Abstract
The deleterious effects of microgravity on lymphocytes have been demonstrated in previous studies. However, research on the effects of microgravity on human natural killer (NK) cells remains exceedingly limited. In this study, we demonstrated that NK cell cytotoxicity was significantly decreased under simulated microgravity (SMG) conditions (p<0.05). Several processes, including apoptosis, receptor expression, and cytokine secretion, were investigated in human NK cells under SMG. We observed decreased cytotoxicity, concurrent with increased apoptosis and necrosis, in NK cells after exposure to SMG (p<0.05). Additionally, interferon (IFN)-γ and perforin expression decreased significantly, and the expression of granzyme-B was only slightly reduced. Meanwhile, SMG selectively inhibited the expression of certain surface receptors on NK cells. Specifically, the expression of NKG2A and NKG2D were significantly downregulated under SMG, but the expression of NKp30 and NKp44 was not affected. We also found that interleukin (IL)–15 alone or in combination with IL-12 could counteract the inhibition of NK cell cytotoxicity under SMG. Our findings indicate that human NK cells were sensitive to SMG, as reflected by their decreased cytotoxicity. Factors such as increased early apoptosis and late apoptosis/necrosis and the decreased expression of INF-γ, cytolytic proteins, and cell surface receptors may be responsible for the loss of cytotoxicity in human NK cells under SMG. A combination of IL-12 and IL-15 may be useful as a therapeutic strategy for overcoming the effects of microgravity on human NK cells during long space missions. Key Words: Simulated microgravity (SMG)—Natural killer (NK) cells—Cytotoxicity. Astrobiology 13, 703–714.
1. Introduction
Since the 1960s, hundreds of astronauts have spent time in space and have consistently shown an increased risk of infection and an overall decrease in immune function (Hawkins and Zieglschmid, 1975; Taylor et al., 1986; Taylor, 1987; Nicogossian and Pool, 1989). The mechanisms underlying these abnormalities are unclear, but many immunological studies performed on astronauts postflight have described decreases in the number and function of circulating leukocytes, as well as a depressed proliferative response of in vitro mitogen-stimulated T cells (Taylor and Dardano, 1984; Meehan et al., 1992; Sonnenfeld et al., 1992; Stowe et al., 2003).
When humans travel into outer space, factors such as microgravity, cosmic radiation, narrow living space, and disturbed circadian rhythm affect the normal physiological functions. Among these factors, microgravity has been recognized as the major cause of immune dysfunction (Wang et al., 2011).
Human T lymphocytes exposed to microgravity show almost no activation in comparison to control cells in a normal Earth environment (Bechler et al., 1986; Cogoli et al., 1993; Pippia et al., 1996). The suppression of phytohemagglutinin (PHA)–stimulated and antigen-specific lymphocyte activation under simulated microgravity (SMG) has also been observed (Cooper and Pellis, 1998; Cooper et al., 2001).
Because of the high cost and scarce opportunities for spaceflight, SMG is widely used for space life studies (Herranz et al., 2013). Rotating wall vessel (RWV) bioreactors and clinostats can suspend cell cultures by rotating them around a horizontal axis at a constant velocity to achieve a time-averaged gravity vector of 10−2g at a rotational speed of 30 rpm; these conditions are used extensively to study the effects of microgravity on immune cells in vitro (Shi et al., 2012). Intriguingly, previous studies have shown that the proliferation of lymphocytes and the secretion of certain types of cytokines were significantly inhibited after exposure to SMG (clinostat or RWV) (Maier, 2006; Martinelli et al., 2009).
Microgravity simulated by RWVs or clinostats may cause some side effects at the cellular level. For example, SMG “disturbed the ultrastructural level in the root of a small plant” (Hensel and Sievers, 1980) and “resulted in a transient process of cell rounding,” “increased cortical actin cytoskeleton,” “decreased actin stress fibers and caused the disappearance of focal adhesions.” (Moes et al., 2010). SMG also increased apoptosis rates (Jessup et al., 2000). These effects may be related to certain characteristics of clinostation itself, such as shear stress and vibration (Herranz et al., 2013). However, RWVs and clinostats are still recognized as valuable tools for the simulation of microgravity in suspension cell cultures because similar results are obtained under SMG and true microgravity conditions (reviewed by Herranz et al., 2013).
Natural killer (NK) cells are large, granular, cytotoxic lymphocytes that can lyse virus-infected or oncogenically transformed cells and promote cytokine secretion without requiring prior immunization (Smyth et al., 2005). They are an important link between the innate and adaptive immune systems and play a pivotal role in anti-inflammatory mechanisms, tumor surveillance, and the regulation of autoimmune disorders (Vivier et al., 2004). The effect of microgravity on the activity and function of NK cells during spaceflight is a particularly interesting problem. Pioneering research demonstrated decreases in the activity and induced interferon (IFN) production of NK cells in the lymphocytes of astronauts after a 7-day spaceflight (Talas et al., 1983a). Similar decreases were also reported in both the cytotoxicity and percentage of NK cells after a 21-day spaceflight (Konstantinova et al., 1995). A study in rats showed that, after long-term spaceflights, the percentage of NK cells is significantly reduced, and NK cell activity is suppressed by 20–85% compared to preflight data (Rykova et al., 1992). Nevertheless, there are also some conflicting results in the literature. Buravkova reported that the cytotoxicity and IFN production of NK cells did not change between “control” and “flight” groups, and changes were not observed in samples that underwent clinorotation in a ground laboratory (Buravkova et al., 2004). These studies suggest that the role of microgravity in modulating NK cell activity has not yet been fully elucidated, and the current literature on the activity and function of NK cells under microgravity conditions, studied either in space or via ground-based SMG, remains quite limited.
The main objective of this study was to systematically investigate the effects of SMG on primary human NK cells. The cytotoxicity, apoptosis, receptor expression, and cytokine secretion levels of NK cells after exposure to SMG were evaluated.
Interleukins are recognized as important stimulants of the bioactivity of NK cells (Fehniger et al., 2000; Marcenaro et al., 2005; Szczepanski et al., 2010). Interleukin (IL)–2, IL-12, and IL-15 were added to the SMG culture system, and we expected to find that certain interleukins could counteract the effects of microgravity on human NK cells.
2. Materials and Methods
2.1. Preparation of primary human NK cells
Primary human NK cells were prepared with the peripheral blood mononuclear cell (PBMC)–based ex vivo expansion method (Huang et al., 2008). Briefly, peripheral venous blood (∼10 mL) was collected in heparinized tubes from healthy donors (n=12) according to the informed consent guidelines of the Ethics Committee of the Hospital of Northwestern Polytechnical University. Blood samples were diluted 2-fold with sterile phosphate-buffered saline (PBS) and overlaid onto Histopaque-1077 (Sigma, St. Louis, MO, USA) and centrifuged at 400g for 30 min at room temperature. The PBMCs were collected and washed twice with PBS, followed by resuspension in RPMI-1640 media (Gibco, Rockville, MD, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, Rockville, MD, USA), 100 U/mL IL-2 (PeproTech, Rocky Hill, NJ, USA), and 100 μg/mL penicillin and streptomycin (Genview, Carlsbad, CA, USA). The PBMCs were co-cultured with equal numbers of stimulating cells (irradiated genetically modified K562 cells, prepared as described by Imai et al., 2005). Half the culture solution was changed every 3 days after the second week, and 1×105 ex vivo expanded NK cells were collected after 3 weeks of culture, stained with CD3/CD16+CD56 [LeuTM-4/11c+19 that contained FITC-labeled CD3 (Leu-4) and PE-labeled CD16 and PE-labeled CD56] monoclonal antibodies (mAbs) along with isotype-matched controls (IgG1-FITC/IgG2-PE) (BD Biosciences, San Jose, CA, USA). The percentage of NK cells (CD56+CD16+CD3−) among the PBMCs was analyzed by flow cytometry (BD FACSCalibur, San Jose, CA, USA). K562 cells (human myelogenous leukemia cells) were purchased from the China Center for Type Culture Collection, Wuhan, China (CCTCC Number GDC037), and stimulated cells were maintained in RPMI-1640 cell media supplemented with 10% FBS containing 100 μg/mL of penicillin and streptomycin and cultured under routine conditions at 37°C in 5% CO2 atmosphere.
