Abstract
Summary
Lipid droplets (LDs) are dynamic organelles that collect, store, and supply lipids [1]. LDs have a central role in the exchange of lipids occurring between the cell and the environment, and provide cells with substrates for energy metabolism, membrane synthesis, and production of lipid-derived molecules such as lipoproteins or hormones. However, lipid-derived metabolites also cause progressive lipotoxicity [2]; accumulation of reactive oxygen species (ROS), endoplasmic reticulum stress, mitochondrial malfunctioning, and cell death [2]. Intracellular accumulation of LDs is a hallmark of prevalent human diseases including obesity, steatosis, diabetes, myopathies, and arteriosclerosis [3]. Indeed, non-alcoholic fatty liver disease is the most common cause of abnormal hepatic function among adults [4, 5]. Lipotoxicity gradually promotes cellular ballooning and disarray, megamitochondria, and accumulation of Mallory’s hyaline in hepatocytes and inflammation, fibrosis, and cirrhosis in the liver. Here, using confocal microscopy, serial-block-face scanning electron microscopy, and flow-cytometry we show that LD accumulation is heterogeneous within a cell population and follows a positive skewed distribution. Lipid availability and fluctuations in biochemical networks controlling lipolysis, fatty acid oxidation, and protein synthesis, contribute to cell-to-cell heterogeneity. Critically, this reversible variability generates a subpopulation of cells that effectively collect and store lipids. This high-lipid subpopulation accumulates more LDs, more ROS, and reduces the risk of lipotoxicity to the population without impairing overall lipid homeostasis, since high-lipid cells can supply stored lipids to the other cells. In conclusion, we demonstrate fat storage compartmentalization within a cell population and propose that this is a protective social organization to reduce lipotoxicity.
Results and Discussion
LD accumulation is cell-to-cell heterogeneous
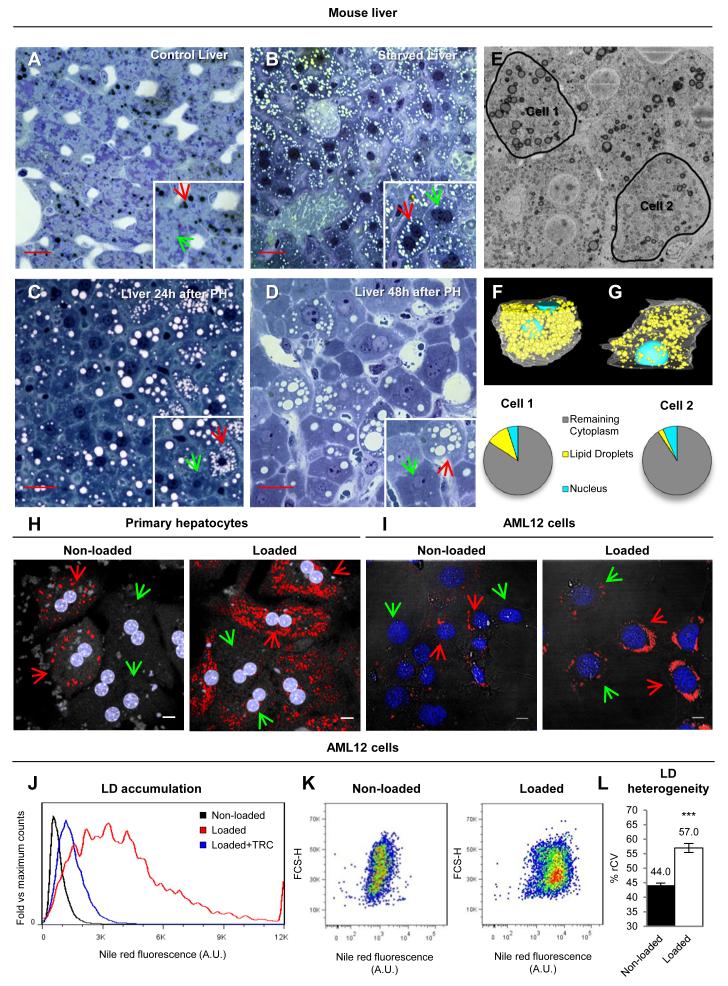
The liver is essential for the body’s fat metabolism by storing and processing lipids, continuously accumulating lipids and thus persistently threatened by lipotoxicity. While poorly understood, there are likely cellular mechanisms to simultaneously deal with the beneficial and harmful aspects of intracellular lipids. Here, to investigate whether such mechanisms exist in hepatic cells, we first examined LD distribution in mice liver sections. In control livers, there were relatively few LDs (Fig.1A). However, some hepatocytes clearly accumulated LDs (black globular structures, red arrows) whereas others completely lacked LDs (green arrows). Under starvation or after a partial hepatectomy, LDs accumulated (white globular structures, Figs.1B, 1C and 1D). In both cases, cell-to-cell differences in LD size and number were evident and the highest variability occurred 48h after hepatectomy. To further characterize the heterogeneous LD accumulation of untreated livers, we used a new technique, serial block face scanning electron microscopy (3View). LDs were clearly distinguished by their electron density (Fig.1E). The analysis of 1000 consecutive sections (approximately 50μm depth), revealed the presence of LDs in all hepatocytes and demonstrated high variability between them (supplemental Movie1). As an example of cell-to-cell heterogeneity, we selected two neighbouring hepatocytes for a detailed segmentation analysis (Fig.1E and supplemental Movie2 and Movie3). The comparison of the volumetric occupancy of LDs demonstrated striking differences; 10.9% (97.7 μm3) of the cytoplasmic volume of Cell 1 was occupied by LDs, whereas only 2.6% (27.6 μm3) of the volume of Cell 2 was occupied by LDs (Figs.1F and 1G).
