on endothelial inflammation. These results suggest that nutrient composition of dietary formula as reflected in the GI may differently influence acute postprandial inflammation in non-diabetic subjects.
Keywords: Diabetes-specific dietary formula, glycemic index, endothelial inflammation
Introduction
The glycemic index (GI) was proposed in 1981 as a system for classifying carbohydrate-containing foods according to postprandial glycemic response, and can be standardized by the amount of absorbable carbohydrates [1]. GI values range from low (< 55), to medium (55-69), to high (> 70), with lower GI values representing slower digestion rates and frequently lower insulin demands [2]. Currently GI values of 2,487 foods were complied to form the international GI table, and are available in the online appendix at http://dx.doi.org/10.2337/dc08-1239 [3]. The concept of GI can be applied to whole meals or overall diet by calculating from the GI of constituent foods [4].
The major objectives of diabetes care are to reduce hyperglycemia and reduce the risk of complications, particularly cardiovascular disease (CVD) [5]. Whether the GI approach has clinical relevance in diabetes care has been a topic of debate because of the lack of both metabolic and epidemiologic evidence. With regard to the first objective, several lines of evidence have collectively provided strong support for a role of dietary GI in improving glycemic control and reducing insulin resistance of both diabetes and non-diabetes in recent years [5]. However, questions about optimal nutritional approach for the prevention of cardiovascular complication among diabetes still remains a topic of intense controversy. Several pieces of evidence support that an exaggerated postprandial state result in oxidative and inflammatory stress, which in turn may contribute to the endothelial dysfunction [6,7], and ultimately cardiovascular complications in diabetics [8,9]. Ceriello [6] reviewed the epidemiological data and preliminary results of intervention studies and suggested postprandial "hyperglycemic spikes" of diabetic subjects may be relevant to the onset of cardiovascular complications. Whether the GI approach is relevant to reduce the risk of cardiovascular complication in diabetes, however, has been debated [10].
Currently, there are several different dietary formulas on the market specially designed for diabetic patients, with a tendency to contain higher proportions of monounsaturated fatty acids (MUFAs) and/or dietary fiber compared to the pre-existing formulas, thereby improving postprandial glycemic control [11]. In light of the elevated risk of CVD that diabetics face, it is urgent that this risk factor be considered when formulating diabetes-specific dietary formula [12], but there has been limited research concerning the direct relation of the GI and endothelial function. In this study, as a first step to investigate this important issue, we aimed to evaluate whether the nutrient composition of dietary formula as reflected in the GI is predictive of postprandial endothelial inflammation in non-diabetic subjects. To address this aim, we calculated the mean GI and compared glucose, insulin, and markers of endothelial inflammation responses in non-diabetic subjects who consumed a high-MUFA dietary formula (MF), a high-fiber dietary formula (FF), and a control dietary formula (CF). Furthermore, we explored potential relationships between glycemic response and inflammatory markers.
Subjects and Methods
Subjects and study design
This was a double-blinded, randomized and crossover study. Subjects were recruited through advertisements on the university campus and screened using the following exclusion criteria: fasting blood glucose > 6.1 mmol/L, smoking, medication, food allergy or intolerance, dieting, physical or mental illness, history of anemia, family history of diabetes, exercising for more than 30 min per session more than three times a week, and history of blood donation in the preceding eight weeks or an intention to donate blood in the following four weeks. Eight subjects (4 males and 4 females, 25.0 ± 0.7 years) were enrolled in the trial upon providing informed consent and underwent four 3-h meal glucose tolerance tests. On the first session, subjects consumed a standard solution containing 50 g glucose in 100 mL (Taejoon Pharm, Seoul, Korea). For the following sessions, each subject was randomly assigned to receive one of three dietary formulas (MF, FF, and CF). Random allocation was performed by assistant and sequentially numbered containers to blind for the investigators and subjects. Treatment visits were scheduled approximately 1 week apart. During the study period, subjects were asked to maintain their regular diet and lifestyle. Ethics approval for the trial was obtained from the institutional review board of Seoul St. Mary's Hospital, Catholic University, Korea (Reference # KC08HISV0351) and the study was performed in accordance with the principles relating to the Declaration of Helsinki.