2.2. NK cell exposure to SMG
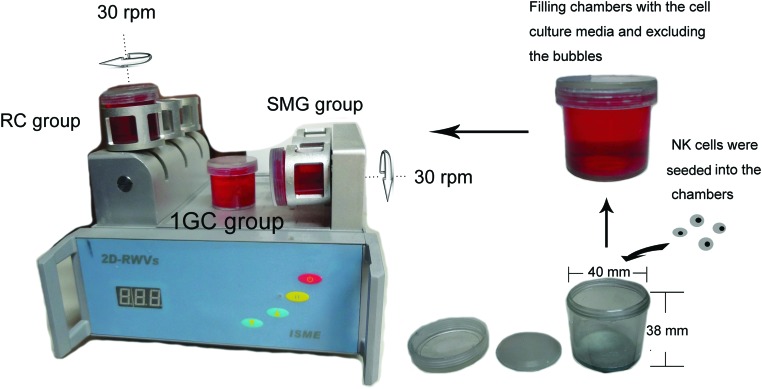
A 2-D RWV (developed by the China Astronaut Research and Training Center) was utilized for the microgravity simulation. The 2-D RWV and SMG protocol is shown in Fig. 1. The chambers were completely filled with culture media and rotated around the horizontal or vertical axis at 30 rpm to achieve a time-averaged gravity vector of 10−2g. The cells grown in vertically rotated chambers served as a control to exclude the effects of clinorotation itself, including the shear stress and vibration (Shi et al., 2012).
FIG. 1.
The 2-D RWV SMG system. The clinostat is equipped with computerized temperature and motor controls. Cells were initially seeded into the chambers. The chambers were filled with complete medium, and care was taken to exclude air bubbles. These chambers were rotated at 30 rpm around the horizontal or vertical axis. The 1GC controls were static control cultures kept in the same room as the clinostat. Color images available online at www.liebertonline.com/ast
Primary human NK cells were randomly divided into three groups: NK cells from the SMG group were rotated around a horizontal axis in the 2-D RWV (a time-averaged gravity vector of 10−2g with a revolution speed of 30 rpm, SMG group), NK cells in the “rotation control group” or “RC group” were rotated around a vertical axis at the same velocity, and NK cells in the “1GC group” were cultured in a normal 1g state. All three groups of primary NK cells were cultured in IL-2-free RPMI-1640 media supplemented with 10% FBS and 100 μg/mL penicillin and streptomycin. The 2-D RWV culture system was maintained at 37°C in a 5% CO2 atmosphere.
2.3. NK cell cytotoxicity
Cytotoxicity was determined by evaluating the rate at which NK cells killed K562 cells. Primary NK cells were seeded in three groups and cultured as required for the SMG, RC, and 1GC groups separately. NK cells (8×105) were taken from each group at 12, 24, 48, and 72 h, respectively. All collected samples were washed three times with PBS, resuspended in 400 μL of IL-2-free RPMI-1640, and transferred to 96-well plates (Costar, New York, USA) (100 μL per well, four wells for each group). K562 cells (4×104 in 100 μL of RPMI-1640) were added to two wells for each group with a 5:1 ratio of effector (NK cells) versus target (K562 cells) (E:T). Subsequently, 100 μL RPMI-1640 was added to the two remaining wells of each group as the NK cell control condition (effect cell control). Concurrently, 100 μL K562 cell suspension (4×104 cells) was mixed with 100 μL RPMI-1640 as the K562 control condition (target cell control). The plate was incubated for 4 h at 37°C in a 5% CO2 atmosphere. To determine the number of viable cells for cell proliferation and cytotoxicity assays, 20 μL Cell Counting Kit-8 (CCK-8, Dojindo, Japan) was added to each well and incubated for 2 h at 37°C in a 5% CO2 atmosphere. CCK-8 contains WST-8, which is similar to MTT and can be used as a sensitive colorimetric assay for live cells. Optical density (OD) values were recorded at 450 nm in a Microplate Research Reader (Bio-Tek Synergy HT). The killing rate of the NK cells was calculated with the following formula (Chen et al., 2012):
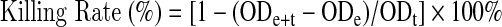 |
(ODe, OD450 of NK cell wells; ODt, OD450 of K562 cell wells; ODe+t, OD450 of NK cell wells plus K562 cell wells).
2.4. Apoptosis and receptor expression analysis by flow cytometry
Natural killer cells (5×105) were taken from each group, centrifuged, and washed three times with PBS; then 1×105 cells were labeled with Annexin V-FITC and PI (Annexin V-FITC Apoptosis Detection Kit, Beyotime Institute of Biotechnology, Haimen, China) following the manufacturer's instructions to identify apoptotic NK cells. The remaining pellets were divided into four groups (1×105 cells/tube) and labeled with PE-conjugated mouse anti-human NKG2A (R&D Systems, Minnesota, USA), NKG2D, NKp30, or NKp44 (BD, Biosciences, California, USA), respectively, according to the manufacturer's instructions. All samples were analyzed by flow cytometry.
2.5. Quantification of secretion levels of IFN-γ, perforin, and granzyme-B
Natural killer cells were co-cultured with K562 cells, and the levels of secreted cytokines and cytolytic proteins were evaluated as previously described (Denman et al., 2012). After treatment with SMG, RC, and 1GC, NK cells (2×106) were taken from each group, washed three times with PBS, resuspended in 2 mL of IL-2-free RPMI-1640, and added to a 24-well plate. K562 cells (4×105) were added to each well to achieve an E:T ratio of 5:1. The plate was then incubated for 4 h at 37°C in a 5% CO2 atmosphere. The cells (NK cells and K562 cells) were centrifuged, and the supernatants were collected. IFN-γ, perforin, and granzyme-B in the co-culture supernatants were quantified with the corresponding ELISA kit (BD, Bioscience, California, USA) according to the manufacturer's specifications. The ELISA assays were performed twice for each sample. OD values were recorded at 450 nm. A standard curve was created by using the OD values of standard samples with Curve Expert 13.0 software. The quantities of IFN-γ, perforin, and granzyme-B in the supernatant were then calculated from these standard curves.
2.6. Real-time quantitative PCR analysis
Relative quantification of mRNAs was performed by real-time quantitative reverse transcription (RT)-PCR with the SYBR Green random mixing method to test the mRNA levels of three cytokines: IFN-γ, perforin, granzyme-B, and four receptors related to NK cell cytotoxicity: NKG2A, NKG2D, NKp30, and NKp44. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal control. The 2−ΔΔCt method was used to calculate relative changes in gene expression (Livak and Schmittgen, 2001). All primers used here (Table 1) exhibited a PCR amplification efficiency similar to that of the GAPDH primers (data not shown). Total RNA was extracted from 1×107 NK cells for each group with TRIzol (TRIzol reagent, Invitrogen, Carlsbad, CA, USA). RT-PCR was performed with the TransStart Top Green qPCR SuperMix kit (TransGen Biotech, Beijing, China) according to the manufacturer's instructions. Briefly, reaction mixtures were incubated for 30 min at 48°C, followed by 40 cycles of PCR at 94°C for 5 s, 55°C for 15 s, and 72°C for 10 s. At the end of 40 cycles, a melting curve analysis was performed to confirm the presence of only a single amplified product of the expected size.
Table 1.
