Abstract
A standardized profiling method based on liquid chromatography with diode array and electrospray ionization mass spectrometric detection (LC–DAD–ESI/MS) was applied to establish the phenolic profiles of 41 green teas and 25 fermented teas. More than 96 phenolic compounds were identified that allowed the teas to be organized into five groups. Epigallocatechin gallate (EGCG) was the major phenolic component of green tea made from mature leaves (group 2), while green tea made from the younger buds and leaves (group 1) contained lower flavonoid concentrations. Partially fermented teas (group 3) contained one-half the EGCG content of the green tea. Fully fermented black teas (group 4) had a trace of EGCG, but contained theaflavins. Highly overfermented black tea (group 5) contained only trace amounts of flavonol glycosides and theaflavins. Over 30 phenolics are new for tea, and this is the first phenolic profile to simultaneously detect C- and O-glycosylated flavonoids, catechins, proanthocyanidins, phenolic acid derivatives, and purine alkaloids.
Keywords: Tea, Camellia sinensis and varieties, flavonoids, phenolic acid derivatives, LC-DAD-ESI/MS, phenolic component profiles
INTRODUCTION
Tea is made from the dried fresh (green tea) or enzymatically oxidized (oolong, black tea) young buds and leaves of varieties of Camellia sinensis L. (Theaceae). This plant was originally found in South China but is now common to many other countries, such as Japan, India, Sri Lanka, Indonesia, Australia, Kenya, and Chile. On the basis of production procedures, tea has been divided into green tea (nonfermented with initial dry heating or steaming to destroy the enzymes); oolong tea, including tikuanyin tea (partially fermented by using the existing enzymes in the fresh leaves for oxidation); and black tea (usually called red tea in China) (fully fermented) (1–4). Puerh tea is made from the fresh dried and partially or fully fermented leaves of Camellia sinensis var. assamica (Masters) Kitamura, grown in the Yunnan Province of China (5–7).
Tea has been an important human drink for over 1000 years and is considered to have many health benefits for the human body. It is now one of the most widely consumed beverages in the world (1–4). Tea has also been used as a traditional medicine in China, India, and some other Asian countries. Some tea products have been used as health foods and herbs, too (1–8).
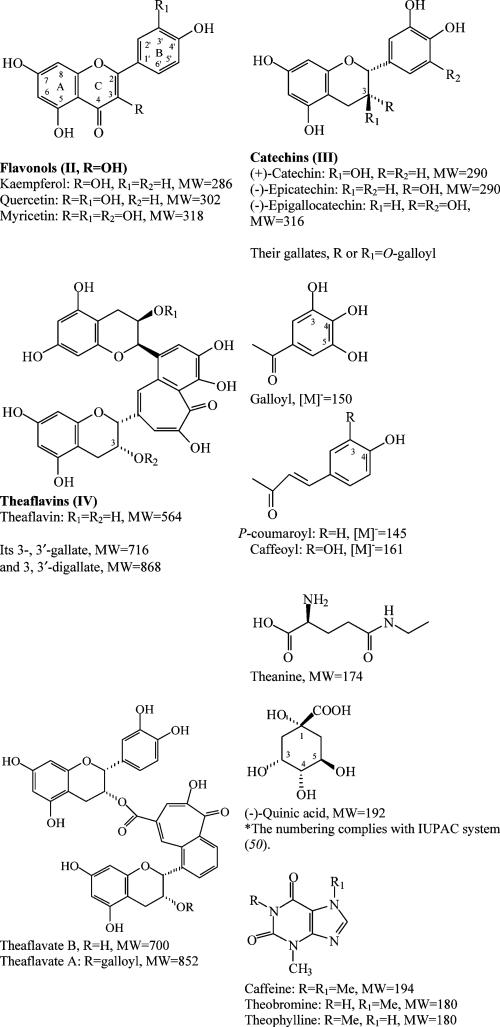
Many chemical studies have been carried out on teas. It has been shown that teas contain purine (xanthine) alkaloids, phenolic compounds (mainly catechins, O-glycosylated flavonols, C-glycosylflavones, proanthocyanidins, phenolic acids, and their derivatives) (Figure 1), terpenoids, and other compounds (fatty acids, essential oils, amino acids, etc.). Oolong and black teas also contain the fermented oxidation products of catechins, theaflavins, and polymeric thearubigins (1–31). Biological studies have indicated that epigallocatechin gallate (EGCG), one of the main tea phenolic components, is the most beneficial to human health. Many of the other phenolic components, however, also have demonstrated health benefits. Thus, tea is a popular drink that is beneficial to human health (1–8, 32–36).
Figure 1.
Structures of tea flavonoids, other phenolics and other compounds.
Many studies of tea phenolic components using liquid chromatography (LC) or mass spectrometric detection (LC–MS) have been reported (13–18, 36–45). None, however, has detected more than 50 phenolic components or simultaneously determined all the previously mentioned tea phenolic components in a single chromatographic run (13–18, 36–45).
As a part of a project to systematically identify glycosylated flavonoids and other phenolic compounds in food plants, we used a standardized profiling method based on liquid chromatography with diode array and electrospray ionization/mass spectrometric detection (LC–DAD–ESI/MS) (46) to examine 41 green and 25 fermented teas. This profiling method was able to detect C- and O-glycosylated flavonoids, catechins, proanthocyanidins, phenolic acid derivatives, and purine alkaloids in a single chromatographic run. More than 96 tea phenolic compounds were detected in the tea samples, including over 30 for the first time to tea. The phenolic profiles of the teas made it possible to divide the teas into five groups.
MATERIALS AND METHODS
Standard Compounds and Other Chemicals
Quercetin dihydrate, rutin trihydrate, kaempferol, (+)-catechin, (–)-epicatechin, (–)-gallocatechin, (–)-epigallocation, (–)-epicatechin 3-gallate, (–)-catechin 3-gallate, (–)-epigallocatechin 3-gallate, (–)-gallocatechin 3-gallate, gallic acid, chrologenic acid (i.e., 5-caffeoylquinic acid), theanine, caffeine, theobromine, and theophylline were obtained from Sigma Chemical Co. (St. Louis, MO). Quercetin 3-O-glucoside, quercetin 3-O-galactoside, quercetin 3-O-rhamnoside, myricetin 3-O-glucoside, kaempferol 3-O-glucoside, kaempferol 3-O-rutinoside, vitexin, vitexin 2″-rhamnoside, isovitexin, saponirin (isovitexin 6″-glucoside), and myricetin were purchased from Extrasynthese (Genay, Cedex, France). Theaflavin and theaflavin 3,3′-digallate were obtained from Chromadex, Inc. (Irvine, CA).
HPLC grade methanol, acetonitrile, formic acid, acetic acid, and NaOH were purchased from VWR International, Inc. (Clarksburg, MD). HPLC grade water was prepared from distilled water using a Milli-Q system (Millipore Laboratory, Bedford, MA).
Two phenolic acids, 3- and 4-caffeoylquinic acid, were prepared by the isomerization of chlorogenic acid (300 mg) and separated by C18 column chromatography (46).
Plant Materials
A total of 66 teas were collected from around the world: 41 green teas, 14 partial fermented teas, 8 black teas, and 2 overfermented teas (Table 1). Among the 41 green teas, 14 were made from the fresh leaves of the spring season of 2007 growing in different places in China with quite different altitudes, and the rest were grown in Japan, India, and Sri Lanka. The fermented teas were from the same four countries (Table 1).
Table 1.
