SUMMARY
Type 1 diabetes is caused by autoimmune-mediated β cell destruction leading to insulin deficiency. The histone deacetylase SIRT1 plays an essential role in modulating several age-related diseases. Here we describe a family carrying a mutation in the SIRT1 gene, in which all five affected members developed an autoimmune disorder: four developed type 1 diabetes, and one developed ulcerative colitis. Initially, a 26-year-old man was diagnosed with the typical features of type 1 diabetes, including lean body mass, autoantibodies, T cell reactivity to β cell antigens, and a rapid dependence on insulin. Direct and exome sequencing identified the presence of a T-to-C exchange in exon 1 of SIRT1, corresponding to a leucine-to-proline mutation at residue 107. Expression of SIRT1-L107P in insulin-producing cells resulted in overproduction of nitric oxide, cytokines, and chemokines. These observations identify a role for SIRT1 in human autoimmunity and unveil a monogenic form of type 1 diabetes.
INTRODUCTION
Diabetes is classified into two main categories based on the clinical presentation and pathophysiology (American Diabetes Association, 2010). Type 1 diabetes is characterized by autoimmune β cell destruction. Conversely, type 2 diabetes is caused by a relative insulin deficiency in the face of insulin resistance. Although polymorphisms in multiple genes have been linked to overall susceptibility to diabetes, single gene defects leading to type 1 diabetes have been identified only in the case of AIRE and FOXP3, which are associated with the development of complex phenotypes. Mutations in AIRE (Finnish-German APECED Consortium, 1997; Nagamine et al., 1997) interfere with the process of immune tolerance to self-antigens and result in autoimmune polyendocrinopahy, candidiasis, ectodermal dystrophy (APCED). Alternatively, mutations in FOXP3 (Bennett et al., 2001) result in defective development of regulatory T (Treg) cells, which leads to a rapidly fatal condition called immunedysregulation, polyendocrinopathy, enteropathy, x-linked (IPEX) syndrome. Interestingly, recent evidence suggests that SIRT1 modulates FOXP3 and thereby influences Treg cell development, which is essential for the maintenance of immune homeostasis and protection against autoimmunity (Beier et al., 2011; van Loosdregt et al., 2010).
Early studies in budding yeast helped to identify sirtuin deacetylases as key enzymes that coordinate the organism's response to calorie intake and the regulation of life span (Banks et al., 2008; Cantó et al., 2009; Haigis and Guarente, 2006; Milne et al., 2007; Westphal et al., 2007; Yoshizaki et al., 2009a). Subsequently, the mammalian sirtuin SIRT1 has received much attention for its role in regulating metabolism and its role in protecting against age-related diseases (Haigis and Sinclair, 2010). Interestingly, SIRT1 is prominently expressed in β cells and regulates insulin secretion (Bordone et al., 2006). In mice, targeted overexpression of SIRT1 in β cells enhances insulin secretion (Moynihan et al., 2005) and protects from apoptosis (Tang et al., 2011). In parallel, SIRT1 also directly modulates insulin sensitivity in peripheral tissues (Sun et al., 2007). More recently, several reports have described a role for SIRT1 in the regulation of cytokine production (Stein et al., 2010; Yang et al., 2010) and maintenance of T cell tolerance (Beier et al., 2011, 2012; van Loosdregt et al., 2010; Zhang et al., 2009a). These results support a previous study demonstrating that SIRT1-deficient mice exhibit sera-containing antibodies against nuclear antigens and develop autoimmune conditions (Sequeira et al., 2008). Furthermore, the SIRT1 activator Resveratrol has been shown to prevent and treat the spontaneous type 1 diabetes which normally develops in nonobese diabetic (NOD) mice (Lee et al., 2011).
