Abstract
Trophocytes and fat cells of queen honeybees have been used for delayed cellular senescence studies, but their oxidative stress and anti-oxidant enzyme activities with advancing age are unknown. In this study, we assayed reactive oxygen species (ROS) and anti-oxidant enzymes in the trophocytes and fat cells of young and old queens. Young queens had lower ROS levels, lower superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) activities, and higher thioredoxin reductase (TR) activity compared to old queens. These results show that oxidative stress and anti-oxidant enzyme activities in trophocytes and fat cells increase with advancing age in queens and suggest that an increase in oxidative stress and a consequent increase in stress defense mechanisms are associated with the longevity of queen honeybees.
Introduction
Aging is a complicated process that leads to decreasing cellular proliferative potential and increasing cellular deterioration, causing a progressive decline in biological function and an increased incidence of age-associated diseases.1 Studies on the biology of aging not only contribute to the understanding of aging mechanisms but also provide insight into age-associated diseases and possible treatment strategies.
The oxidative stress hypothesis indicates that aging results from the accumulation of oxidative damage and that life span is determined by the rate at which oxidative damage occurs.2 Cellular oxidative stress results when the generation of reactive oxygen species (ROS) exceeds the capacity of cellular anti-oxidant defenses to remove these toxic species. ROS include a diverse variety of chemical species, including superoxide (O2• −), hydroxyl radicals (OH•), and hydrogen peroxide (H2O2). ROS are mainly produced through reactions of the mitochondrial electron transport chain, the oxidation of polyunsaturated fatty acids, and nitric oxide generation.3 In addition, nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) oxidase (NO) and xanthine oxidase (XO) also produce superoxide and H2O2. ROS can damage lipids, proteins, carbohydrates, and nucleic acids. The subsequent dysfunction of organelles as well as the damage to cellular integrity and functionality that result from these molecular insults lead to cellular senescence.4 An intricate anti-oxidant defense system that includes catalase (CAT), glutathione peroxidase (GPx)/glutathione reductase (GR) system, superoxide dismutase (SOD), and the thioredoxin peroxidase (TPx)/thioredoxin reductase (TR) system has evolved to neutralize the burden of ROS production. CAT, which is abundant in peroxisomes but less prevalent in mitochondria and the endoplasmic reticulum, converts H2O2 to water and O2. GPx, which is present in the cytoplasm and mitochondrial matrix, removes H2O2 by coupling its reduction to water with the oxidation of glutathione to glutathione disulfide. Subsequently, GR reduces glutathione disulfide to glutathione. GPx can also reduce other peroxides, such as fatty acid hydroperoxides. SODs are metal-containing enzymes that catalyze the removal of superoxide to generate H2O2. Cu,Zn-SOD is present in the cytoplasm and nucleus, whereas Mn-SOD is primarily located in mitochondria.5 TPx, which is present in the cytoplasm, mitochondrial matrix, and nucleus, removes H2O2 by coupling its reduction to water with the oxidation of reduced thioredoxin to oxidized thioredoxin. Subsequently, TR reduces oxidized thioredoxin to reduced thioredoxin.6,7
Despite the presumptive link between oxidative stress and aging, recent studies have shown that increased oxidative stress promotes the longevity and metabolic health of organisms8,9 and that mitochondrial oxidative stress is not causal with respect to aging.10–13 These phenomena highlight the concept of mitohormesis, in which increased mitochondrial metabolism and ROS formation induce an adaptive response that increases stress resistance and extends the life span.8,9
Trophocytes and fat cells of honeybees (Apis mellifera) attach to one another to form a single layer of cells around each segment of abdomen. The ease with which these cells can be isolated from the abdomen and manipulated as well as the fact that no cell division occurs during adulthood make them attractive targets for cellular senescence studies in honeybees.14–18 Aging-related molecule assays in the trophocytes and fat cells of young or old workers and young or old queens showed that the trophocytes and fat cells of queens had longevity-promoting mechanisms that prolonged life span and could serve as target cells for the study of delayed cellular senescence.14,15 If trophocytes and fat cells of queens are to be used for longevity-promoting mechanisms studies, it is important to investigate the changes in oxidative stress and anti-oxidant enzyme activities in young and old queens. Such data could provide clues for understanding delayed cellular senescence mechanisms in future aging studies. In this study, the levels of ROS and the activities of anti-oxidant enzymes were evaluated in the trophocytes and fat cells of young and old queens to clarify the relationship of oxidative stress and aging in queen honeybees.
Materials and Methods
Queen honeybees
The queens were purchased from a single commercial breeder (Hsinchu, Taiwan). As described in a previous study,15 young (2-month-old) and old (16-month-old) queens were mated with drones and were able to lay eggs. They were collected from different hives on the same dates for the following studies. Young queens have dense and light yellowish-brown fuzz and light yellowish-brown epidermis. However, old queens have sparse and dark brown fuzz and dark blackish-brown epidermis.
ROS assays in trophocytes and fat cells
Individual queens of each group were dissected, and their abdominal trophocytes and fat cells were detached from the cuticle in honeybee saline (156.4 mM NaCl, 2.7 mM KCl, 1.8 mM CaCl2, 22.2 mM glucose, pH 7.3).19 Trophocytes and fat cells were used for ROS assays.