The Tested Teas of Each Tea Group
| Group 1, 5 Teas |
| yinhou tea (T1, white tea) (Songyang, Zhejiang Province) |
| longding tea (T3) (Kiahua, Zhejiang Province) |
| jiming gong tea (first grade) (T5) (Chengkou, Chongqing City) |
| jiming gong tea (second grade) (T7) (Chengkou, Chongqing City) |
| Tenren premium white tea (T49) |
| Group 2, 29 Green Teas and 7 Decaffeinated Green Teas |
| Yunnan green tea (T2) (Yunnan Province) |
| donting longjiang tea (T4) (Jiangsu Province) |
| jiming gong tea (sixth grade) (T6) (Chengkou, Chongqing City) |
| longjing tea (T8) (Hangzhou, Zhepang) |
| omei green tea (T9) (Emei mountain, Sichuan Province) |
| wild green tea (T10) (Dabie mountain, Anhui Province) |
| yuyun gong tea (second grade) (T11) (Chongqing city) |
| maofeng tea (made in 2007) (T12) (Yellow mountain, Anhui Province) |
| mingqian green tea (T13) (Sichuan Province) |
| maojian tea (T14) (Xinyan, Henan Province) |
| Fujian jasmine tea (T20) (Fujian Province) |
| China zhu tea (T21) |
| Stash premium green tea (T22) |
| Celestial Seasonings green tea (T23) |
| wufeng tea (T34) |
| maofeng tea (made in 2006) (T35) (Yellow mountain, Anhui Province) |
| Japanese green teas (T37) |
| Tenren pilochun teas (T38) (Taiwan) |
| Japanese sencha (T42) |
| Japanese banchan (T43) |
| Japanese genmaicha (T44) |
| Tenren pilochun teas (T51) |
| Tenren Japanese green teas (T53) |
| Tenren green tea (T54) |
| Earl Grey green tea (T57) |
| Harris green tea (T59) |
| Tetley green tea (T64) |
| Jiangxi maofeng (T65) (Jiangxi Province) |
| Jiangxi pilochua tea (T66) (Jiangxi Province) |
| decaffeinated green tea 1 (T40) |
| decaffeinated green tea 2 (T55) |
| decaffeinated green tea 3 (T58) |
| decaffeinated green tea 4 (T60) |
| decaffeinated green tea 5 (T61) |
| decaffeinated green tea 6 (T62) |
| decaffeinated green tea 7 (T63) |
| Group 3, 14 Teas |
| Yunnan puerh tea (T15) (Yunnan Province) |
| tikuanyin tea (made in 2004) (T16) (Fujian Province) |
| tikuanyin tea (made in 2007) (T17) (Fujian Province) |
| tikuanyin tea (made in 2005) (T18) (Fujian Province) |
| Taiwan penfeng tea (T19) |
| Foojoy oolong tea (T24) (Fujian Province) |
| Fujian shuisien tea (T25) (Fujian Province) |
| Foojoy wuyi oolong tea (T26) (Fujian Province) |
| Fujian oolong tea (T27) (Fujian Province) |
| Fujian tikuanyin tea (T28) (Fujian Province) |
| Foojoy shoumei white tea (T29) (Fujian Province) |
| wuyi rock tea (T36) (Fujian Province) |
| Tenren pouching tea (T50) (Taiwan) |
| Tenren tenwu tea (oolong) tea (T52) |
| Group 4, 9 Teas |
| Yunnan black tea (T30) (Yunnan Province) |
| Foojoy China black tea (T31) |
| China black tea (T39) |
| Sri Lanka Irish breakfast tea (T41) |
| India Earl Grey tea (T45) |
| Assam tea (T46) |
| English breakfast tea (T47) |
| Japanese Kukicha twig tea (T48) |
| Darjeeling black tea (T56) |
| Group 5, 2 Teas |
| Yunnan tou tea (T32) (Yunnan Province) |
| Yunnan citsebeeng tea (T33) (Yunnan Province) |
Seventeen green teas [yinhou tea (T1), Yunnan green tea (T2), longding tea (T3), donting longjiang tea (T4), jiming gong tea (first grade, T5; sixth grade, T6; second grade, T7), longjing tea (T8), omei green tea (T9), wild green tea (T10), yuyun gong tea (grade 2, T11), two maofeng tea (T12 and T35), mingqian green tea (T13), maojian tea (T14), wufeng tea (T34), and Jiangxi maofeng (T65)], and 6 fermented teas [Yunnan puerh tea (T15), three anqi tikuanyin (2004, T16; 2007, T17; 2005, T18), wuyi rock tea (T36), and Jiangxi pilochua tea (T66)], were bought from the local tea stores in Shanghai, Hungzhou, Kunming, and Chongqing, China.
Japanese sencha (T42), banchan (T43), genmaicha (T44), kukicha twig (T48), Sri Lanka Irish breakfast tea (T41), India Earl Grey tea (T45), Assam tea (T46), and English breakfast tea (T47) were obtained from Starwest Botanicals (Rancho Cordova, CA) as a gift. Earl Grey green tea (T57) and Darjeeling black tea (T56) were bought from Frontier Natural Products Corp (Norway, IA; origin: India).
Stash premium green tea (T22), Celestial Seasonings green tea (T23), Harris green tea (T59), Tetley green tea (T64), China black tea (T39), Taiwan penfeng tea (T19), Fijian jasmine tea (T20), China Zhu tea (T21), Foojoy oolong tea (T24), Fujian shuisien tea (T25), Foojoy wuyi oolong tea (T26), Jujian oolong tea (T27), Fujian tikuanyin tea (T28), Foojoy shoumei white tea (T29), Yunnan black tea (T30), Foojoy China black tea (T31), Yunnan tou tea (T32), Yunnan citsebeeng tea (T33), 8 Tenren teas [green tea (T54), tenwu tea (T52), pouching tea (T50), premium white tea (T49), two pilochun teas (T38, T51), two Japanese green teas (T37 and T53)], and 7 decaffeinated green teas [1 (T40), 2 (T55), 3 (T58), 4 (T60), 5 (T62), 6 (T61) and 7 (T63)] were bought from local tea or food stores in Maryland.
Extracts
Prior to extraction, each of the teas was finely powdered and passed through a 20-mesh sieve. Each powdered tea (100 mg) was extracted with 5.00 mL of methanol–water (60:40, v/v) using a FS30 Ultrasonic sonicator (40 KHz, 100 W) (Fisher Scientific, Pittsburgh, PA) for 60 min at room temperature. The slurry mixture was centrifuged at 2500 rpm for 15 min (IEC Clinical Centrifuge, Damon/IEC Division, Needham, MA). The supernatant was filtered through a 17 mm (0.45 μm) PVDF syringe filter (VWR Scientific, Seattle, WA). Finally, 30 μL of the extract was injected onto the chromatographic column (46).
The components of the teas were also analyzed as consumed. For one tea from each group, a boiling water infusion was made from 50 mg of powder in 5.00 mL of HPLC grade water heated at 90 °C water bath for 10 min. The extracts were filtered, and 60 μL of each water infusion was injected onto the column.
Acidic Hydrolyzed Extracts
The filtered extracts (0.50 mL) were mixed with concentrated HCl (37%, 0.10 mL) and heated in a capped tube at 85 °C for 2 h. Then, 0.40 mL of methanol was added to the mixture, and the solution was sonicated for 10 min. The solution was refiltered prior to HPLC injection (46).
Alkali Hydrolyzed Extracts
The filtered extract (2.00 mL) was dried, and the residue was mixed with 0.30 mL of 2 N NaOH and kept at room temperature under a N2 atmosphere for 18 h. Then, 0.10 mL of HCl (37%) was added to the reaction mixture to bring the pH to 1.0, and 0.60 mL of MeOH was added. The solution was filtered prior to HPLC injection (46).
LC–DAD–ESI/MSD Conditions
The system used consisted of a quaternary pump with a vacuum degasser, a thermostatted column compartment, an autosampler, a diode array detector (DAD), and a single-quad mass spectrometer (MSD) from Agilent Technologies (Palo Alto, CA). A 250 × 4.6 mm i.d., 5 μm Symmetry C18 column with a 20 × 3.9 mm i.d., 5 μm sentry guard column (Waters Corp., Milford, MA) was used at a flow rate of 1.0 mL/min. The column oven temperature was set at 25 °C. The mobile phase consisted of a combination of A (0.1% formic acid in water) and B (0.1% formic acid in acetonitrile). The gradient increased linearly from 10 to 26% B (v/v) at 40 min, to 65% B at 70 min, to 100% B at 100 min, and held at 100% B to 105 min. The DAD was set at 350, 310, and 270 nm for real-time monitoring of the peak intensities. UV spectra were continuously recorded from 190 to 650 nm for plant component identification. Mass spectra were simultaneously acquired using electrospray ionization in the positive and negative ionization (PI and NI) modes at low and high fragmentation voltages (100 and 250 V) over the range of m/z 100–2000. A drying gas flow of 13 L/min, a drying gas temperature of 350 °C, a nebulizer pressure of 50 psi, and capillary voltages of 4000 V for PI and 3500 V for NI were used. The LC system was directly coupled to the MSD without stream splitting (46).
