Sir,
It is a well-established fact that endocrine disorders such as hypothyroidism, hyperthyroidism, and parathyroid disorders can cause hair loss. In thyroid dysfunction, other than scalp hair, hair on other parts of the body may also be affected, such as eyebrows and body hair. Diffuse hair loss is sometimes the presenting symptom of hypothyroidism.[1] It is well-known that thyroid hormone is essential for the development and maintenance of the hair follicle. Trichograms from the parietal and occipital areas in a study showed increased dysplastic and broken hairs strengthening the view that alopecia in thyroid disease is not caused by changes within hair cycle, but probably by impaired hair quality.[2]
Although, there are many studies relating to thyroid and hair loss they are all based on a univariate analysis. Here, we have attempted to study multiple variables of alopecia with relation to thyroid disorder.
All patients who attended the clinic from December 2007 to December2009 (25 months) with the complaints of hair loss of any part of the body were seen and classified into diffuse alopecia, alopecia areata, androgenetic alopecia, cicatricial alopecia, alopecia totalis, alopecia universalis, madarosis, diminished facial hair (moustache and beard), and diminished body hair based on clinical diagnosis. They were again categorized sex wise and age wise and the relationship of each group to thyroid disorder was studied. Any associated clinical condition with each type of alopecia was noted and again their relation to thyroid dysfunction was recorded. All patients were tested for thyroid stimulating hormone (TSH) and auto antibodies to thyroid peroxidase (TPOAb) by electro-chemiluminescence immunoassay, a third generation assay with a sensitivity of 0.001 mIU/L. The reference range of test values for TSH are as follows: Euthyroid: 0.4-4, hyperthyroidism: <0.1, subclinical hypothyroidism: 4-20, hypothyroidism: >20. TPOAb values below 60 U/ML were considered normal. Definitions used were: Euthyroid: Normal values of TSH and TPOAb, hypothyroid: Patients with an increased TSH and TPOAb and those with raised TSH but normal TPOAb, hyperthyroid: Patients with subnormal TSH values and normal TPOAb and those with subnormal TSH and raised TPOAb, subclinical: Patients with normal TSH but raised TPOAb. Data were analyzed using the Statistical Package for the Social Sciences (SPSS) Version 16.0 (IBM Inc, USA).
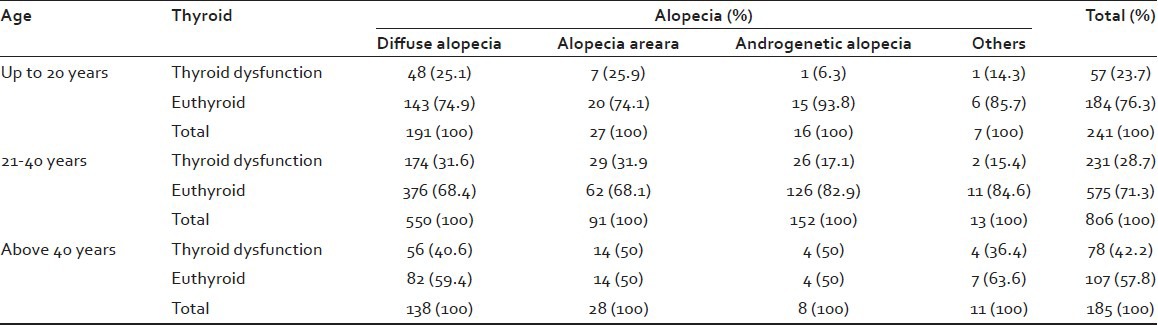
A total of 1232 patients were seen during a period of 25 months. The main types of alopecia seen were diffuse alopecia (71.35%), alopecia areata (11.8%), and androgenetic alopecia (14.29%). The others comprised of alopecia totalis (0.16%), alopecia universalis (0.41%), cicatricial alopecia (0.32%), madarosis (0.89%), diminished/absent facial hair (0.57%), and diminished body hair (0.16%). Since, the sample size of these was small they were clubbed together as “others” in the analysis. A significant difference between females and males was seen in diffuse alopecia and androgenetic alopecia. In the case of alopecia areata, no significant difference was noticed between females and males [Table 1]. It was found that there was a significant difference in thyroid dysfunction with respect to sex (P < 0.001). Initially patients were analyzed on a decade basis that is, 0-10 years, 11-20 years, 21-30 years, 31-40 years, 41-50 years, and >50 years. However, as the number of patients in some of the groups were nil or very small, they were regrouped into three groups for ease of analysis into 0-20 years, 21-40 years, and more than 40 years. More number of patients with complaints of alopecia was seen in the 21-40 age groups. Of this diffuse alopecia was the commonest (44.64%) followed by androgenetic alopecia. In the age groups of 0-20 years and 21-40 years thyroid dysfunction was seen more in alopecia areata and diffuse alopecia, showing almost equal prevalence, whereas in the group above 40 years thyroid dysfunction was noted more in alopecia areata and androgenetic alopecia (50%). Here, again there was a significant difference in thyroid dysfunction with respect to age (P < 0.001) [Table 2]. It was seen that as age advanced the thyroid dysfunction associated with alopecia also increased and this was reflected in all types of alopecia.