Interventions
The dietary formulas evaluated in this study differed primarily in the composition of carbohydrate and fat and were defined as follows: Nucare (Daesang Co., Seoul, Korea) defined as the control formula (CF), Nucare DM (Daesang Co., Seoul, Korea) defined as the high-fiber formula (FF), and Glucerna SR (Abbott Lab, St-Laurent, Quebec, Canada) defined as the high-MUFA formula (MF). Each formula had same protein levels (9.9-10.0 g/serving), was similarly low in saturated fatty acids (0.5-1.4 g/serving), and did not contain trans fatty acids. The volume administered to each volunteer was dependent on the dietary formula received, such that available glucose was standardized to 50 g. The resulting volume, nutritional composition, and mean GI of each formula are summarized in Table 1.
Table 1.
Administered amount and nutrient composition of dietary formulas1)
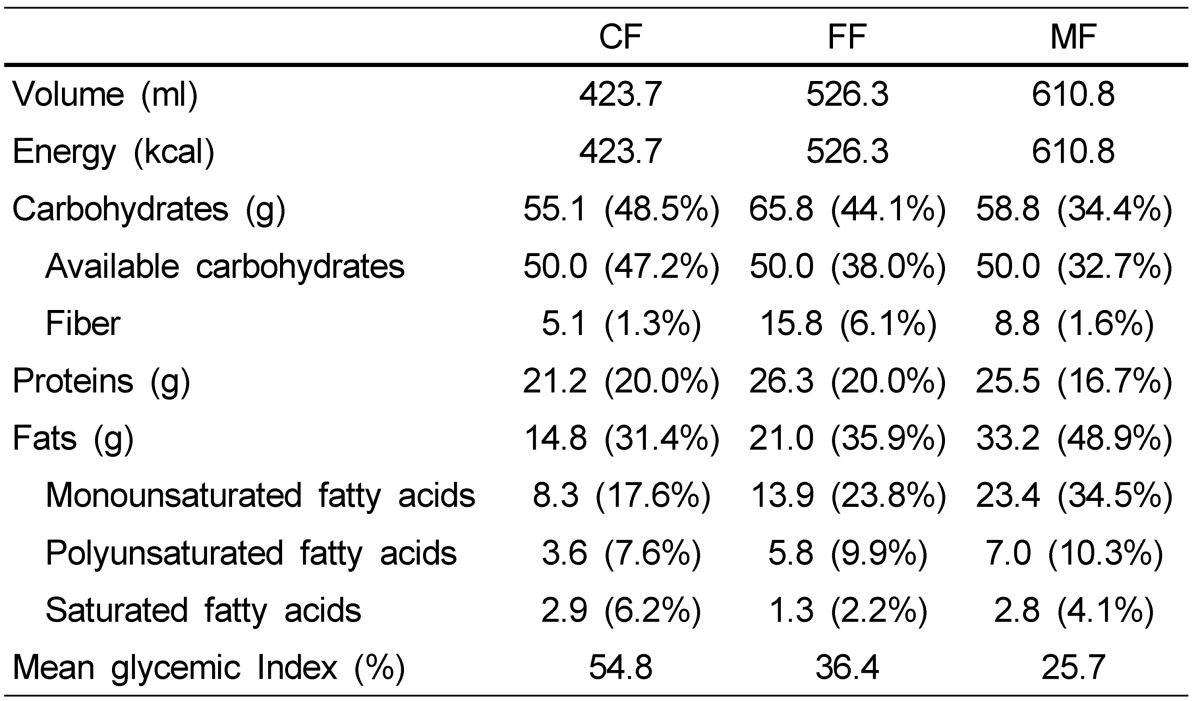
1) CF, control formula; FF, high-fiber formula; MF, high-MUFA formula
Each session began with the measurement of capillary blood glucose level and collection of venous blood sample via a venflon catheter inserted in the antecubital vein for insulin and inflammation markers (for sessions 2-4 only) at fasting (t = 0) and 30, 60, 120, and 180 min after consuming test solutions.