RESULTS AND DISCUSSIONS
Identification of Tea O-Glycosylated Flavonols
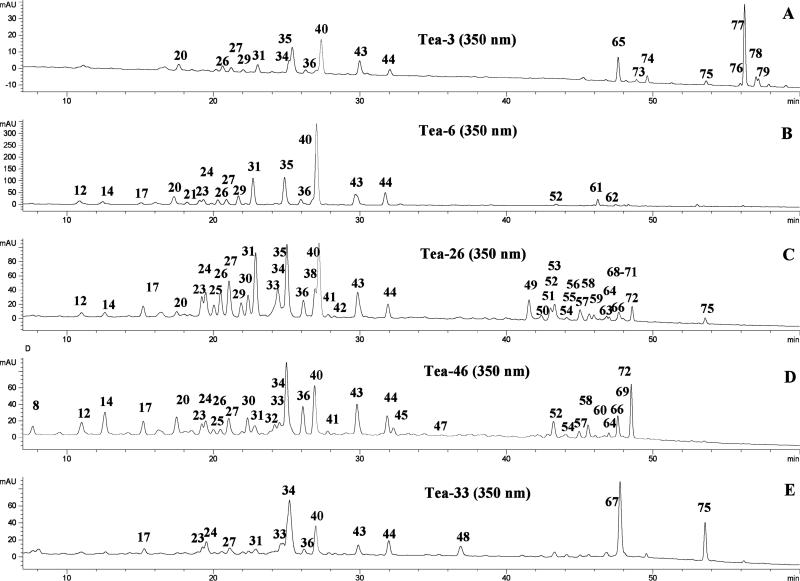
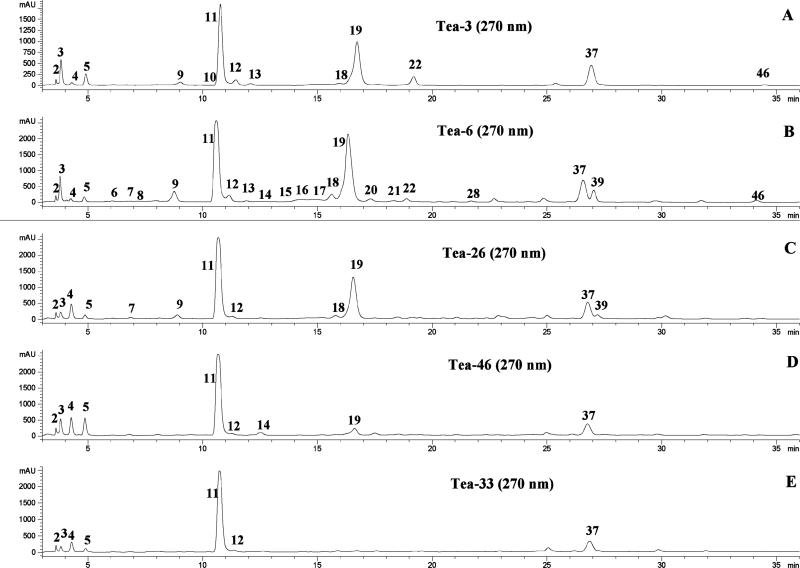
LC chromatograms recorded at 350 and 270 nm are shown in Figures 2, and 3 respectively, for the tea extracts from five tea groups. The retention times (tR), wavelengths of maximum absorbance (λmax), protonated/deprotonated molecules ([M + H]+/[M – H]–), and major fragment ions (PI/NI), including the aglycones ([A + H]+/[A – H]–), are listed in Tables 2–4. Peak identification was based on analysis of the data in these tables, direct comparison with standards, and comparison to published literature. Comparison to standards constituted positive identification. All other identifications were considered provisional. In Tables 2–4, the superscript “a” denotes compounds identified from previous reports of their existence in tea (Camillia sinensis or its varieties), and “b” denotes compounds positively identified by comparison to standards.
Figure 2.
LC chromatograms (350 nm) of the extracts (100 mg/5.0 mL of solvent, 30 μL injected) from five tea groups: (A) T1, (B) T6, (C) T26, (D) T46, and (E) T33. The peaks listed in Table 4, and some of the labeled peaks are much stronger in other tea samples.
Figure 3.
LC chromatograms (270 nm) of the extracts (100 mg/5.0 mL of solvent, 30 μL injected) from five tea groups: (A) T1, (B) T6, (C) T26, (D) T46, and (E) T33. The peaks listed in Tables 2 and 3, and some of the labeled peaks are much stronger in other tea samples.
Table 2.
The Purine Alkaloids, Theanine, and Phenolic Acid Derivatives in Tea Samples
| peak no. | tR (min) | [M + H]+/[M – H] (m/z) | PI/NI aglycone, other ions (m/z) | UV λmax (nm) | identification |
|---|---|---|---|---|---|
| 3 Purine Alkaloids and One Amino Acid | |||||
| 1c | 2.90c | 175/173 | –/– | – | theaninea,b |
| 5 | 4.80 | 181/– | –/– | 274 | theobrominea,b |
| 7 | 6.90 | 181/– | –/– | 274 | theophyllinea,b |
| 11B | 10.88 | 195/– | –/– | 268 | caffeinea,b |
| 15 Phenolic Acid Relatives | |||||
| 2Ad | 3.60 | –/331 | –/169 | 276 | galloylglucose |
| 2B | 3.69 | –/343 | –/191, 169 | 276 | 3-galloylquinic acida |
| 3 | 3.90 | –/343 | –/191, 169 | 276 | 5-galloylquinic acida |
| 4A | 4.38 | –/343 | –/191, 169 | nde | 4-galloylquinic acida |
| 4B | 4.48 | –/169 | –/– | 274 | gallic acida,b |
| 8 | 7.63 | –/353 | –/191, 179 | 240, 326 | 3-caffeoylquinic acida,b |
| 10 | 9.53 | –/483 | –/331, 169 | 276 | 1, 6-digalloylglucose |
| 12A | 11.05 | –/337 | –/191, 163 | – | 3-p-coumaroylquinic acida |
| 12B | 11.10 | –/353 | –/191, 179 | 240, 326 | 5-caffeoyloylquinic acida,b |
| 13 | 12.13 | –/183 | –/169 | 276 | gallic acid methyl ester |
| 14A | 12.52 | –/353 | –/191, 179 | 240, 326 | 4-caffeoylquinic acida,b |
| 17B | 15.50 | –/337 | –/191, 163 | 310 | 5-p-coumaroylquinic acid |
| 20 | 17.40 | –/337 | –/191, 163 | 310 | 4-p-coumaroylquinic acida |
| 22B | 19.14 | –/635 | –/483, 169 | 276 | 1,2,6-trigalloylglucose |
| 45B | 33.10 | –/515 | –/353, 191, 179 | 240, 326 | 3,5-dicaffeoylquinic acida |
Previously reported in tea.
Positively identified by direct comparison with standard.
Not shown in Figure 3, since this peak appeared before 3 min. Its retention time is 4.67 min with 0–10% B in 20 min.
T3 contained high concentrations of this compound.
nd, not determined.
Table 4.
O-Glycosylated Flavonols, C-Glycosylated Flavones, and Flavones in Tea Samples
| peak no. | tR (min) | [M + H]+/[M – H] (m/z) | PI/NI aglycone, other ion (m/z) | UV λmax (nm) | identification |
|---|---|---|---|---|---|
| 19 O-Glycosylated Flavonols | |||||
| 21B | 18.65 | 789/787 | 481, 319/– | nd | myricetin 3-O-galactosylrutinoside |
| 25 | 19.38 | 627/625 | 481, 319/– | 262, 356 | myricetin 3-O-rhamnosylglucosidea |
| 26 | 20.63 | 481/479 | 319/– | 262, 356 | myricetin 3-O-galactosidea |
| 27A | 21.22 | 481/79 | 319/– | 262, 356 | myricetin 3-O-glucosidea,b |
| 29 | 22.05 | 773/771 | 611, 465, 303/– | 256, 354 | quercetin 3-O-galactosylrutinosidea |
| 31 | 23.06 | 773/771 | 611, 465, 303/– | 256, 354 | quercetin 3-O-glucosylrutinosidea |
| 32 | 24.16 | 757/755 | 465, 303/– | 256, 354 | quercetin 3-O-dirhamnosylglucosidea |
| 33B | 24.69 | 611/– | 465, 303/– | 256, 354 | quercetin 3-O-rhamnosylgalactosidea |
| 34B | 25.16 | 611/609 | 465, 303/– | – | rutina,b |
| 35 | 25.31 | 757/755 | 449, 287/– | – | kaempferol 3-O-galactosylrutinosidea |
| 36 | 26.29 | 465/– | 303/– | 258, 270sh, 354 | quercetin 3-galactosidea,b |
| 38 | 27.10 | 465/– | 303/– | 258, 270sh, 354 | quercetin 3-O-glucosidea,b |
| 40 | 27.40 | 757/755 | 595, 449, 287/– | 266, 348 | kaempferol 3-O-glucosylrutinosidea |
| 41 | 28.01 | 595/593 | 449, 287/– | 266, 348 | kaempferol 3-O-rhamnosylgalactosidea |
| 42 | 29.09 | 741/739 | 595, 449, 287/– | 266, 348 | kaempferol 3-O-xylosylrutinosidea |
| 43A | 30.05 | 595/– | 449, 287/– | 266, 348 | kaempferol 3-O-rutinosidea,b |
| 43B | 30.12 | 449/– | 287/– | – | kaempferol 3-O-galactosidea |
| 44 | 32.20 | 433/– | 287/– | 266, 348 | kaempferol 3-O-glucosidea,b |
| 45A | 32.70 | 449/– | 303/– | – | quercetin 3-O-rhamnosidea,b |
| 7 C-Glycosylated Flavones | |||||
| 17A | 15.42 | 595/593 | 577, 475, 457/– | nd | 6,8-C-diglucosylapigenina |
| 23 | 19.39 | 565/563 | 547, 475, 445, 355/– | 272, 336 | apigenin 6-C glucosyl-8-C-arabinosidea |
| 24 | 19.63 | 565/563 | 547, 475, 445, 355/– | 272, 336 | apigenin 6-C-arabinosyl-8-C-glucosidea |
| 27B | 21.89 | 565/563 | 547, 475, 445, 355/– | nd | apigenin 6-C-pentosyl-8-C-hexoside |
| 30 | 22.55 | 595/– | 433, 313/– | 272,336 | vitexin 2″-glucoside or isomer |
| 33A | 24.69 | 579/– | 433, 313/– | 270,338 | vitexin 2″-O-rhamnosideb |
| 34A | 25.05 | 535/533 | 517, 499, 475, 415/– | – | apigenin 6,8-C-dipentosidea |