Figure 1. Hepatic LD heterogeneity.
(A to D) Control (A), starved (B), and 24h (C) and 48h after partial hepatectomy (D) mice liver sections stained with methylene blue. LDs are the black (A) or white (B to D) rounded organelles. Red and green arrows indicate cells with high- or low-lipid content. Scale bars=50μm. (E to G) 3View microscopy of control mice liver. Representatives stack showing the two selected cells (black lines, E) and segmentation analysis of Cell 1 (F) or 2 (G). Translucent white lines = plasma membrane, light blue = nuclei, yellow = lipid droplets. (H and I) Primary hepatocytes (H) and AML12 cells (I) in standard media (left) or loaded for 24h with FAs (50 μg/ml for hepatocytes and 175μg/ml for AML12, right), stained with Nile red (LDs, red) and Hoechst (nucleus, blue). Red and green arrows indicate cells with high- or low-lipid content. Scale bars=10μm. (J to L). Representative histogram (linear scale, J) of Nile Red fluorescence of control cells (Non-loaded, black), cells loaded with FA (Loaded, red) or cells with FA and 10μM Triacsin C (Loaded+TRC, blue). (K) Representative dot plots (logarithmic scale) of Nile Red fluorescence versus forward scatter (FCS-H) in non-loaded (left) and loaded cells (right). (L) %rCV of Nile Red fluorescence of Non-loaded and Loaded cells. Data represent mean ± SEM in at least 10 independent experiments.
To analyse whether cell-to-cell heterogeneity reflects the zonal distribution of hepatocytes within the liver, primary hepatocytes were isolated, labelled with Nile Red, and analysed by microscopy. Only some of these hepatocytes accumulated LDs (left panel, Fig.1H). When these hepatocytes were identically treated for 24h with fatty acids (FAs, albumin-bound oleate), all accumulated LDs but a high heterogeneity in LD number and size was observed (right panel, Fig.1H). Therefore, although liver architecture is a likely source of some variability, heterogeneity seems to be essentially regulated by intracellular factors.
Extent of heterogeneity in LD content
LD accumulation has traditionally been studied in adipocytes, where cell-to-cell differences have been reported during progression of adipogenesis [6]. Variability has also been occasionally mentioned when LDs were promoted under laboratory conditions in clonal yeast, fibroblasts, and tumour cell lines [7-9]. Histological scoring of steatotic livers has also revealed hepatocyte-to-hepatocyte heterogeneity in human biopsies [10]. However, such variability has been masked by commonly used experimental techniques, and the degree, sources, and potential utility of cell-to-cell heterogeneity have received little attention.
To quantify LD accumulation cell-by-cell, we used flow-cytometry. Starting from previous protocols [8], we optimized the approach to measure the LD content of individual cells. Due to cellular aggregation, this method was not reliable for primary hepatocytes, so instead we analysed AML12 cells (alpha mouse liver 12) [11]. Microscopy analysis demonstrated that AML12 also exhibited high heterogeneity before and after the loading with FAs (Fig.1I). To quantify these differences, AML12 were loaded with FAs, labelled with Nile Red, and neutral lipid accumulation by individual cells was determined via flow-cytometry in 1×104 cells per experiment. As expected, FA-treatment increased the average LD content (red line, Fig.1J) and it was reduced with Triacsin C (blue line, Fig.1J) a potent inhibitor of LD formation (Fig.S1 and supplemental text). The relative increment measured by flow cytometry was identical to the result obtained with an enzymatic method (Fig.S1 and supplemental text).
