Abstract
Treatment wetlands have become an attractive option for the removal of nutrients from municipal wastewater effluents due to their low energy requirements and operational costs, as well as the ancillary benefits they provide, including creating aesthetically appealing spaces and wildlife habitats. Treatment wetlands also hold promise as a means of removing other wastewater-derived contaminants, such as trace organic contaminants and pathogens. However, concerns about variations in treatment efficacy of these pollutants, coupled with an incomplete mechanistic understanding of their removal in wetlands, hinder the widespread adoption of constructed wetlands for these two classes of contaminants. A better understanding is needed so that wetlands as a unit process can be designed for their removal, with individual wetland cells optimized for the removal of specific contaminants, and connected in series or integrated with other engineered or natural treatment processes. In this article, removal mechanisms of trace organic contaminants and pathogens are reviewed, including sorption and sedimentation, biotransformation and predation, photolysis and photoinactivation, and remaining knowledge gaps are identified. In addition, suggestions are provided for how these treatment mechanisms can be enhanced in commonly employed unit process wetland cells or how they might be harnessed in novel unit process cells. It is hoped that application of the unit process concept to a wider range of contaminants will lead to more widespread application of wetland treatment trains as components of urban water infrastructure in the United States and around the globe.
Key words: : critical review, municipal wastewater, pathogens, trace organic contaminants, treatment wetlands
Introduction
Attempts by scientists to estimate the value of ecosystem services provided by natural wetlands rank them among the most valuable land on earth (Costanza et al., 1997). They have been called “nature's filters,” and the role that natural wetlands play in water purification is a part of the justification for their protection and restoration. In an attempt to harness these properties, treatment wetlands have been built for a wide range of applications to improve water quality, including treatment of industrial and municipal wastewater, as well as stormwater, agricultural runoff, and acid mine drainage (EPA, 1993; Vymazal, 2009; Malaviya and Singh, 2012). Wetlands are becoming an increasingly popular option with water agencies because of their low operation cost, energy consumption, and environmental impact (Gearheart, 1999; Fuchs et al., 2011). In addition, wetlands provide ancillary benefits, such as the creation of aesthetically appealing green spaces and wildlife habitats (Fleming-Singer and Horne, 2006). Wetlands specifically designed for treatment of municipal wastewater effluents have been used for at least five decades for the removal of suspended solids, biochemical oxygen demand (BOD), nutrients, metals, and pathogens (Mitsch and Gosselink, 2007; Kadlec and Wallace, 2009; Vymazal, 2010).
Many of the treatment wetlands built in the second half of the twentieth century consisted of relatively small plots of land, typically less than 5 hectares (EPA, 2000b; Kadlec, 2012). More recently, the size of treatment wetlands has expanded with systems covering as much as 475 hectares and treating up to 2.5×105 m3/day (60 MGD) of wastewater effluents, or effluent-dominated river water (Table 1). The main purpose of these large wetland systems is typically a combination of nutrient removal and habitat creation. Increasingly, the removal of trace organic contaminants and pathogens is also invoked as a benefit. This new trend, coupled with the continued construction of smaller treatment wetlands, indicates that treatment wetlands are becoming an important part of urban water infrastructure. Despite their increasing popularity, many barriers still prevent them from realizing their full potential for improving water quality and enhancing aquatic habitat.
Table 1.
Examples of Large, Full-Scale Treatment Wetlands in the United States
| Wetland name | Location, year started | Size (ha) | Flow (m3/day) | Stated purpose |
|---|---|---|---|---|
| Easterlya | Orlando, FL; 1987 | 475 | 8×104 | Nutrient removal from wastewater before release to the sensitive St. Johns River; wildlife habitat |
| Pradob | Riverside, CA; 1992 | 200 | 2.5×105 | Nitrate removal from effluent-dominated Santa Ana River before Prado dam, which is used for aquifer recharge; wildlife habitat |
| George W. Shannonc | Tarrant County, TX; 2002 | 180 | 4×105 | Suspended solid and nutrient removal from effluent-dominated Trinity River before reservoirs; wildlife habitat; education |
Florida Department of Environmental Protection, 2012.
Orange County Water District 2012.
Tarrant Regional Water District, 2012.
One of the most significant barriers for the use of treatment wetlands is the difficulty of designing wetlands with predictable performance. Compared with mechanical unit treatment processes, the ecological, transport, and transformation processes occurring in treatment wetlands are even more complex and are not fully understood. For some constituents, researchers have made progress in understanding the detailed transformation mechanisms, including models that account for the complexity (e.g., Wang and Mitsch, 2000; Howell et al., 2005). However, such complex models cannot be used for design purposes, because they are very difficult to parameterize. Nonetheless, the insights provided by mechanistic research can provide the foundation for designing unit process treatment wetlands, with each unit process tailored to the treatment of a specific set of contaminants, by identifying the most important parameters controlling performance. This unit process approach is not meant to undervalue the complexity of wetland ecosystems. Rather, by optimizing specific transformation mechanisms in unit process cells, they can be more easily integrated with other mechanical or natural treatment systems to provide treatment trains with predictable performance.
For example, mechanistic research coupled with studies of full-scale systems has led to robust design approaches for unit process wetlands for denitrification (Kadlec, 2012). Such denitrification wetlands can be used to treat nitrified effluents from mechanical wastewater treatment plants (Table 1), or they can be staged after shallow aerobic nitrification wetlands (Hammer and Knight, 1994; Vymazal, 2007). Similarly, hybrid wetlands comprising a vertical flow cell and a cell with calcite media have been shown to be effective at removing both BOD and phosphorus from wastewater (Arias et al., 2003). In addition, deep detention ponds for particle removal and anaerobic digestion of solids before vegetated wetlands and slow sand filters have been suggested to provide efficient treatment of municipal wastewater (Horne and Fleming-Singer, 2005).
Despite the increasing use of unit process wetlands for nutrient and BOD removal, current understanding of removal mechanisms in wetlands for certain classes of contaminants has not yet been translated into the design of unit process wetlands. This critical review focuses on the application of surface flow unit process wetlands to the removal of two such classes of contaminants, trace organic compounds and pathogens, from wastewater effluents and effluent-dominated river water. Trace organic contaminants are an emerging concern, due to their negative effects on aquatic ecosystems and the inability of conventional wastewater treatment plants to provide adequate removal. Pathogens and indicator organisms, on the other hand, are an historical concern, but removal by wetlands is often poor.
This article starts with a review of hydraulics in surface flow constructed wetlands, given their central role in treatment performance. Next, the reported removals of trace organic contaminants and pathogens in wetlands is summarized, followed by a review of the main removal mechanisms such as sorption and sedimentation, biotransformation and predation, and photolysis and photoinactivation. Gaps in knowledge are identified for future research that can lead to identifying the controlling factors so that effective unit process wetlands and treatment trains can be developed. The final section provides suggestions for how these treatment mechanisms can be enhanced in commonly employed unit process wetland cells or how they might be harnessed in novel unit process cells. It is hoped that the application of the unit process concept to a wider range of contaminants will lead to more widespread application of wetland treatment trains as components of urban water infrastructure in the United States and around the globe.
Discussion
Hydraulics of surface flow wetlands
Inefficiencies in hydraulics are a major barrier to optimizing the removal of contaminants in treatment wetlands, including trace organics and pathogens. Theoretically, the most effective wetland design would employ plug flow conditions to ensure that all water receives an equal amount of time for treatment. However, in practice, plant growth rapidly results in conditions that deviate from ideal. In particular, hydraulic short-circuiting can dramatically decrease the overall performance of a wetland cell. Since this limitation to wetland treatment has been recognized for decades, models have been developed to account for the effects of dispersion due to vegetation, wind, and wetland boundaries (Kadlec, 1994). While these models are an improvement over ideal reactor models and offer an insight into flow patterns in wetlands, the complex effects of heterogeneous and dynamic flow patterns are more difficult to model accurately.
