Abstract
Chemotherapeutic drugs can enhance an immune response of the host against the tumor in addition to killing cancer cells by direct cytotoxicity. Therefore, the combination of chemotherapy and immunotherapy is a promising approach for eliminating tumors, particularly in advanced stages. A strategic medication is to use a bispecific antibody format that is capable of recruiting polyclonal T cells around antibody-target-expressing tumor cells. Recently, we have constructed a bispecific antibody, anti-CD3×anti-CD19, in a diabody configuration. In this study, we measured B7 family members B7.1 (CD80) and B7.2 (CD86) expressed on a CD19+ human leukemia cell line, Nalm-6, stimulated by cytosine arabinoside (Ara-C). We found that a low concentration of Ara-C could upregulate CD80 expressed on CD19+ Nalm-6 cells. The cytotoxicity of T lymphocytes against Nalm-6 cells in vitro and in vivo mediated by the anti-CD3×anti-CD19 diabody with or without a low dose of Ara-C was compared. The combination of the anti-CD3×anti-CD19 diabody and Ara-C showed the greatest effectiveness in enhancing the cytotoxicity of T cells against the tumor cells in vitro and in vivo. Activated T cells expressed higher levels of CD25 and CD69 and released more interleukin 2. Both perforin/granzyme B system and Fas/FasL pathway were involved in the diabody-induced T-cell cytotoxicity. Moreover, the activated T cells could upregulate ICAM-3 expression on Nalm-6 cells, and inhibition of LFA-1–ICAM-3 interaction impaired cytotoxicity of T cells. It was noted that Ara-C could upregulate CD80 expressed on two of five specimens of acute B lymphoblastic leukemia patient-derived cells. Cytotoxicity of T cells against these two patient-derived cells was enhanced in the presence of the anti-CD3×anti-CD19 diabody. These findings indicate that treatment strategy using both cytotoxic lymphocyte-based immunotherapy and chemotherapy may have synergistic effects.
Li and colleagues demonstrate that low concentrations of the chemotherapy agent cytosine arabinoside (Ara-C) can upregulate expression of the costimulatory molecule CD80 on a human leukemia cell line as well as in some patient-derived acute B-lymphoblastic leukemia cells. Polyclonal T cells targeting the leukemic cells via a CD19×CD3 bispecific antibody demonstrate increased activation and more effective killing when the target cells are pretreated with Ara-C, indicating a potential mechanism for synergistic activity of immunotherapy and a conventional chemotherapeutic agent.
Introduction
Chemotherapeutic drugs are traditionally considered to kill cancer cells via direct cytotoxicity. Moreover, some studies have shown that chemotherapeutic drugs might also enhance the immune response of the host against the tumor. For example, administration of cyclophosphamide at low doses has shown to restore an efficient immune response against tumor cells in the tumor-bearing mice (Sharabi et al., 2010). In rats, low-dose combretastatin A4 phosphate has shown to enhance the immune response of tumor hosts against experimental colon carcinomas (Badn et al., 2006). In addition, we found that cytosine arabinoside (Ara-C) enhanced B7 expression on leukemic cells and promoted T-cell cytotoxicity against tumors (Yang et al., 2009). As more and more evidence suggests that the therapeutic efficacy of some chemotherapeutic drugs relies on their capability to interact with the immune system, the development of appropriate combinations of chemotherapy and immunotherapy represents a promising approach for eliminating cancer cells. Currently, the application of chemoimmunotherapy has been successful in some cases in clinical trials. For young patients with diffuse large-B-cell lymphoma with a good prognosis, anti-CD20 rituximab plus the CHOP-like chemotherapy regimen has proven to have better long-term outcomes than the CHOP-like-regimen-alone treatment (Pfreundschuh et al., 2011).
However, with the exception of macrophage and natural killer cells (Richards et al., 2008; Qu et al., 2010), another important class of immune cells that have the potential to eradicate even large tumors is the T-cell population, which cannot be recruited by monoclonal antibodies because it lacks Fcγ receptors (Nagorsen and Baeuerle, 2011). Therefore, during the past two decades, T cells recruited by bispecific antibodies have been exploited both as cancer therapeutics and as cancer immunodiagnostics. Promising imaging diagnostics and therapies have been demonstrated in several clinical trials (Baumann et al., 2011; Girgis et al., 2011); they might have the potential to circumvent some of these escape mechanisms. Diabodies are the smallest bispecific antibodies in size, and the distance between the two antigen-binding sites is sufficient to link two cells. This compact size contributes to low immunogenicity and high tumor penetration (Holliger et al., 2003).
Recently, we have constructed a new anti-CD3×anti-CD19 bispecific antibody that has a diabody configuration (Li et al., 2012). One arm of this diabody targets the CD19+ cell lineage, whereas the other targets CD3+ cells, such as T lymphocytes. Our results showed that CD19 is an excellent target for immunotherapy because it is expressed by virtually all developmental stages of B-cell lineages (except stem cells) (Otero and Rickert, 2003). CD19 is associated with enzymatic or signaling activity and is present on the cell surface without being released into the circulation (Otero et al., 2003). By binding one arm of the diabody with CD3, a common T-cell signaling protein, while the other arm binds to a tumor-associated antigen-CD19 on the target cells, the anti-CD3×anti-CD19 diabody is theoretically capable of recruiting a polyclonal T cell around tumor cells that express the antibody targets.
Osada et al. (2010) have used chemotherapy to sensitize tumor targets through cytotoxicity mediated by bispecific antibodies that directed to T cells. Tretter et al. (2003) reported that taxanes could sensitize BiAb killing. Therefore, we expected to find a chemotherapy agent that could enhance the anti-CD3×anti-CD19 dimer cytotoxicity. In a previous study (Yang et al., 2009), we found that CD80 (B7.1) and CD86 (B7.2) expressed on K562, and K562/A02 cells treated with Ara-C were significantly enhanced when compared with untreated cells. The B7 family members B7.1 and B7.2 interact with CD28 and constitute an essential T-cell co-stimulatory pathway in the initiation of antigen-specific cell-mediated immune response (Pentcheva-Hoang et al., 2004). Therefore, we hypothesized that Ara-C should be capable of upregulating B7 family members expressed on the CD19+ malignant cells and that cytotoxicity would be increased when the anti-CD3×anti-CD19 dimers were applied to recruit T lymphocytes around the Ara-C-stimulated CD19+ tumor cells.
The aims of the study were to detect the B7 family members B7.1 and B7.2 expressed on a CD19+ human leukemia cell line, Nalm-6, or B lymphoblastic leukemia (B-ALL) patient-derived cells pretreated with Ara-C, and to determine whether the combination of diabody and Ara-C could enhance the T cells killing the tumor cells more effectively. The mechanism of T-cell activation mediated by the anti-CD3×anti-CD19 diabody in Nalm-6 tumor-bearing mice was also investigated. The results showed that the tumor cells were not just passive targets for T-cell retargeting therapy; instead, the diabody allows active participation of the CD19+ target cells in the functional activation of T cells.
Materials and Methods
Many of the techniques described in this article are similar to those presented in one of our previously published studies (Barrett et al., 2011—currently the second reference in this article).
Cell lines
The human leukemia cell line Nalm-6 was established in our laboratory. Five samples of B-ALL patient-derived cells were obtained from Institute of Hematology, Chinese Academy of Medical Sciences (Tianjin, China) with informed consent. Cells were maintained in RPMI 1640 (Gibco) containing 10% of fetal bovine serum (FBS) and grown at 37°C with 5% CO2.