The histograms and dot plots demonstrated a high heterogeneity in the cellular LD content (Figs.1J and 1K), with approximately 100-fold maximal cell-to-cell differences in LD levels. To measure heterogeneity, we calculated the % robust coefficient of variation (%rCV), defined as %rCV = ((rSD/median) × 100). Intuitively, the %rCV measures the average deviation of an individual cell from the population median. In FA-loaded cells the overall %rCV was 57±9% (Fig.1L), although the most loaded 15% of the hepatocytes (arbitrary cut-off, see later Fig.4) showed an average %rCV with respect the whole population median of 205±30%. In contrast, the standard deviation between %rCV of independent experiments was only 9%, suggesting that overall the %rCV is relatively constant. When compared with other cell lines, heterogeneity was particularly high in hepatic cells (Fig.S2). Thus, the extent of heterogeneity is different between different cell types, suggesting that it is potentially controllable.
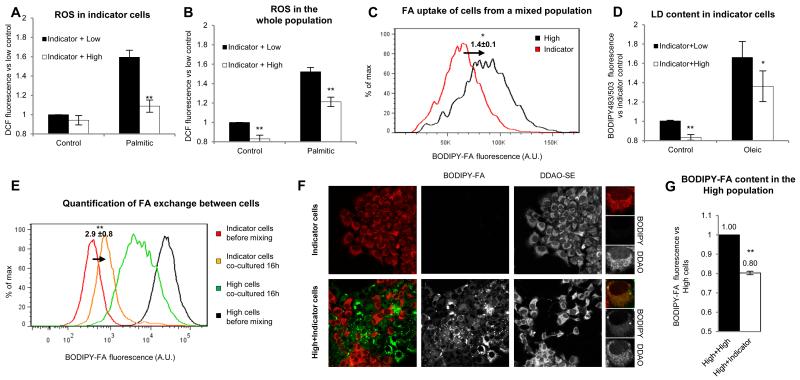
Figure 4. High-lipid cells are population advantage.
(A-D) Non-loaded AML12 cells were stained with Cell Trace DDAO-SE (Indicator cells) and mixed for 16 h with non-loaded cells (Low, black bars) or cells loaded 24h with 500μg/ml of FA to generate high-lipid cells (High, white bars). ROS levels (as in 3F) in the indicator subpopulation (A) and the whole population (B) in cells treated 16h after mixing with control medium or supplemented with 50μg/ml of palmitic acid. Statistical significance was calculated versus the indicator mixed with the low population (black bars). (C) Representative histogram and mean FA uptake of indicator (red line) and high-lipid cells (black line) co-cultured 16h in control medium. Statistical significance was calculated versus indicator cells. (D) Analysis of LD content in indicator cells co-cultured with low-lipid (black bars) or high-lipid cells (white bars) incubated for 16h in control medium or in medium supplemented with 50μg/ml FA (oleic). Statistical significance was calculated versus the indicator mixed with the low population. (E-G) Indicator cells were co-cultured for 16h with high-lipid cells with a BODIPY-FA accumulated in LDs (High, see supplemental and Fig.S4). (E) Histogram of BODIPY-FA content analysed in high-lipid cells and indicator cells before mixing (black and red lines respectively) and after mixing (green and orange lines respectively). The mean fold increase ±SEM of BODIPY-FA in indicator cells is indicated. Statistical significance was calculated versus indicator cells before mixing (red line). (F) The BODIPY-FA in the indicator cells, before and after mixing as in E, was analysed by microscopy. (G) BODIPY-FA fluorescence in high-lipid cells (loaded as in E) was measured after 16h with indicator (white bar) or high-lipid cells (black bar). Statistical significance was calculated versus the high-lipid population. Data represent the mean ± SEM of at least 4 independent experiments.