Primer Pairs Used for Real-Time PCR Analysis
| Primer | Sequence (5′ → 3′) | Amplicon size (bp) |
|---|---|---|
| IFN-γ | F: TGCAGGTCATTCAGATGTAGC | 248 |
| R: GGACATTCAAGTCAGTTACCG | ||
| Perforin | F: GGGACAATAACAACCCCATCT | 174 |
| R:GGAATTTTAGGTGGCCATGAT | ||
| Granzyme-B | F:AGATCGAAAGTGCGAATCTGA | 138 |
| R: TTCGTCCATAGGAGACAATGC | ||
| NKG2A | F: TCATTGTGGCCATTGTCCTGAGG | 291 |
| R: AGCACTGCACAGTTAAGTTCAGC | ||
| NKG2D | F: ATCGCTGTAGCCATGGGAATCCG | 213 |
| R: AGACATACAAGAGACCTGGCTCTC | ||
| NKp30 | F: TGAGATTCGTACCCTGGAAGG | 235 |
| R: CACTCTGCACACGTAGATGCT | ||
| NKp44 | F: TCCAAGGCTCAGGTACTTCAA | 163 |
| R: GATTGTGAATCGAGAGGTCCA | ||
| GAPDH | F: TCCTGCACCACCAACTGCTTAGC | 254 |
| R: ACACGGAAGGCCATGCCAGTGAGC |
2.7. Recovery of NK cell cytotoxicity
Three groups (SMG, RC, and 1GC) of NK cells were prepared. NK cells (8×106) from each group were washed three times with PBS and resuspended in 2 mL of RPMI-1640 (with 100 U IL-2) for further culture in a 24-well plate. NK cells (2×106) were sampled from each group at 0, 3, 5, and 7 days, respectively, for cytotoxicity evaluations.
2.8. The effects of interleukins on NK cells after exposure to SMG
Interleukin (IL)–2, IL-12, and IL-15 (PeproTech, Rocky Hill, NJ, USA) were tested to determine whether any of these factors could counteract the effects of SMG on human NK cells. NK cells were handled as described above, and interleukins were added to the NK cell culture medium. Four interleukin-treated groups (IL-2 group, IL-12 group, IL-15 group, and IL-12+IL-15 group) and a blank control group were prepared. The final concentrations of each interleukin were as follows: IL-2, 500 U/mL; IL-12, 10 ng/mL; IL-15, 10 ng/mL. Each interleukin treatment group was then subdivided into SMG, RC, and 1GC groups. After treatment, NK cells were sampled from each group, and their cytotoxicities were determined.
2.9. Statistics
Statistical analysis was performed with SPSS 16.0 statistical software. Data were presented as the mean±SD (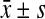 ). The results were evaluated by ANOVA. For multiple comparisons, the least significant difference (LSD) and Student–Newman–Keuls (SNK) tests were used to evaluate the significance of differences between groups. Statistical significance was defined as p<0.05.
). The results were evaluated by ANOVA. For multiple comparisons, the least significant difference (LSD) and Student–Newman–Keuls (SNK) tests were used to evaluate the significance of differences between groups. Statistical significance was defined as p<0.05.
3. Results
3.1. Primary human NK cells
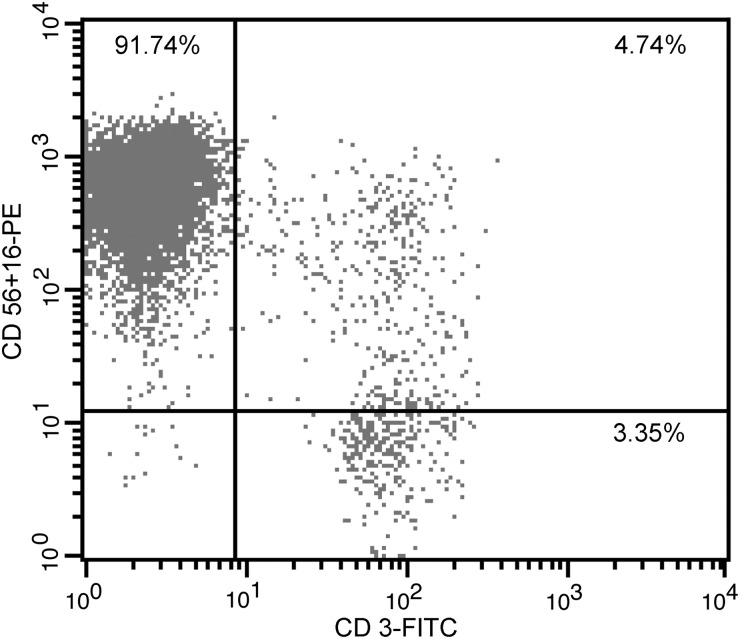
Primary NK cells were harvested after a 21-day ex vivo expansion, stained with CD56+16-PE and CD3-FITC mAbs, and analyzed by flow cytometry. The percentage of NK cells (CD56+16+CD3−) was determined (Fig. 2). The mean percentage of NK cells was 90.17±1.45% (n=4; Table 2).
FIG. 2.
Flow cytometry assay of primary human NK cells. Flow cytometry assay of primary human NK cells from one donor; 91.74% of the cells were NK cells. PBMCs were co-cultured with stimulating cells and harvested after 21 days of ex vivo expansion. All pellets were stained with CD56+16-PE and CD3-FITC mAbs and analyzed by flow cytometry. The percentage of NK cells (CD56+16+CD3−) in the PBMC population was tested.
Table 2.
Percentage of NK Cells after ex vivo Expansion (n=4)
| Donor | 1 | 2 | 3 | 4 | mean±SD |
|---|---|---|---|---|---|
| Percentage of NK cells after ex vivo expansion | 91.74% | 89.71% | 91.03% | 88.18% | 90.17±1.45% |
3.2. NK cell cytotoxicity
The cytotoxicity of NK cells was evaluated after 12, 24, 48, and 72 h of exposure to SMG, RC, and 1GC. We found no obvious differences in cytotoxicity between the 12 and 24 h. However, after 48 h of treatment, the cytotoxicity of the SMG group was significantly decreased (68.52±4.13%) in comparison with that of the RC group (75.72±5.48%) or the 1GC group (75.50±5.04%), as indicated in Fig. 3 (p<0.05). After 72 h of SMG treatment, the cytotoxicity in the SMG group decreased even further, to 61.76±4.89%. These results indicated that 48 h is the earliest time at which decreased NK cell cytotoxicity is observed in the SMG group. Consequently, 48 h was considered an appropriate duration of SMG exposure for NK cells in the subsequent experiments.
FIG. 3.
Cytotoxicity of NK cells after exposure to SMG conditions for different durations. SMG-treated NK cells were mixed with K562 cells in an E:T ratio of 5:1. After co-culture for 4 h, CCK-8 was used to detect the remaining vital cells, and the killing rate (%) was calculated using the following equation: [1 − (ODe+t − ODe)/ODt]×100% (ODe, average OD450 of triplicate wells for NK cell control; ODt, average OD450 of triplicate wells for K562 cell control; ODe+t, average OD450 of triplicate wells for NK cells plus K562 cells). The data represent the mean±SD of six independent experiments. Multivariate ANOVA and LSD test, *p<0.05 compared with the RC group; #p<0.05 compared with the 1GC group.
