Abstract
Background
Most neuroimaging studies exploring brain response to different acupoints have been performed in healthy adults.
Objective
The aim of this study was to compare brain responses to acupuncture at local versus distal acupoints in patients with carpal tunnel syndrome (CTS), who have chronic pain, versus healthy controls (HC) and correlate these responses with median nerve function.
Materials and Methods
Brain response to electroacupuncture (EA; 2Hz) was evaluated with event-related functional MRI (fMRI) in patients with CTS (n=37) and age-matched HC (n=30). EA was applied at acupoints local (PC 7 to TW 5) and distal (SP 6 to LV 4) to the CTS lesions.
Results
Brain response in both groups and acupoints included activation of the bilateral secondary somatosensory cortex (S2) and insula, and the contralesional primary somatosensory cortex (cS1). Deactivation was noted in ipsilesional primary somatosensory cortex (S1). A significant difference between local and distal acupoints was found in cS1 for HC, but not CTS. Furthermore, cS1 activation by EA at local acupoints was negatively correlated with median nerve peak sensory latency in HC, but was positively correlated in CTS. No correlation was found for EA at distal acupoints for either group.
Conclusions
Brain response to EA differs between CTS and HC and, for local acupoint stimulation, is associated with median nerve function, reflecting the peripheral nerve pathophysiology of CTS.
Key Words: Functional MRI (fMRI), Median Nerve Sensory Latency
Introduction
Many neuroimaging studies have characterized the brain response to acupuncture stimuli at various acupoints. Most studies have applied functional MRI (fMRI) for noninvasive assessment of acupuncture brain response to stimulation at various acupoints.1 Interestingly, a recent fMRI meta-analysis suggested that brain response to stimulation at different acupoints produces a specific brain response.2 However, only a few individual fMRI studies evaluated more than a single acupoint with direct statistical contrasts corrected for many comparisons.3,4 Hence, acupoint and meridian specificity in brain response has remained a controversial subject.
In addition, most acupuncture neuroimaging studies have characterized brain response to acupuncture in healthy adults as opposed to the responses in patient populations.3,5,6 Acupuncture and Traditional Chinese Medicine are usually not clinically applied to healthy adults but have been widely applied as alternative treatments for patients with chronic pain, including that caused by carpal tunnel syndrome (CTS). CTS is mainly driven by partial deafferentation secondary to compression of the median nerve within the carpal tunnel at the wrist.7 CTS manifests clinically as slowing of median nerve sensory conduction velocity, as well as causing local pain and paresthesia. Unlike functional pain disorders, such as fibromyalgia, nonspecific low-back pain, or irritable bowel syndrome, the peripheral pathophysiology of CTS is well-understood and limited to a focal area. In fact, the current authors' previous studies have noted altered somatosensory processing in the contralateral primary somatosensory cortex (cS1) for tactile stimuli delivered to median-nerve innervated fingers on an affected hand.8 Thus, CTS exemplifies an ideal chronic pain model to differentiate brain response to different acupoints located relative to a discrete, focal lesion.
The current authors' previous studies have broadly characterized brain response to acupuncture in CTS patients.9 In one study, it found that, compared to healthy controls (HC), patients with CTS had more-pronounced deactivation in a number of limbic brain areas as a response to manual acupuncture at acupoint LI 4. However, the sample size for this pilot study was small, and brain response to acupuncture was not evaluated for electroacupuncture (EA) stimulation at more clinically relevant acupoints in the wrist. Moreover, previous studies have not compared brain response to acupuncture stimulation at acupoints local to the CTS lesion (i.e., carpal tunnel) versus acupoints over body locations far from or distal to this lesion. Finally, as CTS is characterized by slowing of electrical impulses over the median nerve, this objective nerve-conduction metric of pathophysiology should be investigated with respect to its role in any differences in brain response in patients with CTS versus HCs.
This cross-sectional study evaluated brain response to EA applied to local (wrist) and distal (leg) acupoints for both patients with CTS and HCs. It was hypothesized that acupuncture delivered to local and distal acupoints in patients with CTS would produce distinctive cortical activation, particularly in somatosensory brain regions. Specifically, as the current authors' previous studies found maladaptive functional neuroplasticity in cS1,8 for the current study, it was hypothesized that cortical activation to acupuncture in cS1 would differ between patients with CTS and HCs, and would correlate with median nerve function.
Materials and Methods
Subjects
Subjects with histories of pain and/or paresthesia in the median nerve>3 months in duration were enrolled. All subjects were acupuncture naïve and were examined by a physiatrist at Spaulding Rehabilitation Hospital for eligibility, which included testing of median and ulnar sensory/motor nerve conduction latency (Cadwell Sierra EMG/NCS Device, Kennewick, WA). Contraindications to MRI; history of diabetes mellitus; cardiovascular, respiratory, or neurological illnesses; rheumatoid arthritis; wrist fracture with direct trauma to the median nerve; current usage of prescriptive opioid medication; severe thenar atrophy; acupuncture treatment (manual, EA, transcutaneous electrical nerve stimulation); nerve entrapment other than median nerve; cervical radiculopathy or myelopathy; generalized peripheral neuropathy; and blood dyscrasia or coagulopathy or current use of anticoagulation therapy were excluded from the study. A total of 67 subjects, including 37 subjects with CTS (48.5±10.0, mean±standard deviation [SD], 30 F) and 30 age-matched HCs (47.5±9.6, 19 females [F]) were eligible for the study. All study protocols were approved by the Massachusetts General Hospital and Partners Human Research Committee, and written informed consent was obtained from all subjects.