Isolation of peripheral blood mononuclear cells and sorting of T lymphocytes
With informed consent, blood samples were collected from healthy volunteers, and peripheral blood mononuclear cells were isolated by Ficoll-Hypaque density-gradient centrifugation. The interphase cells were washed twice, counted, and tested for viability with trypan blue dye. Then, their monocytes were depleted by adherence to plastic flasks for 2 hr. Nonadherent cells were used for T-cell isolation using fluorescence-activated cell sorting. For the sorting of T cells, 1×108 peripheral blood mononuclear cells in 1,000 μl phosphate-buffered saline (PBS) were incubated with 15 μl allophycocyanin-conjugated antihuman CD2 monoclonal antibodies (mAbs; clone RPA-2.10; BD Pharmingen) at 4°C for 60 min. After washing twice with PBS, T lymphocytes were sorted using fluorescence-activated cell sorting (FACSAria II; Becton, Dickinson and Co.), and the collected cells were cultured in complete RPMI 1640 medium (10% FBS) supplied with 50 IU/ml of interleukin 2 (IL-2) for 48 hr.
Inhibition of growth of Nalm-6 cells by Ara-C
Approximately 1×106 Nalm-6 cells/ml were incubated with Ara-C at terminal concentrations of 0, 0.125, 0.25, 0.5, 1, 2, 4, 8, 16, and 32 μM for 72 hr at 37°C. Then, Nalm-6 resuspended in RPMI 1640 (10% FBS) was added to 96-well culture plates at a concentration of 2×106 cells/ml. The MTT solution [3-(4, 5-dimethylthiazole-2-yl)-2, 5-diphenyltetrazolium bromide] was added to each well to reach a final concentration of 400 μg/ml and was further incubated at 37°C in a CO2 incubator (5% CO2) for 4 hr. The reaction resulted in the reduction of MTT by the mitochondrial dehydrogenase of viable cells to a purple formazan product. The MTT–formazan product was dissolved in dimethyl sulfoxide and estimated by measuring the absorbance at 492 nm in an enzyme-linked immunosorbent assay (ELISA) plate reader (Multiskan Ascent; Thermo Fisher Scientific). The assay was performed with triplicated wells, and the average values of cytotoxicity for each condition are shown.
Co-stimulation of molecule expressed on Nalm-6 cells or B-ALL cells
About 1×106 cells/ml Nalm-6 were incubated with Ara-C at the concentration of 0.25 μM for 0, 24, 48, and 72 hr. Nalm-6 cells incubated with PBS served as the control. After being washed in PBS twice, the Nalm-6 cells in all groups (experimental and control groups) were incubated with FITC-conjugated antihuman CD80 mAb (clone L307.4; BD Biosciences) and PE-conjugated antihuman CD86 antibody mAb (clone IT2.2; BD Biosciences), respectively, for 1 hr at 4°C. The stained cells were then analyzed using flow cytometry. B-ALL at 1×106 cells/ml was incubated with Ara-C at the concentration of 0.25 μM for 72 hr and the remaining procedure was same as for Nalm-6 mentioned above. The assay was repeated three times for each condition.
Cytotoxicity test in vitro
Cytolytic activity of T lymphocytes targeted by the diabody was determined using the calcein-release assay as described by Bargou et al. (2008). The CD19+ cell line Nalm-6, B-ALL cells, and those cells stimulated by Ara-C at a concentration of 0.25 μM for 72 hr were prepared as target cells. Briefly, the target cells were resuspended in RPMI 1640 complete medium (10% FBS) at a concentration of 2×106 cells/ml and incubated with 10 μM calcein-AM (Anaspec) for 40 min, after which extracellular calcein-AM was removed by washing twice. For the experiments, quadruplicates of 1×105 labeled target cells and T cells at different E:T cell ratios ranging from 25:1 to 3:1 per well were added to the round-bottom 96-well plates in a final volume of 100 μl. Diabody dilutions of 0.1, 1.0, and 10 pM were then added to the final volume for the assays. Equal concentrations of an anti-CD3 scFv (Xu et al., 2001) plus anti-CD19 scFv (Chen et al., 2005) mixture and the anti-CD3×anti-Pgp diabody (Liu et al., 2009) were established as controls. The plates were centrifuged at 250×g for 4 min and incubated for 4 hr in a humidified incubator at 37°C in 5% CO2. After incubation, the cells were concentrated by centrifugation, and the supernatant was transferred to a new 96-well plate. Calcein fluorescence in the supernatant was determined using a fluorescence plate reader (Fluoroskan Ascent FL; Thermo Fisher Scientific; excitation at 485 nm, emission at 535 nm). The percentage of cytotoxicity was calculated using the following formula: (Fexperimental lysis − Fspontaneous release)/(Fmaximal lysis − Fspontaneous release)×100. Maximal lysis values were obtained by adding 2% Triton X-100 to labeled target cells. Spontaneous release was observed only in the labeled target cells without T cells and antibodies. The assay was repeated four times for each condition.
Upregulation of T-cell activation markers
To determine the T-cell surface activation markers, PE-conjugated antihuman CD25 mAb (clone 2A3; BD Biosciences) and FITC-conjugated antihuman CD69 mAb (clone L78; BD Biosciences) were mixed, and the mixture was used as an antibody mixture. Experimental groups were set up according to cytotoxicity test in vitro. T cells alone and T cells incubated with diabody were added as controls. The ratio of T cells to target cells was 25:1, and the concentration of the antibodies was 10 pM. After incubation with the target cells for 4 hr, the lymphocytes were collected and washed in PBS with 3% FBS and 0.1% NaN3. Then, 50 μl of the antibody mixture was added, and the cells were incubated for 1 hr at 4°C. The cells were washed and the pellets were resuspended in 600 μl PBS. Samples were analyzed using flow cytometry.
Cytokine release
Cytokine concentrations were analyzed in supernatants of activated T cells using a human IL-2 and IL-4 ELISA kit (human IL-2 and IL-4 ELISA kits; NeoBioscience). Experimental groups were set up according to the cytotoxicity test in vitro. Cytokines released by T cells alone served as background. T cells incubated with the diabody were added as controls. Approximately 1×106 T cells were co-cultured with 4×104 target cells, and the concentration of the antibodies was 10 pM in a 96-well plate. After incubation with the target cells for 4 hr, supernatant was removed and analyzed according to the manufacturer's protocol. The measurements were performed on an ELISA plate reader (Thermo Fisher Scientific).
Expression of perforin, granzyme B, and Fas ligand of activated T-cell subpopulation
Isolated T cells at 1×107 and/or Nalm-6 cells at 4×105 pretreated with Ara-C were incubated with or without the diabody at the concentration of 10 pM for 4 hr. Experimental groups were set up according to cytotoxicity test in vitro. T cells alone and T cells incubated with diabody were served as controls. The cells were washed twice in PBS supplemented with 2% bovine serum albumin (BSA) and resuspended in 100 μl fixative (FixPerm kit; Caltag) at room temperature for 15 min. After further washing, the cell pellet was resuspended in 100 μl permeabilization solution (FixPerm kit; Caltag) for 15 min at room temperature and then incubated with FITC-conjugated anti-perforin mAb (cloned δG9; BD Pharmingen) and PE-conjugated anti-Granyme B mAb (cloned CB9; BD Pharmingen) mixture or PE-conjugated anti-FasL mAb (cloned MFL3; BD Pharmingen) for 45 min at 4°C. The cells were then washed twice in PBS-BSA, and analyzed by flow cytometry.
