Abstract
Background
Hospitalized infants may undergo frequent painful procedures with inadequate pain relief. Alternative pain relief interventions are needed.
Objective
The aim of this research was to determine the safety of noninvasive electrical stimulation of acupuncture points (NESAP) in neonates who were receiving routine heel sticks.
Design
This was a descriptive study performed to assess the safety of using a transcutaneous electrical nerve stimulation (TENS) unit to deliver NESAP to neonates.
Setting/Subjects
The subjects were healthy newborn infants<3 days old before hospital discharge.
Intervention
The intervention was NESAP delivered via a TENS unit, administered before, during, and after heel stick. The electrodes of the TENS unit were applied at four acupuncture points. Settings were gradually increased: 6 infants received 1.0 mA, 2 Hz; the second 6 infants received 2.0 mA, 10 Hz; and the last 18 infants received 3.5 mA, 10 Hz.
Main Outcome Measures
Three main measures were used: (1) skin assessment (2) vital signs; (3) pain scores using the Premature Infant Pain Profile (PIPP).
Results
There were no significant changes in vital signs during and after NESAP. There were no changes in PIPP scores in the first 12 infants after initiation of NESAP. A slight but nonsignificant increase in PIPP scores (from 2.65 to 3.5 on a scale of 0–18) occurred in the last 18 infants. There were no adverse events during or after NESAP.
Conclusions
NESAP is safe for infants with low settings on a TENS unit.
Key Words: Neonate, Pain, Noninvasive Electrical Stimulation of Acupuncture Points (NESAP)
Introduction
Newborn infants are frequently subjected to acute pain, when they are in a hospital, which causes short-term physiological instability and long-term alterations in brain development, behavior, and stress responses, potentially leading to a greater vulnerability to chronic pain.1,2 Prevention and amelioration of neonatal pain are worthy clinical goals. Pharmacological analgesic therapy may be associated with a high incidence of potentially harmful, systemic side-effects and may also alter development and increase cell death in the immature brain.3,4 Nonpharmacological therapies, such as sucrose, massage, or kangaroo care, can reduce neonatal pain, but are relatively ineffective against severe acute pain.
Heel sticks for routine blood sampling are commonly applied to infants; however, topical anesthetics are ineffective against heel-stick pain. Safe and effective nonpharmacological analgesia for acute pain in infants—such as that caused by heel sticks—would cause a paradigm shift in neonatal pain management.
Acupuncture is a well-known and effective treatment for acute and chronic pain in adults and children but has been used sparingly for treating pain in newborn infants.5 Noninvasive electrical stimulation at acupuncture points (NESAP) may fulfill several of the hypothetical criteria for ideal analgesia in infants. Previous work in the current authors' laboratory demonstrated the safety of using NESAP with transcutaneous electrical nerve stimulation (TENS) devices on the flanks of neonatal pigs and the safety of using a TENS device with additional monitoring devices. The purpose of this study was to demonstrate that NESAP using a TENS unit was safe in healthy-term infants. The long-term goal is to perform research on the effectiveness of NESAP in preterm neonates and other neonates who undergo painful procedures.
Materials and Methods
This study evaluated the safety of the Empi Select TENS unit and four Empi StimCare electrodes (1.25 inches) in term healthy infants to administer NESAP during a routine heel stick. Infants in this study served as their own controls as they were evaluated for a pain response when the TENS unit was turned on and again when the painful procedure of a heel stick was performed.
Outcome Measures
The main outcome measurements were: (1) skin assessment to evaluate any injury to the skin of the infant at electrode sites; (2) evaluation of any changes in heart rate (HR) and rhythm, oxygen saturation (SaO2) levels, and blood pressure (BP); and (3) assessment of pain using the Premature Infant Pain Profile (PIPP) scale. A fourth outcome measure was (4) analysis of whether electrical activity from the TENS unit interferes with the heart monitor and SaO2 recording.
Study Design
This study was an open-label trial to assess the safety of using electrical stimulation at acupuncture sites in 30 infants who were receiving routine heel sticks. Initial approval was obtained from the institutional review board of the university medical center where the study took place. Two substudies preceded the last safety study, with 6 infants in each substudy and 18 infants in the last study. The first 6 infants received TENS unit stimulation at 1.0 mA, 2Hz. The second 6 infants received TENS unit stimulation at 2.0 mA, 10 Hz. The last 18 infants received TENS unit stimulation at 3.5 mA, 10 Hz.