Short-circuiting is the result of preferential flow paths through a wetland, which are caused primarily by uneven plant distribution and channelized flow (Kjellin et al., 2007; Lightbody et al., 2008). Short-circuiting results in water having a range of residence times in a wetland, reducing the wetland's treatment efficiency (Keefe et al., 2004; Wörman and Kronnäs, 2005). This is especially detrimental for wetlands designed to remove waterborne pathogens, which require reductions in a concentration of several orders of magnitude to provide effective treatment and, thus, are severely compromised by even a modest amount of short-circuiting. To demonstrate this point, consider a wetland that is designed to provide 4-log removal (99.99%) of a pathogen under ideal, plug flow conditions. If just 20% of the flow has one-eighth of the nominal residence time, as observed by Lightbody et al. (2008) in a recently constructed wetland, the actual removal will only be about 1-log (90%).
The degree of short-circuiting in full-scale wetlands is usually evaluated with tracer studies (Martinez and Wise, 2003; Lin et al., 2003). While tracer studies provide an understanding of how far the system deviates from the ideal, more complicated models are necessary to predict contaminant treatment efficiency, as water flowing via different paths may be subjected to diverse biogeochemical conditions, resulting in variable treatment (Kadlec, 2000; Harvey et al., 2005). For example, Keefe et al. (2004) modeled the reactive transport of rhodamine water tracer (WT) in three wetlands using a solute transport model with transient storage. Results showed that rhodamine WT loss rates via photolysis and sorption differed in storage and main channel zones, with sorption mass transfer rates being a factor of two higher in storage zones than in the main channel, and photolysis rates in the storage zones being almost an order of magnitude lower than those occurring in the main channel. Thus, an understanding of both the flow distributions and the removal processes at work in these different wetland zones was necessary to accurately interpret tracer test results.
Although short-circuiting cannot be eliminated, it can be reduced by proper wetland design. Consideration of soil conditions before wetland construction, such as filling ditches that would channelize flow (Martinez and Wise, 2003), can reduce short-circuiting. Baffles can also be used to increase the wetland's length-to-width ratio (aspect ratio), thereby reducing short-circuiting and encouraging plug flow conditions at a lower cost than building long, narrow wetlands (Reed et al., 1995; Persson, 2000; Shilton and Harrison, 2003). Knight (1987) calculated that an aspect ratio of 2:1 would maximize wetland performance while minimizing construction costs. However, other aspect ratios may be appropriate when there is a need to significantly reduce contaminant concentrations (e.g., in the case of pathogens), and further research is needed to determine the optimal aspect ratio in these cases. The use of a subsurface berm or island placed in front of the wetland inlet also may reduce short-circuiting and improve hydraulic performance (Persson, 2000).
Despite careful wetland design, flow irregularities will still develop over time as plants grow. Flow irregularities may be minimized by using deep transverse mixing zones and other structures to break wetlands into multiple smaller cells. These zones improve wetland performance by mixing water that has traveled through different flow paths, as well as by reducing the likelihood that fast flow paths will be aligned (Lightbody et al., 2007, 2009). Breaking a wetland into multiple cells has a similar effect, disrupting high-speed flow paths and ensuring that water is well mixed between cells (Kadlec, 2000; Horne and Fleming-Singer, 2005).
Periodic maintenance can also be used to control short-circuiting. For example, at the Prado Treatment Wetlands in Southern California, emergent plants are removed during maintenance activities (Scott Nygren, Orange County Water District, personal communication, March 13, 2012). This process involves draining the cell, allowing it to dry for several weeks, and using a mower designed for brush removal to cut the plants near the ground surface. At the Easterly Wetlands in Central Florida, wetland plants are occasionally burned to thin the density of accumulated plants.
Incorporating multiple wetland cells is a fundamental component of the unit process wetland design. By linking unit process wetland cells in series, designing cells to have deep zones and baffles, considering the effects of inlet and outlet structures, and providing adequate maintenance, inefficiencies introduced by hydraulic short-circuiting can be minimized. Further research is needed to identify cost-effective maintenance practices that will minimize hydraulic short-circuiting.
Contaminants of concern
Trace organic contaminants
Municipal wastewater effluent typically contains relatively low levels of organic matter (i.e., most wastewater treatment plants achieve BOD<10 mg/L). In addition to the biopolymers and residual organic waste that make up the bulk of the biodegradable organic matter, wastewater effluents also contain an assortment of trace organic contaminants, such as pharmaceuticals and personal care products (Kolpin et al., 2002; Ternes et al., 2004b). Trace organic contaminants in wastewater effluents are an issue of concern due to their potential to cause adverse impacts to aquatic organisms at low concentrations (Daughton and Ternes, 1999; Suárez et al., 2008) as well as their potential to contaminate downstream drinking water supplies (Snyder et al., 2003).
The ability of constructed wetlands to remove trace organic contaminants from wastewater effluents has recently received growing attention (Matamoros and Bayona, 2008). Removal efficiencies for some pharmaceuticals and personal care products in treatment wetlands (Fig. 1) suggest that trace organic contaminants generally fall into one of three groups of removal efficiency. The first group of compounds is removed efficiently (i.e., >60% removal) regardless of wetland design and includes substances such as caffeine and naproxen. The second group, which includes the majority of the compounds in Fig. 1, exhibits partial removal with varying efficiencies depending on wetland design and hydraulic residence times. The final group of compounds, which includes carbamazepine and clofibric acid, are more recalcitrant and exhibit limited removal (i.e., typically <40% removal) irrespective of wetland design. Note that some values were determined from studies in subsurface wetlands, but they are included here because data from surface flow wetlands have not been reported. Optimization of treatment wetlands has the highest potential for enhancing the removal of the compounds in the second group.
FIG. 1.
Averages with standard deviations of pharmaceutical and personal care product removal efficiencies in treatment wetlands reported in recent studies (n=3–16). References: aGray and Sedlak, 2005; bSong et al., 2011; cBreitholtz et al., 2012; dPark et al., 2009; eCamacho-Muñoz et al., 2011; fHijosa-Valsero et al., 2011; gMatamoros and Bayona, 2006; hMatamoros et al., 2007; iMatamoros et al., 2009; jLlorens et al., 2009; kMatamoros et al., 2005; lMatamoros et al., 2008; mWaltman et al., 2006.
Waterborne pathogens
Wastewater effluents contain potentially infectious microorganisms, including viruses, bacteria, protozoan (oo)cysts, and helminth eggs. Removal or inactivation of pathogens is, therefore, necessary before treated effluents are discharged or reused. Treatment wetlands that receive wastewater which has already been disinfected may provide additional treatment of pathogens that are resistant to disinfection (e.g., Cryptosporidium oocysts for chlorine or adenovirus for UV). In this case, a wetland may be used to reduce the chemical disinfection requirements and to provide an additional treatment barrier. Alternatively, treatment wetlands that receive wastewater effluent which has not been disinfected can play a primary role in pathogen attenuation. In this context, Gersberg et al. (1989) suggested that treatment wetlands with hydraulic residence times of 3 to 6 days may be as effective as conventional water treatment systems employing disinfection for the removal of pathogenic bacteria and viruses. Reliance on polishing wetlands for disinfection has the advantage over chlorination of avoiding the production of disinfection byproducts (Buth et al., 2009, 2010).
Most studies on the removal of pathogens in treatment wetlands have measured fecal indicator bacteria rather than actual pathogens. The reported removal efficiency of fecal coliforms by surface wetlands is around 1-log removal (Vymazal, 2005; Kadlec and Wallace, 2009). The few studies that have been conducted with actual pathogens (Fig. 2) show removal efficiencies up to 2-log, with average values around 1-log. The dominant removal mechanisms vary dramatically among pathogen groups. A better understanding of pathogen removal mechanisms, including attachment and sedimentation, predation, and photoinactivation, and their effectiveness for different pathogen groups is needed to improve the ability to design unit process wetlands for disinfection.
