Abstract
Objective
This study assessed whether serum adiponectin could be used as a biochemical marker to differentiate type 1 diabetes mellitus (T1DM) from type 2 diabetes mellitus (T2DM) among young Asian Indians.
Research Design and Methods
We recruited age- and sex-matched individuals with physician-diagnosed T1DM (n=70) and T2DM (n=72). All were 12–27 years of age with a duration of diabetes of >2 years, at a large tertiary-care diabetes center in Chennai, southern India. Age- and sex-matched individuals with normal glucose tolerance (NGT) (n=68) were selected from an ongoing population study. NGT was defined using World Health Organization criteria. Serum total adiponectin was measured by enzyme-linked immunosorbent assay. Receiver operating characteristic (ROC) curves were used to identify adiponectin cut points for discriminating T1DM from T2DM.
Results
Adiponectin levels were higher in T1DM and lower in T2DM compared with the NGT group (9.89, 3.88, and 6.84 μg/mL, respectively; P<0.001). In standardized polytomous regression models, adiponectin was associated with T1DM (odds ratio [OR]=1.131 per SD; 95% confidence interval [CI], 1.025–1.249) and T2DM (OR=0.628 per SD; 95% CI, 0.504–0.721) controlled for age, gender, waist circumference, body mass index, hypertension, glycated hemoglobin, total cholesterol, serum triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, family history of T2DM, and estimated glomerular filtration rate. Using ROC analysis, an adiponectin cut point of 5.1 μg/mL had a C statistic of 0.886 (95% CI, 0.836–0.953), sensitivity of 80.6%, and specificity of 80.6% to differentiate T1DM from T2DM. Using the 5.1 μg/mL cut point, 80.6% of T1DM and 81.8% of T2DM would be correctly classified.
Conclusions
Serum adiponectin is a useful biochemical marker for differentiating T1DM and T2DM among young Asian Indians.
Introduction
Adipose tissue secretes biologically active substances, including adiponectin, leptin, resistin, and tumor necrosis factor-α, which may be associated with various metabolic and vascular diseases.1 Adiponectin is of particular interest as it is an important determinant of whole-body insulin sensitivity and has protective cardiovascular effects.2–4 Earlier reports in Asian Indians have shown that lower adiponectin levels are associated with cardiometabolic risk factors, increased carotid intimal media thickness, and non-alcoholic fatty liver disease.5–7
Experimental studies have indicated anti-inflammatory and anti-atherosclerotic effects of adiponectin.8,9 Adiponectin has also been suggested as an endogenous insulin sensitizer,10 and it may play a role in the regulation of glucose and lipid metabolism.11 However, adiponectin levels paradoxically may increase in several chronic conditions such as type 1 diabetes mellitus (T1DM) (with or without nephropathy),12 chronic heart failure,13 and end-stage renal disease.14
Because adiponectin levels are increased in T1DM and decreased in type 2 diabetes mellitus (T2DM), we hypothesized that measurement of adiponectin may be a useful tool in the classification of diabetes.
Research Design and Methods
Recruitment of subjects
We recruited age- and sex-matched individuals with T1DM (n=70) and T2DM (n=72) registered at Dr. Mohan's Diabetes Specialties Centre, a large tertiary-care diabetes center at Chennai, southern India. Subjects were between the ages of 12 and 27 years with a duration of diabetes of >2 years. Age- and sex- matched subjects with normal glucose tolerance (NGT) (n=68) were selected from an ongoing population based study in Chennai. In total, 210 subjects were included in the study, of whom 50% (n=105) were male and 50% (n=105) were female; of these participants, 1.9% (n=2) were in the prepubertal stage and 2.8% (three of the 105 females) had clinical symptoms of polycystic ovarian syndrome.
Diabetes was defined as a fasting plasma glucose (FPG) level of ≥126 mg/dL (7.0 mmol/L) and/or 2-h post-load glucose level of ≥200 mg/dL (11.1 mmol/L), a past medical history of diabetes (self-reported diabetes under treatment by a physician), or drug treatment for diabetes (insulin or oral hypoglycemic agents).15 NGT was defined as an FPG level of <100 mg/dL and a 2-h post load plasma glucose of <140 mg/dL.15 T1DM was defined as abrupt onset of symptoms like polyuria, polydipsia, or unexplained weight loss, ketonuria or past history of diabetic ketoacidosis, lack of insulin reserve as shown by C-peptide assay (fasting, <0.3 pmol/mL; stimulated, <0.6 pmol/mL), and requirement of insulin from the time of diagnosis for control of hyperglycemia.16,17 T2DM was defined as absence of ketosis, good β-cell reserve as shown by C-peptide assay (fasting, ≥0.6 pmol/mL; stimulated, ≥1.6 pmol/mL), absence of pancreatic calculi on abdomen X-ray, and lack of requirement of insulin for at least 2 years after diagnosis.17
The Modification of Diet in Renal Disease study equation18,19 was used to calculate estimated glomerular rate (eGFR):
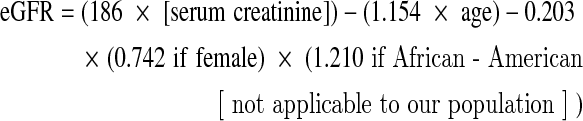 |
Patients with kidney dysfunction, cardiovascular disease (coronary artery disease, stroke, peripheral vascular disease), history of cancer, severe mental disorders (schizophrenia, dementia), hypothyroidism, or history of any known infectious or inflammatory diseases, based on medical history, were excluded.