Inclusion/Exclusion Criteria
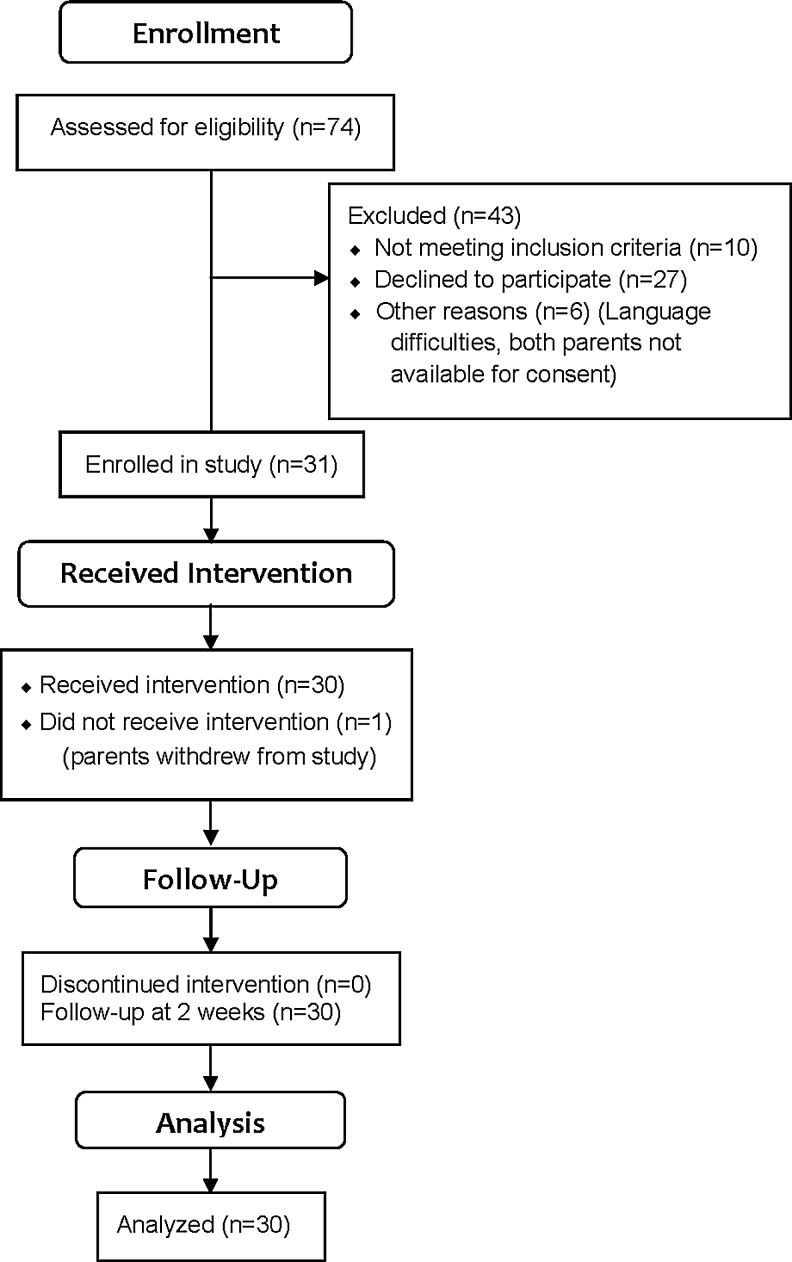
Infants in a newborn nursery who were estimated to be 37–42 weeks' gestational age, <3 days old, with normal neurologic assessment were eligible for the study. Infants born to mothers with diabetes, preeclampsia, or systemic inflammatory conditions were excluded. Infants were excluded from the study if there was suspected or confirmed neuromuscular disease, congenital anomalies, sepsis, or congenital heart defects; or if any infant had an Apgar score of ≤5 at 5 minutes, or a cord blood pH of <7.0. Infants were also excluded if they were receiving mechanical ventilation or any analgesic treatment. In addition, they were excluded if there was documented maternal opiate use prior to delivery or a positive drug screen based on a review of the mother's medical record. Infants were not considered if they had birth trauma to the lower extremities, dermatologic conditions in the area of electrode placement, known allergy to the adhesive, or multiple heel sticks in the previous 24 hours. A total of 31 infants were enrolled in the study. Parents withdrew 1 infant after giving consent, but this withdrawal occurred before the heel-stick procedure was completed. Figure 1 presents a participant flow diagram.