| 28 Acylated Glycosylated Flavonols | |||||
| 47 | 35.22 | 783/781 | 287/– | 266, 348 | kaempferol 3-O-acetyl-dirhamnosylhexoside |
| 49 | 41.90 | 1065/1063 | 303/– | 266, 310 | quercetion 3-O-acylglycoside |
| 50A | 42.49 | 1049/1047 | 287/– | 266, 310 | kaempferol 3-O-acylglycoside |
| 50B | 42.63 | 1035/1033 | 303/– | 266, 310 | quercetin 3-O-acylglycoside |
| 50C | 42.76 | 1051/1049 | 303/– | 266, 310 | quercetin 3-O-acylglycoside |
| 51 | 43.43 | 919/917 | 303/– | 266, 310 | quercetion 3-O-p-coumaroylglucosylrhamnosylglactoside |
| 52 | 43.56 | 903/901 | 303/– | 266, 310 | quercetin 3-O-p-coumaroylglucosylrutinoside |
| 53 | 43.66 | 1065/1063 | 303/– | 266, 310 | quercetion 3-O-acylglycoside |
| 54 | 44.19 | 887/885 | 287/– | 266, 310 | kaempferol 3-O-p-coumaroyldirhamnosylhexosidea |
| 55 | 44.69 | 887/885 | 287/– | 266, 310 | kaempferol 3-O-p-coumaroyldirhamnosylhexoside |
| 56A | 44.73 | 1049/1047 | 287/– | 266, 310 | kaempferol 3-O-di-p-coumaroylrhamnosyldiihexoside |
| 56B | 44.96 | 1051/1049 | 303/– | 266, 310 | quercetin 3-O-acylglycoside |
| 57 | 45.31 | 887/885 | 287/– | 266, 310 | kaempferol 3-O-p-coumaroyldirhamnosylhexoside |
| 60 | 46.35 | 1065/1063 | 303/– | 266, 310 | quercetion 3-O-acylglycoside |
| 61 | 46.59 | 903/901 | 287/– | 266, 310 | kaempferol 3-O-p-coumaroylglucosylrhamnosylglactoside |
| 62 | 46.89 | 595/593 | 287/– | 266, 310 | kaempferol 3-O-p-coumaroylglucoside |
| 63 | 47.05 | 1049/1047 | 287/– | 266, 310 | kaempferol 3-O-p-coumaroylrhamnosyldihexoside |
| 64 | 47.22 | 903/901 | 287/– | 266, 310 | kaempferol 3-O-p-coumaroyldirhamnosylhexoside |
| 65 | 47.75 | 595/593 | 287/– | 266, 310 | kaempferol 3-O-6″-p-coumaroylglucoside |
| 68 | 48.15 | 887/885 | 287/– | 266, 310 | kaempferol 3-O-p-coumaroyldirhamnosylhexosidea |
| 70 | 48.35 | 741/739 | 287/– | 266, 310 | kaempferol 3-O-p-coumaroylglycoside |
| 71 | 48.48 | 903/901 | 287/– | 266, 310 | kaempferol 3-O-p-coumaroylglucosylrhamnosylglactoside |
| 73 | 49.07 | 595/593 | 287/– | 266, 310 | kaempferol 3-O-p-coumaroylglucoside |
| 74 | 49.80 | 595/593 | 287/– | 266, 310 | kaempferol 3-O-p-coumaroylhexoside |
| 76 | 56.13 | 741/739 | 287/285 | 266, 316 | kaempferol 3-O-di-p-coumaroylhexoside |
| 77 | 56.42 | 741/739 | 287/285 | 266, 316 | kaempferol 3-O-2″,6″-di-p-coumaroylglucoside |
| 78 | 57.19 | 741/739 | 287/285 | 266, 316 | kaempferol 3-O-di-p-coumaroylhexoside |
| 79 | 57.37 | 741/739 | 287/285 | 266, 316 | kaempferol 3-O-di-p-coumaroylhexoside |
| 3 Flavonols | |||||
| 48 | 36.90 | 319/317 | –/– | 266, 368 | myricetina,b |
| 67 | 47.84 | 303/301 | –/– | 266, 368 | quercetina,b |
| 75 | 53.17 | 287/285 | –/– | 266, 368 | kaempferola,b |
Previously reported in tea.
Identified by direct comparison with a standard.
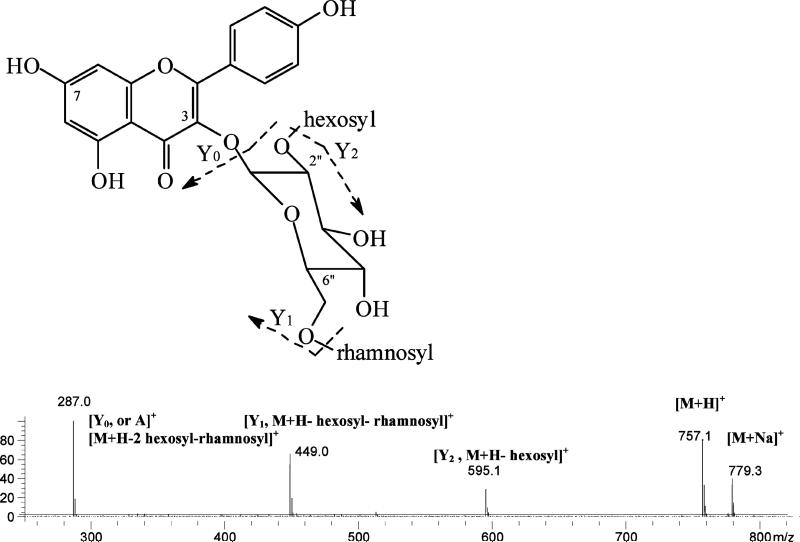
The identification process can be illustrated by considering peaks 35 (tR 25.31 min) and 40 (tR 27.40 min) in Figure 2. Their MS data, [M + H]+/[M – H]– at m/z 755/753, [A + H]+/[A – H]– at m/z 287/285, and PI fragment ions at 595 (loss of the third glycosyl, a hexosyl, from the glycoside) and 449 (loss of the secondary glycosyl, a rhamnosyl) (Table 4) suggested these peaks were kaempferol–hexosylrhamnosylhexoside (Figure 4) (46, 47). The UV data (λmax 266sh and 348 nm, respectively) and retention times strongly suggested that the glycosides had a sugar at the 3-position of kaempferol (46, 48).
Figure 4.
Mass spectrum of kaempferol 3-O-2″-hexosyllrhamnosylglucoside (peak 35 in Figure 2) obtained from the PI 100 V MS chromatogram.
These peaks were finally identified as kaempferol 3-O-2″-galactosylrutinoiside (peak 35) and kaempferol 3-O-2″-glucosylrutinoiside or kaempferol 3-O-3‴-glucosylrutinoiside (the glucosyl at the 3-postion of the rhamnosyl) (peak 40), since these three flavonoids have been previously reported in tea (3, 4, 11–15). Peak 35, with a galactosyl group, eluted earlier than peak 40, with a glucosyl group (46). These are the only flavonoids reported in tea that matched the observed retention times (elution order) and UV and mass data for peaks 35 and 40 (Table 4).
In a similar manner, peaks 29 and 31 (tR 22.05 and 23.06 min, λmax 256, 266sh and 354 nm, [M + H]+/[M – H]– at m/z 773/771, and PI mass ions at m/z 611, 465, and 303) were identified as quercetin 3-O-3‴-glucosylrhamnosylgalactoside and quercetin 3-O-3‴-glucosylrutinoside, respectively(Table 4).Each was previously reported in tea (3, 4, 12–16). Peaks 25, 26, 27A, 32, 33B, 34B, 36, 38, 41, 42, 43A, 43B, 44, and 45A were identified as listed in Table 4. These flavonoids have all been previously reported in tea (3, 4, 12–16). In some cases, their identification was confirmed by direct comparison with standards as indicated in Table 4.
On the basis of biogenetic consideration, the remaining peaks 21B (tR 18.65 min, PI mass ions at m/z 789, 627, 481, and 319) was provisionally identified as myricetin 3-O-galactosylrutinoside. This compound was not previously reported in tea.
The identification of the tetrahydroxy-, pentahydroxy-, and hexahydroxy-flavonol glycosides presented above is further confirmed by the fact that kaempferol, quercetin, and myricetin are the only aglycones detected in the hydrolyzed extracts of the group 5 teas (data not shown). Furthermore, the three flavonols were also detected in some of the fully fermented tea extracts, particularly in the overfermented teas (Figure 2E).
Identification of Tea Acylated O-Glycosylated Flavonols
Table 4 lists 28 acylated flavonol glycosides detected and identified in the tea samples. Only one (peak 47) of them contained an acetyl group. The remaining compounds contained one or two p-coumaroyl group or other phenolic acyls.