FIG. 2.
Averages with standard deviations of pathogen removal efficiencies in surface flow wetlands receiving nondisinfected influent. References: aMandi et al., 1996; bMandi et al., 1998; cReinoso et al., 2008; dFalabi et al., 2002; eGerba et al., 1999; fQuiñónez-Díaz et al., 2001; gHerskowitz, 1986; hHill and Sobsey, 2001; iSong et al., 2010; jKarpiscak et al., 2001; kKadlec and Wallace, 2009.
Removal mechanisms of trace organic contaminants
Sorption
Sorption of trace organic contaminants encompasses two distinct processes. Adsorption involves the interaction of a compound with a surface, typically via ion exchange or surface complexation, while absorption entails partitioning into a particle-associated organic phase. Sediments and biofilms in treatment wetlands provide numerous surfaces that may be capable of sorbing trace organic contaminants. If the contaminants exhibit a high affinity for a surface, they will eventually be buried as decaying plant litter accumulates in the wetland. If the sorbent does not degrade, the contaminants will remain in the litter layer until it is removed as a part of wetland maintenance activities. If the contaminants are weakly associated with the sorbent, or if the sorbent (e.g., plant litter) degrades, the process may simply slow the movement of contaminants through a wetland, providing more time for other transformation processes to occur.
Although sorption is assumed to be important to trace organic contaminant fate in treatment wetlands (Imfeld et al., 2009), a few studies have investigated it specifically. In these studies, log Kow, a measure of a contaminant's hydrophobicity, generally predicts which contaminants are most susceptible to absorption. For example, in a bulrush-dominated surface flow wetland with a 30 cm deep gravel bed, the hydrophobic phthalate esters and fragrance molecules (log Kow>4) were absorbed to organic matter in the gravel bed as well as suspended particles (Reyes-Contreras et al., 2011). Absorption was also found to slow the movement of two steroid hormones (log Kow≈4) relative to a conservative tracer in a densely vegetated surface flow wetland (Gray and Sedlak, 2005).
Removal of chemical contaminants by absorption may be more important in subsurface flow wetlands, where flowing water encounters higher densities of particulate organic matter. For example, partial removal of the recalcitrant contaminant carbamazepine (log Kow≈2.5) by absorption and negligible sorptive removal of less hydrophobic contaminants was observed in a study of a subsurface flow treatment wetland (Matamoros et al., 2005).
Studies of pesticide sorption in agricultural wetlands show a similar dependence on contaminant hydrophobicity (Kruger et al., 1996; Moore et al., 2002; Reichenberger et al., 2007). For example, sorption of the herbicide atrazine (log Kow=2.75) to soil, litter, peat, and sediments from three Midwest wetlands was well described for all sorbents by an organic carbon-normalized distribution coefficient (KOC=760 L/kg OC) (Alvord and Kadlec, 1995). Given this distribution coefficient, a wetland with about 2 kg/m2 litter, containing about 40% organic carbon (Alvord and Kadlec, 1995), and a depth of 40 cm, would be capable of absorbing more than 60% of atrazine from the aqueous phase. This suggests that absorption in wetlands could be significant for compounds with a log Kow greater than about 2.5, provided that the system is designed properly. For comparison, absorption of trace organic contaminants in activated sludge treatment plants is usually unimportant for compounds with log Kow values less than about 4 (Ternes et al., 2004a; Wick et al., 2009).
Less hydrophobic compounds have been found to adsorb via specific ionic interactions with activated sludge in treatment plants (Stuer-Lauridsen et al., 2000; Golet et al., 2003) and soils (Tolls, 2001). In wetlands, the relatively hydrophilic fluorescent dye, rhodamine WT, sorbs significantly to plants and sediments (Lin et al., 2003; Keefe et al., 2004). At neutral pH values, rhodamine WT contains both positively and negatively charged functional groups and is, thus, likely to be adsorbed via specific interactions with charged functional groups on the sorbents (Kasanavia et al., 1999). Therefore, adsorption of ionic trace organic contaminants in treatment wetlands may be an important loss mechanism for certain compounds. However, additional research is needed to assess the overall importance of this phenomenon and ways in which it could be enhanced through wetland design.
Certain types of wetland vegetation may increase the removal of trace organic contaminants by sorption. For example, it has been suggested that wetlands dominated by bulrush (e.g., Scirpus spp.) are conducive to sorption of trace organic contaminants due to the large amounts of spongy peat formed by decomposing plants (Horne and Fleming-Singer, 2005). Duckweed (Lemna spp.), a floating macrophyte often present in open waters in treatment wetlands, sorbs trace organic contaminants such as halogenated phenols (Tront et al., 2007) and pharmaceuticals and personal care products, including fluoxetine, ibuprofen, and triclosan (Reinhold et al., 2010). However, duckweed grows in a thin layer near the water surface and the relatively small mass of the plant in wetlands likely precludes it from removing a significant fraction of the trace organic contaminants as water passes through a wetland.
Water chemistry also affects sorption of chemical contaminants in constructed wetlands (Hussain and Prasher, 2011). In particular, the pH of wetland water will affect the sorption of contaminants by changing their speciation. This phenomenon has been observed in wastewater treatment plant sludge for the acidic pharmaceuticals diclofenac (pKa=4.6) and ibuprofen (pKa=3.5), which absorbed to primary sludge to a greater extent than to secondary sludge, because a greater fraction of the pharmaceuticals were in their uncharged form at the lower pH value (pH=6.6 in primary versus pH=7.5 in secondary) (Ternes et al., 2004a). Basic contaminants, such as those containing amine functional groups (e.g., the β-blockers), have pKa values near 9 and are positively charged at neutral pH values. Consequently, their sorption is likely controlled by specific interactions, as was observed in a study by Yamamoto et al. (2009). Increasing the pH of wetland water could increase the fraction of the uncharged forms of the compounds, resulting in enhanced sorption by hydrophobic interactions. Further research is needed to determine the potential for enhancing sorption in treatment wetlands through the use of natural processes that alter pH values (i.e., photosynthesis and microbial respiration).
Biotransformation
Microorganisms play a prominent role in the attenuation of trace organic contaminants in constructed wetlands (Matamoros et al., 2008; Hijosa-Valsero et al., 2010) due to the diversity of microorganisms and enzymatic activities present (D'Angelo, 2003). In surface flow wetlands, biofilms found on roots, stalks, and detritus are more important to biotransformation than planktonic microorganisms (Gagnon et al., 2007; Truu et al., 2009). Thus, it is not surprising that properties affecting biofilm growth, such as the attachment matrix, hydraulic conditions, and composition of the wastewater effluents, can strongly influence microbial ecology and contaminant transformation rates in these systems (Truu et al., 2009).
In vegetated treatment wetlands, the density and type of plants affect microbial community dynamics (Ibekwe et al., 2006; Calheiros et al., 2009) by providing labile forms of organic carbon, surfaces for biofilm growth, and oxygen gradients (Reddy and D'Angelo, 1997). The ability of decaying plants to create anoxic zones in surface and subsurface flow constructed wetlands is important for the transformation of trace organic contaminants, because some compounds are more readily transformed under aerobic conditions (e.g., ibuprofen) while others (e.g., tonalide and galaxolide) are more readily transformed under anaerobic conditions (Hijosa-Valsero et al., 2010). Further, anoxic, nitrogen-reducing surface flow wetlands have been shown to be capable of transforming certain trace organic contaminates, including atenolol, naproxen, and triclosan, possibly through amide hydrolysis and reductive dehalogenation (Park et al., 2009).
Plant biomass and DOC from plant litter, and to a lesser degree, residual organic carbon from wastewater effluent, provide an important energy source and create selective pressure for microbial community structure and function in wetland systems (Shackle et al., 2000; Gutknecht et al., 2006). Biotransformation of pharmaceuticals in wastewater effluents can be affected by both the abundance and source of organic carbon derived from decaying aquatic plants. For example, gemfibrozil and sulfamethoxazole, two compounds that are poorly removed in wastewater treatment plants, showed better removal in the presence of labile organic carbon derived from wetland plants than in the presence of the labile dissolved organic carbon in wastewater effluents (Lim et al., 2008). Other macrophyte characteristics, such as surface area and litter properties, can also affect microbial density (Bastviken et al., 2005), which may correlate with trace organic contaminant removal rates.