FIG. 1.
Participant flow diagram.
Consent
The parents of eligible infants received a detailed description of this study procedures together with a brochure describing the electrical stimulation at acupuncture sites proposed for the study. The consent process was initiated prior to the scheduled blood sampling, so that parents would have enough time to determine the risks and benefits of the proposed study and have the opportunity to discuss the study with study personnel. Parents gave written consent for their infants to be enrolled in the study.
Procedures
Data collection was initiated, starting at 20 minutes prior to the heel-stick procedure. All study procedures were performed in a treatment room to minimize extraneous stimulation from the environment. The clinical approach for heel stick was standardized for all infants. Each infant was placed under a warmer and unbundled to expose one foot. Continuous HR, respiration rate (RR0, and SaO2 were monitored prior to the initiation of NESAP, throughout the duration of NESAP, and after return to the infant's room. The infant's BP was assessed before, during, after NESAP and after return to his or her room. Noninvasive acupuncture was applied, using four self-adhesive electrodes to the baby's lower extremities at the following acupuncture points: Zusanli (ST 36); Sanyinjiao (SP 6); Taixi (KI 3); and Kunlun (BL 60).6–9 The placement of the electrodes was determined by an anesthesiologist who had acupuncture experience. The TENS unit was turned on and the infant received NESAP for 10 minutes prior to the heel stick. NESAP continued for the duration of the heel stick and for 5 minutes afterward. A heel-warming device was applied for approximately 2 minutes prior to the heel stick. The heel stick was performed using an automated lancet and the total amount of blood collected was recorded.
Infant responses to the use of the NESAP and the painful procedure were assessed using the PIPP, significant changes in crying or in vital signs, and clinical observations. The PIPP score includes assessment of contextual, physiological, and behavioral parameters, and has been extensively validated for pain assessment in preterm and term infants.10 The physiological component of the PIPP is scored by calculating increases in heart rate from baseline and decreases in oxygen saturation from baseline. Characteristic facial expressions were analyzed and timed to give a score for the behavioral portion of the PIPP. A digital video camera was used to record facial expressions of infants beginning ∼1 minute before the TENS unit was turned on and continuing throughout the process of lancing and squeezing the heel. PIPP scores were provided every 30 seconds throughout the heel-stick procedure and for 2 minutes following the procedure during the recovery phase. The duration of the procedure was also recorded to be used as a covariate when comparing the PIPP scores between groups. Reliability of scoring was measured by simultaneous assessment of all videotapes by 2 research assistants who scored the facial expressions (behavioral) portion of the PIPP.
PIPP scores were provided every 30 seconds throughout the heel-stick procedure according to the following phases: (A) baseline – 60 seconds; (B) heel preparation – 30 seconds; (C) heel lance and beginning squeeze – 30 seconds; (D) heel squeeze – 30–120 seconds, and (E) recovery – 120 seconds. A mean PIPP score was calculated for each phase that lasted 60–120 seconds and therefore contained more than one PIPP score.
Additional monitoring for adverse events from the time of enrollment before NESAP, during NESAP, and after NESAP until discharge included assessments for: seizure activity; emesis; color change; skin integrity and redness at the electrode sites; muscle-tone changes; significant changes in crying; and clinical observations. Frequency of urination was also monitored using wet-diaper counts from time of enrollment until discharge. A written note was provided to the parents to inform their pediatricians that the infants had participated in the study. A neonatologist who was present during the NESAP treatment and who monitored the infants during this procedure provided the family with a 2-week follow-up phone call to discuss any potential issues related to the study.