Causes of heterogeneity in LD content
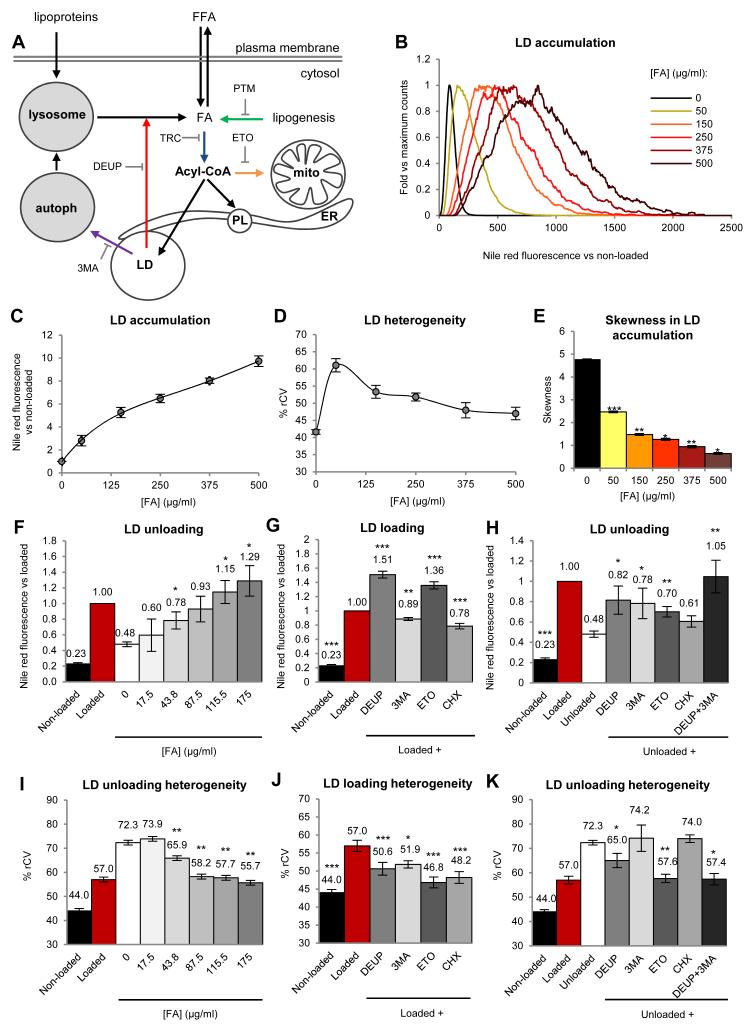
Next, we analysed how environmental and intracellular factors determine heterogeneity (Fig.2A). To assess how lipid availability contributes to heterogeneity, AML12 cells were incubated for 24h with increasing FA doses. As expected, overall LD accumulation was proportional to lipid availability (Figs.2B and 2C). The %rCV was maximal at low concentrations and progressively decreased with higher doses (Fig.2D). Linear histograms reveal that with moderate concentrations of FAs (up to 250μg/ml) the LD content of the population followed a positive skewed distribution, suggesting possible collective mechanisms to increase the mass of the distribution in low-lipid cells, by preferential accumulation of lipids in a reduced number of high-lipid cells (Fig.2B). In contrast, higher doses of FAs progressively decreased asymmetry (as measured by skewness, Fig.2E), the relative percentage of cells in the low lipid range, and thus the final %rCV.
Figure 2. Causes of LD heterogeneity.
(A) Biochemical networks (black) and interfering drugs (colour arrows) related to LD metabolism (PL, phospholipid; Mito, mitochondria; ER, endoplasmic reticulum; autoph, autophagosome). (B-E) Quantification of LD content and heterogeneity of AML12 cells treated 24h with the indicated doses of FAs. Representative histograms (linear scale, B), average LD accumulation compared to non-loaded cells (C), %rCV (D), and skewness coefficient (E) of the distributions. (F and I) FA loaded cells were incubated for additional 16h in a FA-free medium containing the indicated concentrations of FAs. LD accumulation (F) compared to loaded cells (red bar) and the %rCV (I) was calculated. Statistical significance was calculated versus unloaded control cells (white bars). (G and J) LD accumulation (G) and %rCV (J) of non-loaded cells and cells loaded with FA in the presence of 500μM DEUP, 10mM 3MA, 100μM etomoxir (ETO) or 10μg/ml cycloheximide (CHX). Statistical significance was calculated versus loaded cells (red bars). (H and K) LD accumulation (H) and %rCV (K) of non-loaded cells, loaded cells, and cells additionally unloaded 16h in a FA-free medium and the drugs detailed in G. Statistical significance was calculated versus unloaded cells (white bars). Data represent mean ± SEM in at least 3 independent experiments.
We also analysed whether LD unloading contributes to heterogeneity. Thus, FA-loaded cells were subsequently incubated in a FA-free media for 16h. As expected the LD levels were reduced but interestingly the %rCV increased significantly (white bars, Figs.2F and 2I). Importantly, LD unloading and heterogeneity were highly reduced by relatively low concentrations of FAs in the media. Thus, systems with prolonged circulating lipids – e.g. obese individuals- will not only increase LD formation but also reduce LD unloading and heterogeneity.