It is known that drugs like statins, peroxisome proliferator-activated receptor-γ agonists, and angiotensin receptor blockers could affect adiponectin levels.20 Therefore, we collected data on medication use. Of the T1DM patients, 70 (100%) were on insulin therapy, four (5.5%) were on antihypertensive therapy, and two (2.8%) were on statin therapy. Of the patients with T1DM, none was taking a peroxisome proliferator-activated receptor-γ agonist or angiotensin receptor blocker. Of the patients with T2DM, 65 (90%) were on oral antidiabetes drugs, seven (10%) were receiving therapy with insulin and oral antidiabetes drugs, six (9%) were on antihypertensive therapy, and five (7%) were on statin therapy. Among the patients with T2DM, six (8.3%) and two (2.7%) were taking peroxisome proliferator-activated receptor-γ agonists and angiotensin receptor blockers, respectively.
Institutional ethics committee approval was obtained prior to the start of the study. Written informed consent was obtained from the parents and also assent from the study subjects who were younger than 18 years of age.
Anthropometric measurements
Anthropometric measurements, including weight, height, and waist circumference, were obtained using standard methods.21 Body mass index (BMI) was calculated as weight (kg)/height squared (m2). Blood pressure was recorded from the right arm with the subject in a sitting position to the nearest 2 mm Hg with a mercury sphygmomanometer (Deluxe BP apparatus; Diamond®, Pune, India). Two readings were taken 5 min apart, and the mean of the two was taken as the blood pressure.
Biochemical tests
FPG (hexokinase method), serum cholesterol (cholesterol oxidase–peroxidase–amidopyrine method), serum triglycerides (glycerol phosphate oxidase–peroxidase–amidopyrine method), high-density lipoprotein (HDL) cholesterol (direct method; polyethylene glycol-pretreated enzymes), and creatinine (Jaffe's method) levels were measured using a Hitachi-912 Autoanalyzer (Hitachi, Mannheim, Germany). Low-density lipoprotein (LDL) cholesterol was calculated using the formula of Friedewald et al.22 The intra- and interassay coefficients of variation for the biochemical assays ranged between 3.1% and 7.6%.
Glycated hemoglobin (HbA1c) was measured by high-pressure liquid chromatography using the Variant™ machine (Bio-Rad, Hercules, CA). Fasting and stimulated (post-breakfast) C-peptide levels were estimated by the electroluminescence method on an Elecsys2010 device (Hitachi).
Glutamic acid decarboxylase (GAD) antibody and tyrosine-phosphatase-like transmembrane protein (IA-2) were measured on a plate reader (model 680; Bio-Rad) using an enzyme-linked immunosorbent assay kit (Euro Immun, Lubeck, Germany). Subjects who had serum GAD- or IA-2-specific autoantibody levels of ≥10 IU/mL were classified as GAD- or IA-2-positive, respectively. Our laboratory is certified by the College of American Pathologists and the National Accreditation Board for Testing and Calibration of Laboratories.
Adiponectin was measured by quantitative sandwich enzyme-linked immunosorbent assay (R&D Systems, Abingdon, United Kingdom). The values were expressed in μg/mL units. The intra- and interassay coefficients of variation were <5 and <10%, respectively.
Statistical analysis
Student's t test or one-way analysis of variance (with Tukey's Highly Significant Difference) was used to compare groups for continuous variables, and the χ2 test or Fisher's exact test was used to compare proportions. Standardized polytomous logistic regression analysis was done using T1DM, T2DM, and NGT as the dependent variables and adiponectin as the independent variable, adjusting for age, gender, waist circumference, BMI, hypertension, HbA1c, total cholesterol, serum triglycerides, HDL cholesterol, LDL cholesterol, and family history of T2DM.
Receiver operating characteristic (ROC) curves were plotted for adiponectin to discriminate T1DM from T2DM. Sensitivity, specificity, positive and negative predictive values, and accuracy for discriminating T1DM from T2DM were calculated for various adiponectin cut points. The C statistic, or the area under the ROC, was estimated, and by interpolation from the area under the curve, the point closest to the upper-left corner, which maximized sensitivity and specificity, was selected as the optimal cut point; this identified the highest number of subjects with or without T1DM.23 All analyses was done using the Windows-based SPSS statistical package (version 12.0; SPSS, Inc., Chicago, IL).