Dihydroethidine (HET) was used to evaluate ROS, including superoxide, hydroxyl radicals, and H2O2.20–22 The trophocytes and fat cells from one young or old queen were incubated with 5 μM HET (D7008; Sigma, Saint Louis, MO) for 10 min at 37°C and then stained with 0.2 μM Hoechst 33342 for 5 min at 37°C (H3570; Invitrogen, Carlsbad, CA). ROS (red fluorescence) was detected by observing cells at room temperature under a confocal laser scanning microscope (Leica TCS SP2; Leica, Wetzlar, Germany) at an excitation wavelength of 520 nm and an emission wavelength of 610 nm; nuclei (blue fluorescence) were detected at excitation and emission wavelengths of 350 and 461 nm, respectively.22,23
2′,7′-Dichlorodihydrofluorescein-diacetate (H2DCF-DA) was used to evaluate ROS, including H2O2, hydroxyl radicals, and peroxyl radicals.22,23 The trophocytes and fat cells from one young or old queen were incubated with 5 μM H2DCF-DA (D6886; Sigma) for 20 min at 37°C and then stained with 0.2 μM Hoechst 33342 (Invitrogen) for 5 min at 37°C. Confocal laser scanning microscopy and image analyses were performed as described above using excitation and emission wavelengths of 498 nm and 522 nm, respectively, to detect ROS (green fluorescence).22–24 The nuclei were detected as described for HET.
The average intensities and areas of red fluorescence (ROS) and green fluorescence (ROS) in the cells were determined using QWin image processing and analysis software (version 2.5, Leica, Wetzlar, Germany). The ratio of average intensity/area of red and green fluorescence in the cells represented ROS levels, respectively. Both the HET and H2DCF-DA experiments were biologically replicated four times and used a total of four young and four old queens.
Supernatant preparations of trophocytes and fat cells
Trophocytes and fat cells were isolated from one young or old queen, homogenized in 500 μL of phosphate-buffered saline containing protease inhibitors (11697498001; Roche Applied Science, Indianapolis, IN), and centrifuged at 5,000×g for 10 min at 4°C. The resulting supernatant was collected and assayed immediately. The protein concentration was determined using a protein assay reagent (500-0006; Bio-Rad Laboratories, Hercules, CA) by monitoring the wavelength of 595 nm at room temperature.
ROS assays in supernatants
A total of 10 mM H2DCF-DA (2 μL) was added to 200 μL of fresh supernatant (described above), and the fluorescence was monitored at room temperature for 120 min at 5-min intervals at excitation and emission wavelengths of 485 nm and 530 nm, respectively.20 The ROS levels are expressed as DCF min−1 mg−1 of protein. This experiment was biologically replicated six times in queens and used a total of six young and six old queens.
NO activity assay
NO activity was measured as previously described.25 Briefly, a final 200 μL of reactive solution containing 5 μL of fresh supernatant (described in Supernatant preparations of trophocytes and fat cells, above), 5 μL of NADPH (1 mM), and 2 μof lucigenin (20 μM) initiated the reaction followed by immediate measurement of chemiluminescence in a ultraviolet-visible (UV/VIS) spectrophotometer (Spectramax M2, Molecular Devices, NY) for 12 min. Appropriate blanks were established. The resultant chemiluminescence was normalized to the blank. The specific activity was expressed as micromole min−1 mg−1 of protein. This experiment was biologically replicated six times and used a total of six young and six old queens.
XO activity assay
XO activity was measured using the XO Assay Kit (ab102522; Abcam, Cambridge, MA). Briefly, trophocytes and fat cells were isolated from one young or old queen, homogenized in 60 μL of assay buffer, and centrifuged at 16,000×g for 10 min at 4°C. The protein concentration was determined (described in Supernatant preparations of trophocytes and fat cells, above). According to the manufacturer's instructions, 50 μL of supernatant was incubated at 37°C in the reaction mixture for 30 min and the measurement of fluorescence was performed in a microplate reader at 570 nm. XO supplied in the kit was used as the standard, and XO activity was determined by comparing the fluorescence of supernatants with that of standards. The specific activity was expressed as unit mg−1 of protein. This experiment was biologically replicated six times and used a total of six young and six old queens.
CAT activity assay
CAT assays were performed as previously described26 with slight modifications. Briefly, 890 μL of 50 mM potassium phosphate buffer (pH 7.0), 100 μL of 30% H2O2, and 10 μL of fresh supernatant (described in Supernatant preparations of trophocytes and fat cells, above) were mixed at 25°C. A blank was prepared with 900 μL of 50 mM potassium phosphate buffer and 100 μL of 30% H2O2. The rate of absorbance change (ΔA/min) at 240 nm, indicating the decomposition of H2O2, was recorded, and the activities were calculated using the molar extinction coefficient of H2O2 at 240 nm (43.59 L/mol-cm). One unit of CAT corresponds to the amount of enzyme that degrades 1 μmol H2O2 per minute at 25°C. The specific activity was expressed as micromole min−1 mg−1 of protein. This experiment was biologically replicated eight times and used a total of eight young and eight old queens.
GPx activity assay
GPx activity was measured as previously described.27 Briefly, 100 μL of fresh supernatant (described in Supernatant preparations of trophocytes and fat cells, above) was incubated with 875 μL of coupling reagent (50 mM Tris-HCl, pH 7.6, 2 mM EDTA, 1 mM NaN3, 0.1 mM reduced glutathione, 0.2 mM NADPH, and 1 U/mL GPx) for 2 min at 25°C, and the reaction was initiated by adding 25 μL of 1 mM H2O2. Then, the ΔA/min at 340 nm was recorded; the ΔA/min of a blank, in which the fresh supernatant was replaced with 100 μL of Tris-HCl buffer, was also recorded. The net ΔA/min of the samples after subtracting the blank rate was used to calculate the GPx activity using the molar extinction coefficient of NADPH at 340 nm (6,220 L/mol-cm). The specific activity was expressed as nanomole min−1 mg−1 of protein. This experiment was biologically replicated six times and used a total of six young and six old queens.