Analysis
Infants were monitored for signs of behavioral distress or changes in vital signs (HR, BP, and RR changes of greater than 20% from baseline; or an SaO2 of <90%) after the TENS unit was turned on but preceding the heel stick. Infants were also monitored for possible additional adverse events including apnea >20 seconds, seizure activity or twitching, emesis, color change, skin redness, or break in skin integrity, change in muscle tone, or significant change in crying, with an increase from the baseline PIPP score of 4 or more indicating pain10,11 before the heel stick. Infants were monitored for possible adverse events from time of enrollment before NESAP and during NESAP. Infants who had any adverse events before NESAP would have been withdrawn from the study. For infants having adverse events during NESAP, electrical stimulation would have been terminated, and the infant would have been withdrawn from the study to receive immediate care. Infants were under the care of a neonatologist and other experienced health care providers throughout the duration of electrotherapy and after completion of the procedure.
Results
Table 1 presents demographic data for the 30 infants who had data collected. There were no significant changes in infant vital signs, including HR, RR, BP, or SaO2, after the initiation of TENS stimulation, compared with baseline. Table 2 summarizes the vital signs data for the 30 infants in the study.
Table 1.
Demographic Data (n=30)
| Gender | Race | Ethnicity | Birth weight (g) (±SD) | Gestational age (weeks) (±SD) |
|---|---|---|---|---|
| Male=12 | African American=12 | Non-Hispanic=30 | ||
| Female=18 | Asian=1 | 3399.7 | 39.44 | |
| White=17 | (440.98) | (1.042) |
SD, standard deviation.
Table 2.
Vital Sign Changes During TENS Interventiona
| Means | Before NESAP (±SD) | After 5 minutes of NESAP (±SD) | 5 minutes after end of heel stick, at end of NESAP (±SD) | Upon return to mother/infant room (±SD) |
|---|---|---|---|---|
| HR | 136.55 (11.86) | 130.57 (14.98) | 132.7 (14.79) | 132.8 (14.8) |
| RR | 42.7 (7.95) | 40 (5.8) | 41.5 (7.56) | 42 (9.88) |
| Systolic BP | 84.97 (13) | 84 (12) | 83.76 (13.4) | 83 (9.67) |
| Diastolic BP | 46.9 (12.7) | 44 (11.76) | 46.5 (11.3) | 44.8 (8) |
| Mean BP | 59.6 (11.67) | 57.56 (11.24) | 58.9 (10.4) | 57.5 (7.54) |
| SaO2% | 97.9 (1.55) | 97 (2.18) | 96.4 (2.3) | Not taken in mother/infant room |
Phase 1 summary of vital signs across NESAP and heel stick (n=30), with 6 infants receiving mA 1, Hz 2, 6 infants receiving mA 2, Hz 10, and 18 infants receiving mA 3.5, Hz 10.
TENS, transcutaneous electrical nerve stimulation; NESAP, noninvasive electrical stimulation of acupuncture points; SD, standard deviation; HR, heart rate; RR, respiratory rate; BP, blood pressure, SaO2, oxygen saturation.
In the first 12 infants (1.0 mA, 2 Hz, n=6; 2.0 mA, 2 Hz, n=6), there were no changes in mean PIPP scores before and after initiating the TENS treatment (see Table 3). The mean PIPP score prior to treatment with the TENS unit was 1.75 (standard deviation [SD]: 1.2), and the mean after initiation of the TENS intervention was 1.75 (SD: 1.2).
Table 3.
PIPP Scoring in Phase 1a
| Before NESAP (±SD) | After NESAP started (±SD) | Code Ab(±SD) | Code Bb(±SD) | Code Cb(±SD) | Code Db(±SD) | Code Db(±SD) | Code Db(±SD) | Code Eb(±SD) | Code Eb(±SD) | Code Eb(±SD) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1.75* | 1.75* | 1.75* | 3.25 | 9.2 | 8.45 | 6.2 | 7.0 | 3.75 | 3.08 | 3.17 |
| (1.2) | (1.2) | (1.2) | (1.8) | (4.17) | (4.48) | (4.14) | (4.4) | (3.33) | (3.31) | (3.48) |
Mean composite PIPP scores with SD for phase 1 of NESAP and heel-stick procedure (n=12), with 6 infants receiving mA 1, Hz 2, and 6 infants receiving mA 2, Hz 10.