Acupuncture Procedure
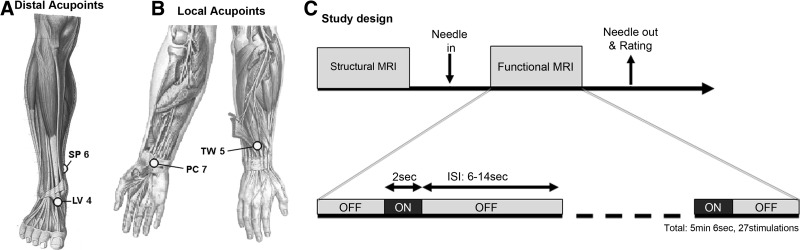
MRI-compatible titanium needles, 0.2 mm in diameter and 35–50 mm in length (DongBang Aucpuncture Inc. Boryeong, Korea), were used. Acupuncture was performed by a licensed acupuncturist trained to needle in the scanner environment. Subjects were randomized to receive either local or distal acupuncture stimulation. Acupuncture needles were inserted and placed to elicit the De Qi sensation. During the fMRI session, in each subject, EA was performed between acupoints either local (PC 7 to TW 5 in the wrist that was more affected by CTS and the dominant hand in HCs) or distal (SP 6 to LV 4 in the leg contralateral to the hand that was more affected by CTS and the dominant hand in HCs) to the lesion (Fig. 1A and B). Both PC 7 and TW 5 have been noted for relieving hand and/or wrist pain or paresthesias in the fingers.10 The distal acupoints SP 6 and LV 4 were chosen on the ankle opposite to the wrist CTS lesion to mimic one of the mirror acupuncture styles commonly used to treat wrist pain/tingling.11 For both local and distal pairs of acupoints, needles were stimulated at 2Hz using a current-constant biphasic EA device (HANS LH202H, Neuroscience Research Center, Peking University, Beijing, China). A frequency of 2 Hz for EA was chosen, as this frequency was also used in clinical treatments for the current authors' ongoing longitudinal study and was also used in the current authors' previous, successful pilot study of acupuncture for CTS.9,12 For practical reasons, a lower frequency of stimulation was chosen, as higher EA frequencies were more likely to induce paresthesias when piloting stimulation parameters in patients who had CTS during those previous studies. In all cases, EA intensity was set to deliver moderately strong—but not painful—stimulation prior to the scan.
FIG. 1.
Acupoints and schematic scan session. Electroacupuncture was performed at both (A) local (PC 7 to TW 5) and (B) distal (SP 6 to LV 4) acupoints. (C) Study protocol and event-related design. MRI, magnetic resonance imaging; sec, seconds; ISI, interstimulus interval; min, minutes.
Data Acquisition
Structural imaging data were acquired by a multiecho MPRAGE T1-weighted pulse sequence (TR=2530 ms, TE1/TE2=1.64/30.0ms, TI=1200 ms, flip angle=7°, field of view [FOV]=256×256, slices=176, sagittal acquisition, spatial resolution=1×1×1 mm3). fMRI data were acquired by using a gradient echo BOLD T2*-weighted pulse sequence (TR/TE=2000/30 ms, FOV=200×200 mm, 32 axial slices parallel to the anterior/posterior commissural plane, voxel size=3.125×3.125×3.6 mm, flip angle=90°) on a 3.0T Siemens Trio (Siemens Medical, Erlangen, Germany) equipped with 32-channel head coil. Subjects lay supine in the scanner with earplugs to attenuate gradient noise. Subjects were informed to expect intermittent acupuncture stimulation (on and off), and were instructed to close their eyes and focus on the stimulus, as spatial attention might influence brain response and should be controlled. EA at either local or distal acupoint pairs was applied to subjects who had CTS, depending on group randomization. HC subjects experienced EA at both local and distal at the separate sessions, which were at least 1 week apart. An event-related design was used (2-second stimulation events with randomized interstimulus interval (ISI), 6–12 seconds, and total scan time of 5 minutes and 6 seconds, Fig. 1C). After the fMRI scan, all subjects were asked to rate the intensity of sensations using the Massachusetts Acupuncture Sensation Scale (MASS),13 which was used to evaluate differences in both individual (0; none, 10 very strong) and summed sensations using the MASS Index (MI).13 Anxiety was assessed on a scale from −5 (very relaxed) to 5 (very anxious).
Data Analysis
Demographic and clinical data were compared between groups with a Student's t-test at a significance level of p<0.05 (SPSS version 10.0.7, Chicago, SPSS, Inc). Psychophysical responses to EA and EA stimulus intensity were analyzed with a 2×2 factorial analysis of variance (ANOVA) with factors GROUP (CTS and HC) and SITE (local and distal). Correlations between De Qi sensation (via MI) and median nerve peak latency (PL) were calculated within-group.
fMRI data were preprocessed using the FMRIB software Library (FSL v. 4.1), Freesurfer (v. 5.1.) and AFNI (v. 2.). fMRI data were first co-registered to each subject's structural MRI data (Freesurfer, bbregistration).14 Preprocessing included slice timing correction, motion correction, high pass filtering (cutoff period=50 seconds), and spatial Gaussian smoothing (full width at half maximum [FWHM]=5 mm; FSL, Feat). Preprocessed fMRI data were then analyzed using a general linear model for all subjects (FSL, Feat), contrasting on and off periods of EA stimulation. The resultant parameter estimates from all subjects were nonlinearly transformed to standard Montreal Neurological Institute (MNI) space (FSL, FNIRT) to perform further group analyses. Registration was ensured by visualization with AFNI software. To explore laterality of response, parameter estimates for subjects who had CTS and whose most-affected hand was the left hand (and hence local EA was performed on the left hand) had their fMRI parameter estimates mirrored or flipped across the midsagittal plane before passing them up to the group analysis. This allowed evaluation directly of responses found with respect to the study research questions in brain areas known to be lateralized relative to the stimulated side (i.e., S1, primary motor cortex [M1], ventro–postero–lateral thalamus).