We next used specific inhibitors of intracellular processes related to LD accumulation to determine the pathways that contribute to heterogeneity (Fig.2A and supplemental text). Inhibition of lipases (DEUP) or mitochondrial FA uptake (ETO) increased the LD content and reduced cell-to-cell heterogeneity (Figs.2G, 2J and S3). In contrast, inhibition of protein translation (CHX) reduced both the LD content and heterogeneity, pinpointing differences in the FA-promoted protein synthesis as an important source of variability. Inhibition of autophagy (3MA) slightly decreased LD levels and the %rCV, suggesting that under these conditions LDs are not significantly metabolized by this mechanism but are supplied with lipids from autophagy. Other factors such as the phase of the cell cycle and the synthesis of new FAs did not affect heterogeneity (Fig.S3 and supplemental text).
To evaluate heterogeneity sources during unloading, previously FA-loaded cells were incubated 16h in FA-free media but in the presence of the inhibitors. The unloading of LDs was now highly reduced when lipolysis (DEUP) and autophagy (3MA) were inhibited, suggesting that LD unloading is conducted by both mechanisms (Figs.2H and 2K). Inhibition of mitochondrial FA uptake (ETO) and protein synthesis (CHX) also reduced LD unloading. The inhibition of lipases or mitochondrial FA uptake decreased heterogeneity. In contrast, inhibition of protein synthesis or autophagy had no effect on the %rCV, suggesting that these mechanisms are activated proportionally to the LD content of each cell. Thus, LD storage and heterogeneity are overall regulated by lipid availability although they are also determined by the oxidation of FAs, lipolysis, and the synthesis of new protein.
Heterogeneity in LD content is regulated by reversible mechanisms
Phenotypic heterogeneity within a cell population is well-known yet poorly understood. When identical cells - or the same cell over time- respond differently to an identical stimulus, it is often caused by stochasticity in gene expression, which can reflect fluctuations of transcription and/or translation of genes and proteins [12]. In principle, stochasticity provides a mechanism for different cells in an identical population to sample different physiological states without need for irreversible genetic mutations, making it likely that at least some of the population survives/functions when faced with different types of unexpected challenges.
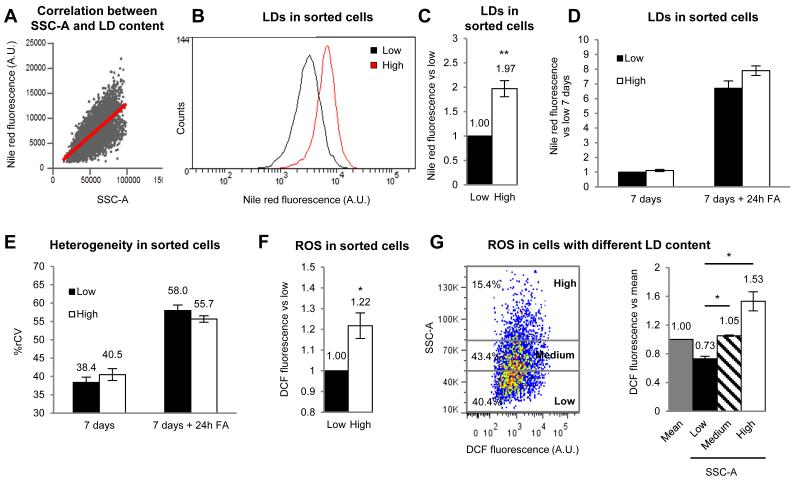
Thus, we analysed whether the heterogeneity is reversible, as expected from stochastic biochemical networks. After FA-loading, the high- and low-lipid loaded cells of the population were separated by flow-cytometry using side scatter (SSC-A) as the sorting criteria. This SSC-A reflects the cell’s inner complexity and is proportional to LD levels (Fig.3A and supplemental text)[13]. With this method the separated high-lipid cells had double the lipid content of the low-lipid cells (Figs.3B and 3C). Next, both subpopulations were independently re-cultured during 7 days in standard media to metabolize LDs, eliminating the majority of LDs (Fig 3D, “7 days”). Finally, cells were again FA-loaded and the LD content was quantified. At an overall population level, the original high- and low-lipid cells were indistinguishable, and accumulated equivalent LDs (Fig 3D, “7 days +24h FA”). Importantly, the %rCV was identical for each group of newly cultured cells that had originated from different subpopulations, and was identical to the %rCV of the whole population before sorting (Fig.3E). Thus, as expected from a process regulated by stochasticity, the cell’s capacity to accumulate LDs heterogeneously is variable and reversible over time. It is likely, then, that this reversibility allows exchanging the risk of lipotoxicity between different cells of the population over time.