Outcome measurements
Finger prick blood samples were taken for capillary blood glucose analysis by using Accu-Chek aviva (Roche Diagnostics, Rotkreuz, Switzerland). Blood was drawn into K2 EDTA evacuated tubes (BD Biosciences, San Jose, CA, USA) and centrifuged immediately. Plasma insulin, soluble intercellular adhesion molecule-1 (sICAM-1), nitrotyrosine (NT), and free fatty acid (FFA) were measured by a radioimmunoassay kit (TFB Inc., Tokyo, Japan), a human sICAM-1 ELISA kit (Cat. # BMS201TENCE, Bender MedSystems, Vienna, Austria), a NTassay kit, chemiluminescence detection (Cat. # 17-376, Millipore, St. Charles, MO, USA), and a FFA quantification kit (Cat. # K612-100, Biovision, Mountain View, CA, USA), respectively.
Sample size estimation and statistical analysis
The sample size was designed to detect a difference among groups in the postprandial glucose response with 95% confidence interval and 80% power.
Plasma glucose, insulin, sICAM-1, NT, and FFA concentrations were normalized to the t = 0 baseline value for each individual due to high individual variability. The area under the curve (AUC) for each variable was calculated by the trapezium rule [13]. The mean GIs were determined according to the following formula: GI (%) = (AUC for glucose response in given test formula) / (AUC for glucose response in a standard glucose solution) × 100, where the test formula and standard glucose solution each contained 50 g of available carbohydrate.
Data were analyzed with a general linear model for repeated measures. Individual time points and AUCs were analyzed by analysis of variance (ANOVA) with post hoc Bonferroni multiple comparison tests. For further exploratory analysis, Pearson's correlation was used to examine the relative contribution of the mean GI to AUCs of insulin, sICAM-1, NT, or FFA. Significance was set at P < 0.05 for all statistical analyses. Data were expressed as mean ± SD. All statistical analyses were performed using the Statistical Analysis Systems package version 9.2 (SAS Institute, Cary, NC, USA).
Results
Effect of nutrient composition of dietary formula on postprandial glucose and insulin response
The baseline characteristics of subjects are shown in Table 2. The mean GI values of all dietary formulas were different despite an equal amount of available carbohydrate, but all below 55 (MF < FF < CF) according to the method of calculating GI (Table 1). Plasma glucose and insulin responses after ingestion of three dietary formulas evaluated are shown in Fig. 1A and 1B. Although the peak postprandial glucose response was consistently observed 30 min after consumptions for all study formulas, the magnitude of the peak was significantly lower in subjects that received the MF (P = 0.012), an effect that was also observable but not significant in AUC calculations (P = 0.056) (Fig. 1A). The apparent superiority of the MF, however, must be interpreted with caution. While plasma glucose levels for the CF and FF returned to or below baseline at t = 180 min, the MF remained modestly above baseline. Plasma insulin concentrations were similarly affected, as peak concentration was significantly lower for the MF at t = 30 min compared to the CF and FF (P = 0.004), but failed to return to baseline by t = 180 min. Insulin AUC for insulin was also significantly low for the MF (P = 0.038) (Fig. 1B).
Table 2.
Baseline characteristics

Fig. 1.