Codes: A, after 10 minutes of continuous NESAP but before heel stick; B, heel cleaning; C, heel stick; D, heel squeeze; E, recovery.
Scores reflect behavioral state as described above. There were no indications of pain or discomfort.
PIPP, Premature Infant Pain Profile; NESAP, noninvasive electrical stimulation of acupuncture points; SD, standard deviation.
In the last 18 infants, some infants moved their legs for a few seconds, indicating that they probably felt the stimulation. The mean baseline PIPP score in these 18 infants was 2.56 (SD: 1.89) before the TENS unit was turned on and 3.5 (SD: 2.36) after initiating the TENS stimulation (see Table 4). Increases in PIPP scores lasted from 30 to 90 seconds, and then the scores returned to baseline or lower. The maximum increase in PIPP scores was 3 above baseline. Increases in PIPP scores occurred in the first 30–90 seconds of TENS therapy, and then PIPP scores returned to baseline or lower. Table 5 provides a summary of the maximum increase and duration of PIPP score per patient.
Table 4.
PIPP Scoring in Phase 2a
| Before NESAP (±SD) | After NESAP started (±SD) | Code Ab(±SD) | Code Bb(±SD) | Code Cb(±SD) | Code Db(±SD) | Code Db(±SD) | Code Db(±SD) | Code Eb(±SD) | Code Eb(±SD) | Code Eb(±SD) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2.56c | 3.5c | 1.6c | 3.1c | 8.6 | 8.89 | 8.7 | 8.5 | 5.67c | 5.44c | 4.3c |
| (1.98) | (2.35) | (1.37) | (2.3) | (4.42) | (4.23) | (4.4) | (4.3) | (3.9) | (3.95) | (3.6) |
Mean composite PIPP scores with SD for phase 2 of NESAP and heel-stick procedure (n=18) with infants receiving mA 3.5, Hz 10.
Codes: A, after 10 minutes of continuous NESAP but before heel stick; B, heel cleaning; C, heel stick; D, heel squeeze; E, recovery.
Scores reflect behavioral state as described above. There were no indications of pain or discomfort.
PIPP, Premature Infant Pain Profile; NESAP, noninvasive electrical stimulation of acupuncture points; SD, standard deviation.
Table 5.
PIPP Scores Before and After TENS Unit Activated at 3.5 mA (n=18)
| Baseline PIPP | PIPP after TENS unit turned on at 3.5 mA | Maximum increase in PIPP score | Duration of PIPP increase in seconds |
|---|---|---|---|
| 2 | 5 for 60 sec | 3 | 60 |
| 3 | 3 | 0 | 0 |
| 2 | 2 | 0 | 0 |
| 0 | 2 for 30 sec, 1 for 30 sec | 2 | 60 |
| 3 | 3 | 0 | 0 |
| 2 | 2 | 0 | 0 |
| 2 | 2 | 0 | 0 |
| 2 | 4 for 30 sec, 3 for 30 sec | 2 | 60 |
| 3 | 4 for 30 sec, 6 for 30 sec | 3 | 60 |
| 0 | 0 | 0 | 0 |
| 3 | 6 for first 60 sec | 3 | 60 |
| 3 | 3 | 0 | 0 |
| 9 | 10a | 1 | 30 |
| 3 | 5 for 30 sec, 6 for 60 sec | 3 | 90 |
| 2 | 2 | 0 | 0 |
| 3 | 3 | 0 | 0 |
| 3 | 3 | 0 | 0 |
| 1 | 1 | 0 | 0 |
After 30 seconds on TENS, this infant's PIPP score started to decrease. After 60 seconds, PIPP was 2. After 90 seconds, PIPP was 1.
PIPP, Premature Infant Pain Profile; TENS, transcutaneous electrical nerve stimulation; sec, seconds.
There were no adverse events, such as color changes, skin abnormalities, feeding difficulties, or alterations in urine output, either at the time of testing or at the 1 week follow-up. Two infants spat up immediately following the heel stick. These incidents were not related to the TENS unit; they were normal events noted routinely in newborns. All infants had normal neurological assessment results before NESAP, and there were no neurological changes noted after NESAP or by pediatricians after discharge.