Figure 3. Cell sorting of high and low-lipid cells.
(A) Correlation between Nile Red fluorescence and SSC-A in AML12 cells. (B and C) Representative histogram (B) and mean LD content (C) of high- and low-lipid cells sorted by SSC-A and stained with Nile Red. (D and E) LD accumulation (D) and %rCV (E) of cells sorted as in B and maintained 7 days in normal medium and additionally treated for 24h with 175μg/ml FA. (F) ROS in sorted cells measured as DCF fluorescence compared to low-lipid cells. (G) Representative dot plot of the SSC-A (LDs) versus DCF fluorescence (ROS) in the whole population of cells. Cells are classified (arbitrary cut-off) in low- (40.4% of the population), medium- (43.4%) or high-lipid cells (15.4%). Mean DCF fluorescence in each type of cells compared to mean of the population. Statistical significance was calculated versus low cells (black bars). Data represent the mean ± SEM of at least 4 independent experiments.
High-lipid cells are an advantage for the population
The existence of cells specialized in fat storage is conserved from flies to humans suggesting that fat compartmentalization has been a positive trait during evolution. In worms, in the absence of an authentic adipose tissue, C. elegans compartmentalizes the fat in the intestine [14]. Further, we have confirmed (data not shown) the existence of a high heterogeneity in clonal populations of unicellular organisms such as the yeast Saccharomyces cerevisiae [7]. In humans, when such compartmentalization is impaired, a series of complex metabolic effects (commonly known as lipodystrophies) are elicited in the rest of cells [15]. Lipodystrophies are associated with early multi-organ complications especially in cardiovascular and hepatic tissues that lead to diabetes and metabolic syndromes [3]. Therefore, similar to adipose tissue, we hypothesized that the existence of high-lipid cells within a cell population might potentially reduce lipotoxicity to the average cell without impairing overall lipid homeostasis.
To explore the cell-to-cell distribution of toxic metabolites, we first separated the cells into high- and low-lipid subpopulations by flow-cytometry, and measured ROS levels in each subpopulation. ROS levels were higher in high-lipid cells (Fig.3F). Importantly, cell-to-cell correlation studies on the whole population demonstrated that ROS levels were proportional to LD levels (Fig.3G). Single cell analysis demonstrated that the 15% of cells with the highest LD levels (arbitrary cut-off) accumulate much more than 15% of the overall lipids, and also have 53% more ROS than the mean of the population (distribution in Fig 3G left and averages of ROS for each group of cells shown in Fig. 3G right). Thus, this small percentage of high-lipid cells allows 40% of the population to have low lipids and a significant reduction of ROS (nearly 30%), which was similar to the ROS levels of these cells before the addition of FAs (data not shown). These results suggest that LD compartmentalization reduces toxic metabolites in most cells and thus is an advantage for the population.
To directly test this idea of ROS protection due to high-lipid cells, we created a set of initially low-lipid cells to act as ‘indicator’ cells. These cells, cultured in standard conditions, were labelled with the fluorescent Cell Trace DDAO-SE (far red emission). The, ‘indicator’ cells were mixed with either i) additional unlabelled low-lipid cells or ii) unlabelled high-lipid cells generated by treatment with 500μg/ml of FAs for 24h (see Figs. 2B to 2E). Then, the mixed cell population was co-cultured for 16h, in either normal media or in the presence of lipotoxic palmitic acid. Finally, ROS levels were measured only in ‘indicator’ cells (Fig.4A) or in the whole population (Fig.4B). Importantly, the ‘indicator’ cells had less ROS when co-cultured with high-lipid cells (white bar in palmitic, 4A) than with low-lipid cells (black bar in 4A), showing that indeed the presence of the high-lipid cells can decrease ROS in the low lipid cells. Surprisingly, the presence of high-lipid cells was also protective during palmitic incubation for the population as a whole, since total ROS levels were significantly reduced when high-lipid cells were present in the culture (white bar in palmitic, 4B). The beneficial effects of high-lipid cells were also seen when cells were in standard media (white bars in control, Fig.4B). Thus, high-lipid cells protect not only low-lipid cells but importantly reduce ROS levels to the entire population. Based on these findings, we suggest that high-lipid cells activate systems to reduce ROS accumulation, but these mechanisms will need to be tested in future work.