Postprandial plasma glucose (A) and insulin (B) responses in non-diabetic subjects (n = 8/group) to a control dietary formula (CF, ●), a high-fiber dietary formula (FF, ○), and a high-MUFA dietary formula (MF, ▼), standardized to 50 g available glucose. Data are represented as percentages of the baseline concentration, and curves represent changes in plasma concentrations for 180 min following the ingestion of each dietary formula. The corresponding AUC was calculated using the trapezoidal method. Data points (mean ± SD) and bars with different superscripts are significantly different (P < 0.05) by ANOVA with the post-hoc Bonferroni multiple comparison tests.
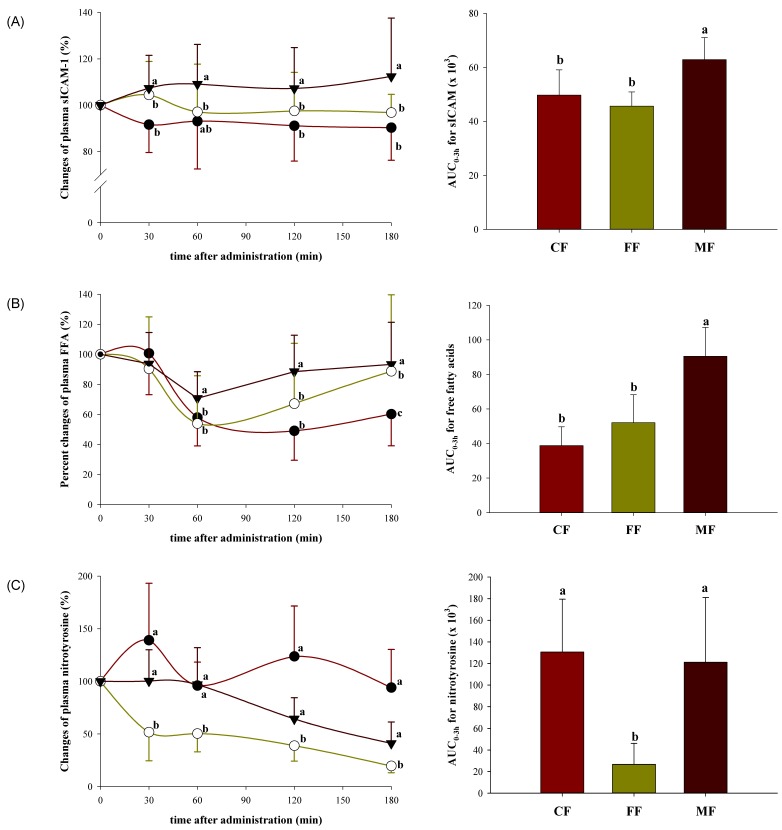
Effect of nutrient composition of dietary formula on postprandial endothelial inflammation
Circulating sICAM-1, NT, and FFA responses were analyzed at 0, 30, 60, 120, and 180 min and the results are shown in Fig. 2A, 2B, and 2C. These markers were significantly and differentially affected by the formulas examined under an equal amount of available carbohydrate. The sICAM-1 levels were significantly elevated for the MF at all time points compared to the CF and FF (P = 0.002, 0.007, 0.003, and < 0.001 at t = 30, 60, 120, and 180 min, respectively). The sICAM-1 AUCs were 1.2 to 1.4-fold higher for the MF compared with the FF and CF (P < 0.001) (Fig. 2A). Similarly, circulating FFA levels were significantly higher for the MF compared to the CF or FF after 60 min (P < 0.001 at t = 60, 120, and 180 min); further, FFA AUCs were 1.7 to 2.3-fold higher for the MF compared with the FF and CF (P < 0.001) (Fig. 2B). Circulating NT levels also varied significantly over time (P < 0.001), but showed different pattern as compared to sICAM-1 and FFA, demonstrating significantly lower level for the FF at all time points compared with the CF or MF (P < 0.001, 0.002, 0.001, and 0.001 at t = 30, 60, 120, and 180 min, respectively). Similarly, NT AUCs for the MF and CF were statistically equivalent, but 5.8 to 8.2-fold higher compared to the FF (P < 0.001) (Fig. 2C).