Results
The characteristics of the study subjects are shown in Table 1. Compared with NGT subjects, BMI and waist circumference were higher in T2DM and lower in T1DM. GAD and IA-2 were present in 62.8% and 35.1%, respectively, of T1DM patients and 7.1% and 8.3%, respectively, of T2DM patients. Either GAD or IA-2 positivity was present in 67.2% of T1DM patients and 12.5% of T2DM patients, respectively.
Table 1.
Baseline Characteristics of Study Subjects
| Parameter | NGT (n=68) | T1DM (n=70) | T2DM (n=72) |
|---|---|---|---|
| Age (years) | 21±3.5 (12–26) | 21±3.5 (13–26) | 21±3.6 (14–27) |
| Male [n (%)] | 34 (50.0) | 35 (50.0) | 36 (50.0) |
| Body mass index (kg/m2) | 24±5.0 | 21±3.3a | 26±4.4bc |
| Waist circumference (cm) | 80.8±11.7 | 74.0±11.0a | 88.6±14.5bc |
| Blood pressure (mm Hg) | |||
| Systolic | 121±16 | 117±12 | 123±13 |
| Diastolic | 76±10 | 72±7 | 76±9 |
| Fasting plasma glucose (mg/dL) | 85±7 | 192±94b | 145±55bc |
| Glycated hemoglobin (%) | 5.4±0.4 | 9.2±1.8b | 7.9±2.1bc |
| Total cholesterol (mg/dL) | 158±31 | 163±36 | 154±32 |
| Serum triglycerides (mg/dL)d | 85.7 | 74.4 | 118.0b |
| HDL cholesterol (mg/dL) | 40±8.6 | 47±12b | 37±7.5c |
| LDL cholesterol (mg/dL) | 100±24 | 97±25 | 90±27 |
| C-peptide (pmol/mL) | |||
| Fasting | 1.40±0.25 | 0.29±0.07b | 1.20±0.45bc |
| Stimulated | 3.2±0.9 | 0.35±0.20b | 2.8±1.0bc |
| GAD positivity [n (%)] | 1 (1.4) | 44 (62.8) | 5 (6.9) |
| IA-2 positivity [n (%)] | 1 (1.4) | 25 (35.1) | 6 (8.3) |
| Family history of T2DM [n (%)] | 41 (60.2) | 21 (30.0) | 54 (75) |
| Serum adiponectin (μg/mL) | 6.84±2.7 (2.2–16.4) | 9.89±4.4 (3.2–20.5)b | 3.88±1.2 (1.02–7.99)bc |
| Treatment [n (%)] | |||
| Insulin alone | — | 70 (100) | — |
| OHA alone | — | — | 65 (90.0) |
| Insulin+OHA alone | — | — | 7 (10.0) |
| Antihypertensive | — | 4 (5.5) | 6 (9.0) |
| Statin therapy | — | 2 (2.8) | 5 (7.0) |
Data are mean±SD values (range) or n (%) as indicated.
P<0.01, bP<0.001 compared with normal glucose tolerance (NGT).
P<0.001 compared with type 1 diabetes mellitus (T1DM).
Geometric mean.
GAD, glutamic acid decarboxylase; IA-2, tyrosine-phosphatase-like transmembrane protein; OHA, oral antidiabetes drug; T2DM, type 2 diabetes mellitus.
Adiponectin levels were higher in T1DM and lower in T2DM compared with the NGT group (9.89, 3.88, and 6.84 μg/mL, respectively; P<0.001). In a separate analysis, we excluded those receiving peroxisome proliferator-activated receptor-γ agonists and angiotensin receptor blocker treatment, as these may affect adiponectin levels, and found similar results (data not shown).
To examine the influence of BMI on the association between adiponectin levels and type of diabetes, study subjects were stratified by tertiles of BMI. In all tertiles of BMI, adiponectin level was associated with diabetes type even after controlling for age, gender, waist circumference, hypertension, HDL cholesterol, LDL cholesterol, serum triglycerides, and total cholesterol (Fig. 1). In regression analysis also, adiponectin was associated with diabetes type independent of BMI.
FIG. 1.
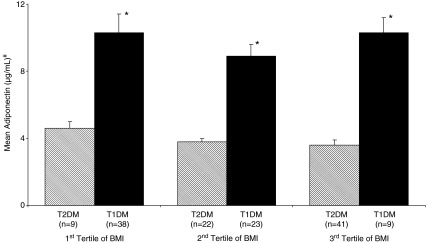
Mean adiponectin levels in tertiles of body mass index (BMI) among type 1 and type 2 diabetes mellitus (T1DM and T2DM, respectively) patients. Data are mean±SEM values. #Adjusted for age, gender, waist circumference, systolic and diastolic blood pressure, high- and low-density cholesterol, serum triglycerides, and total cholesterol. *P<0.001 compared with T2DM of the respective tertile.