SOD activity assay
SOD activity was measured using the SOD Assay Kit-WST (S311; Dojindo, MD). Briefly, 200 μL of the supernatant (described in Supernatant preparations of trophocytes and fat cells, above) or 200 μL of the SOD standard solution was applied to each well of a 96-well plate, and 200 μL of the WST working solution was added. After mixing, 20 μL of enzyme working solution was added, and each sample was incubated at 37°C for 20 min. The total SOD activity was obtained by measuring the absorbance at 450 nm using a microplate reader (DU-70; Beckman, NJ). Mn-SOD activity was measured by adding 4 μL of 0.33 M sodium cyanide to the mixture, and the Cu,Zn-SOD activity was calculated through subtracting the Mn-SOD activity from the total SOD activity.28 The specific activity was expressed as unit mg−1 of protein. This experiment was biologically replicated six times and used a total of six young and six old queens.
TR activity assay
TR activity was measured using the Thioredoxin Reductase Assay Kit (CS0170; Sigma, Saint Louis, MO) according to the manufacturer's instructions. This assay is based on the reduction of 5,5′-dithiobis(2-nitrobenzoic) acid (DTNB) with NADPH to 5-thio-2-nitrobenzoic acid (TNB), which produces a strong yellow color that is measured at 412 nm. Briefly, 14 μL of the supernatant (described in Supernatant preparations of trophocytes and fat cells, above) or 10 μL of the supernatant (described in Supernatant preparations of trophocytes and fat cells, above) and 4 μL of inhibitor solution were mixed with 180 μL of working buffer and 6 μL of DTNB in 96-well plate. The absorption was measured at 412 nm using an enzyme-linked immunosorbent assay (ELISA) plate reader for 1 min. TR activity was calculated through subtracting the rate of DTNB reduction in the presence of sample plus an inhibitor of TR from the rate of DTNB reduction in the presence of sample. The specific activity was expressed as unit μg−1 protein. This experiment was biologically replicated six times and used a total of six young and six old queens.
Statistical analysis
Differences in mean values between the two age groups were examined using two-sample t-tests. A p value of less than 0.05 was considered statistically significant.
Results
ROS levels increase with age
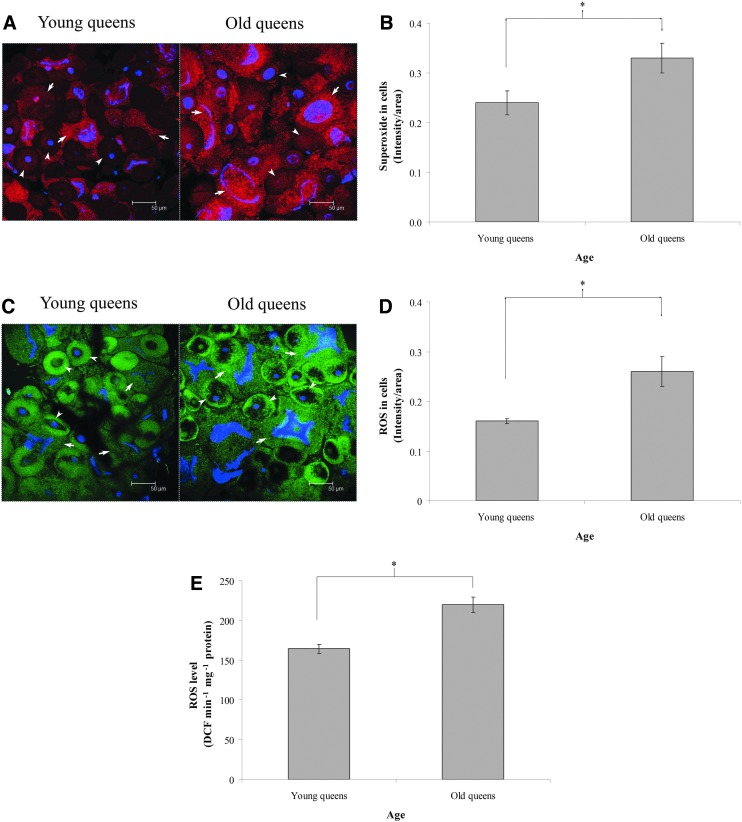
ROS, determined through measuring the levels of superoxide, hydroxyl radicals, and H2O2,20–22 showed a change with the queen's age. The trophocytes and fat cells of young queens exhibited lower ROS levels than old queens (Fig. 1A). The average intensity/cellular area of ROS in old queens was significantly higher compared to young queens (n=4, p<0.05; Fig. 1B), demonstrating that ROS levels increase with age in queens.
FIG. 1.
Reactive oxygen species (ROS) levels in the trophocytes and fat cells of young and old queens. (A) Red fluorescence indicates the presence of ROS in young and old queens. Purple fluorescence indicates nuclei. Arrows point to trophocytes. Arrowheads point to fat cells. Scale bar, 50 μm. (B) Quantification of ROS in young and old queens. Bars represent mean±standard error of the mean (SEM) (n=4). (C) Green fluorescence indicates the presence of ROS in young and old queens. Blue fluorescence indicates nuclei. Arrows point to trophocytes. Arrowheads point to fat cells. Scale bar, 50 μm. (D) Quantification of ROS in young and old queens. Bars represent mean±SEM (n=4). (E) The ROS levels in the supernatants of the trophocytes and fat cells of young and old queens. Bars represent mean±SEM (n=6). Asterisks indicate statistical significance as determined by two-sample t test (*) p<0.05).
In addition, ROS, determined through measuring the levels of H2O2, hydroxyl radicals, and peroxyl radicals,22,23 also showed a change with the queen's age. The results showed that ROS levels in the trophocytes and fat cells of queens increased with age (Fig. 1C). The average intensity/cellular area of ROS in old queens was significantly higher compared to young queens (n=4, p<0.05; Fig. 1D), indicating that overall oxidative stress increases with age in queens.