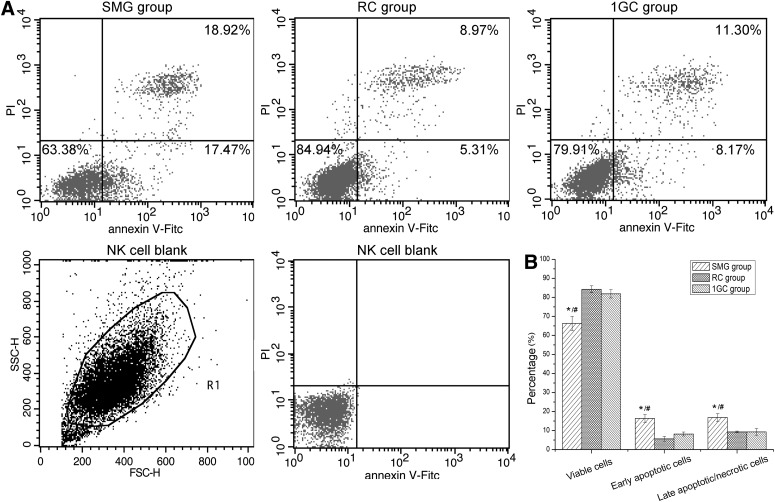
3.3. Apoptosis analysis of NK cells
Annexin V/PI flow cytometry analysis of NK cells after 48 h of exposure to SMG treatment is shown in Fig. 4. The early apoptosis rates of NK cells (Annexin V+/PI−) for the SMG group were markedly increased (16.34±2.04%) compared to those of the RC (5.64±1.26%, p<0.05) and 1GC (8.16±1.04%, p<0.05) groups. The late apoptosis/necrosis rate of NK cells (Annexin V+/PI+) in the SMG group was also increased (16.84±2.02%) compared to that of the RC group (9.27±0.45%) and 1GC group (9.30±1.75%). The proportions of viable cells in the three groups were 66.30±3.73% (SMG), 84.24±1.82% (RC), and 81.87±2.19% (1GC).
FIG. 4.
Apoptosis and necrosis of NK cells after 48 h of exposure to SMG. NK cells were stained with FITC-conjugated Annexin V and PI, followed by flow cytometry analysis. (A) A representative flow cytometry assay of NK cells from one donor. The percentages of viable (Annexin V−/PI−), early apoptotic (Annexin V+/PI−), and late apoptotic/necrotic (Annexin V+/PI+) populations of NK cells are shown. (B) The percentages of apoptotic cells are summarized in the bar graph; each column represents the mean±SD of four independent experiments. One-way ANOVA and LSD test, *p<0.05 compared with the RC group; #p<0.05 compared with the 1GC group.
3.4. Immunoassays for IFN-γ, perforin, and granzyme-B secreted by NK cells
The level of IFN-γ and perforin secretion was altered after exposure to SMG treatment (Fig. 5). The INF-γ concentration in the supernatant of the SMG group was significantly decreased, to 238.02±23.57 pg/mL, in comparison to 732.29±38.34 pg/mL in the RC group and 770.73±37.64 pg/mL in the 1GC group (p<0.05). The perforin concentration in the SMG group was 427.75±27.91 ng/mL, which was also clearly decreased in comparison to both the RC group (580.55±54.37 ng/mL) and the 1GC group (554.25±26.60 ng/mL) (p<0.05). Granzyme-B was not detectable in the culture supernatant of any of the three groups.
FIG. 5.
IFN-γ and perforin secretion levels of NK cells after 48 h of exposure to SMG. NK cells were stimulated with K562 cells for 4 h, supernatants were collected, and the concentrations of IFN-γ and perforin were detected using the appropriate ELISA kits. Each sample was tested twice. The data represent the mean±SD of four independent experiments. One-way ANOVA and LSD test, *p<0.05 compared with the RC group, #p<0.05 compared with the 1GC group.
3.5. Quantitative real-time PCR analysis
The gene expression profiles of the NK cells differed greatly between the SMG group and the two control groups. The quantitative real-time PCR results presented in Table 3 show that the IFN-γ mRNA level was decreased in the SMG group to only one-tenth of the level in the RC group and one-third of the level in the 1GC group (p<0.05). Similarly, the perforin mRNA level in the SMG group was clearly decreased compared to the levels in the RC and 1GC groups (p<0.05). Furthermore, the expression levels of the cell surface receptors NKG2A and NKG2D were significantly downregulated in the SMG group compared with the RC and 1GC groups (p<0.05). However, the mRNA levels of NKp30 and NKp44 were not significantly different among the three groups.
Table 3.
Gene Expression Profiles of IFN-γ, Perforin, Granzyme-B, NKG2D, NKG2A, NKp30, and NKp44 in NK Cells after 48 h Exposure to SMG
| Gene | ΔΔCt (compared with RC) | ΔΔCt (compared with 1GC) |
|---|---|---|
| IFN-γ | 3.53±0.75 | 1.96±0.33 |
| 2−ΔΔCt=2−3.53=0.1(fold)* | 2−ΔΔCt=2−1.96=0.3(fold)# | |
| Perforin | 2.09±0.97 | 2.52±0.43 |
| 2−ΔΔCt=2−2.09=0.2(fold)* | 2−ΔΔCt=2−2.52=0.2(fold)# | |
| Granzyme-B | 1.09±0.6 | 0.96±0.51 |
| 2−ΔΔCt=2−1.09=0.5(fold) | 2−ΔΔCt=2−0.96=0.5(fold) | |
| NKG2A | 2.34±0.3 | 2.05±0.76 |
| 2−ΔΔCt=2−2.34=0.2(fold)* | 2−ΔΔCt=2−2.05=0.2(fold)# | |
| NKG2D | 2.9±0.78 | 2.36±0.27 |
| 2−ΔΔCt=2−2.9=0.1(fold)* | 2−ΔΔCt=2−2.36=0.2(fold)# | |
| NKp30 | (−)0.28±0.45 | 0.23±0.61 |
| 2−ΔΔCt=20.28=1.2(fold) | 2−ΔΔCt=2−0.23=0.9(fold) | |
| NKp44 | 0.51±0.21 | 0.99±0.94 |
| 2−ΔΔCt=2−0.51=0.7(fold) | 2−ΔΔCt=2−0.99=0.5(fold) |
Real-time quantitative (RT)-PCR using the SYBR Green random mixing method was employed to test the mRNA levels. GAPDH served as internal standard control. The data represent the mean±SD of six independent experiments. One-way ANOVA and LSD test, *p<0.05 compared with the RC group, #p<0.05 compared with the 1GC group.
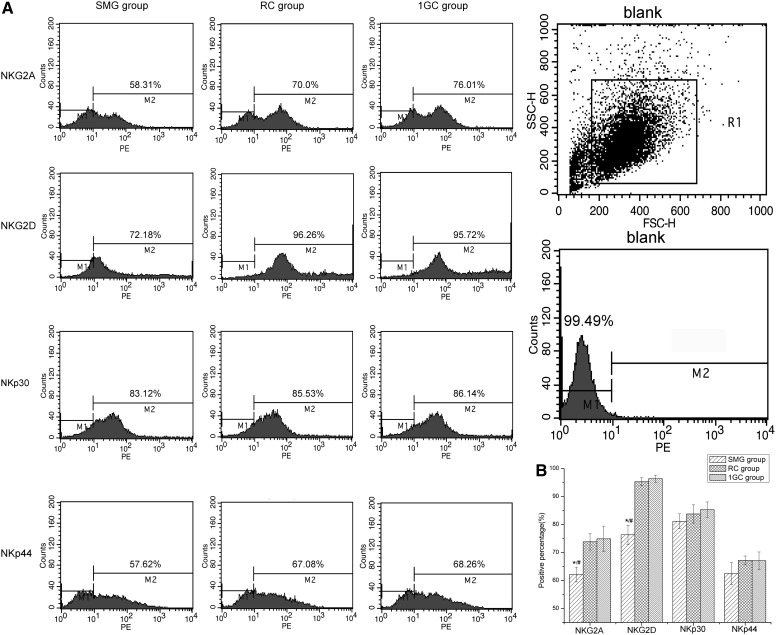
3.6. Cytofluorimetric analysis of NK cells
Cytofluorimetric analysis revealed that the percentages of NK cells expressing the NKG2A receptor were 62.12±2.64%, 73.84±2.95%, and 74.89±4.53% in the SMG, RC, and 1GC groups, respectively. There was a significant decrease in NKG2A receptor expression in the SMG group relative to the two control groups (p<0.05). NKG2D receptor expression was also distinctly decreased in the SMG group. The percentage of NKG2D-expressing cells in the SMG group was 76.33±3.43%, compared with 95.3±1.47% in the RC group and 96.37±1.29% in the 1GC group (Fig. 6B). No differences in NKp30 or NKp44 expression were observed among the three groups.