Standardized polytomous logistic regression analysis was done with NGT, T1DM, and T2DM as the dependent variables and adiponectin as the independent variable (Table 2). Every 1 SD increase in adiponectin was associated with an increased risk of T1DM (odds ratio [OR]=1.186; 95% confidence interval [CI], 1.092–1.298; P<0.001). This association remained statistically significant even after adjusting for age, gender, waist circumference, BMI, hypertension, HbA1c, total cholesterol, serum triglycerides, HDL cholesterol, LDL cholesterol, family history of T2DM, and eGFR (OR=1.131; 95% CI, 1.025–1.249; P<0.001). Every 1 SD decrease in adiponectin was associated with an increased risk of T2DM (OR=0.654; 95% CI, 0.543–0.773; P<0.001). Even after adjusting for age, gender, waist circumference, BMI, hypertension, HbA1c, total cholesterol, serum triglycerides, HDL cholesterol, LDL cholesterol, family history of T2DM, and eGFR, this negative association remained significant with T2DM (OR=0.628; 95% CI, 0.504–0.721; P<0.01).
Table 2.
Polytomous Regression for Type 1 Diabetes Mellitus and Type 2 Diabetes Mellitus Compared with Normal Glucose Tolerance
| |
|
Odds ratio (95% CI) |
|
|
|---|---|---|---|---|
| Adiponectin | NGT (reference) | T1DM | T2DM | P value |
| Unadjusted | 1 | 1.186 (1.092–1.298) | 0.654 (0.543– 0.773) | <0.001 |
| Adjusted for | ||||
| Age | 1 | 1.185 (1.091–1.296) | 0.656 (0.542–0.777) | <0.001 |
| Age and gender | 1 | 1.182 (1.088–1.295) | 0.652 (0.535–0.772) | <0.001 |
| Age, gender, and BMI | 1 | 1.153 (1.063–1.256) | 0.661 (0.540–0.779) | <0.001 |
| Age, gender, BMI, and waist circumference | 1 | 1.132 (1.051–1.228) | 0.654 (0.537–0.771) | <0.001 |
| Age, gender, BMI, waist circumference, and hypertension | 1 | 1.130 (1.046–1.222] | 0.657 (0.539–0.772) | <0.001 |
| Age, gender, BMI, waist circumference, hypertension, HbA1c, and total cholesterol | 1 | 1.127 (1.042–1.217) | 0.658 (0.541–0.776) | <0.001 |
| Age, gender, BMI, waist circumference, hypertension, HbA1c, total cholesterol, and serum triglycerides | 1 | 1.124 (1.038–1.211) | 0.651 (0.535–0.766) | <0.001 |
| Age, gender, waist circumference, BMI, hypertension, HbA1c, total cholesterol, serum triglycerides, and HDL cholesterol | 1 | 1.122 (1.035–1.208) | 0.656 (0.539–0.769) | <0.001 |
| Age, gender, waist circumference, BMI, hypertension, HbA1c, total cholesterol, serum triglycerides, HDL cholesterol, and LDL cholesterol | 1 | 1.121 (1.015–1.211) | 0.655 (0.519–0.769) | <0.001 |
| Age, gender, waist circumference, BMI, hypertension, HbA1c, total cholesterol, serum triglycerides, HDL cholesterol, LDL cholesterol, and family history of T2DM | 1 | 1.136 (1.032–1.266) | 0.636 (0.502–0.741) | <0.001 |
| Age, gender, waist circumference, BMI, hypertension, HbA1c, total cholesterol, serum triglycerides, HDL cholesterol, LDL cholesterol, family history of type 2 diabetes, and eGFR | 1 | 1.131 (1.025–1.249) | 0.628 (0.504–0.721) | <0.01 |
BMI, body mass index; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NGT, normal glucose tolerance; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
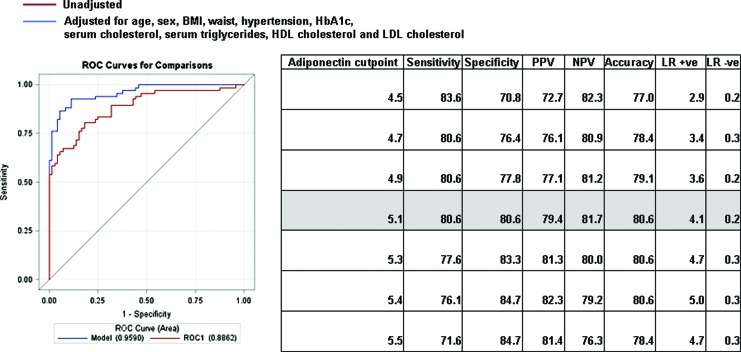
ROC curves were constructed to derive the cut point for adiponectin levels with the best sensitivity and specificity to identify T1DM from T2DM. Figure 2 shows the C statistic for the unadjusted and adjusted models of the adiponectin in predicting T1DM. An adiponectin cut point of ≥5.1 μg/mL had a C statistic of 0.886 (95% CI, 0.836–0.953; P<0.001), indicating the high ability of adiponectin to discriminate T1DM from T2DM. The C statistic improved to 0.959 when adjusted for age, gender, waist circumference, BMI, hypertension, HbA1c, total cholesterol, serum triglycerides, HDL cholesterol, and LDL cholesterol. The sensitivity and specificity of this adiponectin cutoff of ≥5.1 μg/mL were 80.6% and 80.6%, respectively, for identifying subjects with T1DM. The positive predictive value was 79.4%, the negative predictive value was 81.7%, and the accuracy was 80.6%. Using this adiponectin cut point of ≥5.1 μg/ml, 80.6% of those with T1DM were correctly identified; using a value of <5.1 μg/mL, 81.8% of those with T2DM were correctly identified.