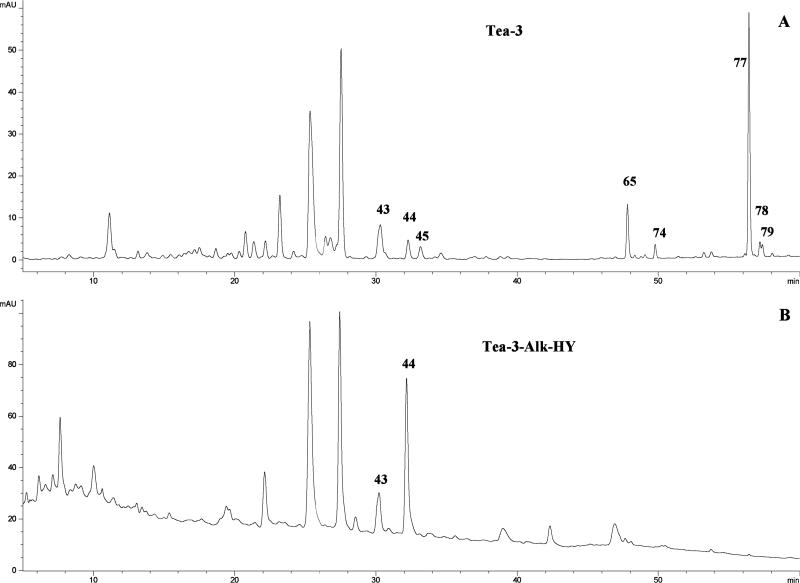
The main characteristics of the p-coumaroylglycosylated flavonols are the shift of absorption band II to a λmax of 310–316 nm and PI/NI molecular ions 146 amu larger than their parent glycosides for mono-p-coumaoylated glycosides and 292 amu larger for di-p-coumaroylated glycosides (39, 40). Peak 65 (tR 47.80 min, λmax 266, 312 nm, PI mass ions at m/z 595, 449, 287) and peak 77 (tR 56.44 min, λmax 266, 312 nm, PI mass ions at m/z 739, 449, 287) were the main peaks in several extracts of the teas of group 1. These compounds were converted into kaempferol 3-O-glucoside (peak 44) (Figure 5) upon alkaline hydrolysis. Thus, these two peaks were identified as kaempferol 3-O-6″-p-coumaroylglucoside (peak 65), and kaempferol 3-O-6″,2″ (or 6″,4″)-di-p-coumaroylglucoside (peak 77), respectively.
Figure 5.
LC chromatograms of (A) T3 and (B) T3 after alkaline hydrolysis. Kaempferol 3-O-glucoside (peak 44) was confirmed to be the parent glycosides of its mono- and di-p-coumaroyl derivatives (peaks 65 and 77).
Several other acylated glycosides were provisionally identified as listed in Table 4 on the basis of similar logic. However, many of these were not present in sufficient concentration to permit their parent glycosides to be clearly seen upon alkaline hydrolysis. Some of them are the minor isomers of more concentrated compounds with the acyl group at positions other than 6″ of the glucosyl (or other hexosyl). Thus, their structures cannot be positively determined by LC–MS analysis (46), and their identification was only provisional as listed in Table 4. In some cases, no parent compounds could be observed following alkaline hydrolysis (Figure 5). However, their LC–MS data and the fact that they all disappeared supported that they were acylated flavonol glycosides.
Another 10 acylated glycosylated flavonols, mainly p-coumaroylated, were detected in the tested teas. They are not listed in Table 4, since they were generally isomers of some of the listed compounds, appearing in the same chromatographic range (40–60 min, Figure 2) and were much less concentrated, providing much smaller peaks.
To date, only three or four acylated glycosides have been reported as conjugates of kaempferol and quercetin with [M – H]– at m/z 885 and 901, respectively (13, 16). Thus, 24 of the listed acylated glycosides in Table 4 should be new for tea.
Identification of Tea C-Glycosylated Flavones
Seven C-glycosylated apigenins were detected in tea extracts (Table 4 and Figure 2). The first two, peaks 30 (tR 22.55 min, [M + H]+ at m/z 595) and 33A (tR 24.69 min, [M + H]+ at m/z 579) had important PI fragment ions at m/z 433 and 313 due to the loss first of 162 amu and 146 amu, respectively, and then 120 amu to provide C-hexosyl (46, 47). Furthermore, they were converted into vitexin (apigenin 8-C-glucoside) by loss of their O-glycosyls during acid hydrolysis. These data suggested they were vitexin 2″-O-glucoside (or its 6″ isomer) and vitexin 2″-O-rhamnoside, respectively. The identification of vitexin was obtained by comparison with a standard.
The remaining five C-glycosylated apigenins were identified as C-diglycosylated apigenins; four had been previously isolated from tea (7, 10, 12, 33). Peak 34A (tR 25.05 min) has PI ions at m/z 533, 517 (due to loss of water), 499 (due to loss of two waters showing the existence of two pentosyl at C-6 and C-8), 475 (due to loss of 60 amu from one pentosyl), 415 (due to loss of two pentosyls of 60 amu) (46). This compound has not been previously reported in tea. Peak 17A (tR 15.42 min, [M + H]+ at m/z 595) had PI ions at m/z 577 (due to loss of one water), 559 (due to loss of two waters showing the existence of two glycosyls), and was identified as 6,8-C-diglucosylapigenin.
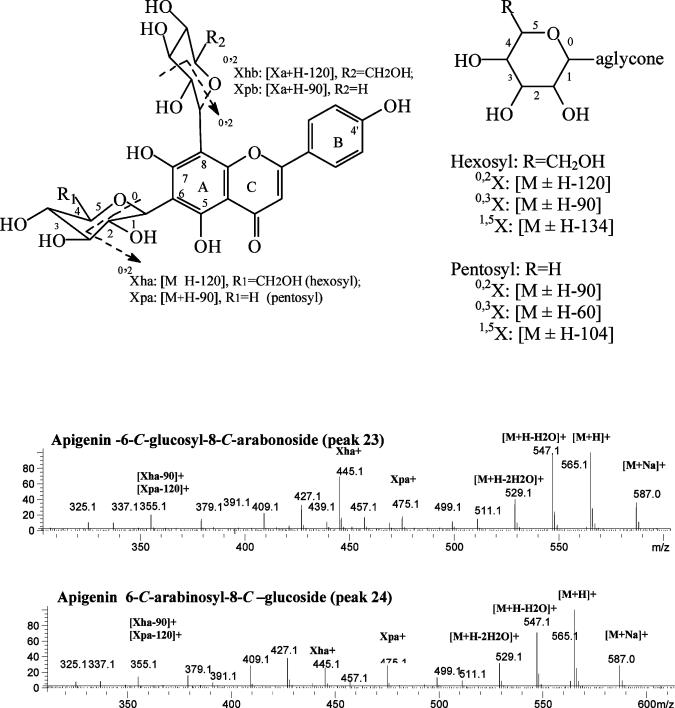
Peaks 23 (tR 19.39 min) and 24 (tR 19.63 min) were identified as apigenin 6-C-arabinosyl-8-C-glucoside (isoschaftoside) and apigenin 6-C-glucosyl-8-C-arabinoside (schaftoside), respectively. Both of them had PI ions at m/z 563, 547, 475, 445, and 355. However, the ratios of the relative intensity of the ions at m/z 475 (loss of 90 amu for the existence of pentosyl) to the ion at 445 (loss of 120 amu for the existence of the hexosyl) are quite different (38, 39) (Figure 6). On the basis of the fact that peak 23 had an intensity ratio of 82:18 for ions for m/z 445:475, it was identified as apigenin 6-C-arabinosyl-8-C-glucoside, while peak 24 had a ratio of 29:26 for ions m/z 445: 275, and was identified as apigenin 6-C-glucosyl-8-C-arabinoside. These identifications are based on the same reasoning that was used to identify them in the seeds of quince (49).
Figure 6.
Mass spectra of apigenin-6-C-glucosyl-8-C-arabinoside (peak 23 in Figure 2C) and apigenin 6-C-arabinosyl-8-C-glucoside (peak 24 in Figure 2C) obtained from the PI 250 V MS chromatogram.
Identification of Tea Catechins, Theaflavins and Proanthocyanidins
Biologically, the most important tea flavonoids are the catechins and their oxygenated products, the theaflavins. They have been the subject of many analytical studies to optimize chromatography for their separation, identification, and quantification (13–18, 30–45). As shown in Figure 3, catechins are the main phenolic components of tea, especially green tea, and (–)-epigallocatechin gallate and (–)-epicatechin gallate are the most prominent. (–)-Epicatechin and (–)-epigallocatechin are the main phenolics, and catechin, gallocatechin, catechin gallate, and gallocatechin gallate are present at relatively low concentrations. These compounds were detected in many tea samples, and they were identified by direct comparison with standards (Table 3).
Table 3.