The ROS levels in trophocytes and fat cells were confirmed through assaying ROS levels in cellular supernatants again. The mean values of ROS were 164.27±5.80 and 219.67±9.61 DCF min−1 mg−1 protein in young and old queens, respectively (n=6, p<0.05; Fig. 1E), demonstrating that ROS increase with age in queens.
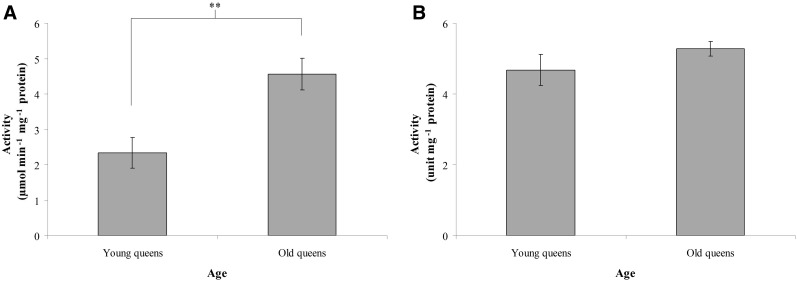
The activities of NO and XO
To evaluate ROS production, we assayed the activities of NO and XO. The NO activity was 2.33±0.44 μmol min−1 mg−1 protein in young queens and 4.56±0.44 μmol min−1 mg−1 protein in old queens (n=6, p<0.01; Fig. 2A), indicating that NO activity increases with age in queens. This result demonstrated that ROS production increases with age in queens. The XO activity was 4.68±0.44 unit mg−1 protein in young queens and 5.28±0.20 unit mg−1 protein in old queens (n=6, p>0.05; Fig. 2B), indicating that XO activity is not significantly different between young and old queens.
FIG. 2.
The activities of nicotinamide adenine dinucleotide phosphate hydrogen (NADHP) oxidase (NO) (A) and xanthine oxidase (XO) (B) in the trophocytes and fat cells of young and old queens. Bars represent mean±standard error of the mean (SEM) (n=6). Asterisks indicate statistical significance as determined by two-sample t test (**) p<0.01).
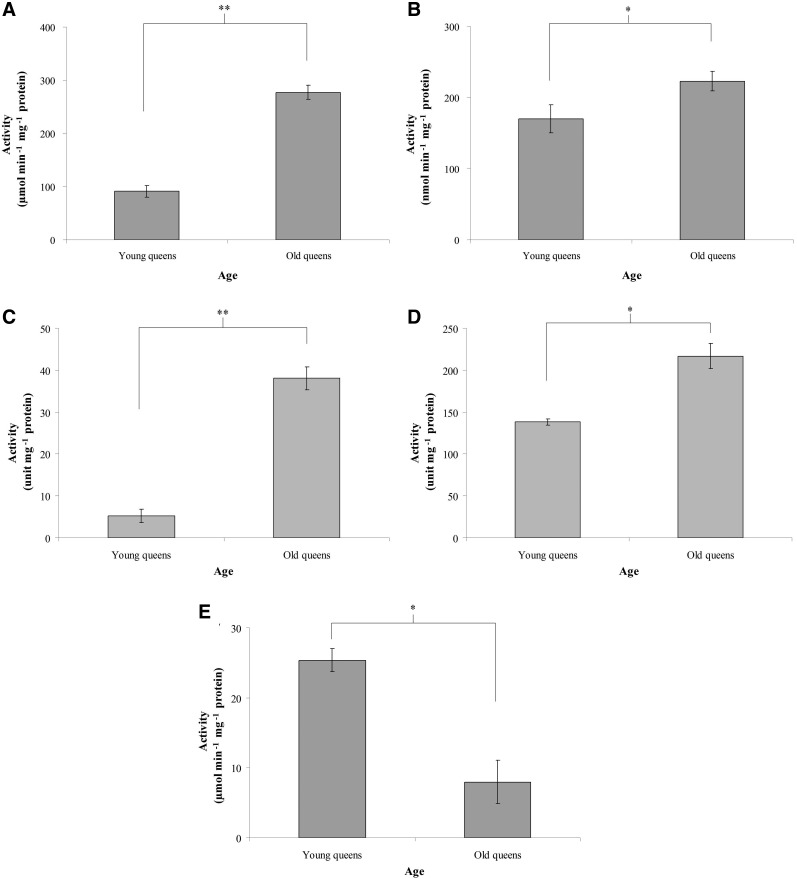
The activities of CAT, GPx, Mn-SOD, Cu,Zn-SOD, and TR
The CAT activity was 91.31±10.98 μmol min−1 mg−1 protein in young queens and 277.18±12.99 μmol min−1 mg−1 protein in old queens (n=8, p<0.01; Fig. 3A), and the GPx activity was 169.95±19.57 nmol min−1 mg−1 protein in young queens and 223.22±13.98 nmol min−1 mg−1 protein in old queens (n=6, p<0.05; Fig. 3B). The Mn-SOD activity was 5.19±1.57 units/mg protein in young queens and 38.04±2.74 units/mg protein in old queens (n=6, p<0.01; Fig. 3C), and the Cu,Zn-SOD activity was 138.26±3.60 units/mg protein in young queens and 216.77±14.99 units/mg protein in old queens (n=6, p<0.05; Fig. 3D). The TR activity in trophocytes and fat cells was 25.39±1.65 μmol min−1 mg−1 protein in young queens and 7.97±3.10 μmol min−1 mg−1 protein in old queens (n=6, p<0.05; Fig. 3E). These results showed that the activities of CAT, GPx, Mn-SOD, and Cu,Zn-SOD increased and TR decrease with age in the trophocytes and fat cells of queens.
FIG. 3.