Fig. 2.
Postprandial plasma sICAM-1 (A), NT (B), and FFA (C) responses in non-diabetic subjects (n = 8/group) to a control dietary formula (CF, ●), a high-fiber dietary formula (FF, ○), and a high-MUFA dietary formula (MF, ▼), standardized to 50 g available glucose. Data are represented as percentages of the baseline concentration, and curves represent changes in plasma levels for 180 min following the ingestion of a dietary formula. The corresponding AUC was calculated using the trapezoidal method. Data points (mean ± SD) and bars with different superscripts are significantly different (P < 0.05) by ANOVA with the post-hoc Bonferroni multiple comparison tests.
Pearson correlations revealed that changes in sICAM-1 paralleled changes in NT (r = 0.355, P = 0.046) and FFA (r = 0.434, P = 0.013), while glucose response was not correlated to changes in sICAM-1 or NT. In fact glucose response was negatively correlated to changes in FFA (r = -0.473, P = 0.006).
Discussion
While the metabolic and hormonal changes in the overnight fasted state has received the clinical focus for many years, the postprandial state began to receive more attention in recent years [14]. Genetic factors may be important on what happens in the postprandial phase, but the composition of meals may be regarded to be more important. In this study, the CF provided low in fiber and fats compared with the FF and MF, respectively and thus increased the GI in non-diabetic subjects despite an equal amount of available carbohydrate. In consideration of the glycemic response for patients with diabetes, two different types of dietary formulas were designed: one with high-fiber and the other with high-MUFA. The FF and MF provided 6.1% of energy as fiber and 23.8% of energy as MUFA versus 1.6% of energy as fiber and 34.5% of energy as MUFA, respectively, resulting in improvement of postprandial glycemic response in non-diabetic subjects as compared with the CF. In particular, the MF was highly efficient in reducing postprandial glycemic response. The same result was observed with another study of our lab in subjects with impaired glucose tolerance (unpublished data). Recently, Yokoyama et al. [15] compared the effects of high MUFA (49.3% of energy as fats and 31.5% of energy as carbohydrate) versus high-carbohydrate (30.8% of energy as fats and 53.4% of energy as carbohydrate) enteral formula on postprandial glucose and insulin response in diabetic and non-diabetic subjects, concluding that a high-MUFA formula may suppress postprandial elevation of plasma glucose concentration and reduce the burden on pancreatic β-cells. A meta-analysis of various studies in patients with type 2 diabetes also showed that high-MUFA formulas (22% to 33% energy) improved glycemic control compared to low-fat, high-carbohydrate (49% to 60% energy) formulas [16]. Thus, it was thought that the strategy to replace energy with MUFA was highly effective with regard to the management of postprandial glycemic response.
In postprandial phase, atherosclerotic risk factors may be adversely modified and a simultaneous postprandial hyperglycemic spike in diabetic patients may augment these phenomena [17]. The postprandial state therefore is again important for developing CVD risks in both non-diabetic and diabetic subjects [18]. The mechanism through which postprandial state exerts its effects may be identified in the production of free radicals and circulating inflammatory factors [19]. Several markers of acute inflammatory stress during the postprandial period have been identified. Among the various markers, intercellular adhesion molecule-1 (ICAM-1) has received particular interest. The soluble form of ICAM-1 (sICAM-1) is stored in the cells and can be quickly overexpressed outside them in response to various stimuli [20]. Their greater expression would imply an increase in the circulating leukocyte adhesion to the endothelium, which is considered important and earlier processes leading to atheromatous lesion [17]. Next, NT has been received attention as an independent predictor of CVD [21]. During the postprandial state, the simultaneous overgeneration of nitric oxide and superoxide favors the production of the peroxynitrite anion, which in turn nitrates amino acids like tyrosine [22]. From the presence of nitrotyrosine, we can infer that peroxynitrite has been increasing. Lastly, while it remains unclear whether fasting or postprandial FFA level is more predictive of cardiovascular risk, high levels of plasma FFA have been correlated to elevated expression of inflammation markers [23].