The rhizosphere associated with wetland plants hosts a unique community of microorganisms within aerobic microzones, due to the release of oxygen and nutrient-rich exudates (Brix, 1997; Kyambadde et al., 2004; Münch et al., 2007; Gagnon et al., 2007). Microbially mediated iron and manganese oxides formed in this region also have the potential for indirect oxidation or enhanced sorption of trace organic contaminants (Mendelssohn et al., 1995; Emerson et al., 1999). Rhizosphere-associated transformation appears to be an important process for trace organic contaminants in subsurface flow wetlands (Zhang et al., 2012). However, the rhizosphere may be less important in surface flow wetlands due to limited contact between the rhizosphere and flowing waters.
Bacteria and fungi transform macromolecules, such as cellulose and lignin, into lower-molecular-weight compounds through the excretion of extracellular enzymes. Enzyme expression studies in constructed wetlands have provided insights into the importance of extracellular enzyme activity to the processing of these recalcitrant forms of organic carbon (Shackle et al., 2000; Wright and Reddy, 2001; Francoeur et al., 2006; Hill et al., 2006; Rier et al., 2007). Many of the extracellular enzymes used to transform cellulose and lignin (e.g., laccases, phenol oxidases, and peroxidases) can also transform recalcitrant trace organic contaminants (Gianfreda and Rao, 2004; Lu et al., 2009). By modifying the quantity and type of carbon sources in constructed wetlands, it may be possible to increase the activity of extracellular enzymes. Since most extracellular enzymes utilize oxygen or hydrogen peroxide as terminal electron acceptors, their activity is expected to be higher in aerobic environments (Sinsabaugh, 2010; Porter, 2011).
Photolysis
In natural waters, photolysis of trace organic contaminants can occur via direct and indirect mechanisms (Schwarzenbach et al., 2003). Direct photolysis occurs when a contaminant absorbs sunlight and undergoes a transformation reaction. Indirect photolysis involves other chemicals, most often NO3− and colored dissolved organic matter (CDOM), which absorb light and produce reactive intermediates that subsequently react with contaminants. With regard to the transformation of organic contaminants, important reactive intermediates include hydroxyl radical (•OH) (Zepp et al., 1987; Brezonik and Fulkerson-Brekken, 1998), singlet oxygen (1O2) (Zepp et al., 1977), excited triplet state DOM (3DOM*) (Canonica et al., 1995; Boreen et al., 2004), organoperoxy radicals, and carbonate radical (•CO3−) (Lam et al., 2003; Canonica et al., 2005).
Treatment wetlands may be conducive to indirect photolysis due to the presence of NO3− in nitrified wastewater effluent, which produces •OH via the reaction:
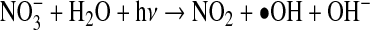 |
CDOM derived from wastewater effluents and decaying plants may also promote indirect photolysis through a variety of mechanisms. For example, CDOM was significant to the removal of the pesticides alachlor and carbaryl in wetland waters via indirect photolysis (Miller and Chin, 2002, 2005). For those reactions where CDOM served as a source of •OH, rates of photolysis were not strongly affected by DOM concentrations because they acted as both a source of CDOM and a •OH scavenger. In addition to generating •OH, CDOM can also serve as a source of the selective oxidants 1O2 and 3DOM*, which have been found to be important to the indirect photolysis of certain trace organic chemicals in the environment (Gerecke et al., 2001; Latch et al., 2003).
Most constructed wetlands are not designed to include shallow open water zones. As a result, few investigators have studied the role of photolysis in trace organic contaminant removal in treatment wetlands. Matamoros et al. (2008) attributed the nearly complete removal of ketoprofen in an engineered treatment wetland with deep (1.5 m) open water zones and a long hydraulic residence time (30 days) to photolysis. While this study demonstrated that photolysis in deep, open ponds can significantly attenuate organic compounds that are particularly susceptible to direct photolysis [ketoprofen has a half-life of 2.5 min under near-surface summer-noon conditions (Lin and Reinhard, 2005)], removal efficiencies would be significantly lower for compounds with longer direct photolysis half-lives. For example, consider sulfamethoxazole, a compound that is relatively susceptible to direct photolysis [half-life of about 2 h under near-surface summer-noon conditions (Lam and Mabury, 2005)], in a 1 m deep wetland with the EPA (2000a) recommended hydraulic residence time of 3 days for open water zones. Given a typical beam attenuation coefficient (α) of 6/m at 330 nm, and ideal plug flow conditions, sulfamethoxazole would exhibit a decrease in concentration of only about 10% due to direct photolysis under daily averaged mid-summer conditions at 40° latitude (Schwarzenbach et al., 2003).
Removal of waterborne pathogens
Attachment and sedimentation
Some pathogens, such as helminth eggs, have settling velocities that are high enough to be removed by sedimentation in treatment wetlands (e.g., ∼0.1 mm/s) (Sengupta et al., 2011). The presence of rhizomes and macrophyte stalks can further enhance sedimentation (Mandi et al., 1996). However, protozoan (oo)cysts and bacteria have much lower settling velocities [e.g., <0.001 mm/s for Giardia cysts (Dai and Boll, 2006)], and viruses are stable in suspension. These organisms will only be removed by sedimentation if they are attached to larger particles. As a result, the removal of cysts such as Giardia in some cases has been correlated with particle removal (Quiñónez-Díaz et al., 2001). However, particle association is not always conducive to pathogen removal. For example, in a study conducted by Boutilier et al. (2009), the association of Escherichia coli with particles appeared to decrease their removal compared with free-floating bacteria. This was because particles with diameters less than 80 μm had settling velocities that were too low to be removed by sedimentation.
In addition to attaching to settling particles, pathogens can be removed by attachment to other surfaces in treatment wetlands. However, the contribution of this mechanism to overall removal is not known. For example, in vegetated surface flow wetlands, viruses were removed by attachment to the biofilm layer of rhizomes and submerged stalks of emergent plants (Gersberg et al., 1987). Biofilms have also been shown to increase the removal of pathogen surrogates (0.1-, 1.0-, and 4.5-μm latex microspheres) in microcosm experiments using surface flow wetland water (Stott and Tanner, 2005). A better understanding of the processes that affect the association of pathogens with particles and surfaces, as well as the size distribution of particles in treatment wetlands, may provide insights into means for enhancing pathogen removal via sedimentation and attachment.
Pathogenic helminth eggs, protozoan (oo)cysts, and viruses that accumulate on wetland surfaces and in sediments will become inactivated over time. In contrast, indicator and pathogenic bacteria have the potential to grow under certain environmental conditions (Ksoll et al., 2007; Ishii et al., 2010), although there is no evidence of growth occurring in treatment wetlands. It should be noted that sloughing or resuspension of sediments has the potential to remobilize viable organisms. In addition, wetland vegetation or sediments that are removed during maintenance activities may contain viable pathogens, which could complicate efforts to reuse the material (e.g., as a soil amendment).
Predation
Predation has the potential to be an important removal mechanism for bacteria and protozoan (oo)cysts in treatment wetlands (Stott et al., 2001; Song et al., 2008), but much less is known about viruses. Grazing of particles in the size range of individual viruses is typically inefficient (Hahn and Höfle, 2001), although viruses attached to larger particles have the potential to be removed by predation. Potential predators of pathogens in treatment wetlands include nematodes, copepods, rotifers, and protozoa (Decamp and Warren, 1998). For example, grazing rates for the ciliated protozoa Paramecium were measured to be 111 E. coli/(ciliate-hour) and 170 Cryptosporidium parvum oocysts/(ciliate-hour) in lab experiments (Decamp and Warren, 1998; Stott et al., 2001). Hence, a population of 2×104Paramecium per liter (Decamp and Warren, 1998) would have the potential to achieve greater than 6-log removal of E. coli or oocysts in 1 h, if these were the only particles in the water. Grazing rates in an actual wetland are expected to be significantly lower, because pathogens would represent a small fraction of the total particles.
