Abstract
Helper-dependent adenoviral vectors (HDAd) have been shown to mediate a considerably longer duration of transgene expression than first-generation adenoviral vectors. We have previously shown that transgene expression from HDAd-transduced hepatocytes can persist at high levels for up to 2.6 years in nonhuman primates following a single-vector administration. Because duration of transgene expression and long-term toxicity are critical for risk:benefit assessment, we have continued to monitor these animals. We report here that transgene expression has persisted for the entire observation period of up to 7 years for all animals without long-term adverse effects. However, in all cases, transgene expression level slowly declined over time to less than 10% of peak values by the end of the observation period but remained 2.3–111-fold above baseline values. These results will provide important information for a more informed risk:benefit assessment before clinical application of HDAd.
Brunetti-Pierri and colleagues report long-term safety and expression data from baboons treated with a helper-dependent adenoviral vector expressing α-fetoprotein from a liver-specific promoter. In contrast to the transient expression observed with first-generation adenoviral vectors, all animals exhibited sustained, albeit declining, transgene expression, with no apparent vector-related toxicity.
Introduction
Because helper-dependent adenoviral vectors (HDAd) are devoid of all viral coding sequences, they can mediate very long-term transgene expression. For example, a single injection of HDAd into rodents can result in lifelong hepatic transgene expression, which is about 2 years (Kim et al., 2001; Toietta et al., 2005). Similarly, in nonhuman primates, we have demonstrated that hepatic transduction by HDAd can also provide up to 2.6 years of high-level transgene expression (Brunetti-Pierri et al., 2006, 2007, 2009, 2012). In addition, hepatic transgene expression for up to 2 years has been reported in the dog following a single injection of HDAd (Brunetti-Pierri et al., 2005a; McCormack et al., 2006). Because the duration of transgene expression impacts risk:benefit assessment, it is important to determine if HDAd-mediated hepatic transgene expression can persist for longer periods than what has been published, especially in a relevant preclinical large animal model. Equally important is to determine if any adverse consequences are associated with long-term hepatic transduction by HDAd. In this study, we report long-term observations of up to 7 years in nonhuman primates following a single administration of HDAd. The results herein will provide important information for assessing risk:benefit of HDAd-mediated, liver-directed gene therapy.
Materials and Methods
Helper-dependent adenoviral vectors
HDΔ21.7E4PEPCK-bAFP-WL contains a liver-restricted baboon alpha-fetoprotein (bAFP) expression cassette. This vector and its production and characterization have been described in detail elsewhere (Palmer and Ng, 2003, 2004; Brunetti-Pierri et al., 2006).
Animals and procedures
Adult male baboons (Papio sp.) were used in this study. Animal experiments were reviewed and approved by Institutional Animal Care and Use Committees of Baylor College of Medicine or Texas Biomedical Research Institute. Three routes of vector administration were used. Method 1 involved direct injection into the surgically isolated liver as detailed previously (Bruentti-Pierri et al., 2006). Method 2 mimicked hydrodynamic injection as detailed previously (Brunetti-Pierri et al., 2007). Method 3 involved direct injection of the vector, via the hepatic artery, into the liver while the hepatic outflow is occluded by a balloon catheter as detailed previously (Bruentti-Pierri et al., 2009). Serum bAFP was measured as previously reported (Brunetti-Pierri et al., 2006). Serum antivector neutralizing antibody (NAB) titers were determined as described elsewhere (Croyle et al., 2001) and expressed as the reciprocal dilution of serum that reduced transduction by 50%. Antivector NABs were undetectable in all animals before vector injection. At necropsy, a wide array of organs (brain, thymus, trachea, lung, heart, aorta, liver, pancreas, stomach, spleen, kidney, testis, skeletal muscle, and bone marrow) was collected for hematoxylin and eosin (H&E) histology.