RESULTS
Description of the Patients
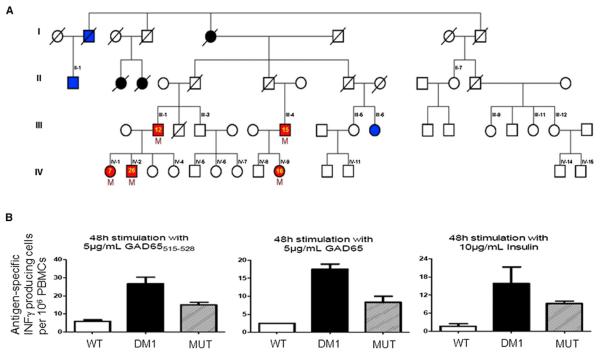
Type 1 diabetes was diagnosed in a 26-year-old Ashkenazi Jewish male on the basis of hyperglycemia, a lean body mass index of 21.5 Kg/m2, signs of β cell autoimmunity (autoantibodies to glutamic acid decarboxylase 1,150 U/L [normal value <10], islet-cell autoantibody-2 3.0 U/L [normal value <1.5]), and insulin dependence (patient IV-2 in Figure 1A). Surprisingly, the patients' sister, father, and a paternal cousin were also diagnosed by their endocrinologists with type 1 diabetes at the ages of 7, 12, and 15 years, respectively (Figure 1A). All affected relatives were lean, displayed autoantibodies to β cells, lacked measurable C-peptide levels, and required insulin injections. The autoimmune nature of the disease was confirmed by assessing β cell antigen-specific T cell activation by peripheral blood mononuclear cells (Figure 1B). Interestingly, mRNA transcript and protein expression levels for several genes involved in T cell homeostasis and regulation were decreased in Th cells. These cells also displayed low basal and induced FOXP3 (see Figures S1A and S1B online).
Figure 1. The Patients' Family Tree and Functional Analysis of Peripheral Blood Mononuclear Cells.
(A) Red symbols indicate family members who developed an autoimmune disease: type 1 diabetes (III-1, III-4, IV-1, IV-2) or colitis (IV-9). The yellow numbered symbols denote the age of onset of the disease. Blue symbols indicate type 2 diabetes, while black symbols indicate an unclear diabetes phenotype. Black numbered symbols identify all family members who were tested for the presence of the mutation, and the letter “M” identifies the ones who were positive. A slash denotes deceased family members. Individuals IV-1 to IV 15, III-1, III-4, III-5, III-9, III-11, and III-12 are lean (body mass index <25 Kg/m2), and II-1 and III-6 have body mass indices of 27 and >40 Kg/m2, respectively.
(B) Peripheral blood mononuclear cells were obtained from healthy control subjects (WT) and from patients with type 1 diabetes carrying wild-type SIRT1 (DM1) or the L107P mutation (MUT). GAD65515–528 peptide, whole GAD65 protein, and insulin elicited antigen-specific IFN-γ production. n = 3 for each of the three groups. All data are presented as mean ± SEM.
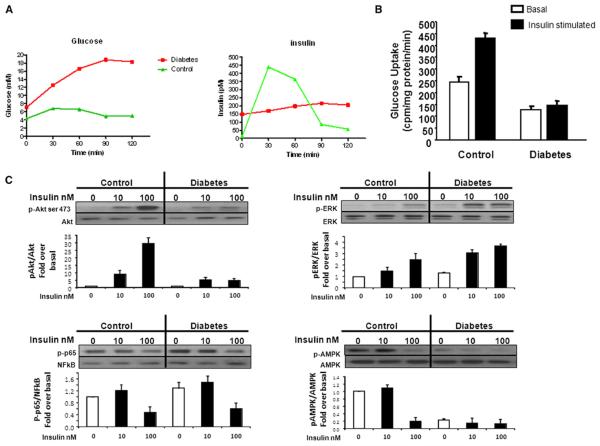
To further characterize the pathology of the index patient, an oral glucose-tolerance test was performed 10 months after the onset of diabetes. As expected, β cell function was severely impaired, with a blunted insulin release following stimulation by an oral glucose load (Figure 2A). Follow-up showed that serum insulin and C-peptide concentrations steadily decreased over time (50% decrease after 1 year, undetectable after 2 years). The patient also presented some resistance to insulin, as revealed by a euglycemic-hyperinsulinemic clamp study (M value 34.1 10−3 mM/min/Kg BW) and a muscle biopsy. The latter yielded myoblasts displaying a reduced glucose uptake in response to insulin along with changes in the phosphorylation levels of the insulin-dependent proteins, Akt, ERK, and AMPK (Figures 2B and 2C).
Figure 2. Impaired Insulin Secretion and Sensitivity in a Patient Carrying a Mutation in the SIRT1 Gene.
(A) Plasma glucose and insulin levels during oral glucose tolerance tests of the index patient with a SIRT1 mutation (red lines) and a control 20-year-old non-affected male family member (green lines).
(B) Impaired insulin stimulated glucose uptake of cultured human skeletal myotubes obtained from biopsies of the vastus lateralis muscle of the index patient with a SIRT1 mutation (diabetes) compared to the control 20-year-old nonaffected male family member (control).