In addition to ciliated protozoa, rotifers may play an important role in the removal of pathogens via grazing due to their dominance in the total zooplankton population in shallow wetlands (Beaver et al., 1998; Fayer et al., 2000; Trout et al., 2002; Proakis, 2003). For example, the rotifer Brachionusplicatilist rapidly removed E. coli from water under laboratory conditions, with an average feeding rate of almost 700 E. coli/(rotifer-hour) (Proakis, 2003). Rotifers from six different genera were also found to ingest Giardia cysts and Cryptosporidium oocysts in simple laboratory experiments (Fayer et al., 2000; Trout et al., 2002). However, ingestion rates in actual wetlands have not been measured, and conditions that promote predation in treatment wetlands are not sufficiently understood.
While predation may provide an effective means for removing indicator organisms and pathogens from water, the viability of the organisms after ingestion is uncertain. In general, protozoan grazing is a major mechanism of bacterial population control (Hahn and Höfle, 2001). However, there is evidence that some pathogenic bacteria are not inactivated as a result of ingestion by protozoan grazers, such as ciliates and amoebas (Barker and Brown, 1994; Meltz Steinberg and Levin, 2007). Indeed, pathogenic bacteria such as Legionella actually colonize free-living amoeba to protect themselves from unfavorable environmental conditions (Thomas et al., 2010). More research is needed on inactivation of the wide range of pathogens of concern in wastewater by the different types of grazers in treatment wetlands.
Photoinactivation
Sunlight-mediated inactivation is one of the most important disinfection mechanisms in waste stabilization ponds (Davies-Colley et al., 2000; Davies-Colley, 2005), suggesting that open water zones are promising for the removal of pathogens in treatment wetlands. There are three main mechanisms of sunlight-mediated inactivation of microorganisms (Davies-Colley et al., 1999). Analogous to direct photolysis of chemical contaminants, the absorption of UVB radiation (280–320 nm) by DNA causes direct damage to cellular DNA, primarily by pyrimidine dimer formation (Jagger, 1985). Similar to indirect photolysis of chemical contaminants, there are also indirect disinfection mechanisms, in which sensitizers absorb light and produce reactive species that damage organisms. Indirect damage may occur due to absorption of sunlight by cell constituents (endogenous sensitizers) (Davies-Colley et al., 1999; Bosshard et al., 2010) or by sensitizers in water (exogenous sensitizers), such as CDOM. 1O2 has been shown to be the most important reactive species produced by exogenous sensitizers during the sunlight-mediated inactivation of MS2 coliphage, which is a model for human enteric viruses (Kohn and Nelson, 2007).
Exogenous inactivation initiated by sensitizers that absorb longer wavelengths, such as CDOM, may be more important than endogenous mechanisms, because the UV wavelengths which contribute to endogenous inactivation (direct and indirect) are readily absorbed in wetland water (i.e., λ=280 to 400 nm). However, not all organisms appear to be susceptible to this mechanism (Davies-Colley et al., 1999). Photoinactivation can also be enhanced by high dissolved oxygen and elevated pH (e.g., greater than pH 9) that result from algal photosynthesis (Curtis et al., 1992; Davies-Colley et al., 1999; Ansa et al., 2011).
The importance of sunlight-mediated disinfection in surface flow treatment wetlands was illustrated when E. coli concentrations declined significantly after a thick bed of floating duckweed (Lemna spp.) had been removed from a newly constructed surface flow wetland (MacIntyre et al., 2006). A better understanding of the sunlight-mediated inactivation mechanisms and their roles in pathogen removal is needed to optimize the design and to enable prediction of the fate of pathogens in surface flow treatment wetlands.
Novel unit process wetlands for removal of trace organic contaminants and pathogens
A growing understanding of wetland hydraulics and contaminant attenuation mechanisms in treatment wetlands provides an opportunity for optimizing the design and operation of wetlands to remove trace organic contaminants and pathogens in a sequence of unit process cells. The individual unit processes incorporated into a wetland treatment train will depend on a variety of considerations, including influent water quality, contaminants of concern, point of discharge, regulations on effluent water quality, and space constraints. Different unit processes will be needed to address the wide range of trace organic contaminants and pathogens, which are removed by different removal mechanisms. Based on the review of removal mechanisms in Removal mechanisms of trace organic contaminants and Removal of waterborne pathogens sections, in this section, we further explore several novel designs for unit process cells that target removal of trace organics and wetlands: a shallow, open-water cell, vegetated wetlands that optimize specific enzymes and biodegradation pathways, and a cell incorporating filter-feeding bivalves.
An example of how these unit processes could be combined in a treatment train for nitrified wastewater effluent is provided in Fig. 3. In this example, the first cell provides treatment of trace organics through direct photolysis, indirect photolysis (including •OH produced from NO3−), and sorption and biotransformation in the thick biofilm layer that forms on the cell's bottom. Inactivation of pathogens also occurs through direct and indirect photoinactivation. Next, in the cattail cell, labile organic matter produced by cattails fuels denitrification as well as biodegradation of trace organics; the anaerobic conditions promote precipitation of metal sulfides; and quiescent conditions promote further settling of particle-associated pathogens. Next, in the bulrush cell, nonlabile organic carbon accumulates and serves as a sorbent for trace organic contaminants. Finally, in the bivalve cell, particle-associated trace organic contaminants and pathogens are ingested, and transformed or inactivated.
FIG. 3.
Example of unit process wetland treatment train, along with key processes occurring in each unit process cell.
A deeper understanding of the specific mechanisms at play will allow the individual cells to be optimized, as well as their sequential order. For example, if DOM produced by vegetated cells is found to be effective at sensitizing the degradation of specific chemicals or pathogens, the photolysis cell could be placed after the vegetated cell. The potential for each of these unit processes is explored in greater detail in subsequent sections.
Shallow, open-water cells
Shallow, open-water wetland cells (Fig. 4) represent a new approach for integrating photochemical processes and aerobic microbes into a unit process wetland. While photolysis can provide a means of removing contaminants, it is difficult to design treatment wetlands for photolysis, because emergent macrophytes and floating plants (e.g., duckweed) shade the water. Furthermore, chromophores in wastewater effluents and wetland water strongly absorb sunlight, especially in the important UV region of the solar spectrum, greatly slowing photochemical reactions at depths of more than about 0.5 m. To circumvent these problems, a shallow, open-water wetland cell can be used. In these cells, concrete or geotextile liners are used on the bottom of the cell to prevent emergent macrophyte growth. Under these conditions, the bottom of the basin is rapidly colonized by a periphyton mat, which can be defined as a consortium of organisms dominated by algae, aerobic bacteria, and other eukaryotes (Wetzel, 1983).
FIG. 4.

Pilot-scale open-water wetland cell located in Discovery Bay, CA. The cell is about 20 cm deep, 400 m2, and has a hydraulic residence time of about 3 days.
A pilot-scale wetland of this design (Fig. 4) allows sunlight to penetrate throughout the water column, which is typically around 20 cm. The growth of periphyton on the bottom of the cell, rather than suspended in the water column as is the case with high rate algal ponds, prevents shading of the water by algae, and the use of relatively high water velocities prevents the accumulation of duckweed on the surface. Inexpensive materials (i.e., wooden boards) are used as baffles to minimize hydraulic short-circuiting.