Catechins, Proanthocyanidins, Theaflavines, and Theaflavates in Tea Samples
| peak no. | tR (min) | [M + H]+/[M – H] (m/z) | PI/NI aglycone, other ion (m/z) | UV λmax (nm) | identification |
|---|---|---|---|---|---|
| 12 Catechins | |||||
| 6 | 5.80 | –/305 | –/– | 272 | gallocatechina,c |
| 9 | 8.90 | –/305 | –/– | 272 | (–)-epigallocatechina |
| 11C | 10.55 | –/633 | –/– | ndd | (–)-methylepigallocatechin gallate |
| –/– | glucoside | ||||
| 12C | 11.38 | –/289 | –/– | 276 | (+)-catechinaa,b |
| 18A | 15.87 | –/289 | –/– | 276 | (–)-epicatechina,b |
| 19 | 16.64 | –/457 | –/– | 276 | (–)-epigallocatechin gallatea,b |
| 21A | 18.62 | –/457 | –/– | 276 | gallocatechin gallatea,b,c |
| 28 | 21.53 | –/273 | –/– | 274 | (–)-epiafzelechina |
| 37 | 26.75 | –/441 | –/– | 278 | (–)-epicatechin gallatea,b |
| 39 | 27.25 | –/441 | –/– | 278 | (+)-catechin gallatea,b |
| 45C | 33.52 | –/455 | –/– | 278 | (–)-methoxyepiafzelechine gallate |
| 46 | 34.50 | –/425 | –/– | 274 | (–)-epiafzelechin gallatea |
| 6 Proanthocyanidins | |||||
| 11A | 10.55 | –/609 | –/– | 272 | gallocatechin dimera |
| 14B | 12.52 | –/577 | –/– | 276 | procyanidin dimera |
| 15 | 13.73 | –/745 | –/– | 276 | gallocatechin catechingallatea |
| 16 | 14.22 | –/865 | –/– | 276 | procyanidin trimera |
| 18B | 16.00 | –/745 | –/– | 276 | gallocatechin-catechingallatea |
| 22A | 19.02 | –/897 | –/– | 278 | digallocatechin-catechina |
| 6 Theaflavins and Theaflavates in Fermented Teas | |||||
| 58 | 45.32 | 565/563 | –/– | 272, 374 | theaflavina,b |
| 59 | 45.99 | 701/699 | –/– | 270, 372 | theaflavate Ba |
| 66 | 47.77 | 717/715 | –/– | 270, 372 | theaflavin-3-gallatea,b |
| 69 | 48.30 | 853/851 | –/– | 278, 396 | theaflavate Aa |
| 72A | 48.67 | 869/867 | –/– | 276, 376 | theaflavin-3,3′-gallatea,b |
| 72B | 48.67 | 717/715 | –/– | 270, 372 | theaflavin-3′-gallatea |
Previously reported in tea.
Positively identified by direct comparison with a standard.
The configuration was not determined.
nd, not determined.
Epiafzelechin (peak 28, tR 21.53 min, [M – H]– at m/z 273, UV λmax at 276 nm) and its gallate (peak 46, tR 34.50 min, [M – H]– at m/z 425, UV λmax at 274 nm) were detected only in green tea extracts. Peak 11C (tR 10.55 min, [M – H]– at m/z 633) was tentatively identified as methylepigallocatechin galate glucoside, and peak 45C (tR 33.52 min, [M – H]– at m/z 455, UV λmax at 278 nm) was identified as methoxyepiafzelechin gallate. They were first detected in green teas, and the latter was detected only in a green tea (Tea 37 from Japan).
It is worth noting that recently, polyphenols and purine alkaloids were identified in 22 tea cultivars from the Fujian and Guangdong Provinces of China (30). In addition, (–)-epigallocatechin 3-(3″-O-methyl)gallate (MW = 472), (–)-epicatechin 3-(3″-O-methyl)gallate (MW = 456), (–)-epicatechin 3,5-digallate (MW = 594), and two unknown catechins (MW = 634, and MW = 636) were detected, especially in the tea cultivars from Guangdong Province (30). Of these, (–)-epicatechin 3-(3″-O-methyl)gallate could be the same as peak 45C. Peak 11C ([M – H]– at m/z 633 with UV λmax at 240, 275 nm) tentatively identified as methylepigallocatechin gallate glucoside, could be the unknown catechin with MW = 634. Peak 22B ([M – H]– at m/z 635, UV λmax at 276 nm) might be the unknown catechin with MW = 636 (30), but this compound was identified as a trigalloylglucose.
The oxidative products of the catechins found in the fermented tea extracts are listed in Table 3. Four theaflavins and two theaflavates (17) were identified: theaflavin (peak 58), theaflavate B (peak 59), theaflavin-3-gallate (peak 66), theaflavate A (peak 69), theaflavin-3, 3′-gallate (peak 72A), and theaflavin-3′-gallate (peak 72B). Their relative concentrations (peak areas) appeared to be proportional to the degree of fermentation. Thus, fully fermented black tea always contained more of the theaflavins than the partially (30–50%) fermented oolong and puerh teas. However, highly overfermented black tea (group 5) contained only trace amounts of theaflavins. It is hypothesized that the theaflavins were further polymerized in these teas.
Six previously reported tea proanthocyanidins (some called theasinensins) (11, 12, 19, 23) were detected in some of the tea extracts. On the basis of the mass data, they were identified as listed in Table 3.
Identification of Tea Phenolic Acids and their Derivatives
Fifteen phenolic acids and their derivatives were detected in the tea extracts and identified as listed in Table 2. Seven of these compounds [galloylglucose (peak 2A, tR 3.69 min, [M – H]– at m/z 331, NI fragment ions at m/z 169 for gallic acid, UV λmax at 276 nm), 1,6-digucosylglucose (peak 10, tR 9.53 min, [M – H]– at m/z 483, NI fragment ions at m/z 169 and 331), 1,2,6-trigalloylglucoside (peak 22B, tR 19.14 min, [M – H]– at m/z 635, NI fragment ions at m/z 169), 3,5-dicaffeoylquinic acid (peak 45B, [M – H]– at m/z 515, NI fragment ions at m/z 353, 191, 179 for quinic and caffeic acids, UV λmax at 326 nm) and 3-p-coumaroylquinic acid (peaks 12A [M – H]– at m/z 337, NI fragment at 163 for p-coumaric acid, UV λmax at 310 nm)] were reported for the first time in tea. The remaining compounds [gallic acid, 5- and 4-p-coumaroylquinic acids, and the six monoacylquinic acids (5-, 3-, and 4-caffeoyl- and galloylquinic acids)] were previously reported in green teas (11, 14), but the latter two have only recently been identified in green tea (14).
Theanine and Purine Alkaloids
The tea-specific amino acid, theanine, was detected in aqueous methanol extracts with MS detection (transparent in the UV) (Table 2). It was present at higher concentrations in the water infusion, since theanine is more soluble in water than in the aqueous methanol. To observe the theanine peak, the standard gradient was altered by appending a step running 0–10% solvent B (see Experimental Section) in 20 min. Under these conditions, the retention time of theanine was 4.67 min.
Caffeine is the major purine alkaloid in tea. Some teas contain as much as 2–4% caffeine on a dry weight basis (2, 30). The caffeine analogues, theobromine and theophylline, were also detected in the LC chromatogram at 270 nm (Figure 3 and Table 2), since their UV λmax falls between 268 and 274 nm.
Decaffeinated tea products are also popular and are readily found on the market. Seven decaffeinated teas (T40, T55, T58, T60, T61, T62, and T63) were analyzed, and it was possible to compare five of them to the original, nondecaffeinated teas (data not shown). Using the LC conditions of this study, it was easy to see that three of the decaffeinated teas were prepared efficiently, that is, only 10–15% of the caffeine remained, and only ~20% of the EGCG was lost. The other two decaffeinated teas were poorly prepared and either contained more than 20% of the original caffeine content or lost more than 20% of the EGCG, or both.
Phenolic Profiles for the Five Tea Groups
The data in Tables 2, 3, and 4 provide detailed information on the individual flavonoid and other phenolic components of tea and are comprehensive phenolic profiles. These profiles can be used to divide teas into five groups. Representative chromatograms for each of the five groups are shown in Figures 2 (350 m) and 3 (270 nm). The catechins and their dimers, gallic acids, and purine alkaloids are detected at 270 nm, and the flavonols, flavones, and the rest of the phenolic compounds are seen at 350 nm. These chromatograms allow the patterns of the five tea groups to be easily seen, and the teas of each group are listed in Table 1.
The first tea group contained three maofeng, or high-grade, teas (T3, T5, and T7) and two white teas (T1, T49). The maofeng, or high-grade, teas consisted of the younger buds and leaves harvested in the early leaf-growing stage. They contained lower concentrations of glycosylated flavonols and EGCG and a higher fraction of acylated flavonol glycosides (Figures 2A, 3A, and 5A) than the common green teas (group 2, discussed later). However, these high-grade teas have much higher prices than that of common teas in China. This is the first study to connect high-grade teas with the degree of acylation of the flavonoid glycosides.