FIG. 6.
Protein expression profiles of NKG2A, NKG2D, NKp30, and NKp44 receptors on NK cells after 48 h of exposure to SMG. (A) Representative flow cytometry analysis of PBMC from one donor. NK cells were stained with PE-conjugated mAbs followed by flow cytometric analysis. (B) Data are for positive percentages and represent the mean±SD of four independent experiments. One-way ANOVA and LSD test, *p<0.05 compared with the RC group, #p<0.05 compared with the 1GC group.
3.7. Recovery of cytotoxicity in SMG-treated NK cells
We found that the cytotoxicity of SMG-treated NK cells recovered after removal from SMG treatment and culture under the 1g condition for 3–5 days (Fig. 7). The cytotoxicity recovered from 66.4±2.21% (0 days) to 74.5±0.87% at 3 days and 75.1±0.59% at 5 days.
FIG. 7.
Recovery of cytotoxicity in NK cells following exposure to SMG treatment. After 48 h of exposure to SMG, NK cells were removed and cultured under normal gravity conditions (1g), and cells were sampled from each group at 0, 3, 5, and 7 days to determine the cytotoxicity of the NK cells. The data represent the mean±SD of four independent experiments. Multivariate ANOVA and LSD test. *p<0.05 compared with the RC group, #p<0.05 compared with the 1GC group.
3.8. The effects of interleukin on the cytotoxicity of NK cells under SMG
These results show that when interleukins were added to NK cell cultures, neither IL-2 nor IL-12 had any visible effect on the cytotoxicity of NK cells. In contrast, IL-15 was found to enhance the cytotoxicity of the NK cells (Fig. 8). The cytotoxicity of the SMG-treated NK cells was increased to 68.51±1.54% after IL-15 treatment, compared to 62.96±3.47% of the SMG-treated NK cells that were not cultured with interleukins (blank control). Treatment with IL-15 increased the cytotoxicity of NK cells in both the RC and 1GC groups to 82.67±2.7% and 83.1±1.86%, respectively, compared to 77.4±1.34% and 76.2±1.67% in the blank control.
FIG. 8.
The effects of interleukins on the cytotoxicity of NK cells under SMG. Interleukins were supplied to each experimental group. The final concentration of each interleukin was IL-2, 500 U/mL; IL-12, 10 ng/mL; and IL-15, 10 ng/mL. The cytotoxicity of NK cells in each group was assayed as previously described. The data represent the mean±SD of six independent experiments. Multivariate ANOVA and LSD test, *p<0.05 compared with blank control.
Combination treatment with IL-15+IL-12 increased the cytotoxicity of the SMG group, RC group, and 1GC group by 8.94%, 6.03%, and 8.5%, respectively, in comparison to the blank controls.
4. Discussion
Spaceflight induces human immune dysfunction accompanied by an accelerated proliferation of pathogenic microbes and may increase the potential risk of infection and malignant transformation in astronauts (Gridley et al., 2009). The effects of spaceflight on the immune system are not caused simply by physical and psychological stress but may also be directly affected by microgravity (Martinelli et al., 2009). Previous studies have shown that microgravity and SMG can affect both the proliferation and viability of lymphocytes, which suggests that their effects occur at the cellular level (Meehan et al., 1992; Taylor, 1993; Cooper and Pellis, 1998; Sastry et al., 2001; Martinelli et al., 2009).
The microgravity-induced changes in the activity and functions of NK cells are neither clearly defined nor fully understood. In the present study, we employed 2-D RWV rotation to simulate microgravity and analyzed a vertically rotated control group to exclude the side effects of clinostation. Cells have been exposed to SMG for different lengths of time (usually 24–72 h) in studies. Martinelli reported that there is a significant decrease in lymphocyte proliferation and viability after 48 h of rotation in a 3-D clinostat (Martinelli et al., 2009). Several studies have shown that cells can respond to SMG within less than 24 h (Sarkar et al., 2000a, 2000b; Simons et al., 2006); moreover, other researchers exposed the cells to SMG conditions for as long as 72 h (Cao et al., 2007). We assayed the cytotoxicity of NK cells after different durations of exposure to SMG conditions (12, 24, 48, and 72 h). The cytotoxicity of the NK cells decreased dramatically after 48 h of SMG treatment. For this reason, we chose 48 h as an appropriate treatment time for subsequent experiments.
The cytotoxicity of NK cells plays a key role in the innate immune system, which acts against tumors and viral infections. Decreased NK cell cytotoxicity has been observed in many patients suffering from tumors or immunosuppressive diseases (Ziegler et al., 1981; Sirianni et al., 1990; Danielou-Lazareth et al., 2013; Reiners et al., 2013). Our results indicate that SMG can significantly inhibit the cytotoxicity of NK cells (p<0.05). Although NK cells from different donors displayed different susceptibilities to SMG, the average cytotoxicity (%) of the SMG group was decreased by 7.20% and 6.98% compared to the RC and 1GC groups, respectively, after 48 h of treatment. Our results indicate that increased rates of apoptosis and necrosis, decreased secretion of IFN-γ and perforin, and downregulated expression of functional cell surface receptors may be responsible for the inhibition of NK cell cytotoxicity under SMG conditions.
(1) Apoptosis: The early apoptosis rates of NK cells in the SMG group were increased by 10.70% and 8.18% compared with those of the RC and 1GC groups, respectively. Similarly, the late apoptosis/necrosis rates of NK cells in the SMG group increased by 7.57% and 7.54% compared with those of the RC and 1GC groups, respectively. This result further validates our theory that microgravity can enhance the apoptosis of human lymphocytes. Lewis et al. (1998) reported that DNA condensation characteristic of apoptosis was observed in 30% of flown human lymphocytes (Jurkat cells) but only 17% of ground control cells; other authors have reported similar results (Mognato and Celotti, 2005; Mognato et al., 2009).
(2) IFN-γ and perforin: IFN-γ is a multifunctional cytokine that promotes both adaptive and innate immune responses (Gridley et al., 2009). Studies of flight crews have generally shown that their lymphocytes have diminished IFN-γ secretion capacities, and SMG treatment is associated with a similar phenomenon (Licato and Grimm, 1999; Crucian et al., 2000; Crucian et al., 2008; Stowe et al., 2011). However, contradictory results have also been reported (Talas et al., 1983b, 1984). Our results show that the IFN-γ secretion levels in NK cells were decreased after 48 h of SMG treatment, to 494.27 pg/mL from 532.71 pg/mL. The perforin levels were also decreased in the SMG group. The concentrations of granzyme-B in the supernatants were too low to be detected by ELISA, possibly because NK cells lyse their target cells by releasing granzyme-B directly into these target cells rather than into the culture supernatant. Furthermore, our RT-PCR results show that the mRNA levels of IFN-γ and perforin were decreased significantly in the SMG group. This indicates that 48 h of SMG treatment strongly affects the IFN-γ and perforin secretion of NK cells.