Inhibition of LFA-1–ICAM3 interaction
Isolated T cells at 1×107 or Nalm-6 cells at 4×105 cells pretreated with Ara-C were incubated with or without the dimer antibody at the concentration of 10 pM for 4 hr. Then, the cells were washed twice in PBS supplemented with 2% BSA and the Nalm-6 cells were characterized by flow cytometry for CD19 (PE-conjugated anti-CD19 mAb, cloned HIB19; BD Pharmingen) and CD50 (FITC-conjugated anti-ICAM3, cloned TU41; BD Pharmingen). Nalm-6 cells and Nalm-6 cells pretreated with Ara-C were served as controls. To block the LFA-1–ICAM-3 interaction, Nalm-6 cells were preincubated with the mixture of anti-ICAM-3 mAb (cloned TU41; BD Pharmingen) and anti-LFA-1 mAb (cloned G43-25B; BD Pharmingen) for 30 min at 37°C. An isotype-matched mAb was used as a control, respectively. Cytotoxicity test was performed as mentioned before. The ratio of E to T was 25:1, and the concentration of diabody was 10 pM. Experiments were performed in triplicate.
In vivo expression of CD80 and CD86 in response to Ara-C
The experiments on the mice were carried out in accordance with our institution's guidelines on animal care and use. Approximately 1×107 Nalm-6 cells were inoculated subcutaneously at the right flank of each 6-week-old female nonobese diabetes/severe combined immune deficiency (NOD/SCID) mouse (Cancer Institute, Chinese Academy of Medical Sciences) 1 day after the application of total body irradiation (400 rad); there were seven mice in each group. Six days after tumor inoculation, when the solid tumors reached sizes of 80–100 mm3, the mice were subcutaneously injected with 1.0 mg/kg/day Ara-C for 3 days. At 24 hr after the last dosing, the mice were euthanized, the tumor xenografts were removed, and Nalm-6 cells were isolated for analysis of CD80 and CD86 expression by flow cytometry.
Tumor growth inhibition in vivo
In total, 98 female 6-week-old NOD/SCID mice (Cancer Institute, Chinese Academy of Medical Sciences) were kept under sterile conditions and given autoclaved food, water, and bedding. For tumor inoculation, 1×107 Nalm-6 cells were injected subcutaneously at the right flank of each mouse on the next day after receiving total body irradiation (400 rad). The mice were divided into 14 groups, and each group consisted of seven animals. Six days after the tumor inoculation, half of the mice (n=49 in 7 groups) were injected subcutaneously with Ara-C at the dose of 1 mg/kg daily for 3 days. The tumors were measured daily using a caliper in two perpendicular dimensions. Treatments were started at 24 hr after the last dosing of Ara-C. For the mice not injected with Ara-C, three groups of them were treated with intravenous injections of three dose levels of the diabody (0.5, 1.0, or 2.0 nM per mouse), combined with preactivated T cells (5×106 cells/mouse) in PBS via the tail vein, every 7 days for 3 weeks. The other four groups of mice received intravenous injections of preactivated T cells (5×106 cells/mouse); a combination of anti-CD3 scFv (2.0 nM/mouse), anti-CD19 scFv (2.0 nM/mouse), and preactivated T cells (5×106 cells/mouse); a combination of the anti-CD3×anti-Pgp (2.0 nM/mouse) diabody and preactivated T cells (5×106 cells/mouse); or PBS, respectively, as controls. The tumors were measured three times per week, and the tumor volumes were calculated using the following formula: V=(L×W2)/2, where L is the longest axis of tumors (in mm) and W is the shortest axis (in mm). Mice were euthanized by cervical dislocation under anesthesia when the tumors reached 3,000 mm3 in size. The mean tumor volume of the treatment groups and the control groups were calculated and analyzed using the Student's t-test.
Statistical analysis
All data were expressed as mean±standard deviation and statistically analyzed by the Student's t-test with SPSS 18.0 software (IBM).
Results
Inhibition of growth of Nalm-6 by Ara-C in vitro
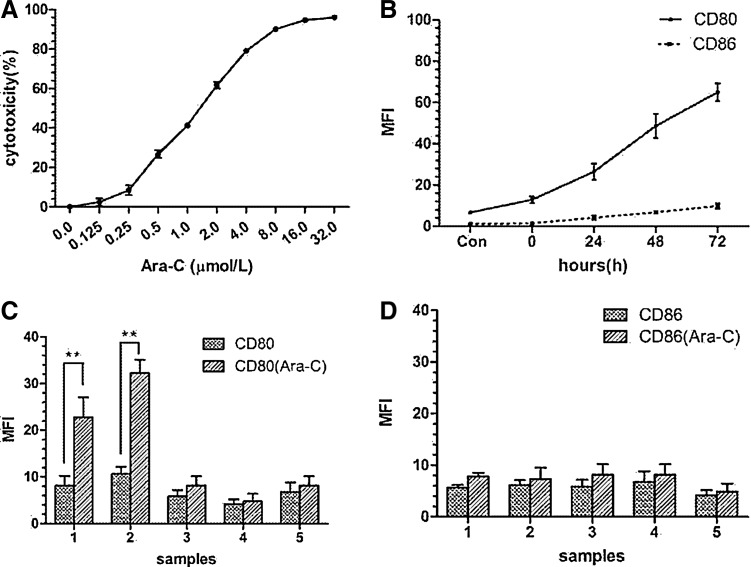
As its concentration increased, the cytotoxicity of Ara-C increased (Fig. 1A). At the concentrations of 0.125, 0.25, 0.5, 1.0, 2.0, 4.0, 8.0, 16.0, and 32.0 μM, the cytotoxicity were 3.967%±1.827%, 9.487%±2.459%, 26.825%±1.887%, 41.375%±0.913%, 61.655%±1.598%, 79.152%±0.616%, 90.088%±0.245%, 94.710%±0.243%, and 96.064%±0.523%, respectively. The value of IC10 (concentration for 10% inhibition of cell growing) was 0.25 μM.
FIG. 1.
Growth inhibition of Nalm-6 cells by Ara-C in different concentrations and expression of CD80 and CD86 on Nalm-6 or B-ALL cells stimulated by Ara-C. (A) With the concentration increased, cytotoxicity of Ara-C increased. IC10 was 0.25 μM. (B) At the Ara-C concentration of 0.25 μM, CD80 increased along with prolonged time, but CD86 displayed no obvious change. (C) Stimulated by Ara-C (0.25 μM) for 72 hr, CD80 expression increased on two of five samples of B-ALL patient-derived cells. (D) CD86 expression displayed no obvious change on these five samples. **p<0.01. Ara-C, cytosine arabinoside; B-ALL, B lymphoblastic leukemia.
Co-stimulation of molecular expression on Nalm-6 cells or B-ALL cells
CD80 and CD86 expressed on Nalm-6 stimulated by Ara-C were tested for time dependence (Fig. 1B). At the time point of 0, 24, 48, and 72 hr, the mean fluorescent intensity (MFI) values were 12.84±1.53, 26.44±3.94, 48.6±5.89, and 64.96±4.30, respectively (p<0.05). The point Con means that no Ara-C was added. So CD80 expression increased with time. However, with additional time, CD86 expression did not change obviously compared with CD80 (MFI=1.345±0.095, 4.150±1.05, 6.675±0.795, and 9.8±1.33, p>0.5; Fig. 1B). For the B-ALL patient-derived cells, after being stimulated by Ara-C at the concentration of 0.25 μM for 72 hr, CD80 expression increased obviously on 2 of 5 samples (Fig. 1C), but no marked change observed for CD86 expression (Fig. 1D).