Photolysis in a shallow, open-water cell with a hydraulic residence time of at least 24 h would likely result in removal of chemical contaminants that are susceptible to direct photolysis, such as NDMA, ketoprofen, and diclofenac. The shallow cell might also be conducive to the removal of certain chemical contaminants by indirect photolysis. Given NO3− concentrations of 20 mg N/L and DOC concentrations of 10 mg/L, which are typical of secondary wastewater effluents, a daily averaged •OH steady-state concentration of about 4×10−16 M would be expected in 20 cm of water during mid-summer at 40° latitude (Zepp et al., 1987; Schwarzenbach et al., 2003). For a typical organic contaminant, which reacts with •OH at near diffusion-controlled rates (i.e., about 7×109/[M·s]), ∼50% removal would be expected after 3 days in the wetland. Both direct and indirect photolysis were found to contribute to the removal of a suite of trace organic compounds in the Discovery Bay pilot system described above (Jasper and Sedlak, 2013).
The water in periphyton-containing wetland cells exhibits diurnal cycles in which dissolved oxygen concentrations and pH values increase during the day due to photosynthesis (Fletcher and Marshall, 1982; Pollard, 2010). In the pilot-scale wetland shown in Fig. 4, the pH typically increases to values of between 9 and 10 within 50 m of the inlet. The alkaline pH conditions in the shallow, open-water cell may affect the rate of direct photolysis of chemical contaminants by changing contaminant speciation (Boreen et al., 2004). Indirect photolysis rates will also be affected by changes in water pH, because •OH is scavenged by inorganic carbon under alkaline conditions forming •CO3−, which may then react with contaminants (Lam et al., 2003). This shift in radical formation could lead to selective oxidation of sulfur-containing compounds, which often exhibit elevated reaction rates with •CO3−(Huang and Mabury, 2000). High concentrations of dissolved oxygen could alter other indirect photolysis pathways, either by quenching intermediate triplet states (Ryan et al., 2011) or by enhancing production of 1O2 (Latch et al., 2003).
Pathogens would also be inactivated in a shallow wetland cell, by both direct and indirect mechanisms. Indirect mechanisms would be especially important due to the high 1O2 concentrations produced by DOM present in wastewater. For example, a daily averaged steady-state 1O2 concentration of about 2×10−14 M would be expected during mid-summer at 40° latitude in 20 cm of water (Haag and Hoigné, 1986; Schwarzenbach et al., 2003). Over a 3 day residence time, this would result in almost 3-log inactivation of MS2 coliphage, which was reported to be inactivated by 1O2 with a second-order rate of 1.3×109/[M·s] (Kohn and Nelson, 2007).
In addition to modifying overlying water chemistry, a periphyton mat could remove chemical contaminants and pathogens through sorption, biotransformation, and predation. For example, researchers have found that periphyton mats present in streams are capable of sorbing, and, in some cases, of transforming trace organic contaminants such as steroid hormones, alkylphenols, nonsteroidal anti-inflammatory drugs, and the cyanotoxin microcystin-RR (Wu et al., 2010; Writer et al., 2011; Dobor et al., 2012). Biotransformation of chemicals may be encouraged by the aerobic conditions encountered at the top of the periphyton mat. In addition, the labile carbon provided by periphyton has been shown to enhance denitrification rates in anoxic wetlands (Sirivedhin and Gray, 2006), and the increased activity of extracellular enzymes, such as the phenol oxidases, which are associated with photosynthesis (Romani et al., 2003; Francoeur et al., 2006; Rier et al., 2007), might also be important. Pathogens have been found to attach to periphyton as well, although detachment at a later time is possible (Ksoll et al., 2007).
Maintenance activities in a shallow, open-water wetland cell may include removing floating vegetation as well as detritus that accumulates in the periphyton mat. Floating vegetation, such as duckweed, is capable of quickly covering a wetland, limiting the effectiveness of photolysis and potentially altering the microbial community in the periphyton mat. The growth of floating vegetation can be limited by ensuring that hydraulic residence times are less than about 3 days (EPA, 2000a) and that the outlet structure allows floating vegetation to leave with the outflow. If floating vegetation grows, the wetland can be periodically flushed by increasing the flow rate into the wetland to wash the floating vegetation out. The slow buildup of particulate matter and detritus from decomposing periphyton will also need to be removed regularly, as over a few years, it may slowly fill in the wetland. This can be accomplished by draining the wetland and removing the dried periphyton mat with a bulldozer. After the old mat is removed, a new periphyton mat will re-grow within weeks on the wetland bottom.
Macrophyte-dominated wetland cells
To enhance contaminant attenuation, vegetated wetland zones can be designed and managed to select for microorganism communities with specific and complimentary composition and functionality. Enhanced attenuation can be achieved through a unit process approach in which different cells are optimized for specific purposes. For example, cells containing cattails provide biomass that is more readily decomposed, while bulrush cells would be expected to exhibit higher activities of extracellular enzymes that are needed to break down the lignin-rich plants (Horne and Fleming-Singer, 2005).
The linking of microbial community dynamics with factors such as plant substrates, temperature, nutrient loading, and dissolved oxygen will allow for wetlands to be actively managed based on environmental indicators. Nutrient availability can also affect microbial community structure and enzyme expression. For example, an abundance of phosphorous can reduce microbial diversity (Ahn et al., 2007) and potentially metabolic diversity. To increase microbial activity or select for reducing conditions, plant biomass harvested during routine maintenance activities can be added back to specific wetland cells. Vegetated wetlands can also be designed to include deep zones to limit plant growth. Other strategies have been suggested, such as raising the water level after plants have senesced to provide additional carbon from the previously un-submerged plant detritus in the winter (Thullen et al., 2005).
Through an increased understanding of wetland microbial communities and the ability to monitor their composition and activity, it may be possible to optimize the performance of unit process cells. Past studies have relied on culture-dependent methods to discern metabolic potential with inherent and often artificial selective pressures (Fortin et al., 2000; Truu et al., 2009) as well as fingerprinting techniques to track spatiotemporal variations in dominant microbes in these systems (Boon et al., 1996; Faulwetter et al., 2009). However, little has been done to thoroughly understand microbial ecology and enzymatic regulation in engineered wetlands. This understanding is necessary to more effectively manage microbial transformation of contaminants and to develop enhanced design and monitoring tools for future engineered wetlands. A suite of culture-independent molecular-based methods such as fluorescent in situ hybridization, quantitative PCR (DeJournett et al., 2007; Bacchetti De Gregoris et al., 2011), high throughput pyrosequencing for phylogenetic analysis, and the generation of metagenomes (Jiang et al., 2011) hold immense promise for future studies. Collectively, these molecular tools can further elucidate the microbial structure and function in constructed wetland systems. Studies that characterize enzyme expression in concert with phylogenetic characterization are important to more effectively track these complimentary but not always synonymous variables (Vilchez-Vargas et al., 2010).
Bivalve filtration wetland cells
As a compliment to contaminant attenuation in shallow, open-water wetlands and macrophyte-dominated wetlands, wetland cells can be built to provide a habitat for organisms that remove contaminants through filter feeding, as shown in Fig. 5. Studies have shown that bivalves such as mussels and clams filter large volumes of water and remove organic particulate matter from the water column (Winter, 1978; Møhlenberg and Riisgård, 1979; Kryger and Riisgård, 1988; Riisgård, 2001). While bivalves occur at low densities in habitats such as coastal estuaries, rivers, and littoral zones of lakes, large populations may be supported in systems with short hydraulic residence times and high primary productivity. Thus, it may be possible to support high densities of filter feeding organisms in a constructed wetland cell where significant concentrations of wastewater- or macrophyte-derived organic matter are available.
FIG. 5.
Schematic of bivalve uptake and removal mechanisms of organic contaminants and pathogens.
While bivalves have not yet been applied for water quality improvement in unit process wetlands, they have been considered for a variety of applications, including drinking water treatment (McIvor, 2004), algae and suspended particulate matter removal from river water (Li et al., 2010), and clarification of secondary municipal wastewater effluent (Haines, 1979). Bivalves have also been considered as a means of removing nutrients from aquaculture wastewater (Buttner, 1986; Shpigel et al., 1997) and, more recently, to remediate surface waters polluted by excessive nutrients in New York City (Cotroneo et al., 2011; NOAA, 2011).