(C) The cell lysates of the myotubes were analyzed by immunoblotting with antibodies to phosphorylated and nonphosphorylated AKT, ERK, NF-κB (p65), and AMP-K. n = 3 myotube subpopulations. All data are presented as mean ± SEM.
Identification of the Mutation
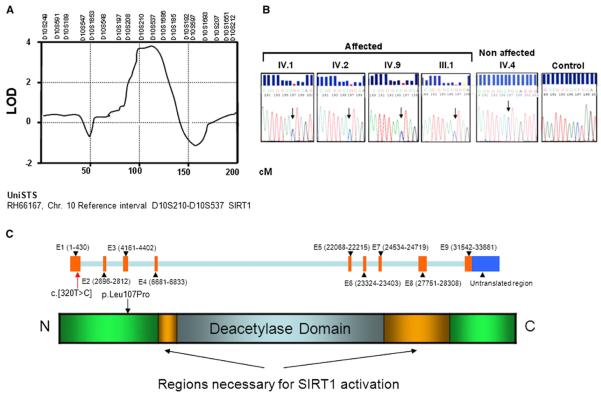
The pattern of inheritance among the affected family members was indicative of an autosomal dominant mutation (Figure 1A). We used three different techniques in order to identify and validate the gene(s) targeted by the inherited mutation: microsatellite genotyping, targeted deep sequencing, and Sanger sequencing of relevant candidate genes. All three techniques converged on a single target gene, SIRT1. Whole-genome microsatellite analysis identified a region on chromosome 10 with a significant LOD score of 4.0 between markers D10S210 and D10S537 corresponding to SIRT1 (Figure 3A and Figure S2A). Although there were segments on chromosomes 4 and 22 that yielded a LOD score of approximately 2, there were no discernible genotype-phenotype correlations for these regions. Direct sequencing of the SIRT1 gene revealed the presence of a T-to-C exchange in exon 1 (c.[320T > C]) present in single copy in the DNA of the affected individuals, leading to a Leucine107Proline mutation in the SIRT1 protein (p. Leu107Pro, Figures 3A and 3B). Based on the microsatellite analysis, exome sequencing and analysis of specific areas from chromosomes 4, 10, and 22 were performed in three patients carrying the mutation (patient III-1, III-4, and IV-2 in Figure 1A) and in three nonaffected subjects from the same family (individuals IV-4, IV-5, and IV-8 in Figure 1A) and confirmed that only this mutation segregates with the phenotype of the patients (Figure S2B). The analysis of chromosome 10 also revealed that a SNP related to insulin activity, in KIAA1274 (Paladin), is present in the family. However, the frequency of this SNP is high (80% homozygote in the family and 60% in a control population), and it is equally distributed across the whole family and independent of the phenotype (Figure S2B). Despite its lack of correlation with the phenotype, this SNP may have helped to precipitate the observed type 1 diabetes, since it has been proposed to inhibit insulin signaling (Huang et al., 2009). To rule out the possibility of a common polymorphism in SIRT1, we searched for the presence of c.[320T > C] in 100 unrelated whites, 90 Ashkenazi Jews, 160 patients with sporadic type 1 diabetes, and 1,900 type 1 diabetes patients, with sib-pair or parent-offspring affected families. None of these groups possessed the c.[320T > C] variant. Moreover, this mutation has not been documented in dbSNP (http://www.ncbi.nlm.nih.gov/snp/), in 1,000 Genomes (http://www.1000genomes.org/), or in the Exome Variant Server (http://evs.gs.washington.edu/EVS/). This demonstrates that the exchange c.[320T > C] is not a common polymorphism. However, there may exist other families with mutations in different regions of SIRT1 that could lead to type 1 diabetes. Of some interest, analysis of data from a genome-wide association study (Barrett et al., 2009) for the SIRT1 locus rs12778366 showed modest evidence of association with type 1 diabetes (p = 0.005). This SNP is in moderate LD with an eQTL signal for SIRT1 (D′ = 1/r2 = 0.5).
Figure 3. Description of the Mutation.
(A) Whole-genome microsatellite linkage analysis. Details of chromosome 10, for which the LOD reached significance (LOD 4.1; whole-genome significance, LOD > 2.2). The length of the chromosome is given in centimorgans (cM). According to the UniSTS database, the region between microsatellite D10S210 and D10S537 contains the SIRT1 gene.
