Abstract
Background:
There is no consensus on the standard treatment options for female pattern androgenetic alopecia (AGA). Efficacy of finasteride in women is controversial. The purpose of this study was to evaluate the clinical efficacy and safety of 5 mg/day oral finasteride in normoandrogenic postmenopausal woman.
Materials and Methods:
A total of 40 normoandrogenic postmenopausal women with AGA was enrolled in this study. They were treated with oral finasteride 5 mg/day for 18 months. Efficacy was evaluated by patient's satisfaction and global photograph assessment. All the 40 patients completed 18 months of finasteride treatment schedule.
Results:
After 6 months, 22 patients referred significant improvement, 12 moderate improvement, and 6 no improvement. Regarding to global photo assessment, 8 patients showed no improvement, 16 showed moderate improvement and 16 showed significant improvements at the 6th month. A slight improvement was observed over time from 6 to 12 and 18 months observation. Maintained libido reduction was referred by four patients and liver enzymes increase was observed in one patient. Older patients were more prone to worse response.
Discussion:
Finasteride 5 mg/day is effective and safe for the treatment of female AGA in postmenopausal women in the absence of clinical or laboratory signs of hyper-androgenism.
Keywords: Female androgenetic alopecia, finasteride, normo-androgenetic, postmenopausal
INTRODUCTION
Diffuse reduction of hair thickness and density of fronto-parietal and crown areas of scalp areas in a woman is called[1] female androgenetic alopecia (AGA). Standard treatments include, topical minoxidil, topical prostaglandins, oral spironolactone cyproterone acetate, flutamide, finasteride, dutasteride, and LASER, however, there is no consensus regarding treatment options. Finasteride, inhibitor of type 2 5α-reductase, inhibits the conversion of testosterone to dihydrotestosterone, resulting in a decrease in serum and scalp dihydrotestosterone levels, believed to be pathogenic in AGA. Oral finasteride 1 mg/day has been shown to be effective in male AGA,[2,3] but ineffective in women.[4] Efficacy of higher doses (2.5 mg or 5 mg/day) in women has remained controversial.[5,6,7,8,9] Yeon et al. showed efficacy and safety of 5 mg/day associated to cyproterone acetate/ethinylestradiol in south Korean premenopausal woman with AGA.[10] The aim of this study was to evaluate the clinical efficacy and safety of 5 mg/day oral finasteride in monotherapy in normoandrogenic postmenopausal European women.
MATERIALS AND METHODS
Patient selection
On the first appointment for hair consultations for patients with AGA, 40 post-menopausal women with no previous treatment for the last 6 months and with normoandrogenism were enrolled in this study. Informed consent for the study was obtained. We obtained 40 volunteers from 121 patients and the main reason for refuse was the need of a pause in any hair treatment for 6 months.
Authors considered normoandrogenism normal levels of total testosterone, free testosterone, Dehydroepiandrosterone (DHEA), delta-4-androstenedione, and 5α- dihydrotestosterone (DHT).
Regimen
Patients were treated with the oral finasteride 5 mg/day for 18 months. They were asked not to use any other treatment, as topical minoxidil. They were explained that improvement would come very late in the study (to improve compliance). Efficacy was evaluated by patient's satisfaction (impairment, none, moderate or highly satisfied with the results) global photograph assessment by two independent dermatologists (major, moderate or no improvement and impairment). They were asked to maintain style, color, and a length of the hair during the study. Digital photograph was taken at the same distance and patient position. At inclusion, medical evaluation and Ludwig score were performed to every patient.
Efficacy was evaluated after 6, 12, and 18 months. Safety evaluation was performed at 0, 6, 12, and 18 months regarding any symptoms and blood tests cell blood count, (CBC), aspartate aminotransferase, (AST), alanine transaminase (ALT), total bilirubin alkaline phosphatase, blood glucose, urea, creatinine, iron, total testosterone, free testosterone, dehydroepiandrosterone sulfate (DHEA-S), delta-4-androstenedione, 5α-DHT, 17β-hidroxiprogesterone, cortisol, prolactin luteinizing hormone (LH), and follicle-stimulating hormone.
There was an efficacy comparison among three age groups: Below 60, 60-70, and over 70 years.
For statistical analysis, Chi-square (c2) test was performed to study the significance of the results, assuming that in a similar group with AGA without treatment the condition will not spontaneously improve within the time (in fact, a population with AGA will spontaneously worsening the condition within the time).