In the process of removing particulate matter, bivalves can also accumulate and, in some cases, transform particle-associated trace organic contaminants. For example, biotransformation of polybrominateddiphenyl ethers and polycyclic aromatic hydrocarbons by a freshwater mussel (Elliptio complanata) was observed after exposure through contaminated algae (O'Rourke et al., 2004; Drouillard et al., 2007). In addition, the transformation of crude oil was accelerated more than ten times in the presence of the mussel Mytilusedilus (Gudimov, 2002). However, recalcitrant contaminants may not be transformed after ingestion and may instead accumulate in the bivalve tissue (Verrengia Guerrero et al., 2002; Drouillard et al., 2007). These contaminants may then be released through the excretion of pseudofeces and feces (Haven and Morales-Alamo, 1966; Hull et al., 2011). Depending on the affinity of the compound for the (pseudo)feces, the contaminants may then desorb and reenter the water column, be consumed by benthic organisms, or be transformed by microbes. Further research is needed to determine whether particle-associated trace organic contaminants commonly present in wastewater effluents, such as the musk fragrances, are effectively removed and transformed by bivalves in a wetland cell.
Bivalves are also capable of ingesting a wide variety of pathogens (Silverman et al., 1995; Graczyk et al., 2003, 2006; Proakis, 2003; Nappier et al., 2008). Given their ability to efficiently remove particles greater than 0.4 μm in diameter from water, bivalves may filter individual bacteria (0.5–2 μm) and protozoan (oo)cysts (2–15 μm), although not individual viruses (20–100 nm). For example, the zebra mussel (Dreissena polymorpha) was found to remove E. coli and other bacteria from pond water at a clearance rate of about 6 L/[g dry tissue·h] (Silverman et al., 1995). Given this rate, a density of 130 mussels/L (∼16 mg dry weight/mussel) would result in 90% clearance of bacteria (1-log removal) over a 3 day residence time. Further research is needed, however, to determine whether bivalve grazing results in pathogen inactivation or just accumulation. Limited studies have shown recovery of viable Giardia spp. and infectious C. parvum oocysts in Macoma spp. and oysters, respectively (Fayer et al., 1998; Graczyk et al., 1999).
Within a unit process wetland, bivalves could be implemented in shallow, open-water cells. Periphyton-derived organic matter or organic matter from wastewater or previous wetland cells would provide sufficient particulate matter for ingestion, and bivalves would enhance contaminant and pathogen removal within the cell. Bivalves could be kept in cages to protect them from predators. Cages would also allow bivalves saturated with recalcitrant contaminants to be easily removed and depurated by exposure to clean water at regular intervals if necessary (Burns and Smith, 1981; Pruell et al., 1986; Peven et al., 1996). Native species should be employed in these wetland cells to avoid invasive species entering receiving surface waters. While further research is necessary to determine appropriate bivalve species, densities, and configurations to maximize treatment efficiency in a unit process wetland, implementation of such a wetland cell could significantly increase attenuation of certain trace organic contaminants and pathogens not effectively removed in other wetland cells, such as particle-associated trace organic contaminants and pathogens that are too small to be removed by sedimentation and which are not susceptible to photoinactivation.
Conclusions
There is growing interest in integrating large-scale treatment wetlands into urban water infrastructure to improve water quality. Treatment wetlands for polishing effluents may offer advantages over mechanical treatment systems due to their low operating cost and potential to remove a variety of difficult-to-treat contaminants. In addition, treatment wetlands offer aesthetic and habitat benefits in urban spaces. However, a better understanding of how to design these natural barriers to provide predictable treatment of target contaminants is needed.
Applying the unit process approach to wetland design has the potential to contribute to more flexible and predictable treatment. It also provides a framework for applying a mechanistic understanding to system optimization. Nonetheless, there are major challenges associated with harmonizing regulations and avoiding unintended consequences associated with large wetland systems. Active management of treatment wetlands and a better understanding of attenuation processes will be required. After we understand what this encompasses, we will have a better idea of how the technology can be compared with other options.
Acknowledgments
This work was supported by the National Science Foundation (NSF) through the Engineering Research Center for Reinventing our Nation's Urban Water Infrastructure (ReNUWIt) EEC-1028968, in addition to NSF grant numbers CBET-0853512 and CBET-1055396, and an NSF Graduate Research Fellowship (to J.T.J.). The authors thank Virgil Koehne, water and wastewater manager of Discovery Bay, CA, for his help in the Discovery Bay treatment wetlands and Dr. Claudia Mueller for her help in creating figures.
Author Disclosure Statement
No competing financial interests exist.
References
- Ahn C. Gillevet P.M. Sikaroodi M. Molecular characterization of microbial communities in treatment microcosm wetlands as influenced by macrophytes and phosphorus loading. Ecol. Indic. 2007;7:852. [Google Scholar]
- Alvord H.H. Kadlec R.H. The interaction of atrazine with wetland sorbents. Ecol. Eng. 1995;5:469. [Google Scholar]
- Ansa E.D.O. Lubberding H.J. Ampofo J.A. Gijzen H.J. The role of algae in the removal of Escherichia coli in a tropical eutrophic lake. Ecol. Eng. 2011;37:317. [Google Scholar]
- Arias C. Brix H. Johansen N.H. Phosphorus removal from municipal wastewater in an experimental two-stage vertical flow constructed wetland system equipped with a calcite filter. Water Sci. Technol. 2003;48:51. [PubMed] [Google Scholar]
- Bacchetti De Gregoris T. Aldred N. Clare A.S. Burgess J.G. Improvement of phylum- and class-specific primers for real-time PCR quantification of bacterial taxa. J. Microbiol. Methods. 2011;86:351. doi: 10.1016/j.mimet.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Barker J. Brown M. Trojan horses of the microbial world. Microbiology. 1994;140:1253. doi: 10.1099/00221287-140-6-1253. [DOI] [PubMed] [Google Scholar]
- Bastviken S.K. Eriksson P.G. Premrov A. Tonderski K. Potential denitrification in wetland sediments with different plant species detritus. Ecol. Eng. 2005;25:183. [Google Scholar]
- Beaver J. Miller-Lemke A. Acton J. Midsummer zooplankton assemblages in four types of wetlands in the Upper Midwest, USA. Hydrobiologia. 1998;380:209. [Google Scholar]
- Boon P. Virtue P. Nichols P. Microbial consortia in wetland sediments. Mar. Freshwater Res. 1996;47:27. [Google Scholar]
- Boutilier L. Jamieson R. Gordon R. Lake C. Hart W. Adsorption, sedimentation, and inactivation of E. coli within wastewater treatment wetlands. Water Res. 2009;43:4370. doi: 10.1016/j.watres.2009.06.039. [DOI] [PubMed] [Google Scholar]
- Boreen A.L. Arnold W.A. McNeill K. Photochemical fate of sulfa drugs in the aquatic environment: sulfa drugs containing five-membered heterocyclic groups. Environ. Sci. Technol. 2004;38:3933. doi: 10.1021/es0353053. [DOI] [PubMed] [Google Scholar]