Table 1.
Alopecia – Sex-wise distribution
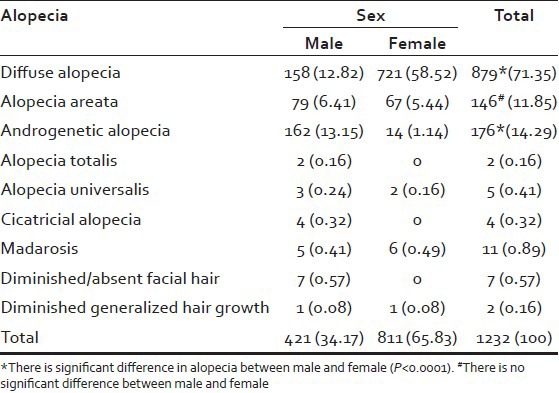
Table 2.
Thyroid, alopecia, age cross-tabulation
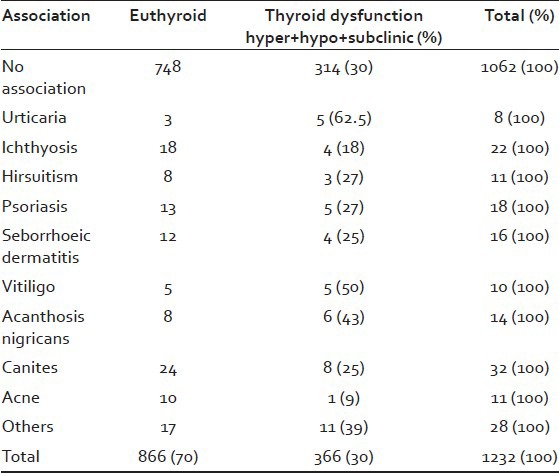
The main associations seen were urticaria, ichthyosis, hirsuitism, psoriasis, seborrheic dermatitis, acanthosis nigricans, premature greying, and acne. Other conditions such as lichen planus, systemic lupus erythematosis steatocystomas, para-psoriasis, facial pigmentation, lichen amyloidosis, herpes genitalis were seen in very small numbers and as the sample size was very small, they were all considered together as “others.” The relationship of these associated conditions with thyroid abnormality is given in Table 3. Although, madarosis is commonly stated to be associated with thyroid dysfunction in our study we found only 11 patients to be havingmadarosis and out of that only 2 patients showed thyroid dysfunction out of which 1 was hypothyroid (1%) and the other was in the subclinical group (1%). Premature greying was the most common association seen. Significant association with alopecia and thyroid dysfunction was found in urticaria (62.5%), vitiligo (50%), acanthosis nigricans (43%), premature greying (25%), hirsuitism (27%), psoriasis (27%), seborrheic dermatitis (25%), and ichthyosis (18%). Other conditions seen associated could be considered incidental as sample size was very small.
Table 3.
Relationship between thyroid dysfunction and clinical associations
The present study has described the different patterns of alopecia seen among a cross-section of alopecia patients and their relation to thyroid dysfunction, age, sex, and associated conditions. Such a comprehensive study is not available in the literature. As this is a descriptive study only there is no scope for comparison with a normal population. It is worth mentioning that a cross-sectional population survey conducted among the residents of urban coastal area of central Kerala, similar to our locality, showed a high prevalence of thyroid dysfunction (19.6%) among the adult population, where females predominated over males (23.6% vs. 13.3%),[3] similar to the results in our study, where females were more among alopecia patients and also with thyroid dysfunction. This is in keeping with other studies of the general population where women were found to have a higher prevalence of thyroid dysfunction. In the higher age group also women prevailed as thyroid dysfunction is more in perimenopausal and postmenopausal women.[4] Thyroid dysfunction is reported in children with alopecia areata and our study also showed a high prevalence of thyroid dysfunction in the group 0-20. Hence, routine screening of all children coming with any type of alopecia is recommended.