Group maps were calculated using a mixed-effects statistical model (FSL, Feat FLAME1). Statistical maps were cluster-corrected for multiple comparisons, using a cluster-forming threshold of z=2.3 and a cluster-size threshold of p<0.05. Difference maps used two-sample Student's t-tests to contrast groups and sites, while a paired Student's t-test was used to contrast brain response to local versus distal EA in HCs (n=14, 7 F).
Significant results from the above analyses were passed on to region of interest (ROI) analysis. To evaluate the influence of median nerve function on brain response in the contralateral primary somatosensory cortex (cS1) to the hand that was more affected by CTS, the most significant voxel within the cS1 cluster was taken from all group maps (distal acupoint stimulation generated cS1 clusters in both CTS and HC subjects, which were just subthreshold, but were clearly somatotopic). The percent of signal change in each voxel was calculated and was correlated with median nerve peak latency for both CTS and HC subjects.
Results
Demographic and Clinical Features
Subjects with CTS reported significant chronicity (Table 1), as symptom duration was 8.5±9.1 (mean±SD) years. Median nerve sensory peak latency was significantly greater in CTS, compared to HC (CTS: 4.8±1.0 ms and HC: 3.4±0.4; mean±SD; p<10–9). There were no differences in ulnar nerve sensory latency between CTS and HC (CTS: 3.3±0.4 ms and HC: 3.3±0.4 mean±SD; p=0.58). Median nerve motor latencies were significantly greater in CTS, compared to HC (CTS: 5.0±1.3 ms and HC: 3.3±0.5 mean±SD; p<10–9). Ulnar nerve motor latencies were not different between CTS and HC (CTS: 2.9±0.4 ms and HC: 3.0±0.4 mean±SD; p=0.8).
Table 1.
Demographic and Clinical Characteristics of Subjects
| Factor | CTS (n=37, 30 F) | HC (n=30, 19 F) | p-Value |
|---|---|---|---|
| Age (years±SD) | 48.5±10.0 | 47.5±9.6 | n.s. |
| Median N. sensory latency (ms) | 4.8±1.0 | 3.4±0.4 | <0.001 |
| Ulnar N. sensory latency (ms) | 3.3±0.4 | 3.3±0.4 | n.s. |
| Median N. motor latency (ms) | 5.0±1.3 | 3.3±0.5 | 0.001 |
| Ulnar N. motor latency (ms) | 2.9±0.3 | 2.9±0.3 | n.s. |
| Symptom duration (year) | 8.5±9.1 | n.s. | n.s. |
CTS, carpal tunnel syndrome; F, females; HC, healthy control; SD, standard deviation, N., nerve; n.s., not significant.
Psychophysical Response to EA
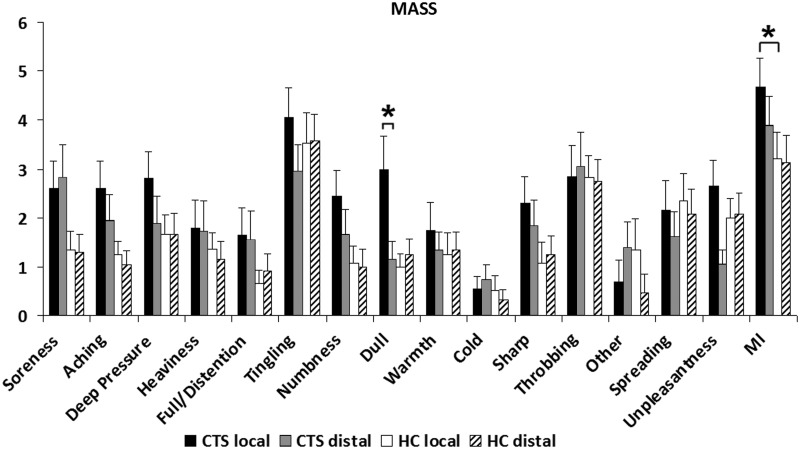
While the percept intensity was a priori matched for all conditions, the evaluation following the scan showed the similar pattern of sensations between groups (Fig. 2). MI (De Qi sensation) scores for the local and distal acupoint stimulation groups were 4.7±2.1 (mean±SD), and 3.9±1.9 for CTS, and 3.3±2.2 and 3.0±1.9 for HC, respectively. ANOVA demonstrated a significant main effect of GROUP (CTS versus HC, F (1, 81)=6.47, p<0.02). There was neither a significant effect of SITE (local versus distal, F (1, 81)=0.96, p=0.33) nor significant interaction between GROUP and SITE (F (1, 81)=0.72, p=0.40). Post hoc testing revealed that there was a significant difference in MI between CTS and HC for local acupoint stimulation (p<0.02). In addition, for individual sensations, there was a significant main effect of GROUP (F (1, 81)=5.70, p<0.02) for dull sensation. There was also a significant interaction effect between GROUP and SITE (F (1, 81)=5.80, p<0.02) for dull sensation. Specifically, dull sensation was similar between local and distal acupoint stimulation for HC, but was significantly greater for local, compared to distal acupoint stimulation (local: 3.0±2.9, mean±SD* and distal: 1.2±1.6; p<0.05) for CTS. However, dull sensation was similar between CTS and HC for distal acupoints. All other comparisons for different MASS sensations were also nonsignificant. Anxiety (CTS: local: −1.0±2.7, mean±SD and distal: −0.6±2.6; HC: local: −1.2±2.7 and distal: −1.8±2.6) also did not differ between groups or sites.