Specific cytotoxicity mediated by the diabody in vitro
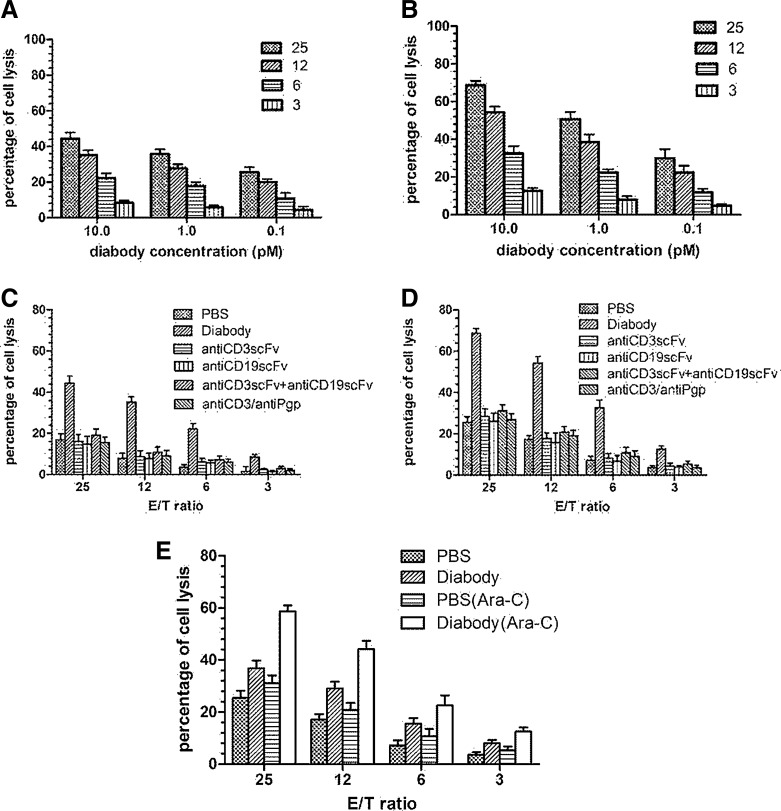
A nonradioactive cytotoxicity assay was performed to determine the ability of the diabody to induce lysing CD19+ tumor cells, Nalm-6, stimulated with or without Ara-C in the presence of human T cells. The diabody appeared to be potent in targeting T cells lysing the Nalm-6 cells (Fig. 2A). Lysis of target cells mediated by the diabody proceeded in a dose-dependent manner. An increase in the ratio of the effector cells to the target cells also resulted in enhanced target-cell cytotoxicity (Fig. 2C). In Nalm-6 cells pretreated with Ara-C, the cytotoxicity of T cells mediated by the diabody increased obviously (Fig. 2B and D). A mixture of equal amount of anti-CD19 scFv and anti-CD3 scFv was much less potent than the diabody in mediating Nalm-6 cell lysis. Because P-gp was not highly expressed on the Nalm-6 cells, the anti-CD3×anti-Pgp diabody was not able to enhance the cytotoxic activity. For the B-ALL patient-derived cells, only the two samples (samples 1 and 2) with high expression of CD80 stimulated by Ara-C were tested for the cytotoxicity. Figure 2E shows the result of sample 1 (Fig. 2E). The concentration of dimer antibody was 10 pM. As the same as Nalm-6 cells, cytotoxic effect was enhanced along with increasing the ratio of the effector cells to the target cells, and the effect was strengthened by the diabody and Ara-C.
FIG. 2.
Cytotoxicity of human T cells, in different E/T ratios mediated by different concentrations of diabody in a nonradioactive cytotoxicity assay. (A) Cytotoxicity of T cells in the presence of Nalm-6 mediated by the diabody. (B) Cytotoxicity of T cells in the presence of Ara-C-stimulating Nalm-6 mediated by the diabody. The diabody was at different concentrations (10, 1.0, and 0.1 pM). E/T ratio ranged from 25:1 to 3:1. (C, D) Lysis of target cells by T cells mediated by PBS, diabody, anti-CD3scFv, or anti-CD19scFv alone; a mixture of anti-CD3scFv and anti-CD19scFv; and anti-CD3×anti-Pgp diabody. The concentration of a variety of antibodies was 10 pM. (C) Target cells were Nalm-6. (D) Target cells were Nalm-6 stimulated by Ara-C. (E) Lysis of B-ALL cells (sample 1) or B-ALL cells (sample 1) stimulated by Ara-C in the presence of diabody (10 pM) or not. E/T ratio ranged from 25:1 to 3:1. Data shown are the mean±SD of experiments performed in quadruplicate. E/T ratio, effector-to-target cell ratio; PBS, phosphate-buffered saline; SD, standard deviation.
Upregulation of activation markers by activated T cells
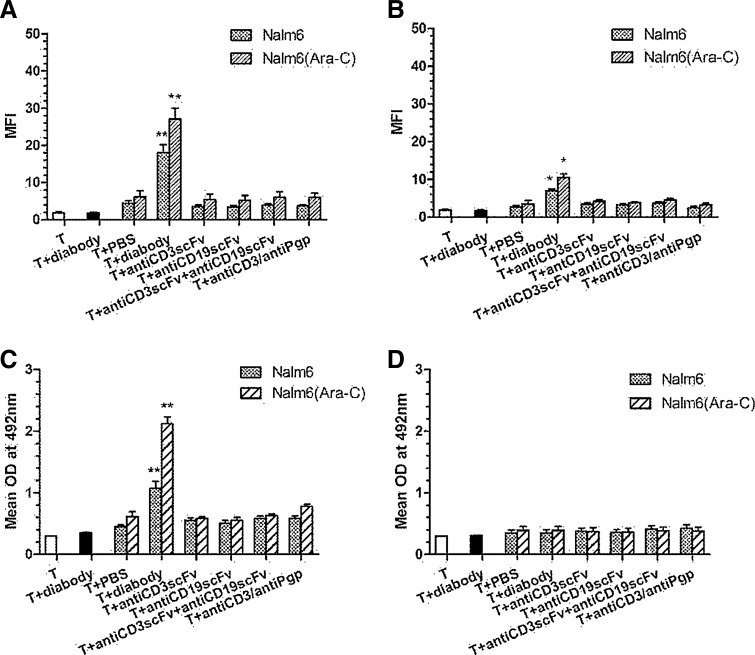
The T cells and T cells mixed with diabody were set up as controls (MFI values for the T cells and T cells mixed with diabody were 1.879±0.221 and 1.785±0.218, respectively). Expression of T-cell activation markers CD25 (Fig. 3A) and CD69 (Fig. 3B) obviously increased in T cells incubated with target cells and the diabody (10 pM) (MFI value were 18.104±2.104 for CD25, p<0.01, and 7.070±0.400 for CD69, p<0.05). Other antibodies, such as the mixture of anti-CD3scFv and anti-CD19scFv or anti-CD3×anti-Pgp diabody, did not increase the expression of T-cell activation markers. Furthermore, the T-cell activation markers increased more when T cells were incubated with the Nalm-6 cells stimulated by Ara-C than with Nalm-6 itself. Increasing of activation markers was marked when T cells were mixed with diabody (MFI values were 27.105±2.895 for CD25, p<0.01, and 10.539±0.95 for CD69, p<0.05) and was limited when T cells were mixed with other antibodies.
FIG. 3.
Activation markers and cytokines release by activated T lymphocytes. The lysis of target cells was mediated by various antibodies at a concentration of 10 pM. Expression levels of T-cell activation markers CD25 (A) and CD69 (B) were significantly increased in case of the T cells incubated with target cells and the diabody. The T-cell activation markers increased at higher level when T cells were incubated with the Nalm-6 cells stimulated by Ara-C than Nalm-6 itself. Cytokine IL-2 significantly increased when T cells were incubated with target cells and the diabody. In addition, Nalm-6 cells stimulated by Ara-C could induce T cells to produce more IL-2 than Nalm-6 itself (C). No elevation of IL-4 compared with base line (D). *p<0.05 and **p<0.01 were compared with controls. T and T+diabody groups are controls.