The activities of catalase (CAT) (A), glutathione peroxidase (GPx) (B), Mn-superoxide dismutase (SOD) (C), Cu,Zn-SOD (D), and thioredoxin reductase (TR) (E) in the trophocytes and fat cells of young and old queens. Bars represent mean±SEM (n=8 in (A), n=6 in (B), n=6 in (C), n=6 in (D), n=6 in (E)). Asterisks indicate statistical significance as determined by two-sample t-test (*) p<0.05; (**) p<0.01).
Discussion
In this study, ROS levels and anti-oxidant enzyme activities in the trophocytes and fat cells of queens were assayed and showed that young queens have lower ROS levels, CAT, GPx, and SODs activities, and higher TR activity than old queens, showing that oxidative stress increases with age in the trophocytes and fat cells of queen honeybees. The increase of oxidative stress might be associated with the cellular longevity-promoting mechanisms of queens.
ROS levels increase with age
ROS are generated as byproducts of energy metabolism in cells.5 ROS levels are higher in the trophocytes and fat cells of old queens compared to young queens. The higher levels of ROS in old queens may be due to higher energy metabolism, which increases the production of superoxide and other ROS.6 This high-energy metabolism in old queens may be closely related to the longevity of queens. This inference is consistent with previous studies showing that: (1) Increased mitochondrial metabolism induced by glaucarubinone extends the life span of Caenorhabditis elegans,29 (2) increased mitochondrial energy metabolism protects against cardiac failure in mice,30 and (3) increased mitochondrial energy metabolism extends the life span of an annual fish (Nothobranchius rachovii) under conditions of ambient temperature reduction, which has been shown to effectively extend the life span of organisms.31
In addition, an increase in ROS levels concomitant with an increase in energy metabolism in the trophocytes and fat cells of old queens is consistent with previous studies indicating that the trophocytes and fat cells of queens had longevity-promoting mechanisms.14,15
NO and XO produced ROS, such as superoxide and H2O2.32,33 NO activity increased with the age of queens, indicating that ROS production also increased with the age of queens. This result is in accord with the results of ROS levels in this study and previous studies showing that NO increased with aging in rat liver, aorta, and myocardium.34–36 Nevertheless, ROS production by mitochondria can significantly surpass the amount of ROS produced by NO.32,37 Therefore, the higher levels of ROS in old queens may be due to higher energy metabolism, which increases the production of superoxide and other ROS.6
Although XO activity increased with aging in human plasma, rat plasma, rat aorta, rat gastrocnemius muscle, mice cerebral cortex, mice liver, mice plasma, mice spleen, and mice thymus,38–40 XO activity did not change with aging in mice kidney, mice lung, long-lived mice liver, long-lived mice kidney, and long-lived mice plasma.39,40 In this study, XO activity is not significantly different between young and old queens. This phenomenon is consistent with a previous study, showing that XO activity is not significantly different between young and long-lived mice.40 The explanation is most likely that XO does not participate in the impairment of trophocytes and fat cells with aging in queens. This inference is in agreement with a previous study showing that XO does not contribute to impaired peripheral conduit artery endothelium-dependent dilatation with aging in human.41
Activities of CAT, GPx, Mn-SOD, Cu,Zn-SOD, and TR
The ROS levels and the activities of CAT, GPx, Mn-SOD, and Cu,Zn-SOD in the trophocytes and fat cells of queens increased with age, indicating that CAT, GPx, Mn-SOD, and Cu,Zn-SOD were responsible for scavenging ROS. The biological function of SOD is to remove superoxide to generate H2O2, which is then scavenged by CAT, the TPx/TR system, and the GPx/GR system. The trophocytes and fat cells of old queens might have a higher energy metabolism, which increases the production of superoxide. The higher level of superoxide induces an increase in the activity of SODs, producing more H2O2, which is then scavenged by CAT, the TPx/TR system, and the GPx/GR system. TPx removes H2O2 by coupling its reduction to water with the oxidation of reduced thioredoxin to oxidized thioredoxin. Subsequently, TR reduces oxidized thioredoxin to reduced thioredoxin using NADPH.6,7 Therefore, TR activity can represent the activity of the TPx/TR system. Likewise, GPx removes H2O2 by coupling its reduction to water with the oxidation of reduced glutathione to oxidized glutathione. Subsequently, GR reduces oxidized glutathione to reduced glutathione using NADPH.6 Therefore, GPx activity can represent the activity of GPx/GR system. The anti-oxidant enzyme activity results of the queens obtained in this study are consistent with those of a previous study, which showed that mated queens exhibited higher CAT activity in the ventriculi and spermathecae and higher SOD activity in muscles and spermathecae compared to virgin queens.42
CAT, the TPx/TR system, and the GPx/GR system are used to scavenge H2O2. CAT activity is higher than TR activity and TR activity is higher than GPx activity in young queens. Likewise, CAT activity is higher than TR activity and TR activity is higher than GPx activity in old queens. This phenomenon suggests that the CAT might be the principal scavenger for H2O2 in queens, with the TPx/TR system and the GPx/GR system playing a secondary role and a third role. The fact that TR activity is higher than GPx activity in queens is consistent with a previous study, showing that TR is higher H2O2 detoxification than GPx in rat brain mitochondria.43 Thioredoxin and glutathione are the substrates of TPx and GPx and are reduced by TR and GR using NADPH,6 which can be supplied through higher cellular energy metabolism. Therefore, the TPx/TR system and the GPx/GR system more actively degrade H2O2 under conditions of higher cellular energy metabolism because of the greater availability of NADPH. This explanation is consistent with the results of previous studies showing that NADPH levels are closely related to anti-oxidant capacity6 and that the injection of reduced glutathione into the antennal lobes of workers prior to treatment with an oxidative stress inducer blocks oxidative stress-mediated inhibitory effects.44 Nevertheless, TR activity is higher in young queens as opposed to CAT and GPx activities, which are lower in young queens, indicating that TR as well as CAT and GPx are regulated in a reverse manner in queens. This phenomenon is consistent with a previous study, showing that TR and GPx are regulated in a contrasting manner in the cancer systems tested.45
The activities of anti-oxidant enzymes have been reported to decrease with age in vertebrates, including in the rat brain,46,47 rat liver,48 mouse brain,49 and fish muscle.50 However, the results of these previous studies are not consistent with our observations in queens, in which the activities of CAT, GPx, Mn-SOD, and Cu,Zn-SOD increased with age. The high activities of anti-oxidant enzymes in queens may be involved in longevity-promoting mechanisms, which is a speculation supported by previous reports that anti-oxidant enzyme activities are elevated in rodents under a calorie-restricted diet.48,51,52 Calorie restriction has been shown to successfully extend the life span of organisms, ranging from ciliates to mammals.53
Taking these findings together, we hypothesize that an increase in metabolism concomitant with an increase in oxidative stress and a consequent increase in stress defense mechanisms are associated with the longevity of queens. This postulate is consistent with previous studies, showing that increased ROS extends the life span of Caenorhabditis elegans,54–58 Saccharomyces cerevisiae,59,60 Schizosaccharomyces pombe,61 and naked mole-rat.62 This postulate is also supported by previous studies, showing that decreasing ROS levels by anti-oxidants and over-expression of ROS scavenge enzymes prevent health-promoting effects of physical exercise in humans and do not increase the life span of mice.63,64
Acknowledgments
This work was supported by CMRPD 1A0492 grant from Chang Gung Memorial Hospital, Linkou, Taiwan.