(B) Sanger sequence analysis of the mutation. DNA sequencing chromatograms obtained by direct sequencing of PCR products showing the presence of the heterozygous c.[320T > C] substitution in exon 1, not present in nonaffected members of the family and normal individuals (control, representative example out of 200 alleles). The roman numerals correspond to those in Figure 1A.
(C) SIRT1 gene structure and predicted protein structure.
Because type 1 diabetes has strong HLA associations with linkage to certain DQ alleles, the HLA genotype was analyzed. The results from this analysis failed to explain the high prevalence of the disease. Also we observed no linkage peak corresponding to the HLA region on chromosome 6p21 (Table S1). Finally, we ruled out mutations in the six MODY genes and in the diabetes-associated TCF7L2, IRS2, and SOCS2 genes (data not shown).
Nonaffected family members were healthy, with normal insulin secretion and action, except for two individuals displaying features typical of type 2 diabetes (advanced age at the onset of the disease, no insulin requirement, obesity, dyslipidemia, and hypertension; patients II-1 and III-6 in Figure 1A). All five family members carrying the mutation were diagnosed with an autoimmune disease, while none of the 16 noncarriers tested were affected (Figure 1A). The segregation of the mutation in the family was in complete agreement with the phenotypes (maximal two-point lod score of 2.4; p < 0.0001 Fisher's exact test). Four members had type 1 diabetes, including a distant member, a cousin of the patients' father. One 18-year-old woman carrying the mutation displayed severe ulcerative colitis which manifested at 16 years of age (patient IV-9 in Figure 1A). This patient required maintenance therapy with the immunosuppressant azathioprine and corticosteroids (repetitively). Increased levels of anti-nuclear antibodies (titer 1:640) and atypical ANCA (titer 1:160) confirmed the autoimmune component of her disease.
Decreased Anti-Inflammatory Activity of SIRT1-L107P
To understand how the mutation could give rise to autoimmune defects, we tested whether any known functions of SIRT1 were altered by the mutation. L107 lies outside of the conserved Sirtuin enzymatic core, in a densely charged region of the protein potentially involved in protein-protein interactions (Autiero et al., 2009a) (Figure 3C). SIRT1-L107P showed a mild decrease in deacetylase activity relative to the wild-type protein (Figure S3A), and there were no changes in protein stability, as assessed by a cycloheximide chase experiment (data not shown). Additionally, mutation of L107P did not affect the subcellular localization of SIRT1 (data not shown). In an effort to identify potential protein-protein interactions affected by the L107P substitution, we performed immunoprecipitation experiments with the wild-type and mutant proteins. We observed that the strongest SIRT1 interactors, identified by mass spectrometry, were not perturbed by the mutation (Figures S3B–S3D). Furthermore, substitution of L107P did not affect the ability of SIRT1 to interact with eIF2α or AROS (data not shown). Therefore, it is likely that protein-protein interactions altered by the substitution are of a transient or context-specific nature.
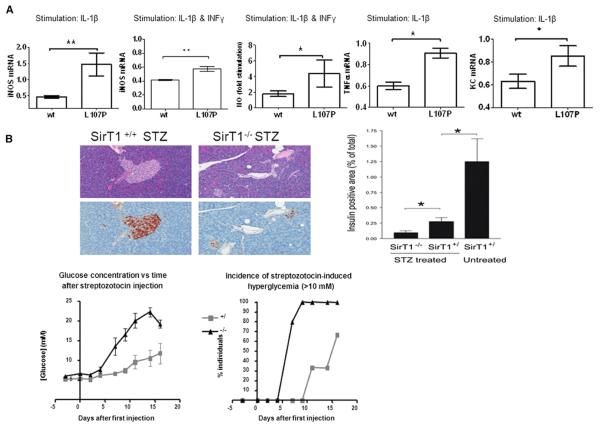
Based on the central role attributed to cytokines and nitric oxide in the development of type 1 diabetes, and on the ability of SIRT1 to suppress nitric oxide production in β cells (Lee et al., 2009), we analyzed the effect of SIRT1-L107P on cytokine production. Stable retroviral transduction of the β cell line MIN6 with SIRT1-L107P led to higher cytokine-induced nitric oxide synthase expression and increased production of nitric oxide relative to cells expressing an equal amount of wild-type SIRT1 protein (Figure 4A and Figure S4A). Moreover, SIRT1-L107P also led to increased expression of the cytokine TNF-α and the chemokine KC, relative to SIRT1 wild-type controls (Figure 4A). In order to validate the importance of SIRT1 in autoimmune destruction of β cells in vivo, we treated wild-type and SIRT1 knockout mice with multiple low doses of streptozotocin to induce pancreatic insulitis (Like and Rossini, 1976). Interestingly, SIRT1 knockout mice displayed increased islet destruction along with early and marked hyperglycemia (Figure 4B). Finally, transduction of myoblasts from healthy individuals with SIRT1-L107P resulted in insulin resistance (Figure S4B) similar to that observed in myoblasts obtained from the index patient (Figure 2B).