Results and Discussion
Because Ad-mediated acute toxicity is dose-dependent, we previously developed three methods of vector administration to achieve preferential, high-efficiency hepatic transduction using low HDAd doses in nonhuman primates. Briefly, Method 1 involved injecting HDAd directly into the surgically isolated liver via the portal vein (Brunetti-Pierri et al., 2006). Method 2 mimicked hydrodynamic injection but without rapid, large-volume injection (Brunetti-Pierri et al., 2007) and was based on our observation that hydrodynamic injection in mice resulted in preferential hepatocyte transduction and reduced extrahepatic vector uptake (Brunetti-Pierri et al., 2005b). Method 3 involved direct injection, via the hepatic artery, into the transiently balloon-occluded liver (Brunetti-Pierri et al., 2009). All animals, vector doses, and routes of administration are summarized in Table 1. Using an HDAd expressing the bAFP from a liver-specific promoter, we showed that high serum bAFP levels were achieved and sustained for up to 2.6 years in all animals by all three methods of vector delivery. With respect to potential clinical application, risk:benefit assessment is affected by the duration of transgene expression; greater risk may be offset by longer therapeutic benefit. For this important reason, we have continued to measure transgene expression levels in these animals.
Table 1.
Summary of Animals, Procedures, Baboon Alpha-Fetoprotein Levels, and Neutralizing Antibody Titers
| Route | Baboon ID | HDAd dose (vp/kg) | Baseline bAFP (ng/ml) | Peak bAFP ng/ml (fold above baseline) | bAFP ng/ml (% of peak, fold above baseline) on the last day in original publication | bAFP ng/ml (% of peak, fold above baseline) on the last day of observation | NAB titers |
|---|---|---|---|---|---|---|---|
| Method 1 | 13947 | 1×1012 | 11.5 | 7,935.4 (690-fold) on day 21 | 2,491 (31.4%, 217-fold) on day 665 | 262 (3.3%, 23-fold) on day 2,569 | 160 on day 1,247 & 160 on day 2,569 |
| 14907 | 3.3 | 10,564.3 (3,201-fold) on day 140 | 4,974 (47.1%, 1,507-fold) on day 560 | 365 (3.5%, 111-fold) on day 2,480 | 640 on day 83 & 320 on day 2,017 | ||
| 12345 | 1×1011 | 27.9 | 1,280.7 (46-fold) on day 84 | 477 (37.2%, 17-fold) on day 560 | 64.6 (5%, 2.3-fold) on day 2,411 | 640 on day 21 & <5 on day 2,411 | |
| Method 2 | 14200 | 1×1011 | 4.2 | 1,675.6 (399-fold) on day 84 | 531.5 (31.8%, 127-fold) on day 413 | 51.8 (3.1%, 12-fold) on day 2,327 | 640 on day 28 & 40 on day 2,327 |
| 14245 | 3 | 725.7 (242-fold) on day 84 | 270.2 (37.2%, 90-fold) on day 413 | 44.4 (6.1%, 15-fold) on day 2,313 | 640 on day 21 & <5 on day 2,313 | ||
| Method 3 | 15225 | 1×1011 | 9.8 | 1,824.5 (186-fold) on day 56 | 245.2 (13.4%, 25-fold) on day 847 | 48.9 (2.7%, 5-fold) on day 1,817 | 1,280 on day 28 & 80 on day 1,817 |
| 15586 | 15.6 | 3,716.5 (238-fold) on day 14 | 625.1 (16.8%, 40-fold) on day 846 | 142 (3.8%, 9-fold) on day 1,823 | 1,280 on day 28 & <5 on day 1,823 | ||
| 16534 | 8.3 | 1,865.9 (225-fold) on day 29 | 311 (16.7%, 37-fold) on day 651 | 33 (1.8%, 4-fold) on day 1,626 | >5,120 on day 28 & 40 on day 1,626 | ||
| 16432 | 19.1 | 1,955.1 (102-fold) on day 176 | 853 (43.6%, 45-fold) on day 547 | 254 (1.3%, 13-fold) on day 1,522 | >500 on day 16 & 10 on day1,522 | ||
| 16105 | 3×1010 | 7.1 | 1,263.1 (178-fold) on day 28 | 136 (10.8%, 19-fold) on day 964 | 50.5 (4%, 7-fold) on day 1,754 | >1,000 on day 28 & 20 on day 1,760 | |
| 16966 | 2.9 | 960.5 (384-fold) on day 28 | 211.6 (22%, 73-fold) on day 963 | 69.7 (7.3%, 24-fold) on day 1,760 | 80 on day 694 &<5 on day 1,760 | ||
| 16997 | 1×1010 | 4.3 | 628 (146-fold) on day 57 | 126 (20%, 29-fold) on day 337 | 37.6 (6%, 9-fold) on day 1,291 | 100 on day 28 & 80 on day 533 |
bAFP, baboon alpha-fetoprotein; NAB, neutralizing antibody.