Author Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Kirkwood TBL. Austad SN. Why do we age? Nature. 2000;408:233–238. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- 2.Harman D. Aging: A theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 3.Miquel J. An update on the mitochondrial-DNA mutation hypothesis of cell aging. Mutat Res. 1992;275:209–216. doi: 10.1016/0921-8734(92)90024-j. [DOI] [PubMed] [Google Scholar]
- 4.Terman A. Brunk UT. Oxidative stress, accumulation of biological ‘garbage’, and aging. Antioxid Redox Signal. 2006;8:197–204. doi: 10.1089/ars.2006.8.197. [DOI] [PubMed] [Google Scholar]
- 5.Halliwell B. Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford University Press; New York: 1999. [Google Scholar]
- 6.Kowaltowski AJ. de Souza-Pinto NC. Castilho RF. Vercesi AE. Mitochondria and reactive oxygen species. Free Radic Biol Med. 2009;47:333–343. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Arnér ESJ. Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 8.Ristow M. Zarse K. How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis) Free Radic Biol Med. 2010;51:327–336. doi: 10.1016/j.exger.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Ristow M. Schmeisser S. Extending life span by increasing oxidative stress. Free Radic Biol Med. 2011;51:327–336. doi: 10.1016/j.freeradbiomed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Jang YC. Perez VI. Song W. Lustgarten MS. Salmon AB. Mele J. Qi W. Liu Y. Liang H. Chaudhuri A. Ikeno Y. Epstein CJ. van Remmen H. Richardson A. Overexpression of Mn superoxide dismutase does not increase life span in mice. J Gerontol A Biol Sci Med Sci. 2009;64:1114–1125. doi: 10.1093/gerona/glp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lapointe J. Stepanyan Z. Bigras E. Hekimi S. Reversal of the mitochondrial phenotype and slow development of oxidative biomarkers of aging in long-lived Mclk1+/− mice. J Biol Chem. 2009;284:20364–20374. doi: 10.1074/jbc.M109.006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y. Ikeno Y. Qi W. Chaudhuri A. Li Y. Bokov A. Thorpe SR. Baynes JW. Epstein C. Richardson A. van Remmen H. Mice deficient in both Mn superoxide dismutase and glutathione peroxidase-1 have increased oxidative damage and a greater incidence of pathology but no reduction in longevity. J Gerontol A Biol Sci Med Sci. 2009;64:1212–1220. doi: 10.1093/gerona/glp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lapointe J. Hekimi S. When a theory of aging ages badly. Cell Mol Life Sci. 2010;67:1–8. doi: 10.1007/s00018-009-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsieh YS. Hsu CY. Honeybee trophocytes and fat cells as target cells for cellular senescence studies. Exp Gerontol. 2011;46:233–240. doi: 10.1016/j.exger.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh YS. Hsu CY. The changes of age-related molecules in the trophocytes and fat cells of queen honeybees (Apis mellifera) Apidologie. 2011;42:728–739. [Google Scholar]
- 16.Hsu CY. Chan YP. The use of honeybees reared in thermostatic chamber for aging studies. Age. 2013;35:149–158. doi: 10.1007/s11357-011-9344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan QWT. Mutti NS. Foster LJ. Kocher SD. Amdam GV. Florian W. The worker honeybee fat body proteome is extensively remodeled preceding a major life-history transition. PLoS One. 2011;6:e24794. doi: 10.1371/journal.pone.0024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chuang YL. Hsu CY. Changes in mitochondrial energy utilization in young and old worker honeybees. (Apis mellifera). Age. 2012 doi: 10.1007/s11357-012-9490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu CY. Ko FY. Li CW. Fann K. Lue JT. Magnetoreception system in honeybees (Apis mellifera) PLoS ONE. 2007;2(4):e395. doi: 10.1371/journal.pone.0000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benov L. Sztejnberg L. Fridovich I. Critical evaluation of the use of hydroethidine as a measure of superoxide anion radical. Free Radic Biol Med. 1988;25:826–831. doi: 10.1016/s0891-5849(98)00163-4. [DOI] [PubMed] [Google Scholar]
- 21.Bindokas VP. Jordán J. Lee CC. Miller RJ. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J Neurosci. 1996;16:1324–1336. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Münzel T. Afanas'ev IB. Kleschyov AL. Harrison DG. Detection of superoxide in vascular tissue. Arterioscler. Thromb Vasc Biol. 2002;22:1761–1768. doi: 10.1161/01.atv.0000034022.11764.ec. [DOI] [PubMed] [Google Scholar]
- 23.Tarpey MM. Wink DA. Grisham MB. Methods for detection of reactive metabolites of oxygen and nitrogen: In vitro and in vivo considerations. Am J Physiol Regul Integr Comp Physiol. 2004;286:R431–R444. doi: 10.1152/ajpregu.00361.2003. [DOI] [PubMed] [Google Scholar]