Cytokines released by activated T cells
Cytokine IL-2 was not produced when the T cells were incubated with the diabody (the value of optical density (OD) was 0.301±0.001 for T cells and 0.355±0.005 for T cells mixed with diabody), but IL-2 was produced when T cells plus the target cells were incubated. Moreover, IL-2 significantly increased when T cells were incubated with target cells and diabody (OD=1.074±0.112, p<0.01). In addition, Nalm-6 cells stimulated by Ara-C induced T cells (OD=2.122±0.225) to produce more IL-2 than Nalm-6 alone (p<0.01) (Fig. 3C). However, IL-4 did not increase compared with the base line, regardless of whether target cells and diabody were added (Fig. 3D).
Expression of perforin, granzyme B, and Fas ligand of activated T-cell subpopulation
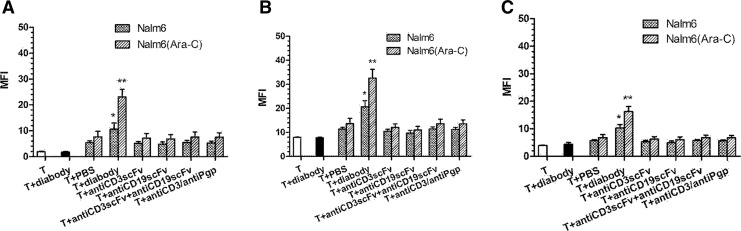
It is well known that T cells kill tumors by the perforin/granzyme B and the Fas/FasL pathways. We observed greater percentage of perforin/granzyme B and FasL-expressing T cells after co-culturing tumors, T cells, and diabody when compared with the control. Furthermore, Nalm-6 cells preincubated with Ara-C stimulated more perforin (MFI=23.11±2.90, p<0.01) (Fig. 4A)/granzyme B (MFI=31.71±3.08, p<0.01) (Fig. 4B), and FasL (MFI=16.30±1.80, p<0.01) (Fig. 4C) expressed by T cells than did Nalm-6 cells alone (MFI=10.61±2.43 for perforin, 20.60±2.61 for granzyme B, and 10.30±1.3 for FasL, p<0.05). These results indicate that both the perforin/granzyme B system and Fas/FasL pathway were involved in diabody-induced T-cell cytotoxicity.
FIG. 4.
Expression of perforin, granzyme B, and Fas ligand of activated T-cell subpopulation. There are greater percentage of perforin/granzyme B and FasL-expressing T cells after co-culturing tumors, T cells, and diabody when compared with the control. Nalm-6 cells preincubated with Ara-C stimulated more perforin (A)/granzyme B (B) and FasL (C) expressed by T cells than Nalm-6 cells alone. However, anti-CD3scFv or anti-CD19scF alone, combination of anti-CD3scFv and anti-CD19scFv, and anti-CD3/anti-Pgp have no effect. *p<0.05 and **p<0.01 were compared with controls. T and T+diabody groups are controls.
Inhibition of LFA-1–ICAM3 interaction
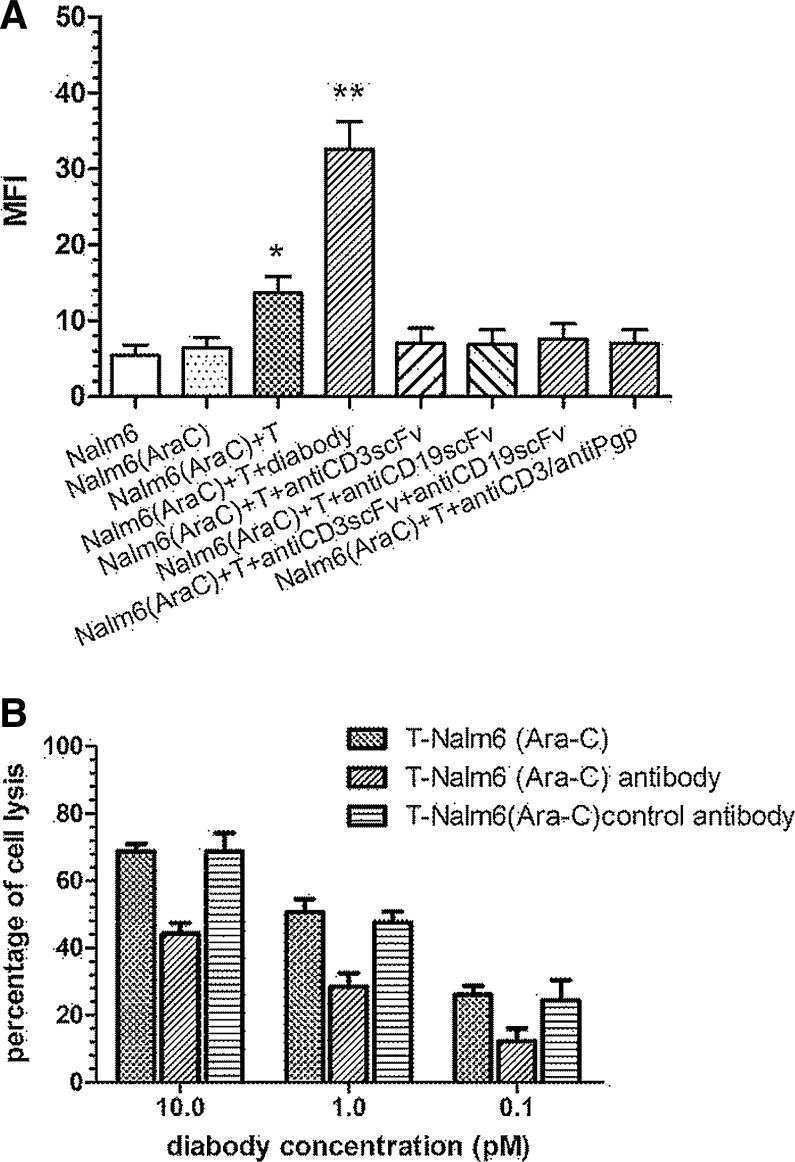
We investigated the functional significance of the involvement of ICAM-3. ICAM-3 expressed on Nalm-6 cells did not change after Nalm-6 cells were stimulated by Ara-C. ICAM-3 increased when Nalm-6 cells stimulated by Ara-C were co-cultured with T cells (MFI=13.65±2.15, p<0.05). When diabody was added, ICAM-3 increased obviously compared with the control (MFI=32.61±3.61, p<0.01) (Fig. 5A). It was shown that inhibition of the LFA-1–ICAM-3 interaction by anti-ICAM-3 mAbs and anti-LFA-1 mAbs reduced T-cell activation nearly to 45% in average (Fig. 5B). An isotype-matched control Ab did not decrease T-cell activation.
FIG. 5.
The functional significance of the involvement of ICAM-3. ICAM-3 expressed on Nalm-6 cells did not change after Nalm-6 cells were stimulated by Ara-C. ICAM-3 increased when Nalm-6 cells were stimulated by Ara-C co-cultured with T cells. When diabody was added, ICAM-3 increased significantly compared with the control (Nalm-6 group is a control) (A). The inhibition of the LFA-1–ICAM-3 interaction by anti-ICAM-3 mAbs and anti-LFA-1 mAbs reduced T-cell activation nearly to 45% on average. An isotype-matched control Ab did not decrease T-cell activation (B). *p<0.05 and **p<0.01 were compared with controls. mAbs, monoclonal antibodies.