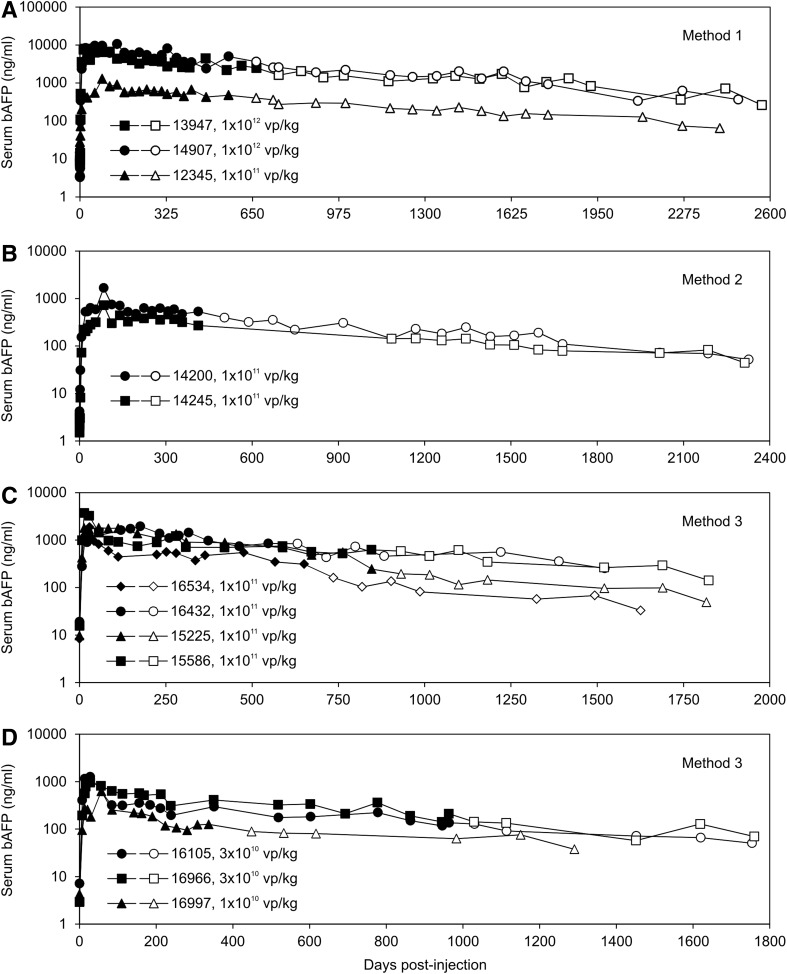
The levels of transgene expression for the first 560–665 days were published for the three animals in which vector was administered by Method 1 (Fig. 1A, closed symbols). We have continued to collect serum and measure transgene expression from these animals for an additional 1,851–1,920 days since the original publication (Fig. 1A, open symbols), for a total of 2,411–2,569 days post-injection. For the two animals in which the vector was administered by Method 2, transgene expression for the first 413 days was published (Fig. 1B, closed symbols), and we have continued to measure transgene expression for an additional 1,900 and 1,914 days (Fig. 1B, open symbols), for a total of 2,313 and 2,327 days. For the seven animals in which the vector was administered by Method 3, transgene expression levels for the first 337–964 days have been reported (Fig. 1C and D, closed symbols). Here, we have measured transgene expression for an additional 790–977 days (Fig. 1C and D, open symbols), for a total of 1,291–1,823 days. Table 1 summarizes transgene expression levels at baseline, at peak, and on the last day of observation. In all cases, transgene expression was detected for all animals for the entire observation period of up to 7 years. However, there was a slow but steady decline in transgene expression in all cases, with final levels ranging from 1.3% to 7.3% of peak values at the end of the observation period, but these still remained 2.3–111-fold over baseline values (Fig. 1 and Table 1). The slow, steady decline in transgene expression over time may be caused by gradual loss of transduced hepatocytes because of physiologic hepatocyte turnover or loss of the extrachromosomal vector genome or a combination of both.