RESULTS
All 40 patients completed 18 months of finasteride treatment schedule (there were no drop outs). At medical evaluation all were classified as having AGA, Ludwig I (15 patients), II (16 patients) or III (9 patients).
Table 1 illustrates the number of patients that referred major, some or no improvement as well as global photo assessment by first (Observation, (OBS) 1) and second dermatologists (OBS 2) at months 6, 12, and 18 [Figure 1]. The data included subjective and objective improvement by age group (<60, 60-70, >70 years).
Table 1.
Clinical assessment by patients and clinicians

Figure 1.
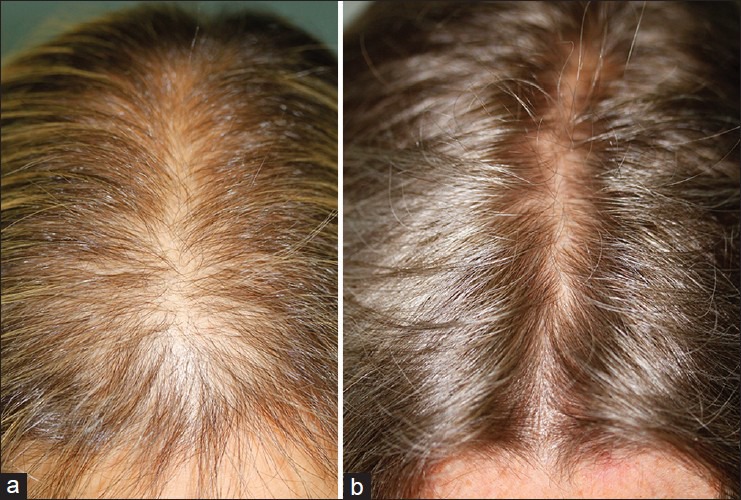
(a) Global photo assessment before (b) treatment and 12 months after
Four patients stated maintained libido reduction and an increase in liver enzymes was observed in one patient and normalized without treatment discontinuation.
The subjective and objective an improvement of patients was statistically significant (c2 linear tendency, P < 0.001) at months 6, 12, and 18 considering that a group of women with AGA will not spontaneously improve their condition. The percentage of highly improved patients was higher in patients <60 years (12/20) and in patients 60-70 years (4/13) than in group >70 years.
DISCUSSION
Up to our knowledge, there is only one study[10] with a large number of patients and 5 mg/day dose favors finasteride efficacy but has a possible bias as patients were also medicated with cyproterone acetate/ethinylestradiol to avoid pregnancy.
Eun et al.[11] reported a randomized, double-blind, placebo-controlled, phase III study enrolling 153 men that were randomized to receive 0.5 mg/day of dutasteride or placebo for 6 months, and they concluded that there was an improvement on hair growth, and it was a well-tolerated treatment on male AGA, even though it was a short duration the study.
There is also a report concerning the improvement of male pattern hair loss in a randomized study in identical twins with 0.5 mg/day of dutasteride for a longer period (12 months).[12]
Olszewska and Rudnicka[13] reported a clinical case of a 46-year-old woman with the AGA non-responsive to minoxidil who was treated initially with finasteride 1 mg/day but to due to limited improvement with this dose she was treated with dutasteride 0.5 mg/day for 12 months with the clinical improvement.
Our results support that 5 mg/day of finasteride is effective for the treatment of AGA in postmenopausal women in the absence of clinical or laboratory signs of hyper-androgenism. The same impression was obtained previously in a study performed with a South Korean population[10] (non-exclusively postmenopausal population) and in a study enrolling over five postmenopausal woman.[8]
One major difference in our study was that the effectiveness cannot be due to oral contraceptives anti-androgenic effect (which may occur in studies involving pre-menopausal populations).
Although an improvement tendency over time can be seen, in most cases improvement both subjective and objective (by photo assessment), was detectable at first observation (6 months). This is essential as probably it is not useful to maintain treatment over 6 months, if a lack of response is observed at that time.
Patients over 70-years are more prone to poor response; in this particular group finasteride might not be the first treatment option.
Authors believe that the lack of efficacy of finasteride in previous studies[4,5,6,7,8,9] is due to a low dose of finasteride (1-2.5 mg/day) and reduced number of patients enrolled.
Oral 5 mg/day finasteride was well tolerated by all patients. Even patients with a libido reduction did not want to discontinue treatment as they considered this adverse effect more tolerable than alopecia.