CD80 and CD86 expression on Nalm-6 cells in vivo
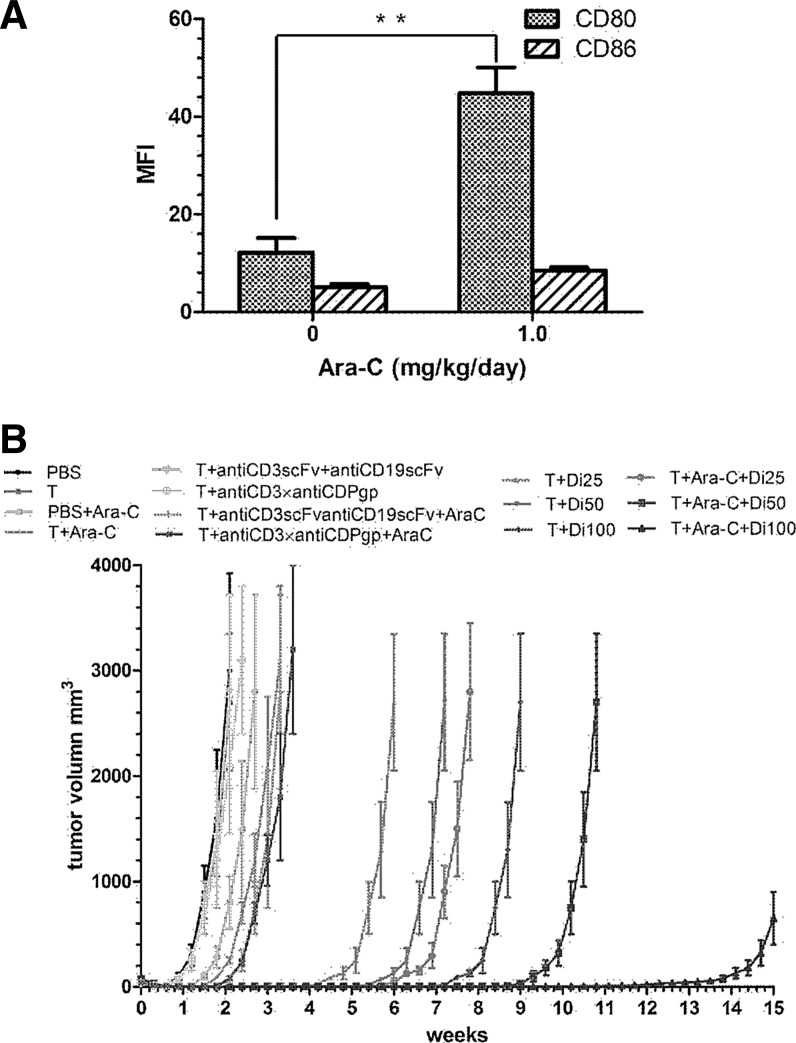
To evaluate the upregulation of CD80 or CD86 in Nalm-6 in vivo, we established a nude mice model bearing Nalm-6 cells. Analysis by flow cytometry revealed that the MFI value of CD80 was elevated from 12.15±3.05 to 44.8±5.3 (p<0.01) when mice were exposed to an Ara-C dose of 1.0 mg/kg/day for 3 days, but CD86 expression was only slightly enhanced, from 5.1±0.6 to 8.45±0.65 (p>0.5) (Fig. 6A).
FIG. 6.
Co-stimulation of molecular expression on Nalm-6 cells in vivo and antitumor effect of the anti-CD3×anti-CD19 diabody in xenotransplanted NOD/SCID nude mice. (A) CD80 expression was enhanced when mice were exposed to Ara-C of 1.0 mg/kg/day for 3 days but slightly enhanced for CD86. **p<0.01. (B) For the Nalm-6 xenotransplanted mice, a mixture of T cells and the diabody in different doses (5, 10, and 20 nM/mouse) were injected intravenously 24 hr later and once a week for 2 weeks. PBS, T cells alone, T cells combined with the mixture of anti-CD3scFv and anti-CD19scFv, and T cells combined with the anti-CD3×anti-Pgp diabody served as control groups.
Tumor inhibition in xenografted nude mice
We used a NOD/SCID mice model bearing the Nalm-6 tumor model to determine whether the diabody combined with Ara-C has a greater antitumor activity than the diabody alone. Three dose levels of the diabody (i.e., 0.5, 1.0, and 2.0 nM/mouse) were selected to test the efficacy of the diabody in the treatment of the xenografts. Animals were euthanized when the tumor size reached approximately 3,000 mm3. The antitumor activity of the diabody with or without Ara-C and that of the control groups are shown in Fig. 6B. No obvious tumor-inhibiting effect was observed in the mice treated with Ara-C alone or with Ara-C combined with T cells. The mice treated with the diabody and with the diabody combined with Ara-C showed significant dose-related antitumor activity. Furthermore, when equivalent doses of diabody and diabody combined with Ara-C were compared, the tumor inhibition of the combination was greater than that of the diabody alone.
Discussion
Chemotherapy drugs typically disrupt fundamental regulatory pathways essential for tumor cell growth and survival, but they usually cause severe toxicity to normal tissues and induce drug resistance. Many standard and high-dose chemotherapy regimens are immunosuppressive, contributing to leukopenia and lymphopenia. Some chemotherapy regimens require the co-administration of glucocorticoids, which can induce further lympholytic and immunosuppressive results (Herold et al., 2006). However, an abundance of evidence suggests that low-dose chemotherapy may augment tumor immunity via a variety of pathways. The critical pathway is one in which chemotherapy can adjust the tumor microenvironment by modulating the expression of tumor antigens, accessory molecules of T-cell activation or inhibition, and molecules involved in antigen processing and presentation (Emens, 2010).
Ara-C can modify the expression of co-stimulatory molecules, namely, CD80 (B7.1) and CD86 (B7.2), on the surfaces of mouse and human leukemic cells. The B7-CD28 signaling pathway is the most potent co-stimulatory pathway facilitating antigen-specific T-cell activation. An assay of human acute myelocytic leukemia cells demonstrated that exposure to doses of Ara-C as low as 0.05 mM led to a marked increase of CD86 and a lesser increase of CD80 (Vereecque et al., 2004). Most of specimens showed enhanced expression of CD80 or CD86, but some subtypes of acute myelocytic leukemia, especially FAB M0 and M1, showed no modification or a decrease of expression. In this study, however, we found that low dose of Ara-C could upregulate CD80 but not CD86 expressed on Nalm-6 cells. The results were confirmed by specimens of B-ALL patient-derived cells. Two of five specimens (about 40%) were detected with increasing expression of CD80 but not CD86. However, the number of five samples is too small, and the indications should be confirmed further by a larger number of samples. Moreover, the low dose of Ara-C administered for 3 days in this study had no side effect on mice but exhibited obvious immunomodulatory potential. The direct immune-modulating effect of Ara-C is dependent on the administrative schedule (i.e., dose and frequency). Although the mechanism of Ara-C that modulates CD80 and CD86 expression is not yet clear, our results indicate that CD80 and CD86 have distinct signaling pathways and different promoters (Fong et al., 1996; Li et al., 1999; Niu et al., 2003).