FIG. 2.
Receiver operator characteristic (ROC) curves showing performance of adiponectin among subjects with type 1 and type 2 diabetes mellitus for unadjusted and adjusted models. BMI, body mass index; HbA1c, glycosylated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LR, likelihood ratio; NPV, negative predictive value; PPV, positive predictive value. Color images available online at www.liebertonline.com/dia
Discussion
An accurate classification of diabetes is necessary for optimal treatment. For those with T1DM, prompt classification is necessary to prevent more severe consequences surrounding uncontrolled blood glucose in the immediate post-diagnosis period and to allow a clinician to immediately start insulin and counsel regarding its proper use. Hence, a tool to simplify classification is needed. This can be at least partly explained by ease of classifying T1DM by a biochemical marker. The results of this study showed that individuals with T1DM have significantly higher, and those with T2DM significantly lower, adiponectin levels, compared with normoglycemic subjects. An adiponectin cut point of 5.1 μg/mL could be used to correctly differentiate over 80% of T1DM and T2DM among young Asian Indians. Additionally, our data suggest that adiponectin had a significant association with diabetes type even after controlling for age, gender, waist circumference, BMI, hypertension, HbA1c, total cholesterol, serum triglycerides, HDL cholesterol, LDL cholesterol, and family history of T2DM.
Studies have reported that higher serum adiponectin levels are seen in patients with T1DM, particularly those with diabetes complications such as nephropathy or cardiovascular disease.12,14 In the FinnDiane study, Forsblom et al.13 demonstrated that high adiponectin levels were associated with 11-year risk of all-cause and cardiovascular mortality in a nationwide multicenter cohort of Finnish adults with T1DM. Hypotheses to explain elevated adiponectin levels in patients with T1DM include a compensatory response to vascular injury,24 decreased clearance due to renal insufficiency,14 effects of subcutaneous insulin treatment,25 and posttranslational modifications (glycosylation) that could occur in persons with T1DM.26 Other possible mechanisms include differences in fat distribution and function between subjects with and without diabetes and alleviation of suppression effect by insulin.27
Significant efforts have been devoted for developing biomarkers that could predict the risk for T1DM.28 Although C-peptide levels do help to differentiate T1DM and T2DM, it may sometimes be difficult to distinguish T1DM and T2DM based on C-peptide cut points as some T2DM patients have low C-peptide in the presence of severe metabolic decompensation, whereas others can develop severe insulinopenia over time. Moreover, patients with T1DM could have higher C-peptide levels during the remission (honeymoon) phase.
In the present study, when T1DM patients were categorized based on fasting and stimulated C-peptide levels, an adiponectin cut point of ≥5.1 μg/mL predicted T1DM with >80% sensitivity and specificity. At the time of diagnosis or presentation, C-peptide levels may be low because of glucotoxicity. Once the glucotoxicity is corrected, repeating the C-peptide measurement often helps in distinguishing T1DM and T2DM as in those with T2DM the values rise substantially.17,29
Although subjects with T1DM do exhibit autoantibodies to islet cells, GAD, zinc transporter 8, insulin, or IA-2,30–32 in some populations (e.g., Asians or Africans) only 50–70% of those with T1DM have these antibodies.17,33,34 Studies have shown that the diagnostic sensitivity of combined autoantibodies to GAD, IA-2, and zinc transporter 8 measurements reached 65.5%.32 However, these autoantibodies can also be detected in a small percentage of patients with T2DM.17,32,35 The results of this study showed that an adiponectin cut point of 5.1 μg/mL could be used to correctly differentiate over 80% of T1DM and T2DM among young Asian Indians, whereas GAD and IA-2 were present only in 62% and 35%, respectively, of our T1DM subjects.