Figure 4. Decreased Anti-Inflammatory Activity of SIRT1-L107P.
(A) MIN6 cells were stably transduced with empty, wild-type (WT), or mutant SIRT1 L107P virus and stimulated with IL-1β or a combination of IL-1β and IFN-γ. iNOS mRNA expression relative to empty virus-transduced cells MIN6 cells, and NO formation following cytokine stimulation expressed as fold of unstimulated cells. Findings were verified with five separately produced sets of sirt1 WT and L107P-overexpressing cell lines each time at least in triplicate (mean ± SD, *p < 0.05; **p < 0.01).
(B) Wild-type and SIRT1-deficient mice were injected with five daily injections of streptozotocin (40 mg/kg). Glycemia and onset of diabetes (serum glucose >10 mM) were monitored for 16 days. Islet histology was visualized by HE and immunostaining for insulin (brown). The proportion of surface area that stained positive for insulin is shown. All data are presented as mean ± SEM. *p < 0.05; n = 6 for +/+ and +/−, and n = 5 for −/−.
DISCUSSION
The present work describes a monogenic form of diabetes with the typical features of type 1 diabetes (autoantibodies to β cells, lean and young at onset of hyperglycemia, rapid disappearance of C-peptide production and insulin dependence) together with insulin resistance. This disease appears to be the consequence of an autosomal-dominant mutation in the SIRT1 gene. Although it is not typical to find insulin resistance in patients with type 1 diabetes, its prevalence may be underestimated (Wentworth et al., 2009). The impaired β cell secretory function and insulin resistance we observed in patients with mutated SIRT1 gene are consistent with data from animal studies (Bordone et al., 2006; Breitenstein et al., 2011; Moynihan et al., 2005; Sequeira et al., 2008; Sun et al., 2007; Yang et al., 2010; Zhang et al., 2009a), which together indicate that SIRT1 may play a pivotal role in preventing autoimmune diseases. It is tempting to speculate that the disease pathology may arise as a combined consequence of β cell impairment and death along with subsequent pathological activation of the immune system. Indeed, in the presence of insulin resistance, stress may accelerate β cell death, which may result in the release of autoantigens together with endogenous “danger signals” (alarmins) capable of promoting pathologic self-antigen presentation (Zhang et al., 2009b). Of particular interest is the ability of SIRT1 to suppress TNFα expression (Yoshizaki et al., 2009b) which is partially ablated by mutation of L107P. Importantly, both type 1 diabetes and ulcerative colitis are strongly associated with this cytokine, and TNF antagonism improves both conditions (Mastrandrea et al., 2009).
Whether or not the L107P mutation is of a dominant nature remains to be confirmed. However, both the analysis of the family tree and the results of the in vitro studies on L107P are suggestive of a dominant mutation. Given that the N-terminal region of SIRT1, encompassing L107P, is thought to be involved in binding to a broad spectrum of other proteins (Autiero et al., 2009b; Dunker et al., 2005), it is conceivable that a point mutation in this region may change the binding affinity toward a subset of binding partners, resulting in an apparent dominant effect.
Together, these data indicate that SIRT1 regulates immune function in humans and that a single amino acid substitution in SIRT1 promotes hyperinflammation and metabolic dysfunction. Molecules that enhance SIRT1 activity may be useful in treating not only this disease but also other inflammatory or autoimmune diseases.
EXPERIMENTAL PROCEDURES
Patients and Study Procedures
We conducted the study in accordance with the ethical guidelines of the Declaration of Helsinki II; the study design was approved by the Zurich regional and institutional review boards. Written informed consent was obtained from all patients.
Oral glucose tolerance tests, euglycemic-hyperinsulinemic clamp studies, and muscle biopsies were performed as described (Larsen et al., 2007).
Mutation Analysis
Genomic DNA was extracted from peripheral blood leukocytes (see the Supplemental Information for more information).