FIG. 1.
Serum bAFP levels in baboons injected with HDAd expressing bAFP from a liver-specific promoter. HDAd was administered by (A) Method 1, (B) Method 2, and (C and D) Method 3. Closed symbols represent data from the original publications. Open symbols represent new, follow-up data. bAFP, baboon alpha-fetoprotein; HDAd, helper-dependent adenoviral vectors.
Administration of HDAd elicits an NAB response against the vector that can prevent successful readministration. We have measured serum NABs at or near the end of the observation period to determine their persistence. NAB titers for baboons injected with the highest dose of 1×1012 vp/kg were 160 on day 2,569 and 320 on day 2,017 postinjection for baboons 13947 and 14907, respectively (Table 1). Thus, at this high dose, NABs remain detectable even up to 7 years postinjection. Animals injected with a lower dose of 1×1011 and 3×1010 vp/kg had lower NAB titers, some of which had declined to undetectable at the end of the observation period, suggesting that successful readministration before complete loss of transgene expression might be possible at these lower vector doses. However, successful readministration of Ad-based vectors may be possible even in the presence of NAB. Specifically, Hodges et al. (2005) showed that using balloon occlusion catheters to achieve local delivery of Ad vectors into the liver of rabbits with preexisting anti-Ad NAB resulted in robust hepatocyte transduction comparable to naive rabbits. Thus, successful readministration in the presence of NAB might be possible with Methods 1 and 3 (but not Method 2) because they involve direct vector injection into the balloon-occluded liver similar to the method of Hodges et al. (2005).
In addition to duration of transgene expression, long-term adverse effects also impact risk:benefit assessment. In this regard, all animals remained in a healthy state throughout the observation period and were in good clinical condition with adequate amounts of fat, normal muscle bulk, and hydration. At the end of the observation, all animals underwent ultrasonography examination of the liver, which revealed normal echogenicity. Baboons 13947, 14907, 16432, and 16534 were necropsied at the end of the observation period, and thorough visual examination during necropsy revealed no significant gross abnormalities in any organ/tissue with the following exceptions. A 3 mm cyst was found in the subcapsular cortex of the left kidney, and the liver had adhesions to the omentum, diaphragm, and duodenum for 13947. A few moderate fibrous adhesions were observed between the liver and the diaphragm of 14907. A 0.5 cm cyst filled with brown, thick fluid was found in the area of the thymus in 16534. The fibrous adhesions observed in 13947 and 14907 may be secondary to the surgical procedure performed as part of Method 1. The renal and thymus cysts are likely incidental findings, not related to our gene therapy. Careful examination of ∼5 mm slices made through the entire liver revealed no macroscopic abnormalities in 13947, 14907, 16432, and 16534. A wide variety of organs (see Materials and Methods) were examined by H&E histology with no significant findings. These results suggest an absence of toxicity at 1,291 to 2,569 days post-HDAd injection.
This study documents for the first time that HDAd can mediate hepatic transgene expression for up to 7 years [which is more than half the life span of most captive baboons (Martin et al., 2002)], following a single administration with no evidence of chronic toxicity in nonhuman primates. However, transgene expression does slowly decline over time. Whether this decline will significantly affect long-term efficacy will be dictated by the underlying disorder. For example, efficacy will be less impacted by this decline for those disorders that require low-level transgene expression for therapeutic benefit. To the best of our knowledge, this is the longest duration of expression from HDAd in any animal model published to date and may provide a better insight of what might be expected in potential human trials. Thus, these results will contribute to a more informed risk:benefit assessment for HDAd-mediated liver-directed gene therapy.