Discussion
A combination of acupuncture and electric current, electroacupuncture (EA), has been widely used for treatment of various diseases and for analgesia.12–15 Evidence suggests that acupuncture activates C-afferent fibers and A-δ fibers, altering transmission of pain signals at the spinal cord, midbrain, and hypothalamus. Stimulation of A-δ and C-afferent fibers in the muscle depresses nociception in the spinal cord. Activation of the hypothalamus triggers release of endorphins and allows for systemic pain relief.9,16
Mechanistically, Cheng suggested that the specific effects of electrical stimulation may be a function of the frequency of electrical stimulation.17 Reports about mice suggest that different receptors appear to be stimulated by different currents, with low frequencies between 2 and 15 Hz activating endorphin pathways, while higher frequencies may lead to release of norepinephrine and/or serotonin without analgesia.18 EA stimulation–induced analgesic effects have been related to frequency-dependent release of specific neuropeptides in the central nervous system. Low frequency (2 Hz) facililtates endorphin and high frequency (100 Hz) facilitates dynorphin, while a 2/100-Hz alternating stimulus promotes maximal release of opioid peptides and produces stronger analgesia.19
Compared to manual acupuncture, EA is more clinically efficacious and provides a more standardized stimulus for scientific study.18 TENS can be applied using self-adhesive electrodes placed on the skin above acupoints without needles to deliver a stimulating current.20 Electrodes fixed to the skin above the selected acupoint targets, using transluscent medical adhesive tape, with a central hole aimed precisely at the selected acupoints, have been used to determine acupoint specificity.21 Electrical stimulation via electrodes and a standard TENS unit may provide an appropriate combination of a noninvasive treatment, as well as providing an adequate analgesic effect.5
Use of gentle electrical stimulation at selected acupuncture points during heel-stick procedures may have a major impact on management of neonatal pain. Innovative features underlying the design of this project include: an analgesic approach for newborn infants; using electrical stimulation at four acupuncture sites; and development of a noninvasive method for applying electrical stimulation.
Conclusions
There were no ill effects from NESAP. These results are consistent with pediatric studies in older children documenting the safety of this device. Further research is needed to determine the effectiveness of the TENS unit to relieve pain in neonates during routine heel sticks, as well as the ideal setting required. In addition, future research investigating the effectiveness of NESAP in infants may lead to a novel therapeutic approach for infants requiring surgical interventions, infants developing opioid tolerance and/or withdrawal, and infants with any highly painful conditions.
Disclosure Statement
No competing financial interests exist.
References
- 1.Anand KJ. Effects of perinatal pain and stress. Prog Brain Res. 2000;122:117–129. doi: 10.1016/s0079-6123(08)62134-2. [DOI] [PubMed] [Google Scholar]
- 2.Porter FL. Wolf CM. Miller JP. Procedural pain in newborn infants: The influence of intensity and development. Pediatrics. 1999;104(1):e13. doi: 10.1542/peds.104.1.e13. [DOI] [PubMed] [Google Scholar]
- 3.Anand KJ. Coskun V. Thrivikraman KV. Nemeroff CB. Plotsky PM. Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiol Behav. 1999;66(4):627–637. doi: 10.1016/s0031-9384(98)00338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall RW. Kronsberg SS. Barton BA. Kaiser JR. Anand KJ. Morphine, hypotension, and adverse outcomes among preterm neonates: Who's to blame? Secondary results from the NEOPAIN trial. Pediatrics. 2005;115(5):1351–1359. doi: 10.1542/peds.2004-1398. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz L. Bauchner H. Blocleer R, et al. Salivary cortisol as an indicator of stress in premature infants: The effect of electric stimulation of acupuncture meridians in blunting this response. J Med Acupunct. 1998/1999;10(2):27–30. [Google Scholar]