Some studies found peripheral circulating cytotoxic T lymphocytes (CTLs) with specificity against leukemic antigen in chronic myeloid leukemia patients and healthy individuals, indicating that a natural antileukemic immune response might exist in vivo (Molldrem et al., 2000; Rezvani et al., 2003). In addition, mouse leukemic cells exposed in vivo to Ara-C were more susceptible to CTL-mediated cell killing (Vereecque et al., 2004). This result suggests that the specific antileukemic activity of CTL, acting in synergy with enhanced sensitivity of leukemic cells to immune-mediated cell killing induced by chemotherapy, might eradicate minimal residual disease (van der Most et al., 2009). Accordingly, we hypothesized that cytotoxicity would be enhanced if CTLs were recruited around the tumor cells. The hypothesis was verified by our results. The combination of the diabody with Ara-C enhanced the ability of T cells to kill the target cells in two ways: first, T cells were recruited by the diabody, resulting in a high density of T lymphocytes around the tumor target; second, CD80 on Nalm-6 cells was upregulated by Ara-C, which enhanced the CD80/CD28 co-stimulatory pathway. Therefore, the diabody was not able to effectively activate T cells without Nalm-6. Furthermore, ICAM-3 was found increasingly expressed on Nalm-6 cells when T cells and target cells converged. However, ICAM-3 was not induced by Ara-C treatment and the changing of ICAM-1 and ICAM-2 was not detected yet (data not shown). We suppose that T cells were activated by Nalm-6 cells stimulated by Ara-C through the CD80/CD28 co-stimulatory pathway, and then Nalm-6 cells also could be activated by the activated T cells inversely, resulting in upregulating ICAM-3 expression on them. The LFA-1–ICAM-3 interaction may enhance the formation of synapse between T cells and target cells and lead to reciprocal signaling within both the target cells and T cells, which enhanced the sensitivity of Nalm-6 cells to be killed by T cells.
Cytotoxic T-lymphocyte antigen-4 (CTLA-4, CD152), the CD28 homolog, mainly expresses on another population of T lymphocytes that express CD4, CD25, and FoxP3 (Zou, 2006). CTLA-4 delivers a downregulatory signal that appears to be critical for the maintenance of immunological homeostasis (Allison, 1994; Chambers et al., 1996). CD4+CD25+ and FoxP3+ regulatory T cells (Tregs) represent 5–10% of the peripheral T lymphocyte pool in mice and humans. They perform two functions: one downregulates the normal immune response, and the other prevents the autoimmunity response (Zou, 2006). In preclinical models, many studies have shown that treatment with a low dose of cyclophosphamide mitigates the influence of CD4+CD25+ Tregs, allowing tumor immunity to emerge (Ghiringhelli et al., 2004). Cyclophosphamide promotes CD4+ T-helper type 1–driven tumor immunity (Ding et al., 2010). Administering a low dose of cyclophosphamide before vaccination can facilitate the recruitment of latent, high-avidity CD8+ T cells that mediate tumor rejection (Ghiringhelli et al., 2004). In advanced cancer patients, metronomic cyclophosphamide depletes CD4+C25+ Tregs and restores T and natural killer effector function (Ghiringhelli et al., 2007). Similarly, we have found that Ara-C is also capable of depleting Tregs and facilitating the diabody to recruit more CD4+ helper T lymphocytes and CTL (data not shown here).
After a TCR-mediated signaling, ligation of CD28 results in the activation of T cells. The activated T cells increase cytokine mRNA transcription, cytokine secretion, and upregulation of several molecules related to T-cell activation, namely, CD25 and CD69. In our study, T cells activated by Ara-C and diabody secreted more IL-2 but not IL-4. Previous studies have indicated that the cytokine secretion profile differs between CD4+ and CD8+ cells. The CD8+ cells have shown to secrete TH1 cytokines, whereas CD4+ cells secrete both TH1 and TH2 cytokines (Zhu and Paul, 2008). Originally, it was hypothesized that the TH1-type response led to tumor regression, whereas TH2 cytokines inhibited a cell-mediated antitumor immune response particularly via IL-4 (Seo and Tokura, 1999). Studies using other cancer models have been published recently that demonstrated the antitumor activity of TH2 cytokines (Yarovinsky et al., 2003). Especially high level of IL-10 within the tumor microenvironment was shown to favor tumor rejection by enhancing cytotoxicity of the T lymphocyte. Thus far, little is known about cytokine secretion of T cells upon incubation with the diabody and target tumor cells, especially in vivo. Also, the differences between CD4+ and CD8+ T lymphocytes in cytokine release and molecules related to the expression of T-cell activation are not yet clear. Therefore, to clarify the mechanism of the combination of diabody plus Ara-C to induce IL-2 while inhibiting IL-4 in both CD4+ and CD8+ T cells, respectively, further investigations are necessary. This synergetic effect still needs to be confirmed in vivo.
In the study, we found that Ara-C could upregulate CD80 expressed on the CD19+ human leukemia cell line Nalm-6 and some specimens of B-ALL patient-derived cells. A combination of the diabody plus Ara-C induced T lymphocytes to exhibit greater cytotoxicity on Nalm-6 cells both in vitro and in vivo and on some B-ALL cells in vitro. Ara-C, one component of the most widely used regimens for treatment of ALL, was used in this study at low doses. The observed advantage in the safety profile of combination immunotherapy (dimer antibody) with chemotherapy (Ara-C) in the preclinical tumor models, with a potent therapeutic effect, has guided us toward developing clinical strategies.
Acknowledgments
This work was supported by grants from the Natural Science Foundation of China (Grant Nos. 30873091 and 30971291), Natural Science Foundation of Tianjin (Grant No. 05YFGZGX02800), and National Science and Technology Major Project (Grant No. 2009ZX09103-720).
Author Disclosure Statement
The authors declare no conflict of interest.