- 24.Nevřelová P. Kolárová H. Bajgar R. Maceček J. Tomečka M. Tománková K. Strnad M. Measurement of reactive oxygen species after photodynamic therapy in vitro. Scripta Medica. 2005;78:281–290. [Google Scholar]
- 25.Cheng SE. Lee IT. Lin CC. Wu WL. Hsiao LD. Yang CM. ATP mediates NADPH mxidase/ROS meneration and COX-2/PGE2 expression in A549 cells: Role of P2 receptor-dependent STAT3 activation. PLoS One. 2013;8(1):e54125. doi: 10.1371/journal.pone.0054125. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Beers RF. Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–140. [PubMed] [Google Scholar]
- 27.Yen H-C. Chen B-S. Hsu Y-T. Effect of anticoagulants and storage of coupling reagent on the activity assay of extracellular glutathione peroxidase in human plasma. J Biomed Lab Sci. 2004;16:6–10. [Google Scholar]
- 28.Kabil H. Partridge L. Harshman LG. Superoxide dismutase activities in long-lived Drosophila melanogaster females: chico genotypes and dietary dilution. Biogerontology. 2006;8:201–208. doi: 10.1007/s10522-006-9065-3. [DOI] [PubMed] [Google Scholar]
- 29.Zarse K. Bossecker A. Müller-Kuhrt L. Siems K. Hernandez MA. Berendsohn WG. Birringer M. Ristow M. The phytochemical glaucarubinone promotes mitochondrial metabolism, reduces body fat, and extends lifespan of Caenorhabditis elegans. Horm Metab Res. 2011;43:241–243. doi: 10.1055/s-0030-1270524. [DOI] [PubMed] [Google Scholar]
- 30.Schulz TJ. Westermann D. Isken F. Voigt A. Laube B. Thierbach R. Kuhlow D. Zarse K. Schomburg L. Pfeiffer AFH. Tschöpe C. Ristow M. Activation of mitochondrial energy metabolism protects against cardiac failure. Aging (Albany) 2010;2:843–853. doi: 10.18632/aging.100234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu CY. Chiu YC. Ambient temperature influences aging in an annual fish (Nothobranchius rachovii) Aging Cell. 2009;8:726–737. doi: 10.1111/j.1474-9726.2009.00525.x. [DOI] [PubMed] [Google Scholar]
- 32.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Rad Biol Med. 2011;51:1289–1301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berry CE. Hare JM. Xanthine oxidoreductase and cardiovascular disease: Molecular mechanism and pathophysiological implications. J Physiol. 2004;555:589–606. doi: 10.1113/jphysiol.2003.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laurent C. Chabi B. Fouret G. Py G. Sairafi B. Elong C. Gaillet S. Cristol JP. Coudray C. Feillet-Coudray C. Polyphenols decreased liver NADPH oxidase activity, increased muscle mitochondrial biogenesis and decreased gastrocnemius age-dependent autophagy in aged rats. Free Radic Res. 2012;46:1140–1149. doi: 10.3109/10715762.2012.694428. [DOI] [PubMed] [Google Scholar]
- 35.Li L. Smith A. Hagen TM. Frei B. Vascular oxidative stress and inflammation increase with age: ameliorating effects of alpha-lipoic acid supplementation. Ann NY Acad Sci. 2010;1203:151–159. doi: 10.1111/j.1749-6632.2010.05555.x. [DOI] [PubMed] [Google Scholar]
- 36.Wang M. Zhang J. Walker SJ. Dworakowski R. Lakatta EG. Shah AM. Involvement of NADPH oxidase in age-associated cardiac remodeling. J Mol Cell Cardiol. 2010;48:765–772. doi: 10.1016/j.yjmcc.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ago T. Matsushima S. Kuroda J. Zablocki D. Kitazono T. Sadoshima J. The NADPH oxidase Nox4 and aging in the heart. Aging. 2010;2:1012–1016. doi: 10.18632/aging.100261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aranda R. Doménech E. Rus AD. Real JT. Sastre J. Viña J. Pallardó FV. Age-related increase in xanthine oxidase activity in human plasma and rat tissues. Free Radic Res. 2007;41:1195–1200. doi: 10.1080/10715760701481461. [DOI] [PubMed] [Google Scholar]
- 39.Vida C. Corpas I. De la Fuente M. González EM. Age-related changes in xanthine oxidase activity and lipid peroxidation, as well as in the correlation between both parameters, in plasma and several organs from female mice. J Physiol Biochem. 2011;67:551–558. doi: 10.1007/s13105-011-0100-8. [DOI] [PubMed] [Google Scholar]
- 40.Vida C. Rodríguez-Terés S. Heras V. Corpas I. De la Fuente M. González E. The aged-related increase in xanthine oxidase expression and activity in several tissues from mice is not shown in long-lived animals. Biogerontology. 2011;12:551–564. doi: 10.1007/s10522-011-9351-6. [DOI] [PubMed] [Google Scholar]
- 41.Eskurza I. Kahn ZD. Seals DR. Xanthine oxidase does not contribute to impaired peripheral conduit artery endothelium-dependent dilatation with ageing. J Physiol. 2006;571:661–668. doi: 10.1113/jphysiol.2005.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weirich GF. Collins AM. Williams VP. Antioxidant enzymes in the honey bee, Apis mellifera. Apidologie. 2002;33:3–14. [Google Scholar]