Studies have reported that adiponectin levels were reduced in those with obesity.4 Our data add to the literature on the influence of BMI on the association between adiponectin levels and type of diabetes. Interestingly, we found that the association between adiponectin levels and type of diabetes is not altered by BMI. Furthermore, the C statistic for adiponectin in predicting type of diabetes was improved even after controlling for confounding factors, indicating the excellent ability of adiponectin to discriminate T1DM from T2DM.
The strength of the study is that the cases and controls were age and sex matched, and diabetes was defined and type-classified using standard methods. One limitation of the study is that although our assay of total adiponectin incorporated the detection of the major polymeric molecular forms of adiponectin in serum, we did not differentiate between specific molecular forms. Another limitation is that GAD, IA-2, or adiponectin measurements could not be done at the time of diagnosis in all cases, as ours is a referral center. Thus, we measured GAD and IA-2 as well as adiponectin when patients were referred to our center. However, we did do adiponectin measurements on some cases of newly diagnosed T1DM and T2DM. We found that at the time of diagnosis, out of 16 T1DM subjects, 12 (75%) had an adiponectin level of ≥5.1 μg/mL, whereas of the 11 newly diagnosed T2DM subjects, eight (72%) had adiponectin levels of <5.1 μg/mL. Although admittedly the sample size is small, this confirms our findings that adiponectin has a good sensitivity to differentiate T1DM from T2DM. It is also possible that although the adiponectin cut point of 5.1 μg/mL may be useful in South Asians, this cut point may well differ in other ethnic groups because of differences in body weight or fat mass distribution.
In summary, our data suggest that because study subjects with T1DM had higher, and subjects with T2DM had lower, adiponectin levels compared with normoglycemic subjects, an adiponectin cut point of 5.1 μg/mL may be a useful tool for differentiating T1DM and T2DM among young Asian Indians, a population that is currently the epicenter of the global diabetes epidemic.36
Acknowledgments
We thank the participants and the staff of Dr. Mohan's Diabetes Specialities Centre and the Madras Diabetes Research Foundation, Chennai, India, for their help with this study. This publication was made possible by the Fogarty International Center and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (grant 1 D43 HD065249). This is the ninth paper (GDRC-9) from the Global Diabetes Research Centre (GDRC), a collaboration between Madras Diabetes Research Foundation, Chennai, India, and Emory University, Atlanta, GA.
Author Disclosure Statement
No competing financial interests exist. V.M. conceived, initiated, supervised, conducted, and commented on all drafts of this article. K.G., S.A., A.A., H.R., R.M.A., and R.U. coordinated the study and monitored all the data entry and work parts of the article. K.G., V.A., P.M., and K.M.V.N. contributed extensively to the interpretative analysis of the data. K.G., S.A., and A.A. had full access to the data, and they take full responsibility for the integrity and accuracy of the data.
References
- 1.Kamada Y. Takehara T. Hayashi N. Adipocytokines and liver disease. J Gastroenterol. 2008;43:811–822. doi: 10.1007/s00535-008-2213-6. [DOI] [PubMed] [Google Scholar]
- 2.Hammarstedt A. Graham TE. Kahn BB. Adipose tissue dysregulation and reduced insulin sensitivity in non-obese individuals with enlarged abdominal adipose cells. Diabetol Metab Syndr. 2012;4:42. doi: 10.1186/1758-5996-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Obata Y. Yamada Y. Takahi Y. Baden MY. Saisho K. Tamba S. Yamamoto K. Umeda M. Furubayashi A. Matsuzawa Y. Relationship between serum adiponectin levels and age in healthy subjects and patients with type 2 diabetes. Clin Endocrinol (Oxf ) 2013;79:204–210. doi: 10.1111/cen.12041. [DOI] [PubMed] [Google Scholar]
- 4.Shanker J. Rao VS. Ravindran V. Dhanalakshmi B. Hebbagodi S. Kakkar V. Relationship of adiponectin and leptin to coronary artery disease, classical cardiovascular risk factors and atherothrombotic biomarkers in the IARS cohort. Thromb Haemost. 2012;108:769–780. doi: 10.1160/TH12-04-0263. [DOI] [PubMed] [Google Scholar]