References
- Allison J.P. CD28–B7 interactions in T-cell activation. Curr. Opin. Immunol. 1994;6:414–419. doi: 10.1016/0952-7915(94)90120-1. [DOI] [PubMed] [Google Scholar]
- Badn W. Kalliomäki S. Widegren B. Sjögren H.O. Low-dose combretastatin A4 phosphate enhances the immune response of tumor hosts to experimental colon carcinoma. Clin. Cancer Res. 2006;12:4714–4719. doi: 10.1158/1078-0432.CCR-05-2807. [DOI] [PubMed] [Google Scholar]
- Bargou R. Leo E. Zugmaier G., et al. Tumor regression in cancer patients by very low doses of a T-cell-engaging antibody. Science. 2008;321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- Baumann K. Pfisterer J. Wimberger P., et al. Intraperitoneal treatment with the trifunctional bispecific antibody Catumaxomab in patients with platinum-resistant epithelial ovarian cancer: a phase IIa study of the AGO Study Group. Gynecol. Oncol. 2011;123:27–32. doi: 10.1016/j.ygyno.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Chambers C.A. Krummel M.E. Boitel B., et al. The role of CTLA-4 in the regulation and initiation of T-cell responses. Immunol. Rev. 1996;153:27–46. doi: 10.1111/j.1600-065x.1996.tb00919.x. [DOI] [PubMed] [Google Scholar]
- Chen S. Rao Q. Wang J.X. Wang M. [Construction and expression of single chain variable fragments (ScFv) against human CD19 antigen] [Article in Chinese] Sheng Wu Gong Cheng Xue Bao (Chin. J. Biotechnol.) 2005;21:686–691. [PubMed] [Google Scholar]
- Ding Z.C. Blazar B. Mellor A., et al. Chemotherapy rescues tumor-driven aberrant CD4+ T cell differentiation and restores an activated polyfunctional helper phenotype. Blood. 2010;115:2397–2406. doi: 10.1182/blood-2009-11-253336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emens L.A. Chemoimmunotherapy. Cancer J. 2010;16:295–303. doi: 10.1097/PPO.0b013e3181eb5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong T.C. Wu Y. Kipps T.J. Identification of a promoter element that regulates tissue-specific expression of the human CD80 (B7.1) gene. J. Immunol. 1996;157:4442–4450. [PubMed] [Google Scholar]
- Ghiringhelli F.M. Larmonier N. Schmitt E., et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur. J. Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- Ghiringhelli F.M. Menard C. Puig P.E., et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector function in end-stage cancer patients. Cancer Immunol. Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis M.D. Kenanova V. Olafsen T., et al. Anti-CA19-9 diabody as a PET imaging probe for pancreas cancer. Surg. Res. 2011;170:169–178. doi: 10.1016/j.jss.2011.03.065. [DOI] [PubMed] [Google Scholar]
- Herold M. McPherson K. Reichardt H. Glucocorticoids in T-cell apoptosis and function. Cell Mol. Life Sci. 2006;63:60–72. doi: 10.1007/s00018-005-5390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliger P. Prospero T. Winter G. “Diabodies”: small bivalent and bispecific antibody fragments. Proc. Natl. Acad. Sci. USA. 2003;90:6444–6448. doi: 10.1073/pnas.90.14.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. Liu Z. Jiang S., et al. T suppressor lymphocytes inhibit NF-kappa B-mediated transcription of CD86 gene in APC. J. Immunol. 1999;163:6386–6392. [PubMed] [Google Scholar]
- Li W. Fan D. Yang M., et al. Disulfide-stabilized diabody antiCD19/antiCD3 exceeds its parental antibody in tumor-targeting activity. Cell. Oncol. (Dordr.) 2012;35:423–434. doi: 10.1007/s13402-012-0101-9. [DOI] [PubMed] [Google Scholar]
- Liu J. Yang M. Wang J., et al. Improvement of tumor targeting and antitumor activity by a disulphide bond stabilized diabody expressed in Escherichia coli. Cancer Immunol. Immunother. 2009;58:1761–1769. doi: 10.1007/s00262-009-0684-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molldrem J.J. Lee P.P. Wang C., et al. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat. Med. 2000;6:1018–1023. doi: 10.1038/79526. [DOI] [PubMed] [Google Scholar]
- Nagorsen D. Baeuerle P.A. Immunomodulatory therapy of cancer with T-cell-engaging BiTE antibody blinatumomab. Exp. Cell Res. 2011;317:1255–1260. doi: 10.1016/j.yexcr.2011.03.010. [Review]. [DOI] [PubMed] [Google Scholar]
- Niu H. Cattoretti G. Dalla-Favera R. BCL6 controls the expression of the B7-1/CD80 costimulatory receptor in germinal center B cells. J. Exp. Med. 2003;198:211–221. doi: 10.1084/jem.20021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada T. Hsu D. Hammond S., et al. Metastatic colorectal cancer cells from patients previously treated with chemotherapy are sensitive to T-cell killing mediated by CEA/CD3-bispecific T-cell-engaging BiTE antibody. Br. J. Cancer. 2010;102:124–133. doi: 10.1038/sj.bjc.6605364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero D.C. Rickert R.C. CD19 function in early and late B cell development. II. CD19 facilitates the pro-B/pre-B transition. J. Immunol. 2003;171:5921–5930. doi: 10.4049/jimmunol.171.11.5921. [DOI] [PubMed] [Google Scholar]
- Otero D.C. Anzelon A.N. Rickert R.C. CD19 function in early and late B cell development: I. Maintenance of follicular and marginal zone B cells requires CD19-dependent survival signals. J. Immunol. 2003;170:73–83. doi: 10.4049/jimmunol.170.1.73. [DOI] [PubMed] [Google Scholar]
- Pentcheva-Hoang T. Egen J.G. Wojnoonski K. Allison J.P. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;21:401–413. doi: 10.1016/j.immuni.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Pfreundschuh M. Kuhnt E. Trümper L., et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12:1013–1022. doi: 10.1016/S1470-2045(11)70235-2. [DOI] [PubMed] [Google Scholar]
- Qu Y.H. Li Y. Wu Y.F., et al. [Influence of FcγRIIIa polymorphism on rituximab-dependent NK cell-mediated cytotoxicity to Raji cells] [Article in Chinese] Zhongguo Shi Yan Xue Ye Xue Za Zhi (Chin. J. Exp. Hematol.) 2010;18:1269–1274. [PubMed] [Google Scholar]
- Rezvani K. Grube M. Brenchley J.M., et al. Functional leukemia-associated antigen-specific memory CD8+ T cells exist in healthy individuals and in patients with chronic myelogenous leukemia before and after stem cell transplantation. Blood. 2003;102:2892–2900. doi: 10.1182/blood-2003-01-0150. [DOI] [PubMed] [Google Scholar]
- Richards J.O. Karki S. Lazar G.A., et al. Optimization of antibody binding to FcgammaRIIa enhances macrophage phagocytosis of tumor cells. Mol. Cancer Ther. 2008;7:2517–2527. doi: 10.1158/1535-7163.MCT-08-0201. [DOI] [PubMed] [Google Scholar]
- Seo N. Tokura Y. Downregulation of innate and acquired antitumor immunity by bystander gammadelta and alphabeta T lymphocytes with Th2 or Tr1 cytokine profiles. J. Interferon. Cytokine Res. 1999;19:555–561. doi: 10.1089/107999099313686. [DOI] [PubMed] [Google Scholar]
- Sharabi A. Laronne-Bar-On A. Meshorer A. Haran-Ghera N. Chemoimmunotherapy reduces the progression of multiple myeloma in a mouse model. Cancer Prev. Res. (Phila) 2010;3:1265–1276. doi: 10.1158/1940-6207.CAPR-10-0138. [DOI] [PubMed] [Google Scholar]
- Tretter C.P. Lewis L.D. Fisher J., et al. Taxanes synergize with the bispecific antibody MDXH447 to enhance antibody-dependent cell-mediated cytotoxicity. J. Chemother. 2003;15:472–479. doi: 10.1179/joc.2003.15.5.472. [DOI] [PubMed] [Google Scholar]
- van der Most R.G. Curie A. Cleaver A., et al. Cyclophosphamide chemotherapy sensitizes tumor cells to TRAIL-dependent CD8+ T cell-mediated immune attack resulting in suppression of tumor growth. PLoS One. 2009;4:e6982. doi: 10.1371/journal.pone.0006982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereecque R. Saudemont A. Quesnel B. Cytosine arabinoside induces costimulatory molecule expression in acute myeloid leukemia cells. Leukemia. 2004;18:1223–1230. doi: 10.1038/sj.leu.2403391. [DOI] [PubMed] [Google Scholar]
- Xu Y. Xiong D. Yang C., et al. [The mutation of anti-CD3 antibody (HIT3a) gene and its expression] [Article in Chinese] Zhonghua Xue Ye Xue Za Zhi (Chin. J. Hematol.). 2001;22:252–255. [PubMed] [Google Scholar]
- Yang M. Fan D.M. Gao Y.D., et al. [The influence of Ara-C on anti-CD3/anti-Pgp mediating T-lymphocytes activities against multi-drug resistant leukemia cells] [Article in Chinese] Zhonghua Xue Ye Xue Za Zhi (Chin. J. Hematol.) 2009;30:812–815. [PubMed] [Google Scholar]
- Yarovinsky T.O. Monick M.M. HunninIghake G.W. Integrin receptors are crucial for the restimulation of activated T lymphocytes. Am. J. Respir. Cell Mol. Biol. 2003;28:607–615. doi: 10.1165/rcmb.2002-0105OC. [DOI] [PubMed] [Google Scholar]
- Zhu J. Paul W.E. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W. Regulatory T cells, tumor immunity, and immunotherapy. Nat. Rev. Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]