FIG. 2.
Acupuncture sensations. The Massachusetts Acupuncture Sensation Scale (MASS) was used to evaluate De Qi and pain response to electroacupuncture. There was a significant main effect of GROUP as shown on the MASS Index (MI [i.e., De Qi sensation]) and both a significant effect of GROUP and a significant interaction effect between GROUP and SITE with respect to dull sensation. *Significant (p<0.05), error bars indicate standard error. GROUP, group assignment to CTS or HC; SITE, local or distal site.
EA stimulus intensities (electrical current) for local and distal acupoints were 1.5±0.7 mA (mean±SD) and 1.9±0.8 mA for CTS and 1.3±0.6 mA, 2.2±0.9mA for HC, respectively. There was a significant main effect of SITE (local versus distal) on EA stimulus intensity (F (1, 81)=16.20, p<0.001). There was neither a significant main effect of GROUP (F (1, 81)=0 .10, p=0.86) nor significant interaction between GROUP and SITE (F (1, 81)=1.90, p=0.17). Post hoc showed a significant difference between local and distal acupoint stimulation for HC (p<0.0001).
Correlation analysis revealed that there was a significantly positive correlation between De Qi sensation (MI) and median nerve sensory latency for the CTS local group (r=0.70, p<0.001). Thus, the more delayed median nerve conduction was, the greater was the De Qi sensation perceived by the subjects in the CTS local group. There were no significant correlations found for any other groups.
Brain Response to EA
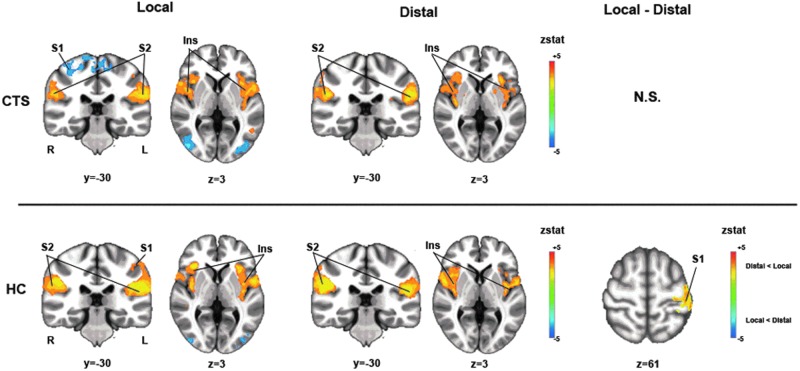
For both groups (CTS and HC) and both acupoints (local and distal), EA activated the bilateral S2, supplementary motor area (SMA), and insula (Fig. 3 and Table 2). Activation was also noted in cS1 and deactivation was noted in the ipsilateral S1 to the more affected hand in CTS and the dominant hand in HC during EA at local acupoints. EA at distal acupoints generated smaller cS1 clusters for both CTS and HC which were just subthreshold, but were clearly somatotopic (see Fig. 4 for these leg area clusters). While no differences between brain response to EA at local and distal acupoints was noted for CTS, for HC, it was noted that cS1, within the somatotopic wrist area, was more activated for local acupoint stimulation (Fig. 3).
FIG. 3.
Brain activation during electroacupuncture (EA) for carpal tunnel syndrome (CTS) and healthy control (HC). For both CTS and HC groups and both local and distal locations, EA activated the bilateral secondary somatosensory cortex (S2) and insula (Ins), as well as the contralateral primary somatosensory cortex (cS1) to the hand that was more affected. There was no significant difference in brain response during EA between local and distal acupoints for CTS, while, for HC, there was significantly greater activation in cS1 (hand area) for the local group compared to the distal group. R, right; L, left; N.S., not significant; zstat, z statistic.
Table 2.