Group 2 contained 36 common (or normal) green teas; among them 29 teas (T2, T4, T6, T8-T14, T20-T23, T34, T35, T37, T38, T42-T44, T51, T53, T54, T57, T59, T64-T66) that are nondecaffeinated green tea, and 7 that are decaffeinated green teas (T40, T55, T58, T60, T61, T62, and T63). The leaves were harvested at a relatively more mature stage. This affected their phenolic content significantly, as shown in Figures 2B and 3B. The teas in group 2 displayed much higher concentrations of EGCG and glycosylated flavonols than the teas in group 1. Much higher catechin and flavonoid concentrations were also found in the common green teas in Yunan province, where the tea trees and their leaves are exposed to much greater levels of sunshine as compared to other locations in China.
Many common green teas contain as much as 20% total phenolics by dry weight. Most of these compounds can be dissolved in hot water. Thus, a tea infusion made from 1 g of tea in 100 mL of hot water (85–90 °C for 10 min) contains most of the tea phenolics, with the exception of some of the acylated flavonol glycosides found in group 1. Green tea is one of very few foods, out of the 360 tested, that is able to supply over 250 mg of flavonoids daily to the consumer (based on consumption of one cup per day of a tea infusion of 250 mL from 2–3 g of green tea). The daily total flavonoid intake of the average tea drinker is 40–60% higher than that of a nontea drinker.
Group 3 was composed of 14 partially fermented (oolong and tikuanyin tea) and some partially fermented puerh teas (T15-T19, T24-T29, T36, T50, and T52). Group 3 contained the same glycosylated flavonols as green tea, considerably less EGCG, but more theaflavins, the oxidation products of EGCG, and other catechins (Figures 2C and 3C).
Group 4 was composed of nine fully fermented black teas (T30, T31, T39, T41, T45, T46-T48, and T56). They were made from fresh tea leaves that were fully fermented. This procedure did not significantly change the glycosylated flavonol content. Most of the catechins, especially EGCG, were oxidized to theavaflavins and other polymers. Thus, they contained only a trace of EGCG and more theavaflavins than Group 3 (Figures 2D and 3D).
Group 5 consisted of two black teas (T32 and T33, tou and chitsebenng tea) that were highly overfermented. This last group contained only a trace of EGCG and theaflavins, few glycosylated flavonoids, and significant amounts of three flavonols produced by partial hydrolysis of some of the glycosides (Figures 2E and 3E). These teas are generally consumed by older people in China. This might be the first time that these teas have been grouped differently from the common black or red teas. The phenolic profiles of these teas make this distinction possible.
The standardized profiling method used in this study is able to detect an extremely wide range of tea phenolic compounds, including the C- and O-glycosylated flavonoids, catechins, proanthocyanidins, phenolic acid conjugates, galloylglucosides, purine alkaloids, and theanine.
ACKNOWLEDGMENT
The authors would like to thank Starwest Botanicals (Rancho Cordova, CA) for the gift of eight tea samples; Professor Hundong Sun, at the Kunmin Institute of Botany, Chinese Academy of Sciences, for three tea samples; Professor Da-Yuan Zhu at the Shanghai Institute of Materai Medica, Chinese Academy of Sciences, for two green tea samples; and Doctor Yu-Lin Hu, at Dupont Stine-Haskell Research Center, Wilmington, DE, for three tea samples.
This research is supported by the Agricultural Research Service of the U.S. Department of Agriculture and by an Interagency Agreement with the Office of Dietary Supplements of the National Institutes of Health.
LITERATURE CITED
- 1.Dufresne CJ, Farnworth ER. A review of latest research finding on the health promotion properties of tea. J. Nutr. Biochem. 2001;12:404–421. doi: 10.1016/s0955-2863(01)00155-3. [DOI] [PubMed] [Google Scholar]
- 2.Chu DC, Chu DC, Juneja LR. Green tea-its cultivation, processing of the leaves for drinking materials and kinds of green tea. General chemical composition of green tea and its infusion. In: Yamamoto T, Juneja LR, Chu DC, Kim M, editors. Chemistry and application of green tea. CRC Press; New York: 1997. pp. 1–22. Chapters 1,2. [Google Scholar]
- 3.Leung AY, Foster S. Tea. In: Leung AY, Foster S, editors. Encyclopedia of common natural ingredients used in food, drugs and cosmetics. 2nd ed. John Wiley & Sons, Inc.; New York: 1996. pp. 489–491. [Google Scholar]
- 4.Encyclopedia of Chinese Medicinal Plants. Vol. 3. Shanghai Press of Science and TechnologyShanghai; China: 1999. Editorial committee of encyclopedia of Chinese medicinal plantsEds.; Tea leaves (#2159). pp. 565–574. [Google Scholar]
- 5.Zhou ZH, Zhang YJ, Xu M, Yang CR. Puerins A and B, two new 8-C substituted flavan-3-ols from Pu-er tea. J. Agric. Food. Chem. 2005;53:8614–8617. doi: 10.1021/jf051390h. [DOI] [PubMed] [Google Scholar]
- 6.Shao W, Powell C, Clifford MN. The analysis by HPLC of green, black and Pu-er teas produced in Yunnan. J. Sci. Food Agric. 1995;69:535–540. [Google Scholar]
- 7.Shao W, Clifford MN, Powell C. A preliminary study on the differences between the black tea and pu'er tea. J. Yunnan Agric. Univ. 1995;10:285–291. [Google Scholar]
- 8.Saito ST, Gosmann G, Saffi J, Presser M, Richter MF, Bergold AM. Characterization of the constituents and antioxidant activity of Brazilian green tea (Camellia sinensis var. assamica IAC-259 cultivar) extracts. J. Agric. Food Chem. 2007;55:9409–9414. doi: 10.1021/jf071796p. [DOI] [PubMed] [Google Scholar]
- 9.Engelhardt UH, Finger A, Kuhr S. Determination of flavone C-glycosides in tea. Z. Lebensm. Unters Forch. 1993;197:239–244. doi: 10.1007/BF01185278. [DOI] [PubMed] [Google Scholar]
- 10.Chiu FL, Lin JK. HPLC analysis of naturally occurring methylated catechins, 3″- and 4″-methyl-epigallocatechin gallate, in various fresh tea leaves and commercial teas and their potent inhibitory effects on inducible nitric oxide synthase in macrophages. J. Agric. Food Chem. 2005;53:7035–7042. doi: 10.1021/jf0507442. [DOI] [PubMed] [Google Scholar]
- 11.Lakenbrink C, Engelhardt UH, Wray V. Identification of two novel proanthocyanidins in green tea. J. Agric. Food Chem. 1999;47:4621–4624. doi: 10.1021/jf9813081. [DOI] [PubMed] [Google Scholar]
- 12.Harborner JB, Baxter H. Camellia sinensis in “Species index”. In: Harborne JB, Baxter H, editors. The handbook of natural flavonoids. Vol. 1. Vol. 2. John Wiley & Sons, Inc.; New York: 1999. pp. 832–833.pp. 823–824. [Google Scholar]
- 13.Del Rio D, Stewart AJ, Mullen W, Burns J, Lean MEJ, Brighenti F, Crozier A. HPLC-MSn analysis of phenolic compounds and purine alkaloids in green and black tea. J. Agric. Food Chem. 2004;52:2807–2815. doi: 10.1021/jf0354848. [DOI] [PubMed] [Google Scholar]
- 14.Clifford MN, Stoupi S, Kuhnert N. Profilling and characterization by LC-MSn of the galloylquinic acids of green tea, tara tannin, and tannic acid. J. Agric. Food Chem. 2007;55:2797–2807. doi: 10.1021/jf063533l. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Sporns P. MALDI-TOF MS analysis of food flavonol glycosides. J. Agric. Food Chem. 2000;48:1657–1662. doi: 10.1021/jf991035p. [DOI] [PubMed] [Google Scholar]
- 16.Stewart AJ, Mullen W, Crozier A. On-line high-performance liquid chromatography analysis of the antioxidant activity of phenolic compounds in green and black tea. Mol. Nutr. Food Res. 2005;49:52–60. doi: 10.1002/mnfr.200400064. [DOI] [PubMed] [Google Scholar]
- 17.Menet MC, Sang S, Yang CS, Ho C-T, Rosen RT. Analysis of theaflavins and thearubigins from black tea extract by MALDI-TOF mass spectrometry. J. Agric. Food Chem. 2004;52:2455–2461. doi: 10.1021/jf035427e. [DOI] [PubMed] [Google Scholar]