- 43.Kudin AP. Augustynek B. Lehmann AK. Kovács R. Kunz WS. The contribution of thioredoxin-2 reductase and glutathione peroxidase to H2O2 detoxification of rat brain mitochondria. Biochim Biophys Acta. 2012;1817:1901–1906. doi: 10.1016/j.bbabio.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 44.Farooqui T. Iron-induced oxidative stress modulates olfactory learning and memory in honeybees. Behav Neurosci. 2008;122:433–447. doi: 10.1037/0735-7044.122.2.433. [DOI] [PubMed] [Google Scholar]
- 45.Gladyshev VN. Factor VM. Housseau F. Hatfield DL. Contrasting patterns of regulation of the antioxidant selenoproteins, thioredoxin reductase, and glutathione peroxidase, in cancer cells. Biochem Biophys Res Commun. 1998;251:488–493. doi: 10.1006/bbrc.1998.9495. [DOI] [PubMed] [Google Scholar]
- 46.Gupta A. Hasan M. Chander R. Kapoor NK. Age-related elevation of lipid peroxidation products: Diminution of superoxide dismutase activity in the central nervous systems of rats. Gerontology. 1991;37:305–309. doi: 10.1159/000213277. [DOI] [PubMed] [Google Scholar]
- 47.Zhu Y. Cavey PM. Ling Z. Age-related changes in glutathione and glutathione-related enzymes in rat brain. Brain Res. 2006;1090:35–44. doi: 10.1016/j.brainres.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pieri C. Falasca M. Marcheselli F. Moroni F. Recchioni R. Marmocchi F. Lupidi G Food restriction in female Wistar rats. V. Lipid peroxidation and antioxidant enzymes in the liver. Arch Gerontol Geriatr. 1992;14:93–99. doi: 10.1016/0167-4943(92)90010-2. [DOI] [PubMed] [Google Scholar]
- 49.Kishido T. Unno K. Yoshida H. Choba D. Fukutomi R. Asahina S. Iguchi K. Oku N. Hoshino M. Decline in glutathione peroxidase activity is a reason for brain senescence: consumption of green tea catechin prevents the decline in its activity and protein oxidative damage in ageing mouse brain. Biogerontology. 2007;8:423–430. doi: 10.1007/s10522-007-9085-7. [DOI] [PubMed] [Google Scholar]
- 50.Hsu CY. Chiu YC. Hsu WL. Chan YP. Age-related markers assayed at different developmental stages of the annual fish Nothobranchius rachovii. J Gerontol A Biol Sci Med Sci. 2008;63A:1267–1276. doi: 10.1093/gerona/63.12.1267. [DOI] [PubMed] [Google Scholar]
- 51.Koizumi A. Weindruch R. Walford RL. Influences of dietary restriction and age on liver enzyme activities and lipid peroxidation in mice. J Nutr. 1987;117:361–367. doi: 10.1093/jn/117.2.361. [DOI] [PubMed] [Google Scholar]
- 52.Xia E. Rao G. Van Remmen H. Heydari AR. Richardson A. Activities of antioxidant enzymes in various tissues of male Fischer 344 rats are altered by food restriction. J Nutr. 1995;125:195–201. doi: 10.1093/jn/125.2.195. [DOI] [PubMed] [Google Scholar]
- 53.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 54.Schulz TJ. Zarse K. Voigt A. Urban N. Birringer M. Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 55.Yang W. Hekimi S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 2010;8:e1000556. doi: 10.1371/journal.pbio.1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Durieux J. Wolff S. Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011;144:79–91. doi: 10.1016/j.cell.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woo DK. Shadel GS. Mitochondrial stress signals revise an old aging theory. Cell. 2011;144:11–12. doi: 10.1016/j.cell.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 58.van Raamsdonk JM. Hekimi S. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000361. doi: 10.1371/journal.pgen.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma PK. Agrawal V. Roy N. Mitochondria-mediated hormetic response in life span extension of calorie-restricted Saccharomyces cerevisiae. Age. 2010;33:143–154. doi: 10.1007/s11357-010-9169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mesquita A. Weinberger M. Silva A. Sampaio-Marques B. Almeida B. Leao C. Costa V. Rodrigues F. Burhans WC. Ludovico P. Caloric restriction or catalase inactivation extends yeast chronological lifespan by inducing H2O2 and superoxide dismutase activity. Proc Natl Acad Sci U S A. 2010;107:15123–15128. doi: 10.1073/pnas.1004432107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zuin A. Carmona M. Morales-Ivorra I. Gabrielli N. Vivancos AP. Ayte J. Hidalgo E. Lifespan extension by calorie restriction relies on the Sty1 MAP kinase stress pathway. EMBO J. 2010;29:981–991. doi: 10.1038/emboj.2009.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andziak B. O'Connor TP. Qi W. DeWaal EM. Pierce A. Chaudhuri AR. van Remmen H. Buffenstein R. High oxidative damage levels in the longest-living rodent, the naked mole-rat. Aging Cell. 2006;5:463–471. doi: 10.1111/j.1474-9726.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- 63.Jang YC. van Remmen H. The mitochondrial theory of aging: Insight from transgenic and knockout mouse models. Exp Gerontol. 2009;44:256–260. doi: 10.1016/j.exger.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 64.Ristow M. Zarse K. Oberbach A. Klöting N. Birringer M. Kiehntopf M. Stumvoll M. Kahn CR. Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]