Summary of Cortical Activation During Acupuncture in CTS and HC
| Group, site, and region | Side | X (mm) | Y (mm) | Z (mm) | p-Value | Cluster size (voxels) | max_z |
|---|---|---|---|---|---|---|---|
| CTS local (nonflipped) | |||||||
| S2 | R | 66 | −20 | 26 | 3.48E–15 | 4813 | 5.2 |
| Anterior insula | R | 36 | 16 | 4 | 3.5 | ||
| Posterior insula | R | 44 | −16 | 19 | 3.58 | ||
| Inferior parietal lobule | R | 58 | −28 | 28 | 4.79 | ||
| Superior temporal gyrus | R | 56 | 12 | 4 | 4.31 | ||
| Middle temporal gyrus | R | 56 | −50 | 3 | 3.18 | ||
| S1 | R | 36 | −26 | 52 | 6.56E–07 | 1671 | −3.78 |
| S2 | L | −62 | −22 | 20 | 4.83E–15 | 4750 | 4.64 |
| S1 | L | −54 | −22 | 44 | 2.74 | ||
| Insula | L | −40 | −2 | −4 | 4.02 | ||
| Cuneus | L | −16 | −90 | 30 | 2.55E–12 | 3603 | −4.05 |
| CTS local (flipped) | |||||||
| S1 | Ipsi | 38 | −26 | 50 | 1.19E–07 | 1885 | −3.84 |
| S1 | Contra | −54 | 20 | 43 | 5.89E–17 | 5566 | 3.79 |
| CTS distal (nonflipped) | |||||||
| Posterior insula | R | 34 | −18 | 16 | 2.6E–10 | 2639 | 4.02 |
| Anterior insula | R | 31 | 25 | −1 | 4 | ||
| Inferior frontal gyrus | R | 45 | 13 | 26 | 2.96 | ||
| Inferior parietal lobule | R | 50 | −26 | 26 | 2.5E–6 | 1394 | 4.47 |
| Superior temporal gyrus | R | 57 | −37 | 17 | 3.07 | ||
| S2 | L | −58 | −26 | 22 | 4.31E–13 | 3653 | 4.41 |
| Middle frontal gyrus | L | −50 | 2 | 15 | 3.0 | ||
| Supramarginal gyrus | L | −55 | −37 | 41 | 3.23 | ||
| Inferior parietal lobule | L | −53 | −34 | 27 | 4.18 | ||
| Anterior insula | L | −32 | 20 | 2 | 3.63 | ||
| Posterior insula | L | −36 | −20 | 12 | 3.69 | ||
| CTS distal (flipped) | |||||||
| M1 | Contra | −52 | −6 | 39 | 1.49E–13 | 3798 | 2.51 |
| HC local (nonflipped) | |||||||
| Inferior parietal lobule | R | 52 | −26 | 26 | 5.52E–13 | 5182 | 4.68 |
| Posterior insula | R | 52 | −40 | 17 | 4.13 | ||
| S2 | R | 60 | −18 | 17 | 4.68 | ||
| Inferior frontal gyrus | R | 34 | 25 | 4 | 4.49 | ||
| Superior temporal gyrus | R | 60 | 10 | 4 | 3.27 | ||
| PCC | 0 | −22 | 28 | 0.0122 | 687 | 3.99 | |
| S2 | L | −62 | −24 | 14 | 2.46E–14 | 5951 | 5.58 |
| Anterior insula | L | −32 | 22 | 5 | 4.14 | ||
| Posterior insula | L | −35 | −18 | 18 | 4.68 | ||
| Superior temporal gyrus | L | −56 | 2 | 5 | 4.49 | ||
| HC local (flipped) | |||||||
| S1 | Contra | −45 | −31 | 45 | 7.68E–15 | 6241 | 3.34 |
| HC distal (nonflipped) | |||||||
| Middle occipital gyrus | R | 42 | −86 | 6 | 0.0141 | 669 | −3.37 |
| Inferior parietal lobule | R | 52 | −28 | 24 | 2.43E–15 | 5042 | 6.18 |
| Superior temporal gyrus | R | 53 | 3 | 5 | 4.65 | ||
| Posterior insula | R | 33 | −21 | 17 | 5.71 | ||
| Anterior insula | R | 33 | 13 | 4 | 3.64 | ||
| Inferior frontal gyrus | R | 56 | 19 | 22 | 2.67 | ||
| Cuneus | L | −26 | −82 | 28 | 1.65E–05 | 1645 | −4.12 |
| Precuneus | L | −20 | −74 | 22 | −3.75 | ||
| Posterior insula | L | −52 | −32 | 20 | 6.33E–11 | 3156 | 5.66 |
| Anterior insula | L | −40 | 14 | 0 | 2.88 | ||
| S2 | L | −59 | −17 | 19 | 4.25 | ||
| Difference map | |||||||
| HC local–distal (flipped) | |||||||
| S1 | Contra | −48 | −34 | 62 | 3.74E–4 | 736 | 3.82 |
Notes: Brain response to electroacupuncture stimulation for each group (CTS and HC) and stimulation site (local and distal). In addition, brain areas known to be lateralized relative to stimulus site (e.g., thalamus, and primary somatosensory and motor cortices) were also evaluated in a R–L flipped analysis in which all subjects were analyzed as if stimuli were applied on a consistent side of the body. The Montreal Neurological Institute (MNI) coordinates (x, y, z) in bold represent the peak of the cluster of activation; p-value” represents the chance this cluster was activated or deactivated by error (type I error); cluster size represents the number of voxels within the cluster; and max_z represents the z-statistic of the peak voxel in the cluster.
CTS, carpal tunnel syndrome; HC, healthy control; S1 and S2, primary and secondary somatosensory cortices, respectively, R, right, L, left, Ipsi, ipsilateral; contra, contralateral to the side that was more affected; M1, primary motor cortex; PCC, posterior cingulate cortex.
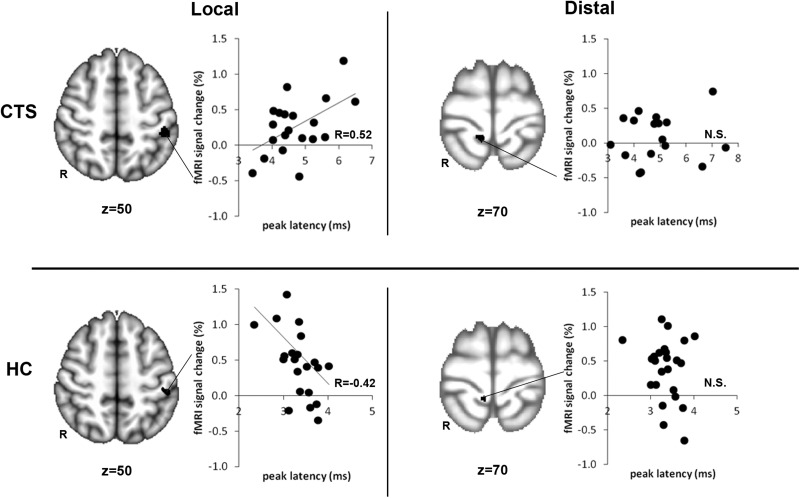
FIG. 4.