Induction of Autoimmune Insulitis in SIRT1 Mice
Wild-type and SIRT1-deficient mice (McBurney et al., 2003) were injected with multiple low-dose streptozotocin as previously described (Like and Rossini, 1976). Accumulation of lymphoid tissue in islets was assessed by histology.
Human Skeletal Muscle Cells
Human skeletal muscle cells were isolated from muscle biopsies by trypsin digestion, grown to confluent myoblasts, and then differentiated into myotubes as previously described (Bouzakri et al., 2003).
Plasmids and Mutagenesis
MSCV-puro containing full-length SIRT1 cDNA was subject to site-directed mutagenesis using the QuikChange II XL Site-Directed Mutagenesis Kit (Stratagene). The following primers were used for the mutagenesis: forward primer, 5′-AGACAATGGGCCGGGCCCGCAGGGCCCATCT-3′; and reverse primer, 5′-AGATGGGCCCTGCGGGCCCGGCCCATTGTCT-3′.
Retroviral Production and Infection
GP2-293 cells were cotransfected with VSVG and MSCV plasmids using FuGENE HD transfection reagent, according to the manufacturer's instructions. Twenty hours after transfection, the media was replaced with fresh DMEM + 10% FBS + 1X pen/strep + 1X glutamine. Virus was harvested between 48 and 72 hr posttransfection. Cells were targeted using filtered viral supernatants in the presence of 5 μg/mL Polybrene (Sigma). Selection with puromycin (0.5 μg/mL) was started 48 hr later.
Cell Culture and Protein Stability Experiments
GP2-293, 293T, and NIH 3T3 were all cultured in high-glucose DMEM media with pyruvate (GIBCO) supplemented with 10% FBS + 1X pen/strep +1X glutamine.
Cycloheximide solution (Sigma) was added to the cells to yield a final dose of 150 μg/mL and left on the cells for the indicated amount of time. Cells were lysed for 40 min in 50 mM Tris (pH 8), 1% NP-40, 1 mM MgCl2, 10% glycerol, and 1 mM DTT + protease inhibitor pellet (Roche).
Immunoprecipitation of SIRT1/SIRT1-L107P and Characterization of Enzymatic Activity and Protein Interactors
Stable 293T cells overexpressing Flag-SIRT1 or Flag-SIRT1 L107P were lysed and subjected to immunoprecipitation using Flag-M2 agarose (Sigma). After washing, bound protein was eluted with 3× Flag peptide (Sigma). Enzymatic activity was assayed using the BIOMOL SIRT1-Fluor de Lys Assay. In parallel, protein complexes were analyzed by mass spectrometry and visualized by western blot.
Production of MIN6 Cell Lines Expressing hSIRT1
MIN6B1 cells were kindly provided by Dr. Jun-ichi Miyazaki, University of Osaka (Miyazaki et al., 1990). Cells were infected with pMSCV-SIRT1-Flag (WT), with pMSCV-SIRT1-Flag (L107P), or with an empty virus as control. Six independent sets of WT, L107P, and empty virus-harboring cell lines were produced. Human Sirt1 expression was analyzed with the TaqMan real-time PCR assay and by western blotting using an anti-human SIRT1 antibody (H-300, Santa Cruz Biotechnology), an anti-mouse SIRT1 antibody (07-131, Millipore, and an anti-FLAG antibody (A9594, SIGMA).
To analyze the induction of iNOS, NO, KC, and TNF-α cells were treated with 1 ng/ml mouse recombinant interleukin-1β alone or in combination with 1 ng/ml interferon-γ (R&D Systems, Inc., Minneapolis, MN, USA). Nitrite concentration was determined using Griess reagent. Real-time PCR data were normalized to 18 s, expressed relative to cells transduced with empty virus, and analyzed with ANOVA and Bonferroni's multiple comparison test.
Statistical Analysis
The experimental data were analyzed with ANOVA and Bonferroni's multiple comparison test, and the results are expressed as means ± SEM, except where noted.
Supplementary Material
ACKNOWLEDGMENTS
We thank the patients and families whose participation made this study possible. This work was supported by grants from the Juvenile Diabetes Research Foundation, the Swiss National Science Foundation, the Zürich Centre for Integrated Human Physiology, the Glenn Foundation for Medical Research, and the Ellison Medical Foundation; by NIA/NIH grants; by an unrestricted grant from Sirtris, a GSK company; and by an NSERC PGS-D Fellowship (to B.P.H.). A.F. is Hertie Senior Research Professor Neuroscience 2009 of the Gemeinnützige Hertie-Stiftung. This research utilizes resources provided by the Type 1 Diabetes Genetics Consortium, a collaborative clinical study sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Human Genome Research Institute, the National Institute of Child Health and Human Development, and the Juvenile Diabetes Research Foundation and supported by U01 DK062418.