Interestingly, most of the endothelial inflammatory factors (sICAM-1, NT, and FFA) measured in this study were significantly higher in the MF compared to the other formulas despite the lowest mean GI value. So it was thought that glucose response might not be representative of endothelial inflammatory response in the postprandial state. Additional dietary components such as fat content may be influential. In fact, the MF had more calories and a higher fat content compared to the FF and CF, as fats provided 48.9% of total caloric content. The FF and CF provided 35.9% and 31.4% of energy as fats, respectively. The results of this study corroborate data from others showing the increases of sICAM-1 and FFA levels following a formula with high fat content [8,24,25]. In a study of Nappo et al. [26], direct comparison of a high-fat and a high-carbohydrate meal on endothelial activation revealed that while ICAM-1 was significantly elevated after both meal types in comparison to a basal value, elevated levels were sustained for a longer period of time following the high-fat meal. Moreover, there are several lines of evidence supporting the adverse effect of high MUFA formulas on atherosclerosis in animals [27,28] and in humans [29]. Excessive dietary MUFA (polyunsaturated : monounsaturated : saturated = 1 : 3.5 : 1 vs 1 : 0.5 : 1) raised plasma lipids, especially triglycerides in healthy subjects [29]. In summary, results reported here buttress existing evidence that dietary fat content is influential and predictive of postprandial inflammatory markers and possible vascular dysfunction.
Although one can argue that this study is consisted of a relatively small number of subjects, we calculated the number of subjects to achieve statistical difference among dietary formulas with 80% power for glycemic response based on our previous study (data not shown). Furthermore, we used the crossover design that can minimize the variability between treatments [19]. Importantly, the present study was conducted in non-diabetic subjects; therefore effects and relationships reported here may provide limited insight into responses and inflammation within the context of diabetes. However the present study gives an idea that the dietary formula specially formulated for diabetic patients should be developed considering its postprandial inflammatory effect as well as glucose response for minimizing the risk of CVD occurrence. A MUFA-rich dietary formula might be suboptimal in terms of the endothelial inflammation, whereas a fiber-rich dietary formula would be the alternative options that might have modest beneficial effects on both glycemic response and endothelial inflammation in management of diabetic patients at high risk of CVD. Further optimization of dietary formulas may be necessary to attenuate both postprandial and chronic hyperglycemia and prevent cardiovascular complications associated with diabetes.
Taken together, while a dietary formula high in MUFA resulted in the lowest postprandial glucose response, it induced high and sustained levels of sICAM-1 and FFA which are indicative of endothelial dysfunction. In the meantime, a dietary formula high in fiber resulted in intermediate postprandial glucose response, but it showed significantly low level of NT. These results are provocative and suggest that nutrient composition of dietary formula differently influenced in terms of the acute postprandial glucose levels and endothelial inflammatory markers in non-diabetic subjects. Future studies are needed on the response changes over time with repeated ingestion in diabetic subjects to better understand the clinical relevance of the GI approach in the cardiovascular complications in diabetes.
Acknowledgements
The authors are grateful to the participants of this study. We also thank the staff of Daesang Co. (Seoul, Korea) for providing the experimental dietary formulas.
Footnotes
This project was supported the 2nd stage of Brain Korea 21 Project and NRF Project (No. 2012M3A9C4048761) funded by the Ministry of Education, Science and Technology.