Brain response within contralateral S1 (cS1) to the hand that was more affected during electroacupuncture (EA) correlated with median nerve sensory latency. For carpal tunnel syndrome (CTS), activation in cS1 during EA at local acupoints was positively correlated with median nerve latency. Thus, the greater the dysfunction (i.e., slower median nerve conduction) was, the greater the activation was in cS1. For HC, activation in cS1 during EA at local acupoints was negatively correlated with median nerve latency. Thus, the greater median nerve conduction (i.e., faster median nerve conduction) was, the greater the activation was in cS1. This relationship did not exist for either CTS or healthy control (HC) during EA at distal acupoints. fMRI, functional magnetic resonance imaging; N.S., not significant; R to left of brain images, right; R in graphs, correlation coefficient.
ROI analyses revealed the influence of median nerve sensory latency on cS1 activation to EA (Fig. 4). For CTS, activation in the cS1 during EA at local acupoints was positively correlated with median nerve sensory latency (r=0.52, p<0.02). Thus, the greater the dysfunction (i.e., slower median nerve conduction) was, the greater was the activation in the cS1. For HC, the opposite was true. Activation in cS1 during EA at local acupoints was negatively correlated with median nerve sensory latency (r=–0.42, p<0 .05). Thus, faster nerve conduction was associated with greater activation in the cS1. Median nerve sensory latency was not correlated to brain response to EA at distal acupoints for either CTS or HC (p>0.1).
Discussion
This study investigated EA-induced brain response at acupoints local to the lesion in subjects who had CTS and compared brain and psychophysical response to that elicited by EA applied distal to CTS lesions. The main finding was that brain response in the cS1 differed between local and distal EA for HC, while it did not differ for CTS. Furthermore, activation in cS1 in response to EA at local acupoints in CTS was positively correlated with median nerve sensory latency but was negatively correlated with median nerve sensory latency in HC. This suggests that subjects who had CTS had a different relationship between median nerve function and cS1 activation in response to EA. Thus, for subjects who had CTS, the greater the dysfunction (i.e., greater latency caused by slower median nerve conduction) was, the greater was the cS1 activation. These correlations were not seen in distal groups, supporting the view that acupuncture response in the brain is directly related to patient-specific pathology (i.e., impaired median nerve conduction).
The results showed that EA at local and distal acupoints produced grossly similar sensations as measured with the MASS scale in both CTS and HC. The only differences were that subjects who had CTS experienced slightly stronger sensations, particularly a “dull” sensation, which was stronger for EA at acupoints local to the lesion. This result may have been caused by the known sensitization of peripheral receptors and neurogenic inflammation local to the lesion in subjects who had CTS.15 Interestingly, greater reported acupuncture-induced sensations might relate to the well-known Ah Shi phenomenon in acupuncture practice. Ah Shi points are locations of increased sensitivity or pressure pain that develop on the body. In this study, subjects who had CTS might have been expected to experience greater sensitivity at local (at the wrist) compared to distal (at the ankle) acupoints. However, if sensitization had reached multiple levels in the spinal cord and brain, leading to central hypersensitivity, greater sensitivity might have been experienced in both local and distal acupoints. There was evidence of both effects (i.e., subjects with CTS reported greater De Qi sensation induced by EA at both local and distal acupoints, compared to HC, and greater dull sensation at local, compared to distal, acupoints). These results support the existence of more-pronounced Ah Shi points in patients who have CTS, both in general but also specifically at the lesion sites.
Brain response to EA at both local and distal acupoints demonstrated mainly activation within somatosensory- and salience-processing regions including bilateral S2, contralateral S1 to the stimulation, SMA, and insula. This was the case for both HC and CTS, which is consistent with previous studies,3,4 such as those showing that EA at different acupoints induced broadly overlapped brain response for healthy adults.16,17 Lack of notable deactivation in limbic brain regions was inconsistent with previous studies18 and with a recent acupuncture fMRI meta-analysis.2 However, the current authors found that EA did produce deactivation in limbic, default mode network brain areas in both CTS and HC, but at a subthreshold level (data not shown). Thus, differences between the results of the current study and prior studies reporting limbic and default mode network (DMN) deactivations (e.g., Hui et al.)18 may have been caused by the current authors' more-conservative threshold with cluster-correction for multiple comparisons, although other differences also included the use of electrical versus manual stimulation and event versus block protocol designs. Previous EA fMRI studies showed that EA at GB 34 produced deactivation in only a rostral segment of the anterior cingulate cortex (ACC),19 or none at all,20 while broad brain activation was noted in multiple areas—such as the postcentral gyrus, insula, thalamus, and prefrontal cortex—in both studies.