- 5.Mohan V. Deepa R. Pradeepa R. Vimaleswaran KS. Mohan A. Velmurugan K. Radha V. Association of low adiponectin levels with the metabolic syndrome—the Chennai Urban Rural Epidemiology Study (CURES-4) Metabolism. 2005;54:476–481. doi: 10.1016/j.metabol.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Gokulakrishnan K. Anjana RM. Indulekha K. Anuradha S. Mohan V. Association of hypoadiponectinemia with non-alcoholic fatty liver disease in urban South Indians—(CURES-81) Indian J Med Res. 2010;132:271–277. [PubMed] [Google Scholar]
- 7.Gokulakrishnan K. Indulekha K. Ganesan S. Anuradha S. Mohan V. Adiponectin and carotid intimal medial thickness in subjects with and without glucose intolerance (CURES-82) Diabetes Technol Ther. 2010;12:109–115. doi: 10.1089/dia.2009.0100. [DOI] [PubMed] [Google Scholar]
- 8.Otabe S. Wada N. Hashinaga T. Yuan X. Shimokawa I. Fukutani T. Tanaka K. Ohki T. Kakino S. Kurita Y. Nakayama H. Tajiri Y. Yamada K. Hyperadiponectinemia protects against premature death in metabolic syndrome model mice by inhibiting AKT signaling and chronic inflammation. J Endocrinol. 2012;213:67–76. doi: 10.1530/JOE-11-0329. [DOI] [PubMed] [Google Scholar]
- 9.Addabbo F. Nacci C. De Benedictis L. Leo V. Tarquinio M. Quon MJ. Montagnani M. Globular adiponectin counteracts VCAM-1-mediated monocyte adhesion via AdipoR1/NF-κB/COX-2 signaling in human aortic endothelial cells. Am J Physiol Endocrinol Metab. 2011;301:E1143–E1154. doi: 10.1152/ajpendo.00208.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziemke F. Mantzoros CS. Adiponectin in insulin resistance: lessons from translational research. Am J Clin Nutr. 2010;91:258S–261S. doi: 10.3945/ajcn.2009.28449C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Q. Yuan B. Lo KA. Patterson HC. Sun Y. Lodish HF. Adiponectin regulates expression of hepatic genes critical for glucose and lipid metabolism. Proc Natl Acad Sci U S A. 2012;109:4568–4573. doi: 10.1073/pnas.1211611109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abi Khalil C. Mohammedi K. Aubert R. Abou Jaoude E. Travert F. Hadjadj S. Fumeron F. Roussel R. Marre M. Hyperadiponectinemia is independent of kidney function, diabetes duration, and control in type 1 diabetic patients without microangiopathy. J Clin Endocrinol Metab. 2011;96:E485–E487. doi: 10.1210/jc.2010-1835. [DOI] [PubMed] [Google Scholar]
- 13.Forsblom C. Thomas MC. Moran J. Saraheimo M. Thorn L. Wadén J. Gordin D. Frystyk J. Flyvbjerg A. Groop PH. FinnDiane Study Group: Serum adiponectin concentration is a positive predictor of all-cause and cardiovascular mortality in type 1 diabetes. J Intern Med. 2011;270:346–355. doi: 10.1111/j.1365-2796.2011.02406.x. [DOI] [PubMed] [Google Scholar]
- 14.Jorsal A. Tarnow L. Frystyk J. Lajer M. Flyvbjerg A. Parving HH. Vionnet N. Rossing P. Serum adiponectin predicts all-cause mortality and end stage renal disease in patients with type I diabetes and diabetic nephropathy. Kidney Int. 2008;74:649–654. doi: 10.1038/ki.2008.201. [DOI] [PubMed] [Google Scholar]
- 15.Alberti KG. Zimmet PZ. Definition, diagnosis, classification of diabetes mellitus, its complications. Part 1: Diagnosis and classification of diabetes mellitus, provisional report of a WHO Consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 16.Fida S. Myers M. Mackay IR. Zimmet PZ. Mohan V. Deepa R. Rowley MJ. Antibodies to diabetes-associated autoantigens in Indian patients with type 1 diabetes: prevalence of anti-ICA512/IA2 and anti-SOX13. Diabetes Res Clin Pract. 2001;52:205–211. doi: 10.1016/s0168-8227(01)00230-3. [DOI] [PubMed] [Google Scholar]
- 17.Amutha A. Datta M. Unnikrishnan R. Anjana RM. Mohan V. Clinical profile and complications of childhood- and adolescent-onset type 2 diabetes seen at a diabetes center in South India. Diabetes Technol Ther. 2012;14:497–504. doi: 10.1089/dia.2011.0283. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS. Bosch JP. Lewis JB. Greene T. Rogers N. Roth D A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 19.Surendar J. Anuradha S. Ashley B. Balasubramanyam M. Aravindhan V. Rema M. Mohan V. Cystatin C and cystatin glomerular filtration rate as markers of early renal disease in Asian Indian subjects with glucose intolerance (CURES-32) Metab Syndr Relat Disord. 2009;7:419–425. doi: 10.1089/met.2008.0084. [DOI] [PubMed] [Google Scholar]