The main limitations of this study were that has been based in subjective and semi-quantitative assessment and had no placebo group. This was a private patient population and is extremely difficult to have informed consent for a tattoo, crucial for reproducibility of quantitative assessment (hair count and density). The majority of our patients with hair disease consultation is referred by other dermatologists and has suffered from many unsuccessful previous treatments. This is the main reason why it becomes almost impossible having a placebo group as all patients want to get treatment.
Multiple studies have confirmed the benefit of 5α-reductase inhibitors in men with AGA. There are few data available regarding treatment of female AGA whit this drugs; our study supports the fact that finasteride 5.0 mg/day improves this condition, and it might be a treatment option prior to switching to a less selective 5α-reductase inhibitor.
Further studies should be performed to a better understanding of the minimal finasteride effective dose as well as to predict a non-responder patient. Long-term adverse effects are also a fundamental concern. Maybe in a near future the test of variant repeat nucleotide sequences in exon 1 of the androgen receptor gene described by Keene et al.[14] will be useful to determine the patients responsiveness to higher doses of finasteride.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ludwig E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97:247–54. doi: 10.1111/j.1365-2133.1977.tb15179.x. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman KD, Olsen EA, Whiting D, Savin R, DeVillez R, Bergfeld W, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride male pattern hair loss study group. J Am Acad Dermatol. 1998;39:578–89. doi: 10.1016/s0190-9622(98)70007-6. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro J, Kaufman KD. Use of finasteride in the treatment of men with androgenetic alopecia (male pattern hair loss) J Investig Dermatol Symp Proc. 2003;8:20–3. doi: 10.1046/j.1523-1747.2003.12167.x. [DOI] [PubMed] [Google Scholar]
- 4.Price VH, Roberts JL, Hordinsky M, Olsen EA, Savin R, Bergfeld W, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43:768–76. doi: 10.1067/mjd.2000.107953. [DOI] [PubMed] [Google Scholar]
- 5.Thai KE, Sinclair RD. Finasteride for female androgenetic alopecia. Br J Dermatol. 2002;147:812–3. doi: 10.1046/j.1365-2133.2002.49084.x. [DOI] [PubMed] [Google Scholar]
- 6.Shum KW, Cullen DR, Messenger AG. Hair loss in women with hyperandrogenism: Four cases responding to finasteride. J Am Acad Dermatol. 2002;47:733–9. doi: 10.1067/mjd.2002.124608. [DOI] [PubMed] [Google Scholar]
- 7.Trüeb RM Swiss trichology study group. Finasteride treatment of patterned hair loss in normoandrogenic postmenopausal women. Dermatology. 2004;209:202–7. doi: 10.1159/000079890. [DOI] [PubMed] [Google Scholar]
- 8.Valsecchi R, Leghissa P, Riva M. Female androgenetic alopecia treated by finasteride: A case forward. Acta Derm Venereol. 2004;84:488–9. [PubMed] [Google Scholar]
- 9.Iorizzo M, Vincenzi C, Voudouris S, Piraccini BM, Tosti A. Finasteride treatment of female pattern hair loss. Arch Dermatol. 2006;142:298–302. doi: 10.1001/archderm.142.3.298. [DOI] [PubMed] [Google Scholar]
- 10.Yeon JH, Jung JY, Choi JW, Kim BJ, Youn SW, Park KC, et al. 5 mg/day finasteride treatment for normoandrogenic Asian women with female pattern hair loss. J Eur Acad Dermatol Venereol. 2011;25:211–4. doi: 10.1111/j.1468-3083.2010.03758.x. [DOI] [PubMed] [Google Scholar]
- 11.Eun HC, Kwon OS, Yeon JH, Shin HS, Kim BY, Ro BI, et al. Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: A randomized, double-blind, placebo-controlled, phase III study. J Am Acad Dermatol. 2010;63:252–8. doi: 10.1016/j.jaad.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Stough D. Dutasteride improves male pattern hair loss in a randomized study in identical twins. J Cosmet Dermatol. 2007;6:9–13. doi: 10.1111/j.1473-2165.2007.00297.x. [DOI] [PubMed] [Google Scholar]
- 13.Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol. 2005;4:637–40. [PubMed] [Google Scholar]
- 14.Keene S, Goren A. Therapeutic hotline. Genetic variations in the androgen receptor gene and finasteride response in women with androgenetic alopecia mediated by epigenetics. Dermatol Ther. 2011;24:296–300. doi: 10.1111/j.1529-8019.2011.01407.x. [DOI] [PubMed] [Google Scholar]