Interestingly, cS1 activation was greater for local acupoint stimulation, compared to distal acupoint stimulation, in HC, but this pattern was not seen in CTS. The site of this cS1 cluster for HC was consistent with the hand representation in S1. Thus, the difference seen for HC was likely caused by the known somatotopy in cS1 following differential cortical representation for different body sites. As the current authors' previous studies demonstrated magnified and overlapping cortical representations in cS1 for subjects with CTS,8 the lack of local versus distal cS1 activation differences noted for CTS may have been caused by diminished surround inhibition and a general disinhibitory response in cS1 for stimulation across different body sites.8,21 From a clinical perspective, the lack of significant differences in brain response to EA at local versus distal acupoints in CTS supports the idea that this pathology can be treated successfully by both stimulation sites. In fact, acupuncture stimulation on the ankle contralateral to the wrist lesion has been advocated by several “mirror” styles of acupuncture treatment.
Lack of differentiation in brain response to different acupoints outside of cS1 is counter to previous studies exploring this effect,2,4 including the current authors' study that was published online last year.3 This adds to the growing controversy in acupoint specificity underlying differential brain response to different acupoints. Methodological details of the current authors' fMRI and acupuncture protocol may play an important role in why an acupoint-specific brain response was not seen outside of cS1. Stimulation of other acupoints, not known to have clinical efficacy in the case of CTS, may have, in fact, produced differential brain responses to local acupoint stimulation in subjects with CTS. Future studies should explore this directly. In addition, the lack of differences noted in the current study may have been caused by more-conservative statistical methods used to contrast acupoint stimulation brain maps as well as use of EA instead of the more typically used manual acupuncture (as was adopted in Napadow et al.3). In addition, it may be that EA is a more pure and deliberate somatosensory system modulation method for acupuncture needle stimulation. EA may thus be more appropriate for disease processes with pronounced disruption of somatosensory processing, such as CTS.
Furthermore, the current authors were intrigued to note that the intensity of activation in cS1 observed during EA at local acupoints was positively correlated with median nerve sensory latency in CTS. That is, the greater the dysfunction (i.e. greater median nerve sensory latency caused by slower median nerve conduction) was, the greater was the cS1 activation. This association was directly opposite to that seen for HC, whose median nerve sensory latencies are within the normal range. In HCs, activation in cS1 during EA at local acupoints was negatively correlated with median nerve sensory latency (i.e., faster median nerve conduction was associated with greater cS1 activation). The positive correlation seen for CTS may be explained by compensation, in that slowing afference from the periphery triggers central processing enhancements or augmentation. Thus, the brain attempts to compensate for slower signaling in the periphery by amplifying the signal in somatosensory processing regions of the brain. This viewpoint is consistent with previous CTS studies demonstrating greater fMRI activation22 or electrophysiological amplification21 in contralateral S1 following affected finger stimulation. S1 activity is known to reflect sensory-discriminative information such as sensation intensity.23,24 In fact, greater MI (i.e., De Qi sensation) was highly correlated with slower median nerve sensory conduction in this study. Taken together, this amplification from slower peripheral conduction afference may have also manifest in stronger sensations. Importantly, the correlation between cS1 response and median nerve conduction was not seen in the distal acupoint stimulation group, where the afference from the leg was intact, suggesting further that this difference between CTS and HC is specifically a result of the peripheral pathology characterizing CTS. It should be noted that different pathologies may show differences in brain processing of acupuncture stimulation. While this question was not explored in the current study, previous studies such as Yang et al.25 showed that traditional acupuncture in patients with acute migraine resulted in higher brain metabolism in the mid-temporal cortex, orbitofrontal cortex, insula, middle frontal gyrus, angular gyrus, PCC and middle cingulate cortex, with lowered brain metabolism in the parahippocampus, S1, and cerebellum, compared to a control acupuncture group. In general, patient populations with disrupted homeostasis may present with altered fMRI responses to acupuncture stimulation in the same way that acupuncture may decrease blood pressure (BP) in a hypertensive patient, increase BP in a hypotensive patient, and have no BP effect in an HC. Future studies should make direct comparisons between different patient populations who have known pathophysiological response characteristics in specific brain areas.
The current study had a few limitations. One limitation was the sample size. An unpaired Student's t-test did not have statistical power to show differences between EA at local and distal acupoints in both CTS and HC. Because of variability of each subject's brain response during EA, further study is warranted with larger sample sizes or use of a crossover study design. Finally, this study examined brain response to EA in a cross-sectional sample of acupuncture-naïve subjects with CTS. Repeated treatments typically performed in the clinical setting might alter brain plasticity and produce different brain responses during EA—an effect that should be evaluated in future studies.
Conclusions
Brain response to EA at local acupoint stimulation is associated with median nerve function differently between CTS and HC, reflecting the median nerve pathophysiology of CTS. This study also suggests that differences in brain response to EA at different acupoints is primarily reflected in a different magnitude of response within the S1 contralateral to the stimulation.
Footnotes
In all cases, the values for Psychophysical Response to EA are mean±SD.
Acknowledgments
The authors would like to thank NCCAM, National Institutes of Health for funding support (R01-AT004714 [Napadow], R01-AT004714-02S1 [Napadow], P01-AT002048 [Rosen, principal investigator for program project]).
Disclosure Statement
There are competing financial interests for any author of this article.