References
- 1.Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34:362–366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 2.Brand-Miller J, Wolever TM, Foster-Powell K, Colagiuri S. The New Glucose Revolution: The Authoritative Guide to the Glycemic Index--The Dietary Solution for Lifelong Health. New York (NY): Marlowe & Company; 2003. [Google Scholar]
- 3.Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care. 2008;31:2281–2283. doi: 10.2337/dc08-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization; Food and Agriculture Organization of the United Nations. Carbohydrates in Human Nutrition. Report of a Joint FAO/WHO Expert Consultation. Rome: Food and Agriculture Organization of the United Nations; 1998. pp. 1–140. [Google Scholar]
- 5.Willett W, Manson J, Liu S. Glycemic index, glycemic load, and risk of type 2 diabetes. Am J Clin Nutr. 2002;76:274S–280S. doi: 10.1093/ajcn/76/1.274S. [DOI] [PubMed] [Google Scholar]
- 6.Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes. 2005;54:1–7. doi: 10.2337/diabetes.54.1.1. [DOI] [PubMed] [Google Scholar]
- 7.De Man FH, Cabezas MC, Van Barlingen HH, Erkelens DW, de Bruin TW. Triglyceride-rich lipoproteins in non-insulin-dependent diabetes mellitus: post-prandial metabolism and relation to premature atherosclerosis. Eur J Clin Invest. 1996;26:89–108. doi: 10.1046/j.1365-2362.1996.114256.x. [DOI] [PubMed] [Google Scholar]
- 8.Rubin D, Claas S, Pfeuffer M, Nothnagel M, Foelsch UR, Schrezenmeir J. s-ICAM-1 and s-VCAM-1 in healthy men are strongly associated with traits of the metabolic syndrome, becoming evident in the postprandial response to a lipid-rich meal. Lipids Health Dis. 2008;7:32. doi: 10.1186/1476-511X-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14:575–585. doi: 10.1016/j.cmet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grundy SM, Howard B, Smith S, Jr, Eckel R, Redberg R, Bonow RO. Prevention Conference VI: Diabetes and Cardiovascular Disease: executive summary: conference proceeding for healthcare professionals from a special writing group of the American Heart Association. Circulation. 2002;105:2231–2239. doi: 10.1161/01.cir.0000013952.86046.dd. [DOI] [PubMed] [Google Scholar]
- 11.Elia M, Ceriello A, Laube H, Sinclair AJ, Engfer M, Stratton RJ. Enteral nutritional support and use of diabetes-specific formulas for patients with diabetes: a systematic review and meta-analysis. Diabetes Care. 2005;28:2267–2279. doi: 10.2337/diacare.28.9.2267. [DOI] [PubMed] [Google Scholar]
- 12.Pradhan AD, Ridker PM. Do atherosclerosis and type 2 diabetes share a common inflammatory basis? Eur Heart J. 2002;23:831–834. doi: 10.1053/euhj.2001.3052. [DOI] [PubMed] [Google Scholar]
- 13.Wolever TM, Jenkins DJ, Jenkins AL, Josse RG. The glycemic index: methodology and clinical implications. Am J Clin Nutr. 1991;54:846–854. doi: 10.1093/ajcn/54.5.846. [DOI] [PubMed] [Google Scholar]
- 14.Lefèbvre PJ, Scheen AJ. The postprandial state and risk of cardiovascular disease. Diabet Med. 1998;15(Suppl 4):S63–S68. doi: 10.1002/(sici)1096-9136(1998120)15:4+<s63::aid-dia737>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama J, Someya Y, Yoshihara R, Ishii H. Effects of high-monounsaturated fatty acid enteral formula versus high-carbohydrate enteral formula on plasma glucose concentration and insulin secretion in healthy individuals and diabetic patients. J Int Med Res. 2008;36:137–146. doi: 10.1177/147323000803600117. [DOI] [PubMed] [Google Scholar]