(3) NK cell activity–related receptors: NK cell immune responses are controlled through a balance of signals mediated through stimulatory and inhibitory receptors (Lanier, 2005). NKG2D, NKp30, and NKp44 act as primary receptors for target cell recognition and trigger the natural cytotoxicity of NK cells against a variety of tumors or target cells (Lanier, 2005; Moretta et al., 2006). NKG2D is a C-type lectin-like surface receptor that triggers NK cell activation by recognizing ligands on tumor cells and plays a critical role in tumor surveillance (Suarez-Alvarez et al., 2009). NKG2A is an inhibitory NK cell receptor that can recognize self MHC class I molecules and thus maintains tolerance to healthy autologous cells (Claus et al., 2009). SMG can downregulate the expression levels of both the stimulatory receptor NKG2D and the inhibitory receptor NKG2A. The decrease in NKG2D expression may explain the depressed cytotoxicity of SMG-treated NK cells. However, the target cells (K562) tested here do not express the MHC class I receptor, which may explain why we did not observe any effect on the cytotoxicity of NK cells despite the decrease in the inhibitory receptor NKG2A.
We were intrigued by the question of whether the inhibition of NK cell cytotoxicity by SMG is reversible. When SMG-treated NK cells were cultured at 1g after culture under SMG, we found that their cytotoxicity was improved. The cytotoxicity recovered quickly, within the first 3 days of culture at 1g, and further recovered between 3 and 5 days of culture at 1g. Ritz et al. (2006) reported that the proliferation of PBMCs gradually recovered after 48 h of exposure to model microgravity. The recovery was only partial at 48 h (75%), but the PBMCs exhibited full recovery at 72 h (Ritz et al., 2006). Together with our results, these data suggest that recovery from the effects of SMG at the cellular level can occur within 72 h of return to the 1g condition.
We also investigated whether any cytokine could restore the activity of NK cells after exposure to microgravity. Certain interleukins, such as IL-2, IL-12, and IL-15, have been shown to have a positive effect on the bioactivity of NK cells (Fehniger et al., 2000; Marcenaro et al., 2005; Szczepanski et al., 2010). One cytokine, IL-15, was recognized to play an essential role in the ontogenesis and proliferation of NK cells, leading to increased differentiation and survival (Carson et al., 1997; Cooper et al., 2002). In this study, three types of interleukins were applied to NK cells from the SMG, RC, and 1GC groups. The cytotoxicity of the NK cells was increased by IL-15 in all three groups, and this effect was enhanced by the further addition of IL-12, especially in the SMG group. This result indicates that IL-15 in combination with IL-12 may be used as a countermeasure to combat the inhibitory effect of SMG on NK cell cytotoxicity. However, treatment with IL-2 or IL-12 alone showed no obvious effect on cytotoxicity in the SMG group. Even further, IL-12 treatment alone slightly decreased the cytotoxicity of the NK cells. This result is consistent with previous reports that prolonged treatment with IL-12 can induce apoptosis in NK cells and therefore decrease the cytotoxicity of NK cell populations (Huang et al., 2011). However, the mechanism that underlies the positive effects of IL-15 in combination with IL-12 should be studied further. In contrast, many studies have reported that IL-2 can enhance the biological activity of NK cells in a dose-dependent manner (Mori et al., 1998; Fehniger et al., 2000; Kikuchi-Maki et al., 2003). Nevertheless, in the present study, we found no visible effect of IL-2 on the cytotoxicity of NK cells in all three groups. We noted that the primary human NK cells were prepared by ex vivo expansion, for which IL-2 was essential and was supplied to the NK cells at a concentration of 100 U/mL; additional IL-2 has no further effect on NK cells.
In conclusion, human NK cells are sensitive to microgravity, and SMG treatment significantly decreases the cytotoxicity of NK cells. This inhibition of cytotoxicity is reversible when cells are either cultured at 1g after SMG or treated with a combination of IL-12 and IL-15. SMG-induced NK cell dysfunction may be associated with severe immunosuppression and enhance the risk of infection and malignant transformation for astronauts. In the future, further exploration of the underlying mechanisms and the development of potential treatments to overcome the negative influence of microgravity may help prevent spaceflight-induced health risks in astronauts during long space missions.
Acknowledgments
We gratefully acknowledge financial support from the National Natural Science Foundation of China (NSFC, Grant No. 30971425) and the Doctorate Foundation of Northwestern Polytechnical University (CX201023).
Author Disclosure Statement
All the authors listed (Qi Li, Qibing Mei, Ting Huyan, Li Xie, Su Che, Hui Yang, Mingjie Zhang, and Qingsheng Huang) certify that the work described is original research that has not been published previously and that no competing financial interests exist.
Abbreviations
1GC, 1g control; CCK-8, Cell Counting Kit-8; E:T, effector versus target; FBS, fetal bovine serum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IFN, interferon; IL, interleukin; LSD, least significant difference; mAbs, monoclonal antibodies; NK, natural killer; OD, optical density; PBMC, peripheral blood mononuclear cell; PBS, phosphate-buffered saline; RC, rotation control; RT-PCR, reverse transcription polymerase chain reaction; RWV, rotating wall vessel; SMG, simulated microgravity; SNK, Student–Newman–Keuls.
References
- Bechler B. Cogoli A. Mesland D. Lymphocytes are sensitive to gravity. Naturwissenschaften. 1986;73:400–403. doi: 10.1007/BF00367278. [DOI] [PubMed] [Google Scholar]
- Buravkova L.B. Rykova M.P. Grigorieva V. Antropova E.N. Cell interactions in microgravity: cytotoxic effects of natural killer cells in vitro. J Gravit Physiol. 2004;11:177–180. [PubMed] [Google Scholar]
- Cao Y.J. Fan X.J. Shen Z. Ma B.H. Duan E.K. Nitric oxide affects preimplantation embryonic development in a rotating wall vessel bioreactor simulating microgravity. Cell Biol Int. 2007;31:24–29. doi: 10.1016/j.cellbi.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Carson W.E. Fehniger T.A. Haldar S. Eckhert K. Lindemann M.J. Lai C.F. Croce C.M. Baumann H. Caligiuri M.A. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J Clin Invest. 1997;99:937–943. doi: 10.1172/JCI119258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. Wang Y. Zhuang Y. Zhou F. Huang L. Mifepristone increases the cytotoxicity of uterine natural killer cells by acting as a glucocorticoid antagonist via ERK activation. PLoS One. 2012;7:e36413. doi: 10.1371/journal.pone.0036413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus M. Greil J. Watzl C. Comprehensive analysis of NK cell function in whole blood samples. J Immunol Methods. 2009;341:154–164. doi: 10.1016/j.jim.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Cogoli A. Bechler B. Cogoli-Greuter M. Criswell S.B. Joller H. Joller P. Hunzinger E. Muller O. Mitogenic signal transduction in T lymphocytes in microgravity. J Leukoc Biol. 1993;53:569–575. doi: 10.1002/jlb.53.5.569. [DOI] [PubMed] [Google Scholar]