Footnotes
SUPPLEMENTAL INFORMATION Supplemental Information includes four figures, one table, and Supplemental Experimental Procedures and can be found with this article at http://dx.doi. org/10.1016/j.cmet.2013.02.001.
REFERENCES
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autiero I, Costantini S, Colonna G. Human sirt-1: molecular modeling and structure-function relationships of an unordered protein. PLoS ONE. 2009a;4:e7350. doi: 10.1371/journal.pone.0007350. http://dx.doi.org/10.1371/journal.pone.0007350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autiero I, Costantini S, Colonna G. Modeling of the bacterial mechanism of methicillin-resistance by a systems biology approach. PLoS ONE. 2009b;4:e6226. doi: 10.1371/journal.pone.0006226. http://dx.doi.org/10.1371/journal.pone.0006226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks AS, Kon N, Knight C, Matsumoto M, Gutiérrez-Juárez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, Julier C, Morahan G, Nerup J, Nierras C, et al. Type 1 Diabetes Genetics Consortium. (2009). Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat. Genet. 41:703–707. doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier UH, Wang L, Bhatti TR, Liu Y, Han R, Ge G, Hancock WW. Sirtuin-1 targeting promotes Foxp3+ T-regulatory cell function and prolongs allograft survival. Mol. Cell. Biol. 2011;31:1022–1029. doi: 10.1128/MCB.01206-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier UH, Wang L, Han R, Akimova T, Liu Y, Hancock WW. Histone deacetylases 6 and 9 and sirtuin-1 control Foxp3+ regulatory T cell function through shared and isoform-specific mechanisms. Sci. Signal. 2012;5:ra45. doi: 10.1126/scisignal.2002873. http://dx.doi.org/10.1126/scisignal.2002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- Bordone L, Motta MC, Picard F, Robinson A, Jhala US, Apfeld J, McDonagh T, Lemieux M, McBurney M, Szilvasi A, et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006;4:e31. doi: 10.1371/journal.pbio.0040031. http://dx.doi.org/10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzakri K, Roques M, Gual P, Espinosa S, Guebre-Egziabher F, Riou JP, Laville M, Le Marchand-Brustel Y, Tanti JF, Vidal H. Reduced activation of phosphatidylinositol-3 kinase and increased serine 636 phosphorylation of insulin receptor substrate-1 in primary culture of skeletal muscle cells from patients with type 2 diabetes. Diabetes. 2003;52:1319–1325. doi: 10.2337/diabetes.52.6.1319. [DOI] [PubMed] [Google Scholar]
- Breitenstein A, Stein S, Holy EW, Camici GG, Lohmann C, Akhmedov A, Spescha R, Elliott PJ, Westphal CH, Matter CM, et al. Sirt1 inhibition promotes in vivo arterial thrombosis and tissue factor expression in stimulated cells. Cardiovasc. Res. 2011;89:464–472. doi: 10.1093/cvr/cvq339. [DOI] [PubMed] [Google Scholar]
- Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunker AK, Cortese MS, Romero P, Iakoucheva LM, Uversky VN. Flexible nets. The roles of intrinsic disorder in protein interaction networks. FEBS J. 2005;272:5129–5148. doi: 10.1111/j.1742-4658.2005.04948.x. [DOI] [PubMed] [Google Scholar]
- Finnish-German APECED Consortium An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat. Genet. 1997;17:399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Guarente LP. Mammalian sirtuins—emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu. Rev. Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SM, Hancock MK, Pitman JL, Orth AP, Gekakis N. Negative regulators of insulin signaling revealed in a genome-wide functional screen. PLoS ONE. 2009;4:e6871. doi: 10.1371/journal.pone.0006871. http://dx.doi.org/10.1371/journal.pone.0006871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- Lee JH, Song MY, Song EK, Kim EK, Moon WS, Han MK, Park JW, Kwon KB, Park BH. Overexpression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor-kappaB signaling pathway. Diabetes. 2009;58:344–351. doi: 10.2337/db07-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Yang H, Tartar DM, Gao B, Luo X, Ye SQ, Zaghouani H, Fang D. Prevention and treatment of diabetes with resveratrol in a non-obese mouse model of type 1 diabetes. Diabetologia. 2011;54:1136–1146. doi: 10.1007/s00125-011-2064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science. 1976;193:415–417. doi: 10.1126/science.180605. [DOI] [PubMed] [Google Scholar]