Thyroid autoimmunity is well documented in alopecia areata. In androgenetic alopecia multilayered interactions between thyroid hormones and androgens may contribute to the development of alopecia. In females, significant hypophyseal hypothyroidism may play a role in androgenetic alopecia.[5]
In the screening tests for thyroid, TSH assay methodology has undergone dramatic improvements that have revolutionized strategies for thyroid testing and firmly established TSH as the first-line thyroid function test to assess thyroid hormone status for most of the clinical conditions.[6] Both thyroglobulin and thyroid peroxidase antibodies are markers of thyroid autoimmunity. They are found in a small percentage of the normal population. TPOAb, in addition to anti-thyroglobulin antibody, was a clinically useful marker for defining autoimmune thyroid diseases and for detecting an underlying autoimmune process in the thyroid. From the clinical point of view thyroglobulin antibody are less prevalent than TPOAb and less useful than TPOAb for prediction of thyroid dysfunction.[7] Raised TPOAb is a good predictor of hypothyroidism and other autoimmune disorders.[8] High levels of thyroid antibodies with raised TSH strongly suggest progression to overt hypothyroidism.[9]
Skin findings observed in thyroid dysfunction at a study at Numune Education and Research Hospital in Ankara, Turkey were chronic urticaria (6.8%), vitiligo (6.8%), diffuse alopecia (6%), acne vulgaris (5%), and acne rosacea (306%). In our study, patients were seen to have significantly higher association of urticaria and vitiligo as co-association of alopecia with thyroid dysfunction.
The present study has its limitations. Screening for thyroid was based on TSH and TPOAb which are good markers for the purpose. It was not followed-up with additional tests as many of the patients could not afford the cost. However, based on our observations, we recommend that all patients coming with alopecia, irrespective of the pattern of alopecia should be screened for thyroid and TSH and TPOAb are good screening tools and cost effective.
ACKNOWLEDGMENT
We thank Mr. Somasekhara Pillai M., Assistant Professor in Statistics, University College, Trivandrum and Prof. Suja, Department of Dermatology and Venereology, Medical College, Trivandrum.
REFERENCES
- 1.Church RE. Hypothyroid hair loss. Br J Dermatol. 1965;77:661–2. [Google Scholar]
- 2.Sterry W, Konrads A, Nase J. Alopecia in thyroid diseases: Characteristic trichograms. Hautarzt. 1980;31:308–14. [PubMed] [Google Scholar]
- 3.Usha Menon V, Sundaram KR, Unnikrishnan AG, Jayakumar RV, Nair V, Kumar H. High prevalence of undetected thyroid disorders in an iodine sufficient adult south Indian population. J Indian Med Assoc. 2009;107:72–7. [PubMed] [Google Scholar]
- 4.Pearce EN. Thyroid dysfunction in perimenopausal and postmenopausal women. Menopause Int. 2007;13:8–13. doi: 10.1258/175404507780456746. [DOI] [PubMed] [Google Scholar]
- 5.Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13:3–126. doi: 10.1089/105072503321086962. [DOI] [PubMed] [Google Scholar]
- 6.Gharib H, Tuttle RM, Baskin HJ, Fish LH, Singer PA, McDermott MT, et al. Subclinical thyroid dysfunction: A joint statement on management from the American Association of Clinical Endocrinologists, the American Thyroid Association, and the Endocrine Society. [Posted 2005 Mar 23];Endocr Pract. 2004 10:497–501. doi: 10.4158/EP.10.6.497. Available from: http://www.medscape.com . [DOI] [PubMed] [Google Scholar]
- 7.Clinical strategies in the testing of thyroid function. Jim Stockigt. [Accessed on Dec 2010]. Available from: http://www.thyroidmanager.org/chapter 6/6b-frame.htm .
- 8.Inukai T, Takemura Y. Anti-thyroid peroxidase antibody. Nihon Rinsho. 1999;57:1819–23. [PubMed] [Google Scholar]
- 9.Scherbaum WA. On the clinical importance of thyroid microsomal and thyroglobulin antibody determination. Acta Endocrinol Suppl (Copenh) 1987;281:325–9. doi: 10.1530/acta.0.114s325. [DOI] [PubMed] [Google Scholar]