- Cooper D. Pellis N.R. Suppressed PHA activation of T lymphocytes in simulated microgravity is restored by direct activation of protein kinase C. J Leukoc Biol. 1998;63:550–562. doi: 10.1002/jlb.63.5.550. [DOI] [PubMed] [Google Scholar]
- Cooper D. Pride M.W. Brown E.L. Risin D. Pellis N.R. Suppression of antigen-specific lymphocyte activation in modeled microgravity. In Vitro Cell Dev Biol Anim. 2001;37:63–65. doi: 10.1290/1071-2690(2001)037<0063:SOASLA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Cooper M.A. Bush J.E. Fehniger T.A. Van Deusen J.B. Waite R.E. Liu Y. Aguila H.L. Caligiuri M.A. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100:3633–3638. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- Crucian B.E. Cubbage M.L. Sams C.F. Altered cytokine production by specific human peripheral blood cell subsets immediately following space flight. J Interferon Cytokine Res. 2000;20:547–556. doi: 10.1089/10799900050044741. [DOI] [PubMed] [Google Scholar]
- Crucian B.E. Stowe R.P. Pierson D.L. Sams C.F. Immune system dysregulation following short- vs long-duration spaceflight. Aviat Space Environ Med. 2008;79:835–843. doi: 10.3357/asem.2276.2008. [DOI] [PubMed] [Google Scholar]
- Danielou-Lazareth A. Henry G. Geromin D. Khaznadar Z. Briere J. Tamouza R. Cayuela J.M. Thieblemont C. Toubert A. Dulphy N. At diagnosis, diffuse large B-cell lymphoma patients show impaired rituximab-mediated NK-cell cytotoxicity. Eur J Immunol. 2013;43:1383–1388. doi: 10.1002/eji.201242733. [DOI] [PubMed] [Google Scholar]
- Denman C.J. Senyukov V.V. Somanchi S.S. Phatarpekar P.V. Kopp L.M. Johnson J.L. Singh H. Hurton L. Maiti S.N. Huls M.H. Champlin R.E. Cooper L.J. Lee D.A. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS One. 2012;7:e30264. doi: 10.1371/journal.pone.0030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger T.A. Bluman E.M. Porter M.M. Mrozek E. Cooper M.A. Van Deusen J.B. Frankel S.R. Stock W. Caligiuri M.A. Potential mechanisms of human natural killer cell expansion in vivo during low-dose IL-2 therapy. J Clin Invest. 2000;106:117–124. doi: 10.1172/JCI6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gridley D.S. Slater J.M. Luo-Owen X. Rizvi A. Chapes S.K. Stodieck L.S. Ferguson V.L. Pecaut M.J. Spaceflight effects on T lymphocyte distribution, function and gene expression. J Appl Physiol. 2009;106:194–202. doi: 10.1152/japplphysiol.91126.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins W.R. Zieglschmid J.F. Clinical aspects of crew health. In: Johnston R., editor; Dietlein R., editor; Berry C., editor. Biomedical Results of Apollo, NASA SP 368. National Aeronautics and Space Administration; Washington, DC: 1975. pp. 43–81. [Google Scholar]
- Hensel W. Sievers A. Effects of prolonged omnilateral gravistimulation on the ultrastructure of statocytes and on the graviresponse of roots. Planta. 1980;150:338–346. doi: 10.1007/BF00384664. [DOI] [PubMed] [Google Scholar]
- Herranz R. Anken R. Boonstra J. Braun M. Christianen P.C. de Geest M. Hauslage J. Hilbig R. Hill R.J. Lebert M. Medina F.J. Vagt N. Ullrich O. Van Loon J.J. Hemmersbach R. Ground-based facilities for simulation of microgravity: organism-specific recommendations for their use, and recommended terminology. Astrobiology. 2013;13:1–17. doi: 10.1089/ast.2012.0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q.S. Li Q. Huang Y. Shang P. Zhang M.J. [Expansion of human natural killer cells ex vivo.] Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2008;24:1167–1169. [PubMed] [Google Scholar]
- Huang Y. Lei Y. Zhang H. Zhang M. Dayton A. Interleukin-12 treatment down-regulates STAT4 and induces apoptosis with increasing ROS production in human natural killer cells. J Leukoc Biol. 2011;90:87–97. doi: 10.1189/jlb.1210674. [DOI] [PubMed] [Google Scholar]
- Imai C. Iwamoto S. Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–383. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessup J.M. Frantz M. Sonmez-Alpan E. Locker J. Skena K. Waller H. Battle P. Nachman A. Bhatti Weber M.E. Thomas D.A. Curbeam R.L. Baker T.L. Goodwin T.J. Microgravity culture reduces apoptosis and increases the differentiation of a human colorectal carcinoma cell line. In Vitro Cell Dev Biol Anim. 2000;36:367–373. doi: 10.1290/1071-2690(2000)036<0367:mcraai>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kikuchi-Maki A. Yusa S. Catina T.L. Campbell K.S. KIR2DL4 is an IL-2-regulated NK cell receptor that exhibits limited expression in humans but triggers strong IFN-gamma production. J Immunol. 2003;171:3415–3425. doi: 10.4049/jimmunol.171.7.3415. [DOI] [PubMed] [Google Scholar]
- Konstantinova I.V. Rykova M. Meshkov D. Peres C. Husson D. Schmitt D.A. Natural killer cells after ALTAIR mission. Acta Astronaut. 1995;36:713–718. doi: 10.1016/0094-5765(95)00161-1. [DOI] [PubMed] [Google Scholar]
- Lanier L.L. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- Lewis M.L. Reynolds J.L. Cubano L.A. Hatton J.P. Lawless B.D. Piepmeier E.H. Spaceflight alters microtubules and increases apoptosis in human lymphocytes (Jurkat) FASEB J. 1998;12:1007–1018. doi: 10.1096/fasebj.12.11.1007. [DOI] [PubMed] [Google Scholar]
- Licato L.L. Grimm E.A. Multiple interleukin-2 signaling pathways differentially regulated by microgravity. Immunopharmacology. 1999;44:273–279. doi: 10.1016/s0162-3109(99)00123-x. [DOI] [PubMed] [Google Scholar]
- Livak K.J. Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maier J.A. Impact of simulated microgravity on cell cycle control and cytokine release by U937 cells. Int J Immunopathol Pharmacol. 2006;19:279–286. doi: 10.1177/039463200601900205. [DOI] [PubMed] [Google Scholar]
- Marcenaro E. Della Chiesa M. Bellora F. Parolini S. Millo R. Moretta L. Moretta A. IL-12 or IL-4 prime human NK cells to mediate functionally divergent interactions with dendritic cells or tumors. J Immunol. 2005;174:3992–3398. doi: 10.4049/jimmunol.174.7.3992. [DOI] [PubMed] [Google Scholar]
- Martinelli L.K. Russomano T. Dos Santos M.A. Falcao F.P. Bauer M.E. Machado A. Sundaresan A. Effect of microgravity on immune cell viability and proliferation: simulation using 3-D clinostat. IEEE Eng Med Biol Mag. 2009;28:85–90. doi: 10.1109/MEMB.2009.933572. [DOI] [PubMed] [Google Scholar]
- Meehan R.T. Neale L.S. Kraus E.T. Stuart C.A. Smith M.L. Cintron N.M. Sams C.F. Alteration in human mononuclear leucocytes following space flight. Immunology. 1992;76:491–497. [PMC free article] [PubMed] [Google Scholar]
- Moes M. Boonstra J. Regan-Klapisz E. Novel role of cPLA(2)alpha in membrane and actin dynamics. Cell Mol Life Sci. 2010;67:1547–1557. doi: 10.1007/s00018-010-0267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mognato M. Celotti L. Modeled microgravity affects cell survival and HPRT mutant frequency, but not the expression of DNA repair genes in human lymphocytes irradiated with ionising radiation. Mutat Res. 2005;578:417–429. doi: 10.1016/j.mrfmmm.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Mognato M. Girardi C. Fabris S. Celotti L. DNA repair in modeled microgravity: double strand break rejoining activity in human lymphocytes irradiated with gamma-rays. Mutat Res. 2009;663:32–39. doi: 10.1016/j.mrfmmm.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Moretta L. Bottino C. Pende D. Castriconi R. Mingari M.C. Surface NK receptors and their ligands on tumor cells. Semin Immunol. 2006;18:151–158. doi: 10.1016/j.smim.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Mori S. Jewett A. Cavalcanti M. Murakami-Mori K. Nakamura S. Bonavida B. Differential regulation of human NK cell-associated gene expression following activation by IL-2, IFN-alpha and PMA/ionomycin. Int J Oncol. 1998;12:1165–1170. doi: 10.3892/ijo.12.5.1165. [DOI] [PubMed] [Google Scholar]
