Abstract
EMBO J (2013) 32 16, 2204–2216. doi:; DOI: 10.1038/emboj.2013.133
Autophagy is a catabolic mechanism that selectively eliminates long-lived proteins, aggregates and damaged organelles and is thereby fundamental in maintaining cellular homeostasis. As a prosurvival mechanism, autophagy is carefully regulated and its dysfunction is associated with cancer development. Work of Huang et al (2013) in this issue of The EMBO Journal identifies the apoptosis inhibitor XIAP as a novel repressor of autophagy—a function that significantly contributes to its tumorigenecity.
The X-linked inhibitor of apoptosis protein (XIAP) belongs to the IAP family of proteins and is a powerful negative regulator of apoptosis, because it directly interacts with and inhibits caspases3, 7 and 9 and promotes their proteasome-dependent degradation (Eckelman et al, 2006). This function makes it a potential oncogene. Indeed, several studies show increased XIAP expression in a variety of human cancers and high levels of XIAP often correlate with poor clinical outcome. In addition, XIAP overexpression confers resistance to multi-agent chemotherapy (Schimmer et al, 2006). However, deletion of XIAP is not toxic to normal cells, as evidenced by the fact that XIAP knockout mice do not have obvious defects in the development or in the regulation of apoptosis (Harlin et al, 2001).
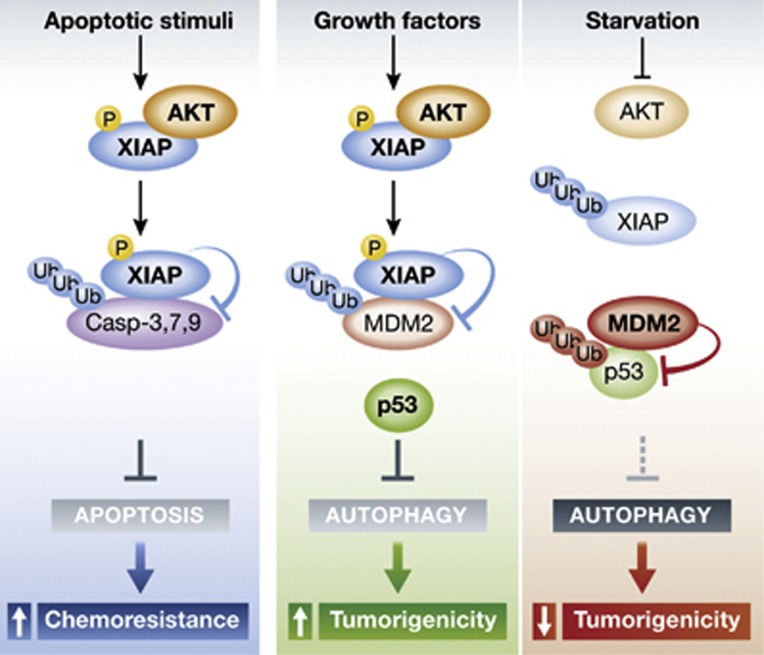
Huang et al (2013) now identify XIAP as a key molecular switch controlling starvation-induced autophagy via an Mdm2/p53-dependent mechanism (Figure 1). XIAP is known to be phosphorylated by Akt and thereby stabilized (Dan et al, 2004). The authors demonstrate that phosphorylated XIAP binds to and ubiquitylates Mdm2, thereby targeting it for proteasomal degradation. This ultimately results in stabilization of cytoplasmic p53, a well-known target of Mdm2. Interestingly, p53 has a dual and conflicting role in the regulation of autophagy. While nuclear p53 promotes the transcriptional activation of autophagy-related genes, cytoplasmic p53 acts as a master repressor of autophagy (Tasdemir et al, 2008a, 2008b). Thus, Huang et al (2013) find that deletion, depletion or inhibition of XIAP in human or murine cell lines result in an increase in basal autophagy. Upon serum starvation, the PI3K/Akt pathway is inhibited leading to a reduction of XIAP phosphorylation. Dephosphorylation of XIAP causes its autoubiquitylation and degradation, allowing Mdm2-dependent degradation of p53 and subsequent autophagy induction (Figure 1).
Figure 1.