References
- 1.Dhond RP. Kettner N. Napadow V. Neuroimaging acupuncture effects in the human brain. J Altern Complement Med. 2007;13(6):603–616. doi: 10.1089/acm.2007.7040. [DOI] [PubMed] [Google Scholar]
- 2.Huang W. Pach D. Napadow V, et al. Characterizing acupuncture stimuli using brain imaging with fMRI—a systematic review and meta-analysis of the literature. PLoS One. 2012;7(4):e32960. doi: 10.1371/journal.pone.0032960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Napadow V. Lee J. Kim J, et al. Brain correlates of phasic autonomic response to acupuncture stimulation: An event-related fMRI study. Hum Brain Mapp. 2012 Apr 14; doi: 10.1002/hbm.22091. e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claunch JD. Chan ST. Nixon EE, et al. Commonality and specificity of acupuncture action at three acupoints as evidenced by fMRI. Am J Chin Med. 2012;40(4):695–712. doi: 10.1142/S0192415X12500528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kong J. Gollub RL. Polich G, et al. A functional magnetic resonance imaging study on the neural mechanisms of hyperalgesic nocebo effect. J Neurosci. 2008;28(49):13354–13362. doi: 10.1523/JNEUROSCI.2944-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong J. Gollub RL. Rosman IS, et al. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci. 2006;26(2):381–388. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiernan MC. Mogyoros I. Burke D. Conduction block in carpal tunnel syndrome. Brain. 1999;122(pt 5):933–941. doi: 10.1093/brain/122.5.933. [DOI] [PubMed] [Google Scholar]
- 8.Napadow V. Kettner N. Ryan A. Kwong KK. Audette J. Hui KK. Somatosensory cortical plasticity in carpal tunnel syndrome—a cross-sectional fMRI evaluation. NeuroImage. 2006;31(2):520–530. doi: 10.1016/j.neuroimage.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 9.Napadow V. Kettner N. Liu J, et al. Hypothalamus and amygdala response to acupuncture stimuli in carpal tunnel syndrome. Pain. 2007;130(3):254–266. doi: 10.1016/j.pain.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deadman P. Al-Khafaji M. Baker K. A Manual of Acupuncture. Ann Arbor: Journal of Chinese Medicine Publication; 1999. [Google Scholar]
- 11.Tan RT-F. Acupuncture 1, 2, 3. San Diego: Self-published; 2007. [Google Scholar]
- 12.Napadow V. Liu J. Li M, et al. Somatosensory cortical plasticity in carpal tunnel syndrome treated by acupuncture. Hum Brain Mapp. 2007;28(3):159–171. doi: 10.1002/hbm.20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong J. Gollub R. Huang T, et al. Acupuncture de qi, from qualitative history to quantitative measurement. J Altern Complement Med. 2007;13(10):1059–1070. doi: 10.1089/acm.2007.0524. [DOI] [PubMed] [Google Scholar]
- 14.Greve DN. Fischl B. Accurate and robust brain image alignment using boundary-based registration. NeuroImage. 2009;48(1):63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de-la-Llave-Rincon AI. Puentedura EJ. Fernandez-de-las-Penas C. New advances in the mechanisms and etiology of carpal tunnel syndrome. Discov Med. 2012;13(72):343–348. [PubMed] [Google Scholar]
- 16.Yan B. Li K. Xu J, et al. Acupoint-specific fMRI patterns in human brain. Neurosci Lett. 2005;383(3):236–240. doi: 10.1016/j.neulet.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Zhang WT. Jin Z. Luo F. Zhang L. Zeng YW. Han JS. Evidence from brain imaging with fMRI supporting functional specificity of acupoints in humans. Neurosci Lett. 2004;354(1):50–53. doi: 10.1016/j.neulet.2003.09.080. [DOI] [PubMed] [Google Scholar]
- 18.Hui KK. Liu J. Makris N, et al. Acupuncture modulates the limbic system and subcortical gray structures of the human brain: Evidence from fMRI studies in normal subjects. Hum Brain Mapp. 2000;9(1):13–25. doi: 10.1002/(SICI)1097-0193(2000)9:1<13::AID-HBM2>3.0.CO;2-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu MT. Sheen JM. Chuang KH, et al. Neuronal specificity of acupuncture response: A fMRI study with electroacupuncture. NeuroImage. 2002;16(4):1028–1037. doi: 10.1006/nimg.2002.1145. [DOI] [PubMed] [Google Scholar]
- 20.Na BJ. Jahng GH. Park SU, et al. An fMRI study of neuronal specificity of an acupoint: Electroacupuncture stimulation of Yanglingquan (GB 34) and its sham point. Neurosci Lett. 2009;464(1):1–5. doi: 10.1016/j.neulet.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Tecchio F. Padua L. Aprile I. Rossini PM. Carpal tunnel syndrome modifies sensory hand cortical somatotopy: A MEG study. Hum Brain Mapp. 2002;17(1):28–36. doi: 10.1002/hbm.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Napadow V. Dhond R. Kennedy D. Hui KK. Makris N. Automated brainstem co-registration (ABC) for MRI. NeuroImage. 2006;32(3):1113–1119. doi: 10.1016/j.neuroimage.2006.05.050. [DOI] [PubMed] [Google Scholar]
- 23.Coghill RC. Sang CN. Maisog JM. Iadarola MJ. Pain intensity processing within the human brain: A bilateral, distributed mechanism. J Neurophysiol. 1999;82(4):1934–1943. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- 24.Derbyshire SW. Jones AK. Gyulai F. Clark S. Townsend D. Firestone LL. Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain. 1997;73(3):431–445. doi: 10.1016/S0304-3959(97)00138-3. [DOI] [PubMed] [Google Scholar]
- 25.Yang J. Zeng F. Feng Y, et al. A PET–CT study on the specificity of acupoints through acupuncture treatment in migraine patients. BMC Complement Altern Med. 2012;12:123. doi: 10.1186/1472-6882-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]