The protective effect of the high-lipid cells could be indirect (e.g. due to cell-cell communication between the two groups). However, it could be also produced by local titration of FAs from the media. This last possibility is likely since the rate of lipid uptake was directly proportional to the cellular levels of LDs (Fig.S4) and when co-cultured, high-lipid cells take up more lipids than ‘indicator’ cells (Fig.4C). To test this possibility, ‘indicator’ cells were co-cultured with low- or high-lipid cells for 16h in the presence of FAs and the resulting LD accumulation was measured. The ‘indicator’ cells accumulated less LDs in the presence of high-lipid cells (Fig.4D), supporting the hypothesis that high-lipid cells reduce accumulation of LDs to low-lipid cells and may in this way decrease overall lipotoxicity.
High-lipid cells supply lipids to the population.
The main function of an adipose tissue is first to collect and store lipids when they are in excess, and then, without impairing overall lipid homeostasis, to provide these lipids to other cells when lipid availability is compromised. Therefore, to test the hypothesis that high-lipid cells are functioning - at a cellular level- as an adipose tissue, we analysed whether the high-lipid cells can supply stored lipids to low-lipid cells. To visualize lipid exchange, we accumulated the BODIPY-FA in the LDs of high-lipid cells (Fig.S5 and supplemental text). Then, these high-lipid cells were co-cultured with ‘indicator’ cells for 16h and the exchange of lipids measured by flow-cytometry. Initially, ‘indicator’ and high-lipid cells were clearly distinguished by the absence or presence of green fluorescence (red and black lines in Fig.4E). However, 16h after co-plating the transfer of the green FA was detected. The new curves (orange for ‘indicator’ cells and green for high-lipid cells) were between the original curves. Thus, ‘indicator’ cells accumulated fluorescent lipids and high-lipid cells with decreased lipids had become a donor population. The exchange was confirmed by fluorescence microscopy (Fig.4F). Although the cellular mechanisms involved in lipid exchange will require further analysis, the unloading of the fluorescent lipid by high-lipid cells was higher in the presence of low-lipid ‘indicator’ cells (white bar, Fig.4G) than in the presence of other unlabelled high-lipid cells (black bar, Fig.4G), suggesting possible mechanisms of cell-to-cell communication between the two groups and potentially the result of increased lipids in the media due to release of FAs from all of the high lipid cells.
Concluding Remarks
Here, we show that cellular LD storage is overall regulated by lipid availability but is also affected by cell-to-cell variable biochemical networks. Indeed, when cells of a clonal population are identically treated with extracellular lipids, the accumulation of LDs follows a heterogeneous and skewed distribution, with most cells having relatively low-lipid accumulation and a few with high lipid storage. As expected from mechanisms regulated by reversible stochastic mechanisms, high-lipid cells are an advantage for the population: when facing toxic lipids or prolonged exposure to FAs, most cells - and intriguingly the whole population- have less toxic metabolites that would be expected for cells with that average lipid accumulation. In addition, the high-lipid cells can supply stored lipids to the low-lipid cells, effectively functioning - at a cellular level- as adipose tissue.
Intriguingly, maximal heterogeneity and skewness occur at relatively low overall levels of lipid availability, consistent with the hypothesis that mechanisms of heterogeneity are overwhelmed when exposed to excess lipids. When compared with other cell lines, heterogeneity is particularly high in hepatic cells suggesting that this protective mechanism could be particularly important for the liver. This appears to have direct implications for NAFLD, an increasingly important chronic liver disease because is strongly correlated with obesity and thus with a persistent lipid exposure [16]. Such a prolonged lipid exposure will not only increase LD formation but will also reduce LD unloading and heterogeneity. In addition, other intracellular heterogeneous factors, such as the mitochondrial activity, also determine the LD content of individual hepatocytes, pinpointing additional risk factors for NAFLD development. Whether mechanisms of heterogeneity in the liver are identical to the mechanisms detailed here for hepatic cells or whether they are also determined by changes in cell physiology due to zonal arrangement will require additional analysis. Importantly, genes and proteins contributing to heterogeneity are likely rate-limiting steps within each network, and are thus a new set of targets to modulate for potential clinical utility, and also to better understand the cellular basis and risk factors for developing NAFLD.
Experimental Procedures
Reagents
Nile red, BODIPY-FLC16, Hoechst-33258, Cell Trace™ Far Red DDAO-SE and BODIPY 493/503 were from Molecular Probes, Invitrogen. Fatty acid free BSA, collagenase type IV, 2′,7′-Dichlorofluorescein diacetate (H2DCFDA), palmitic acid, thymidine, diethylumbelliferyl phosphate (DEUP), 3-methyl-adenine (3MA), platensimycin (PTM), diethyl-p-nitrophenyl phosphate (E600), etomoxir (ETO) and cycloheximide (CHX) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Triacsin C (TRC) was from Santa Cruz Biotechnology (California, USA). Oleic acid and mowiol were from Calbiochem (La Jolla, CA, USA). A detailed explanation of the methods used in this manuscript can be found in the Supplemental Material.