- Mastrandrea L, Yu J, Behrens T, Buchlis J, Albini C, Fourtner S, Quattrin T. Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care. 2009;32:1244–1249. doi: 10.2337/dc09-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurney MW, Yang X, Jardine K, Hixon M, Boekelheide K, Webb JR, Lansdorp PM, Lemieux M. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol. Cell. Biol. 2003;23:38–54. doi: 10.1128/MCB.23.1.38-54.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki J, Araki K, Yamato E, Ikegami H, Asano T, Shibasaki Y, Oka Y, Yamamura K. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology. 1990;127:126–132. doi: 10.1210/endo-127-1-126. [DOI] [PubMed] [Google Scholar]
- Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Méneur C, Permutt MA, Imai S. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–117. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, Krohn KJ, Lalioti MD, Mullis PE, Antonarakis SE, et al. Positional cloning of the APECED gene. Nat. Genet. 1997;17:393–398. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- Sequeira J, Boily G, Bazinet S, Saliba S, He X, Jardine K, Kennedy C, Staines W, Rousseaux C, Mueller R, McBurney MW. sirt1-null mice develop an autoimmune-like condition. Exp. Cell Res. 2008;314:3069–3074. doi: 10.1016/j.yexcr.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Stein S, Lohmann C, Schäfer N, Hofmann J, Rohrer L, Besler C, Rothgiesser KM, Becher B, Hottiger MO, Borén J, et al. SIRT1 decreases Lox-1-mediated foam cell formation in atherogenesis. Eur. Heart J. 2010;31:2301–2309. doi: 10.1093/eurheartj/ehq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307–319. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Tang MM, Zhu QE, Fan WZ, Zhang SL, Li DZ, Liu LZ, Chen M, Zhang M, Zhou J, Wei CJ. Intra-arterial targeted islet-specific expression of Sirt1 protects β cells from streptozotocin-induced apoptosis in mice. Mol. Ther. 2011;19:60–66. doi: 10.1038/mt.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loosdregt J, Vercoulen Y, Guichelaar T, Gent YY, Beekman JM, van Beekum O, Brenkman AB, Hijnen DJ, Mutis T, Kalkhoven E, et al. Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Blood. 2010;115:965–974. doi: 10.1182/blood-2009-02-207118. [DOI] [PubMed] [Google Scholar]
- Wentworth JM, Fourlanos S, Harrison LC. Reappraising the stereotypes of diabetes in the modern diabetogenic environment. Nat. Rev. Endocrinol. 2009;5:483–489. doi: 10.1038/nrendo.2009.149. [DOI] [PubMed] [Google Scholar]
- Westphal CH, Dipp MA, Guarente L. A therapeutic role for sirtuins in diseases of aging? Trends Biochem. Sci. 2007;32:555–560. doi: 10.1016/j.tibs.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Yang Z, Kahn BB, Shi H, Xue BZ. Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J. Biol. Chem. 2010;285:19051–19059. doi: 10.1074/jbc.M110.123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki T, Milne JC, Imamura T, Schenk S, Sonoda N, Babendure JL, Lu JC, Smith JJ, Jirousek MR, Olefsky JM. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol. Cell. Biol. 2009a;29:1363–1374. doi: 10.1128/MCB.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki T, Schenk S, Imamura T, Babendure JL, Sonoda N, Bae EJ, Oh D, Lu M, Milne JC, Westphal C, Bandyopadhyay G, Olefsky JM. SIRT1 inhibits inflammatory pathways in macrophages and modulates insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2009b;298:E419–E428. doi: 10.1152/ajpendo.00417.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lee SM, Shannon S, Gao B, Chen W, Chen A, Divekar R, McBurney MW, Braley-Mullen H, Zaghouani H, Fang D. The type III histone deacetylase Sirt1 is essential for maintenance of T cell tolerance in mice. J. Clin. Invest. 2009a;119:3048–3058. doi: 10.1172/JCI38902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhong J, Yang P, Gong F, Wang CY. HMGB1, an innate alarmin, in the pathogenesis of type 1 diabetes. Int. J. Clin. Exp. Pathol. 2009b;3:24–38. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.