Following a range of apoptotic stimuli, XIAP is phosphorylated by Akt. This modification leads to its stabilization and promotes XIAP-dependent proteosomal degradation of caspases 3, 7 and 9. The result is a block of apoptosis and tumour chemoresistance. Under unstressed conditions, XIAP is phosphorylated by Akt as well, but targets Mdm2 for degradation. This causes the stabilization of cytoplasmic p53 and can inhibit autophagy. Starvation, on the other hand, inhibits Akt and unphosphorylated XIAP undergoes autoubiquitylation and degradation via the proteasome. Subsequently, Mdm2 is stabilized and induces p53 degradation, releasing the block on autophagy.
The discovery of this new XIAP–Mdm2–p53 biochemical axis controlling autophagy expands the role of XIAP in tumour promotion. Autophagy is the main mechanism governing metabolic health and organelle recycling in eukaryotic cells, and its misregulation and malfunction has been linked to tumorigenesis (Mathew et al, 2007). To investigate if the effect of XIAP on autophagy contributes to its oncogenic potential, Huang et al (2013) exploit a xenograft mouse model. In this model, XIAP ablation reduces the tumorigenicity of human cancer cells. Reconstitution of XIAP-deficient cells with either wild-type or a phospho-mimicking mutant of XIAP, which strongly binds to Mdm2, stimulates subcutaneous tumour growth. Conversely, a non-phosphorylatable XIAP mutant that no longer interacts with Mdm2 is unable to induce tumorigenicity. Importantly, the authors also show that a mutant of XIAP that cannot degrade caspases is still able to increase tumorigenesis, indicating that inhibition of apoptosis contributes little, if any, to the tumour-promoting effects of XIAP. Finally, Huang et al (2013) strengthen the biological significance of their findings by demonstrating that the XIAP–Mdm2–p53 signalling cascade correlates with inhibition of autophagy in different type of human primary tumours.
Besides identifying a novel function of XIAP and dissecting the molecular mechanism underlying its action, this work sheds light on the cross-talk between autophagy and apoptosis, two key cellular responses in tumorigenesis. In addition, it places a new piece of the puzzle regarding the role of autophagy in cancer. In recent years, significant effort has been made to dissect the functions of key autophagy genes during cancer progression, in order to target the prosurvival effect(s) of autophagy to prevent cancer cell escape from chemoterapeutic assaults (White, 2012). However, as a powerful inhibitor of autophagy that enhances tumorigenicity, XIAP could now be added to the list of autophagy regulators (such as Beclin 1, UVRAG, Bif-1), whose dysregulation is at the basis of cancer promotion (White, 2012).The double-sided sword of autophagy is still a dangerous weapon that deserves special attention.
Footnotes
The authors declare that they have no conflict of interest.
References
- Dan HC, Sun M, Kaneko S, Feldman RI, Nicosia SV, Wang HG, Tsang BK, Cheng JQ (2004) Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP). J Biol Chem 279: 5405–5412 [DOI] [PubMed] [Google Scholar]
- Eckelman BP, Salvesen GS, Scott FL (2006) Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep 7: 988–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlin H, Reffey SB, Duckett CS, Lindsten T, Thompson CB (2001) Characterization of XIAP-deficient mice. Mol Cell Biol 21: 3604–3608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Wu Z, Mei Y, Wu M (2013) XIAP inhibits autophagy via XIAP-Mdm2-p53 signalling. EMBO J 32: 2204–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R, Karantza-Wadsworth V, White E (2007) Role of autophagy in cancer. Nat Rev Cancer 7: 961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmer AD, Dalili S, Batey RA, Riedl SJ (2006) Targeting XIAP for the treatment of malignancy. Cell Death Diff 13: 179–188 [DOI] [PubMed] [Google Scholar]
- Tasdemir E, Chiara Maiuri M, Morselli E, Criollo A, D’Amelio M, Djavaheri-Mergny M, Cecconi F, Tavernarakis N, Kroemer G (2008a) A dual role of p53 in the control of autophagy. Autophagy 4: 810–814 [DOI] [PubMed] [Google Scholar]
- Tasdemir E, Maiuri MC, Galluzzi L, Vitale I, Djavaheri-Mergny M, D'Amelio M, Criollo A, Morselli E, Zhu C, Harper F, Nannmark U, Samara C, Pinton P, Vicencio JM, Carnuccio R, Moll UM, Madeo F, Paterlini-Brechot P, Rizzuto R, Szabadkai G et al. (2008b) Regulation of autophagy by cytoplasmic p53. Nat Cell Biol 10: 676–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E (2012) Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer 12: 401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]