- Nicogossian A.E. Pool S.L. Medical care and health maintenance in flight. In: Nicogossian A.E., editor; Huntoon C.L., editor; Pool S.L., editor. Space Physiology and Medicine. Lea & Febiger; Philadelphia: 1989. pp. 349–363. [Google Scholar]
- Pippia P. Sciola L. Cogoli-Greuter M. Meloni M.A. Spano A. Cogoli A. Activation signals of T lymphocytes in microgravity. J Biotechnol. 1996;47:215–222. doi: 10.1016/0168-1656(96)01387-9. [DOI] [PubMed] [Google Scholar]
- Reiners K.S. Kessler J. Sauer M. Rothe A. Hansen H.P. Reusch U. Hucke C. Kohl U. Durkop H. Engert A. von Strandmann E.P. Rescue of impaired NK cell activity in Hodgkin lymphoma with bispecific antibodies in vitro and in patients. Mol Ther. 2013;21:895–903. doi: 10.1038/mt.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B.W. Lelkes P.I. Gardner E.M. Functional recovery of peripheral blood mononuclear cells in modeled microgravity. FASEB J. 2006;20:305–307. doi: 10.1096/fj.04-3122fje. [DOI] [PubMed] [Google Scholar]
- Rykova M.P. Sonnenfeld G. Lesnyak A.T. Taylor G.R. Meshkov D.O. Mandel A.D. Medvedev A.E. Berry W.D. Fuchs B.B. Konstantinova I.V. Effect of spaceflight on natural killer cell activity. J Appl Physiol. 1992;73:196S–200S. doi: 10.1152/jappl.1992.73.2.S196. [DOI] [PubMed] [Google Scholar]
- Sarkar D. Nagaya T. Koga K. Nomura Y. Gruener R. Seo H. Culture in vector-averaged gravity under clinostat rotation results in apoptosis of osteoblastic ROS 17/2.8 cells. J Bone Miner Res. 2000a;15:489–498. doi: 10.1359/jbmr.2000.15.3.489. [DOI] [PubMed] [Google Scholar]
- Sarkar D. Nagaya T. Koga K. Kambe F. Nomura Y. Seo H. Rotation in clinostat results in apoptosis of osteoblastic ROS 17/2.8 cells. J Gravit Physiol. 2000b;7:71–72. [PubMed] [Google Scholar]
- Sastry K.J. Nehete P.N. Savary C.A. Impairment of antigen-specific cellular immune responses under simulated microgravity conditions. In Vitro Cell Dev Biol Anim. 2001;37:203–208. doi: 10.1007/BF02577530. [DOI] [PubMed] [Google Scholar]
- Shi F. Wang Y.C. Zhao T.Z. Zhang S. Du T.Y. Yang C.B. Li Y.H. Sun X.Q. Effects of simulated microgravity on human umbilical vein endothelial cell angiogenesis and role of the PI3K-Akt-eNOS signal pathway. PLoS One. 2012;7:e40365. doi: 10.1371/journal.pone.0040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons D.M. Gardner E.M. Lelkes P.I. Dynamic culture in a rotating-wall vessel bioreactor differentially inhibits murine T-lymphocyte activation by mitogenic stimuli upon return to static conditions in a time-dependent manner. J Appl Physiol. 2006;100:1287–1292. doi: 10.1152/japplphysiol.00887.2005. [DOI] [PubMed] [Google Scholar]
- Sirianni M.C. Tagliaferri F. Aiuti F. Pathogenesis of the natural killer cell deficiency in AIDS. Immunol Today. 1990;11:81–82. doi: 10.1016/0167-5699(90)90032-5. [DOI] [PubMed] [Google Scholar]
- Smyth M.J. Cretney E. Kelly J.M. Westwood J.A. Street S.E. Yagita H. Takeda K. van Dommelen S.L. Degli-Esposti M.A. Hayakawa Y. Activation of NK cell cytotoxicity. Mol Immunol. 2005;42:501–510. doi: 10.1016/j.molimm.2004.07.034. [DOI] [PubMed] [Google Scholar]
- Sonnenfeld G. Mandel A.D. Konstantinova I.V. Berry W.D. Taylor G.R. Lesnyak A.T. Fuchs B.B. Rakhmilevich A.L. Spaceflight alters immune cell function and distribution. J Appl Physiol. 1992;73:191S–195S. doi: 10.1152/jappl.1992.73.2.S191. [DOI] [PubMed] [Google Scholar]
- Stowe R.P. Sams C.F. Pierson D.L. Effects of mission duration on neuroimmune responses in astronauts. Aviat Space Environ Med. 2003;74:1281–1284. [PubMed] [Google Scholar]
- Stowe R.P. Sams C.F. Pierson D.L. Adrenocortical and immune responses following short- and long-duration spaceflight. Aviat Space Environ Med. 2011;82:627–634. doi: 10.3357/asem.2980.2011. [DOI] [PubMed] [Google Scholar]
- Suarez-Alvarez B. Lopez-Vazquez A. Baltar J.M. Ortega F. Lopez-Larrea C. Potential role of NKG2D and its ligands in organ transplantation: new target for immunointervention. Am J Transplant. 2009;9:251–257. doi: 10.1111/j.1600-6143.2008.02526.x. [DOI] [PubMed] [Google Scholar]
- Szczepanski M.J. Szajnik M. Welsh A. Foon K.A. Whiteside T.L. Boyiadzis M. Interleukin-15 enhances natural killer cell cytotoxicity in patients with acute myeloid leukemia by upregulating the activating NK cell receptors. Cancer Immunol Immunother. 2010;59:73–79. doi: 10.1007/s00262-009-0724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talas M. Batkai L. Stoger I. Nagy L. Hiros L. Konstantinova I. Rykova M. Mozgovaya I. Guseva O. Kozharinov V. Results of space experiment program “Interferon” I. Production of interferon in vitro by human lymphocytes aboard space laboratory Solyut-6 (“Interferon I”) and influence of space flight on lymphocyte functions of cosmonauts (“Interferon III”) Acta Microbiol Hung. 1983a;30:53–61. [PubMed] [Google Scholar]
- Talas M. Batkai L. Stoger I. Nagy K. Hiros L. Konstantinova I. Kozharinov V. Results of space experiment program “Interferon”. II. Influence of spaceflight conditions on the activity of interferon preparations and interferon inducers (“Interferon II”) Acta Microbiol Hung. 1983b;30:63–67. [PubMed] [Google Scholar]
- Talas M. Batkai L. Stoger I. Nagy K. Hiros L. Konstantinova I. Rykova M. Mozgovaya I. Guseva O. Kozharinov V. Results of space experiment program “Interferon”. Acta Astronaut. 1984;11:379–386. doi: 10.1016/0094-5765(84)90078-x. [DOI] [PubMed] [Google Scholar]
- Taylor G.R. Cell anomalies associated with spaceflight conditions. Adv Exp Med Biol. 1987;225:259–271. doi: 10.1007/978-1-4684-5442-0_24. [DOI] [PubMed] [Google Scholar]
- Taylor G.R. Overview of spaceflight immunology studies. J Leukoc Biol. 1993;54:179–188. doi: 10.1002/jlb.54.3.179. [DOI] [PubMed] [Google Scholar]
- Taylor G.R. Dardano J.R. [Human cellular immune responsiveness following space flight.] Kosm Biol Aviakosm Med. 1984;18:74–80. [PubMed] [Google Scholar]
- Taylor G.R. Neale L.S. Dardano J.R. Immunological analyses of U.S. space shuttle crewmembers. Aviat Space Environ Med. 1986;57:213–217. [PubMed] [Google Scholar]
- Vivier E. Nunes J.A. Vely F. Natural killer cell signaling pathways. Science. 2004;306:1517–1519. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- Wang Y. An L. Jiang Y. Hang H. Effects of simulated microgravity on embryonic stem cells. PLoS One. 2011;6:e29214. doi: 10.1371/journal.pone.0029214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler H.W. Kay N.E. Zarling J.M. Deficiency of natural killer cell activity in patients with chronic lymphocytic leukemia. Int J Cancer. 1981;27:321–327. doi: 10.1002/ijc.2910270310. [DOI] [PubMed] [Google Scholar]