- 16.Garg A. High-monounsaturated-fat diets for patients with diabetes mellitus: a meta-analysis. Am J Clin Nutr. 1998;67:577S–582S. doi: 10.1093/ajcn/67.3.577S. [DOI] [PubMed] [Google Scholar]
- 17.Ceriello A. The post-prandial state and cardiovascular disease: relevance to diabetes mellitus. Diabetes Metab Res Rev. 2000;16:125–132. doi: 10.1002/(sici)1520-7560(200003/04)16:2<125::aid-dmrr90>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Ceriello A, Assaloni R, Da Ros R, Maier A, Piconi L, Quagliaro L, Esposito K, Giugliano D. Effect of atorvastatin and irbesartan, alone and in combination, on postprandial endothelial dysfunction, oxidative stress, and inflammation in type 2 diabetic patients. Circulation. 2005;111:2518–2524. doi: 10.1161/01.CIR.0000165070.46111.9F. [DOI] [PubMed] [Google Scholar]
- 19.Peairs AD, Rankin JW, Lee YW. Effects of acute ingestion of different fats on oxidative stress and inflammation in overweight and obese adults. Nutr J. 2011;10:122. doi: 10.1186/1475-2891-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bevilacqua MP, Nelson RM, Mannori G, Cecconi O. Endothelial-leukocyte adhesion molecules in human disease. Annu Rev Med. 1994;45:361–378. doi: 10.1146/annurev.med.45.1.361. [DOI] [PubMed] [Google Scholar]
- 21.Shishehbor MH, Aviles RJ, Brennan ML, Fu X, Goormastic M, Pearce GL, Gokce N, Keaney JF, Jr, Penn MS, Sprecher DL, Vita JA, Hazen SL. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA. 2003;289:1675–1680. doi: 10.1001/jama.289.13.1675. [DOI] [PubMed] [Google Scholar]
- 22.Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab. 2000;85:2970–2973. doi: 10.1210/jcem.85.8.6854. [DOI] [PubMed] [Google Scholar]
- 23.Pilz S, März W. Free fatty acids as a cardiovascular risk factor. Clin Chem Lab Med. 2008;46:429–434. doi: 10.1515/CCLM.2008.118. [DOI] [PubMed] [Google Scholar]
- 24.Onody A, Csonka C, Giricz Z, Ferdinandy P. Hyperlipidemia induced by a cholesterol-rich diet leads to enhanced peroxynitrite formation in rat hearts. Cardiovasc Res. 2003;58:663–670. doi: 10.1016/s0008-6363(03)00330-4. [DOI] [PubMed] [Google Scholar]
- 25.Shimabukuro M, Chinen I, Higa N, Takasu N, Yamakawa K, Ueda S. Effects of dietary composition on postprandial endothelial function and adiponectin concentrations in healthy humans: a crossover controlled study. Am J Clin Nutr. 2007;86:923–928. doi: 10.1093/ajcn/86.4.923. [DOI] [PubMed] [Google Scholar]
- 26.Nappo F, Esposito K, Cioffi M, Giugliano G, Molinari AM, Paolisso G, Marfella R, Giugliano D. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: role of fat and carbohydrate meals. J Am Coll Cardiol. 2002;39:1145–1150. doi: 10.1016/s0735-1097(02)01741-2. [DOI] [PubMed] [Google Scholar]
- 27.Ceriello A, Quagliaro L, D'Amico M, Di Filippo C, Marfella R, Nappo F, Berrino L, Rossi F, Giugliano D. Acute hyperglycemia induces nitrotyrosine formation and apoptosis in perfused heart from rat. Diabetes. 2002;51:1076–1082. doi: 10.2337/diabetes.51.4.1076. [DOI] [PubMed] [Google Scholar]
- 28.Lada AT, Rudel LL. Dietary monounsaturated versus polyunsaturated fatty acids: which is really better for protection from coronary heart disease? Curr Opin Lipidol. 2003;14:41–46. doi: 10.1097/00041433-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Chang NW, Huang PC. Effects of dietary monounsaturated fatty acids on plasma lipids in humans. J Lipid Res. 1990;31:2141–2147. [PubMed] [Google Scholar]