- 20.Furuhashi M. Ura N. Higashiura K. Murakami H. Tanaka M. Moniwa N. Yoshida D. Shimamoto K. Blockade of the renin-angiotensin system increases adiponectin concentrations in patients with essential hypertension. Hypertension. 2003;42:76–81. doi: 10.1161/01.HYP.0000078490.59735.6E. [DOI] [PubMed] [Google Scholar]
- 21.Deepa M. Pradeepa R. Rema M. Mohan A. Deepa R. Shanthirani S. Mohan V. The Chennai Urban Rural Epidemiology Study (CURES)—study design and methodology (urban component) (CURES-I) J Assoc Physicians India. 2003;51:863–870. [PubMed] [Google Scholar]
- 22.Friedewald WT. Levy RI. Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 23.Hanley JA. McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 24.Frystyk J. Tarnow L. Hansen TK. Parving HH. Flyvbjerg A. Increased serum adiponectin levels in type 1 diabetic patients with microvascular complications. Diabetologia. 2005;48:1911–1918. doi: 10.1007/s00125-005-1850-z. [DOI] [PubMed] [Google Scholar]
- 25.Havel PJ. Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes. 2004;53(Suppl 1):S143–S151. doi: 10.2337/diabetes.53.2007.s143. [DOI] [PubMed] [Google Scholar]
- 26.Richards AA. Stephens T. Charlton HK. Jones A. Macdonald GA. Prins JB. Whitehead JP. Adiponectin multimerization is dependent on conserved lysines in the collagenous domain: evidence for regulation of multimerization by alterations in posttranslational modifications. Mol Endocrinol. 2006;20:1673–1687. doi: 10.1210/me.2005-0390. [DOI] [PubMed] [Google Scholar]
- 27.Fasshauer M. Klein J. Neumann S. Eszlinger M. Paschke R. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2002;290:1084–1089. doi: 10.1006/bbrc.2001.6307. [DOI] [PubMed] [Google Scholar]
- 28.Purohit S. She JX. Risk factors and biomarkers for type 1 diabetes. Int J Clin Exp Med. 2008;1:98–116. [PMC free article] [PubMed] [Google Scholar]
- 29.Snehalatha C. Ramachandran A. Mohan V. Viswanathan M. Pancreatic beta cell response in insulin treated NIDDM patients limitations of a random C-peptide measurement. Diabete Metab. 1987;13:27–30. [PubMed] [Google Scholar]
- 30.Verge CF. Gianani R. Kawasaki E. Yu L. Pietropaolo M. Jackson RA. Chase HP. Eisenbarth GS. Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes. 1996;45:926–933. doi: 10.2337/diab.45.7.926. [DOI] [PubMed] [Google Scholar]
- 31.Hathout EH. Thomas W. El-Shahawy M. Nahab F. Mace JW. Diabetic autoimmune markers in children and adolescents with type 2 diabetes. Pediatrics. 2001;107:E102. doi: 10.1542/peds.107.6.e102. [DOI] [PubMed] [Google Scholar]
- 32.Yang L. Luo S. Huang G. Peng J. Li X. Yan X. Lin J. Wenzlau JM. Davidson HW. Hutton JC. Zhou Z. The diagnostic value of zinc transporter 8 autoantibody (ZnT8A) for type 1 diabetes in Chinese. Diabetes Metab Res Rev. 2010;26:579–584. doi: 10.1002/dmrr.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thai AC. Mohan V. Khalid BA. Cockram CS. Pan CY. Zimmet P. Yeo JP. ASDIAB Study Group: Islet autoimmunity status in Asians with young-onset diabetes (12–40 years): association with clinical characteristics, beta cell function and cardio-metabolic risk factors. Diabetes Res Clin Pract. 2008;80:224–230. doi: 10.1016/j.diabres.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Barker JM. Barriga KJ. Yu L. Miao D. Erlich HA. Norris JM. Eisenbarth GS. Rewers M. Diabetes Autoimmunity Study in the Young: Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY) J Clin Endocrinol Metab. 2004;89:3896–3902. doi: 10.1210/jc.2003-031887. [DOI] [PubMed] [Google Scholar]
- 35.Liu LL. Yi JP. Beyer J. Mayer-Davis EJ. Dolan LM. Dabelea DM. Lawrence JM. Rodriguez BL. Marcovina SM. Waitzfelder BE. Fujimoto WY. SEARCH for Diabetes in Youth Study Group: Type 1 and type 2 diabetes in Asian and Pacific Islander U.S. youth: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32:133–140. doi: 10.2337/dc09-S205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Unwin N, editor; Whiting D, editor; Guariguata L, editor; Ghyoot G, editor; Gan D, editor. Diabetes Atlas. 5th. Belgium: International Diabetes Federation; 2011. [Google Scholar]