Statistical analysis
All data shown in graphs are the mean and SEM, and the statistical significance were determined using the Student’s t test (*P<0.05; **P<0.01; ***P<0.001).
Supplementary Material
Highlights.
LD accumulation follows a skewed distribution within a cell population.
Heterogeneity generates a subpopulation that effectively collect and store lipids.
The high-lipid cell subpopulation reduces lipid-derived toxic metabolites for the overall population.
A subpopulation of high-lipid cells provides lipids to the population in lipid-depleted environments.
Acknowledgments
AP is supported by (BFU2011-23745, CSD2009-00016 from MICINN and Marató de TV3), SPG (GM64624/NIH), FT (BFU2009-13526), CE (BFU2009-10335, CSD2009-00016 and Marató de TV3), MG (CSD2009-00016), RGP is supported by grants from the National Health and Medical Research Council of Australia. The 3View work would not have been possible without the expert assistance of Rick Webb and Robyn Webb at the Centre for Microscopy and Microanalysis at the University of Queensland. We thank Dr. Maria Calvo and Anna Bosch for help with confocal microscopy (SCTUB) and Maria Molinos for technical assistance. We are indebted to the Citomics unit of the IDIBAPS for the technical help.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walther TC, Farese RV., Jr. Lipid droplets and cellular lipid metabolism. Annu Rev Biochem. 2012;81:687–714. doi: 10.1146/annurev-biochem-061009-102430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brookheart RT, Michel CI, Schaffer JE. As a matter of fat. Cell Metab. 2009;10:9–12. doi: 10.1016/j.cmet.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenberg AS, Coleman RA, Kraemer FB, McManaman JL, Obin MS, Puri V, Yan QW, Miyoshi H, Mashek DG. The role of lipid droplets in metabolic disease in rodents and humans. J Clin Invest. 2011;121:2102–2110. doi: 10.1172/JCI46069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 5.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–725. e716. doi: 10.1053/j.gastro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Loo LH, Lin HJ, Singh DK, Lyons KM, Altschuler SJ, Wu LF. Heterogeneity in the physiological states and pharmacological responses of differentiating 3T3-L1 preadipocytes. J Cell Biol. 2009;187:375–384. doi: 10.1083/jcb.200904140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szymanski KM, Binns D, Bartz R, Grishin NV, Li WP, Agarwal AK, Garg A, Anderson RG, Goodman JM. The lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology. Proc Natl Acad Sci U S A. 2007;104:20890–20895. doi: 10.1073/pnas.0704154104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gocze PM, Freeman DA. Factors underlying the variability of lipid droplet fluorescence in MA-10 Leydig tumor cells. Cytometry. 1994;17:151–158. doi: 10.1002/cyto.990170207. [DOI] [PubMed] [Google Scholar]
- 9.Pol A, Luetterforst R, Lindsay M, Heino S, Ikonen E, Parton RG. A caveolin dominant negative mutant associates with lipid bodies and induces intracellular cholesterol imbalance. J Cell Biol. 2001;152:1057–1070. doi: 10.1083/jcb.152.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 11.Wu JC, Merlino G, Fausto N. Establishment and characterization of differentiated, nontransformed hepatocyte cell lines derived from mice transgenic for transforming growth factor alpha. Proc Natl Acad Sci U S A. 1994;91:674–678. doi: 10.1073/pnas.91.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shahrezaei V, Swain PS. The stochastic nature of biochemical networks. Curr Opin Biotechnol. 2008;19:369–374. doi: 10.1016/j.copbio.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Lee YH, Chen SY, Wiesner RJ, Huang YF. Simple flow cytometric method used to assess lipid accumulation in fat cells. J Lipid Res. 2004;45:1162–1167. doi: 10.1194/jlr.D300028-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Mak HY. Lipid droplets as fat storage organelles in Caenorhabditis elegans: Thematic Review Series: Lipid Droplet Synthesis and Metabolism: from Yeast to Man. J Lipid Res. 2012;53:28–33. doi: 10.1194/jlr.R021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vigouroux C, Caron-Debarle M, Le Dour C, Magre J, Capeau J. Molecular mechanisms of human lipodystrophies: from adipocyte lipid droplet to oxidative stress and lipotoxicity. Int J Biochem Cell Biol. 2011;43:862–876. doi: 10.1016/j.biocel.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.