- 6.Wang SM. Kain ZN. White PF. Acupuncture analgesia: II. Clinical considerations. Anesth Analg. 2008;106(2):611–621. doi: 10.1213/ane.0b013e318160644d. [DOI] [PubMed] [Google Scholar]
- 7.Chen L. Tang J. White PF, et al. The effect of location of transcutaneous electrical nerve stimulation on postoperative opioid analgesic requirement: Acupoint versus nonacupoint stimulation. Anesth Analg. 1998;87(5):1129–1134. [PubMed] [Google Scholar]
- 8.Golianu B. Krane E. Seybold J. Almgren C. Anand KJ. Non-pharmacological techniques for pain management in neonates. Semin Perinatol. 2007;31(5):318–322. doi: 10.1053/j.semperi.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Wang SM. Kain ZN. White P. Acupuncture analgesia: I. The scientific basis. Anesth Analg. 2008;106(2):602–610. doi: 10.1213/01.ane.0000277493.42335.7b. [DOI] [PubMed] [Google Scholar]
- 10.Stevens B. Johnston CC. Petryshen P. Taddio A. Premature Infant Pain Profile: Development and initial validation. Clin J Pain. 1996;12(1):13–22. doi: 10.1097/00002508-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Dempsey E. McCreery K. Local anaesthetic eye drops for prevention of pain in preterm infants undergoing screening for retinopathy of prematurity. Cochrane Database Syst Rev. 2011;9:CD007645. doi: 10.1002/14651858.CD007645.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Lin JG. Lo MW. Wen YR. Hsieh CL. Tsai SK. Sun WZ. The effect of high and low frequency electroacupuncture in pain after lower abdominal surgery. Pain. 2002;99(3):509–514. doi: 10.1016/S0304-3959(02)00261-0. [DOI] [PubMed] [Google Scholar]
- 13.Liu S. Zhou W. Liu H. Yang G. Zhao W. Electroacupuncture attenuates morphine withdrawal signs and c-Fos expression in the central nucleus of the amygdala in freely moving rats. Brain Res. 2005;1044(2):155–163. doi: 10.1016/j.brainres.2005.02.075. [DOI] [PubMed] [Google Scholar]
- 14.Wu MT. Hsieh JC. Xiong J, et al. Central nervous pathway for acupuncture stimulation: Localization of processing with functional MR imaging of the brain—preliminary experience. Radiology. 1999;212(1):133–141. doi: 10.1148/radiology.212.1.r99jl04133. [DOI] [PubMed] [Google Scholar]
- 15.Li N. He HB. Wang CW. Yang CM. Observation on therapeutic effect of electroacupuncture at Chengshan (BL 57) and Changqiang (GV 1) on hemorrhoidal pain [in Chinese] Zhongguo Zhen Jiu. 2008;28(11):792–794. [PubMed] [Google Scholar]
- 16.Lang PM. Stoer J. Schober GM. Audette JF. Irnich D. Bilateral acupuncture analgesia observed by quantitative sensory testing in healthy volunteers. Anesth Analg. 2010;110(5):1448–1456. doi: 10.1213/ANE.0b013e3181d3e7ef. [DOI] [PubMed] [Google Scholar]
- 17.Cheng RSS. Neurophysiology of electroacupuncture analgesic. In: Pomeranz B, editor; Sfux G, editor. Scientific Bases of Acupuncture. Berlin: Springer-Verlag; 1989. pp. 119–136. [Google Scholar]
- 18.Wan Y. Wilson SG. Han J. Mogil JS. The effect of genotype on sensitivity to electroacupuncture analgesia. Pain. 2001;91(1–2):5–13. doi: 10.1016/s0304-3959(00)00416-4. [DOI] [PubMed] [Google Scholar]
- 19.Pomeranz B. Paley D. Electroacupuncture hypalgesia is mediated by afferent nerve impulses: An electrophysiological study in mice. Exp Neurol. 1979;66(2):398–402. doi: 10.1016/0014-4886(79)90089-x. [DOI] [PubMed] [Google Scholar]
- 20.Han JS. Acupuncture: Neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003;26(1):17–22. doi: 10.1016/s0166-2236(02)00006-1. [DOI] [PubMed] [Google Scholar]
- 21.Niu C. Hao H. Lu J. Li L. Han Z. Tu Y. A novel uni-acupoint electroacupuncture stimulation method for pain relief. Evid-Based Complement Alternat Med. 2011;2011:209879. doi: 10.1093/ecam/nep104. [DOI] [PMC free article] [PubMed] [Google Scholar]