- 18.Miketova P, Schram KH, Whitney J, Li M, Huang R, Kerns E, Valcic S, Timmermann BN, Rourick R, Klohr S. Tandem mass spectrometry studies of green tea catechins. Identification of three minor components in the polyphenolic extract of green tea. J. Mass Spectrom. 2000;35:860–869. doi: 10.1002/1096-9888(200007)35:7<860::AID-JMS10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto F, Nonaka G-I, Nishioka I. Tannins and related compounds LXIX. Isolation and structure elucidation of B,B′-linked bisflavanoids, theasinensins D-G and oolongtheanin from oolong tea (2). Chem. Pharm. Bull. 1988;36:1676–1684. [Google Scholar]
- 20.Hashimoto F, Nonaka G-I, Nishioka I. Tannins and related compounds. CXIV. Structures of novel fermentation products, theogallinin, theaflavonin and desgalloyltheaflavonin from black tea, and changes of tea leaf polyphenols during fermentation. Chem. Pharm. Bull. 1992;40:1383–1389. [Google Scholar]
- 21.Nonaka G-I, Kawahara O, Nishioka I. Tannins and related compounds. XV. A new class of dimeric flavan-3-ol gallates, theasinensins A and B, and proanthocyanidin gallates from green tea. (1). Chem. Pharm. Bull. 1983;31:3906–3914. [Google Scholar]
- 22.Opie SC, Robertson A, Clifford MN. Black tea thearubigins: their HPLC separation and preparation during in vitro oxidation. J. Sci. Food Agric. 1990;50:547–562. [Google Scholar]
- 23.Haslam E. Thoughts on thearubigins. Phytochemistry. 2002;64:61–73. doi: 10.1016/s0031-9422(03)00355-8. [DOI] [PubMed] [Google Scholar]
- 24.Naef R, Jaquier A, Velluz A, Maurer B. New constituent related to 3-methyl-2,4-nonanedieone identified in green tea. J. Agric. Food Chem. 2006;54:9201–9205. doi: 10.1021/jf061738o. [DOI] [PubMed] [Google Scholar]
- 25.Wang D, Yoshimura T, Kubota K, Kobayashi A. Analysis of glycosidically bound aroma precursors in tea leaves. 1. Qualitative and quantitative analyses of glycosides with aglycons as aroma compounds. J. Agric. Food Chem. 2000;48:5411–5418. doi: 10.1021/jf000443m. [DOI] [PubMed] [Google Scholar]
- 26.Kato M, Shibamoto T. Variation of major volatile constituents in various green teas from Southeast Asia. J. Agric. Food Chem. 2001;49:1394–1396. doi: 10.1021/jf001321x. [DOI] [PubMed] [Google Scholar]
- 27.Ito Y, Sugimoto A, Kakuda T, Kubota K. Identification of potent odorants in Chinese jasmine green tea scented with flowers of Jasminum sambac. J. Agric. Food Chem. 2002;50:4878–4884. doi: 10.1021/jf020282h. [DOI] [PubMed] [Google Scholar]
- 28.Kumazawa K, Masuda H. Identification of potent odorants in different green tea varieties using flavor dilution technique. J. Agric. Food Chem. 2002;50:5660–5663. doi: 10.1021/jf020498j. [DOI] [PubMed] [Google Scholar]
- 29.Pongsuwan W, Fukusaki E, Bamba T, Yonetan T, Yamahara T, Kobayashi A. Prediction of Japanese green tea ranking by gas chromatography/mass spectrometry-based hydrophilic metabolite fingerprinting. J. Agric. Food Chem. 2007;55:231–236. doi: 10.1021/jf062330u. [DOI] [PubMed] [Google Scholar]
- 30.Wang D, Lu J, Miao A, Xie Z, Yang D. HPLC-DAD-ESI-MS/MS analysis of polyphenols and purine alkaloids in leaves of twenty-two tea cultivars in China. J. Food Comp. Anal. In press. [Google Scholar]
- 31.Zhu XL, Chen B, Ma M, Luo XB, Zhang F, Yao SZ, Wan ZT, Yang DJ, Huang HW. Simultaneous analysis of theanine, chlorogenic acid, purine alkaloids and catechine in tea samples with the help of multi-dimension information of on-line high performance liquid chromatography/electrospray-mass spectrometry. J. Pharmaceut. Biomed. Anal. 2004;34:695–704. doi: 10.1016/s0731-7085(03)00605-8. [DOI] [PubMed] [Google Scholar]
- 32.Friedman M. Overview of antibacterial, antitoxin, antiviral and antifungal activities of tea flavonoids and teas. Mol. Nutr. Food Res. 2007;51:116–134. doi: 10.1002/mnfr.200600173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedman M, Mackey BE, Kim H-J, Lee I-S, Lee K-P, Lee S-U, Kozukue E, Kozukue N. Structure-activity relationships of tea compounds against humam cancer cells. J. Agric. Food Chem. 2007;55:243–253. doi: 10.1021/jf062276h. [DOI] [PubMed] [Google Scholar]
- 34.Lambert JD, Yang CS. Cancer chemopreventive activity and bioavailability of tea and tea polyphenolis. Mutat. Res. 2003;523-524:201–208. doi: 10.1016/s0027-5107(02)00336-6. [DOI] [PubMed] [Google Scholar]
- 35.Jie G, Lin Z, Zhang L, LV H, He P, Zhao B. Free radical scavenging effect of pu-erh tea extracts and their protective effect on oxidative damage in human fibroblast cells. J. Agric. Food Chem. 2006;54:8058–8064. doi: 10.1021/jf061663o. [DOI] [PubMed] [Google Scholar]
- 36.Seeram NP, Henning SM, Niu Y, Lee R, Scheuller HS, Heber D. Catechin and caffeine content of green tea dietary supplements and correlation with antioxidant capacity. J. Agric. Food Chem. 2006;54:1599–1603. doi: 10.1021/jf052857r. [DOI] [PubMed] [Google Scholar]
- 37.Yao L, Caffin N, D'Arcy B, Jiang Y, Shi J, Singanusong R, Liu X, Datta N, Kakuda Y, Xu Y. Seasonal variations of phenolic compounds in Australian-grown tea (Camellia sinensis). J. Agric. Food Chem. 2005;53:6477–6483. doi: 10.1021/jf050382y. [DOI] [PubMed] [Google Scholar]
- 38.Si W, Gong J, Tsao R, Kalab M, Yang R, Yin Y. Bioassay-guided purification and identification of antimicrobial components in Chinese green tea extract. J. Chromatogr., A. 2006;1125:204–210. doi: 10.1016/j.chroma.2006.05.061. [DOI] [PubMed] [Google Scholar]
- 39.Zeeb DJ, Nelson BC, Albert K, Dalluge JJ. Separation and identification of twelve catechins in tea using liquid chromatography/atmospheric pressure chemical ionization-mass spec-trometry. Anal. Chem. 2000;72:5020–5026. doi: 10.1021/ac000418f. [DOI] [PubMed] [Google Scholar]
- 40.Dalluge JJ, Nelson BC. Determination of tea catechins. Review. J. Chromatogr., A. 2000;881:411–424. doi: 10.1016/s0021-9673(00)00062-5. [DOI] [PubMed] [Google Scholar]
- 41.Neilson AP, Green RJ, Wood KV, Ferruzzi MG. High-throughout analysis of catechins and theaflavins by high performance liquid chromatography with diode array detection. J. Chromatogr., A. 2006;1132:132–140. doi: 10.1016/j.chroma.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 42.Yanagida A, Shoji A, Shibusawa Y, Shindo H, Tagashira M, Ikeda M, Ito Y. Analytical separation of tea catechins and food-related polyphenols by high-speed counter-current chromatography. J. Chromatogr., A. 2006;1112:195–201. doi: 10.1016/j.chroma.2005.09.086. [DOI] [PubMed] [Google Scholar]
- 43.Lin J-K, Lin C-L, Liang Y-C, Siau S-Y, Juan I-M. Survey of catechins, gallic acid and methylxanthins in green oolong, puerh, and black tea. J. Agric. Food. Chem. 1998;46:3635–3642. [Google Scholar]
- 44.Dalluge JJ, Nelson BC, Thomas JB, Sander LC. Selection of column and gradient elution system for the separation of catechins in green tea using high-performance liquid chromatography. J. Chromatogr., A. 1998;793:265–274. doi: 10.1016/s0021-9673(97)00906-0. [DOI] [PubMed] [Google Scholar]
- 45.Price KR, Rhodes MJC, Baren KA. Flavonol glycoside content and composition of tea infusions made from commercially available teas and tea products. J. Agric. Food Chem. 1998;46:2517–2522. [Google Scholar]
- 46.Lin L-Z, Harnly J. A screening method for the identification of glycosylated flavonoids and other phenolic compounds using a standard analytical approach for all plant materials. J. Agric. Food Chem. 2007;55:1084–1096. doi: 10.1021/jf062431s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cuyckens F, Claeys M. Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectrom. 2004;39:1–15. doi: 10.1002/jms.585. [DOI] [PubMed] [Google Scholar]
- 48.Mabry TJ, Markham KR, Thomas MB, editors. The Systematic Identification of Flavonoids. Springer-Verlag; New York: 1970. The ultraviolet spectra of isoflavones, flavanones and flavanols. pp. 41–45.pp. 165–166. Chapters V and VI. [Google Scholar]
- 49.Ferreres F, Branca M, Silva BM, Andrade PB, Seabra RS, Margarida A, Ferreira MA. Approach to the study of C-glycosyl flavones by ion trap HPLC-PAD-ESI/MS/MS: application to seeds of quince (Cydonia oblonga). Phytochem. Anal. 2003;14:352–359. doi: 10.1002/pca.727. [DOI] [PubMed] [Google Scholar]
- 50.IUPAC Nomenclature of cyclitols. Biochem. J. 1976;153:23–31. doi: 10.1042/bj1530023. [DOI] [PMC free article] [PubMed] [Google Scholar]