Acknowledgments
This study was supported by the National Institutes of Health R01 DK067324 (P.N.) and R00 DK077447 (N.B.-P.), and the Texas Medical Center Digestive Diseases Center Grant DK56338 (M.F.). We thank Allen Tower and Doug Villnave of NuMED for supplying the balloon catheters. We are grateful to Angela Major for H&E histology. This investigation used resources that were supported by the Southwest National Primate Research Center Grant P51 RR013986 from the National Center for Research Resources, National Institutes of Health, and that are currently supported by the Office of Research Infrastructure Programs through P51 OD011133.
Author Disclosure Statement
Dr. Charles Mullins is a paid consultant of NuMED, Inc., and Hopkinton, Inc. The other authors have no conflicts to disclose.
References
- Brunetti-Pierri N., et al. Sustained phenotypic correction of canine hemophilia B after systemic administration of helper-dependent adenoviral vector. Hum. Gene Ther. 2005a;16:811–820. doi: 10.1089/hum.2005.16.811. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N., et al. Increased hepatic transduction with reduced systemic dissemination and proinflammatory cytokines following hydrodynamic injection of helper-dependent adenoviral vectors. Mol. Ther. 2005b;12:99–106. doi: 10.1016/j.ymthe.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N., et al. Improved hepatic transduction, reduced systemic vector dissemination, and long-term transgene expression by delivering helper-dependent adenoviral vectors into the surgically isolated liver of nonhuman primates. Hum. Gene Ther. 2006;17:391–404. doi: 10.1089/hum.2006.17.391. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N., et al. Pseudo-hydrodynamic delivery of helper-dependent adenoviral vectors into non-human primates for liver-directed gene therapy. Mol. Ther. 2007;15:732–740. doi: 10.1038/sj.mt.6300102. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N., et al. Efficient, long-term hepatic gene transfer using clinically relevant HDAd doses by balloon occlusion catheter delivery in nonhuman primates. Mol. Ther. 2009;17:327–333. doi: 10.1038/mt.2008.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti-Pierri N., et al. Balloon catheter delivery of helper-dependent adenoviral vector results in sustained, therapeutic hFIX expression in rhesus macaques. Mol. Ther. 2012;20:1863–1870. doi: 10.1038/mt.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croyle M.A. Chirmule N. Zhang Y. Wilson J.M. “Stealth” adenoviruses blunt cell-mediated and humoral immune responses against the virus and allow for significant gene expression upon readministration in the lung. J. Virol. 2001;75:4792–4801. doi: 10.1128/JVI.75.10.4792-4801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges B.L., et al. Local delivery of a viral vector mitigates neutralization by antiviral antibodies and results in efficient transduction of rabbit liver. Mol. Ther. 2005;12:1043–1051. doi: 10.1016/j.ymthe.2005.06.475. [DOI] [PubMed] [Google Scholar]
- Kim I.H., et al. Lifetime correction of genetic deficiency in mice with a single injection of helper-dependent adenoviral vector. Proc. Natl. Acad. Sci. USA. 2001;98:13282–13287. doi: 10.1073/pnas.241506298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L.J., et al. Lifespan in captive baboons is heritable. Mech. Ageing Dev. 2002;123:1461–1467. doi: 10.1016/s0047-6374(02)00083-0. [DOI] [PubMed] [Google Scholar]
- McCormack W.M., Jr., et al. Helper-dependent adenoviral gene therapy mediates long-term correction of the clotting defect in the canine hemophilia A model. J. Thromb. Haemost. 2006;4:1218–1225. doi: 10.1111/j.1538-7836.2006.01901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer D. Ng P. Improved system for helper-dependent adenoviral vector production. Mol. Ther. 2003;8:846–852. doi: 10.1016/j.ymthe.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Palmer D.J. Ng P. Physical and infectious titers of helper-dependent adenoviral vectors: a method of direct comparison to the adenovirus reference material. Mol. Ther. 2004;10:792–798. doi: 10.1016/j.ymthe.2004.06.1013. [DOI] [PubMed] [Google Scholar]
- Toietta G., et al. Lifelong elimination of hyperbilirubinemia in the Gunn rat with a single injection of helper-dependent adenoviral vector. Proc. Natl. Acad. Sci. USA. 2005;102:3930–3935. doi: 10.1073/pnas.0500930102. [DOI] [PMC free article] [PubMed] [Google Scholar]