- Bosshard F. Bucheli M. Meur Y. Egli T. The respiratory chain is the cell's Achilles' heel during UVA inactivation in Escherichia coli. Microbiology. 2010;156:2006. doi: 10.1099/mic.0.038471-0. [DOI] [PubMed] [Google Scholar]
- Breitholtz M. Näslund M. Stråe D. Borg H. Grabic R. Fick J. An evaluation of free water surface wetlands as tertiary sewage water treatment of micro-pollutants. Ecotoxicol. Environ. Safe. 2012;78:63. doi: 10.1016/j.ecoenv.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Brezonik P.L. Fulkerson-Brekken J. Nitrate-induced photolysis in natural waters: controls on concentrations of hydroxyl radical photo-intermediates by natural scavenging agents. Environ. Sci. Technol. 1998;32:3004. [Google Scholar]
- Brix H. Do macrophytes play a role in constructed treatment wetlands? Water Sci. Technol. 1997;35:11. [Google Scholar]
- Burns K.A. Smith J.L. Biological monitoring of ambient water quality: The case for using bivalves as sentinel organisms for monitoring petroleum pollution in coastal waters. Estuar. Coast. Shelf Sci. 1981;13:433. [Google Scholar]
- Buth J.M. Grandbois M. Vikesland P.J. McNeill K. Arnold W.A. Aquatic photochemistry of chlorinated triclosan derivatives. Environ. Toxicol. Chem. 2009;28:2555. doi: 10.1897/08-490.1. [DOI] [PubMed] [Google Scholar]
- Buth J.M. Steen P.O. Sueper C. Blumentritt D. Vikesland P.J. Arnold W.A. McNeill K. Dioxin photoproducts of triclosan and its chlorinated derivatives in sediment cores. Environ. Sci. Technol. 2010;44:4545. doi: 10.1021/es1001105. [DOI] [PubMed] [Google Scholar]
- Buttner J.K. Corbicula as a biological filter and polyculture organism in catfish rearing ponds. Prog. Fish-Cult. 1986;48:136. [Google Scholar]
- Calheiros C.S.C. Duque A.F. Moura A. Henriques I.S. Correia A. Rangel A.O.S.S. Castro P.M.L. Changes in the bacterial community structure in two-stage constructed wetlands with different plants for industrial wastewater treatment. Bioresour. Technol. 2009;100:3228. doi: 10.1016/j.biortech.2009.02.033. [DOI] [PubMed] [Google Scholar]
- Camacho-Muñoz D. Martín J. Santos J. Aparicio I. Alonso E. Effectiveness of conventional and low-cost wastewater treatments in the removal of pharmaceutically active compounds. Water Air Soil Pollut. 2011;223:2611. [Google Scholar]
- Canonica S. Jans U. Stemmler K. Hoigné J. Transformation kinetics of phenols in water: Photosensitization by dissolved natural organic material and aromatic ketones. Environ. Sci. Technol. 1995;29:1822. doi: 10.1021/es00007a020. [DOI] [PubMed] [Google Scholar]
- Canonica S. Kohn T. Mac M. Real F.J. Wirz J. Von Gunten U. Photosensitizer method to determine rate constants for the reaction of carbonate radical with organic compounds. Environ. Sci. Technol. 2005;39:9182. doi: 10.1021/es051236b. [DOI] [PubMed] [Google Scholar]
- Costanza R. D’Arge R. De Groot R. Farber S. Grasso M. Hannon B. Limburg K. Naeem S. O'Neill R.V. Paruelo J. Raskin R.G. Sutton P. van den Belt M. The value of the world's ecosystem services and natural capital. Nature. 1997;387:253. [Google Scholar]
- Cotroneo C. Csoboth L. Yozzo D.J. Doss T. Will B. McLaughlin J. Working toward nutrient reduction in a eutrophic urban estuary: Ribbed mussel biofiltration in Jamaica Bay, presented at the 4th National Conference on Ecosystem Restoration; Baltimore, MD. 2011. [Google Scholar]
- Curtis T.P. Mara D.D. Silva S.A. Influence of pH, oxygen, and humic substances on ability of sunlight To damage fecal coliforms in waste stabilization pond water. Appl. Environ. Microbiol. 1992;58:1335. doi: 10.1128/aem.58.4.1335-1343.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo E. Wetlands: Biodegradation of Organic Pollutants. In: Bitton G., editor. Encyclopedia of Environmental Microbiology. Hoboken, NJ: John Wiley & Sons, Inc.; 2003. p. 3401. [Google Scholar]
- Dai X. Boll J. Settling velocity of Cryptosporidium parvum and Giardia lamblia. Water Res. 2006;40:1321. doi: 10.1016/j.watres.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Daughton C.G. Ternes T.A. Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ. Health Perspect. 1999;107:907. doi: 10.1289/ehp.99107s6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies-Colley R.J. Pond disinfection. In: Shilton A., editor. Pond Treatment Technology. London: IWA Publishing; 2005. p. 101. [Google Scholar]
- Davies-Colley R.J. Donnison A.M. Speed D.J. Towards a mechanistic understanding of pond disinfection. Water Sci. Technol. 2000;42:149. [Google Scholar]
- Davies-Colley R.J. Donnison A.M. Speed D.J. Ross C.M. Nagels J.W. Inactivation of faecal indicator micro-organisms in waste stabilisation ponds. Water Res. 1999;33:1220. [Google Scholar]
- Decamp O. Warren A. Bacterivory in ciliates isolated from constructed wetlands (reed beds) used for wastewater treatment. Water Res. 1998;32:1989. [Google Scholar]
- DeJournett T.D. Arnold W.A. LaPara T.M. The characterization and quantification of methanotrophic bacterial populations in constructed wetland sediments using PCR targeting 16S rRNA gene fragments. Appl. Soil Ecol. 2007;35:648. [Google Scholar]
- Dobor J. Varga M. Záray G. Biofilm controlled sorption of selected acidic drugs on river sediments characterized by different organic carbon content. Chemosphere. 2012;87:105. doi: 10.1016/j.chemosphere.2011.11.067. [DOI] [PubMed] [Google Scholar]
- Drouillard K.G. Chan S. O'Rourke S. Douglas Haffner G. Letcher R.J. Elimination of 10 polybrominateddiphenyl ether (PBDE) congeners and selected polychlorinated biphenyls (PCBs) from the freshwater mussel, Elliptio complanata. Chemosphere. 2007;69:362. doi: 10.1016/j.chemosphere.2007.05.027. [DOI] [PubMed] [Google Scholar]
- Emerson D. Weiss J.V. Megonigal J.P. Iron-oxidizing bacteria are associated with ferric hydroxide precipitates (Fe-plaque) on the roots of wetland plants. Appl. Environ. Microbiol. 1999;65:2758. doi: 10.1128/aem.65.6.2758-2761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (EPA) Cincinnati, OH: 1993. Subsurface Flow Constructed Wetlands for Waste Water Treatment. [Google Scholar]
- EPA. Constructed Wetlands Treatment of Municipal Wastewaters; Cincinnati, OH: 2000a. [Google Scholar]
- EPA. Guiding Principles for Constructed Treatment Wetlands: Providing for Water Quality and Wildlife Habitat; Cincinnati, OH: 2000b. [Google Scholar]
- Falabi J.A. Gerba C.P. Karpiscak M.M. Giardia and Cryptosporidium removal from waste-water by a duckweed (Lemnagibba L.) covered pond. Lett. Appl. Microbiol. 2002;34:384. doi: 10.1046/j.1472-765x.2002.01104.x. [DOI] [PubMed] [Google Scholar]
- Faulwetter J.L. Gagnon V. Sundberg C. Chazarenc F. Burr M.D. Brisson J. Camper A.K. Stein O.R. Microbial processes influencing performance of treatment wetlands: A review. Ecol. Eng. 2009;35:987. [Google Scholar]
- Fayer R. Graczyk T.K. Lewis E.J. Trout J.M. Farley C.A. Survival of infectious Cryptosporidium parvum oocysts in seawater and eastern oysters (Crassostrea virginica) in the Chesapeake Bay. Appl. Environ. Microbiol. 1998;64:1070. doi: 10.1128/aem.64.3.1070-1074.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayer R. Trout J.M. Walsh E. Cole R. Rotifers ingest oocysts of Cryptosporidium parvum. J. Eukaryot. Microbiol. 2000;47:161. doi: 10.1111/j.1550-7408.2000.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Fleming-Singer M.S. Horne A.J. Balancing wildlife needs and nitrate removal in constructed wetlands. Ecol. Eng. 2006;26:147. [Google Scholar]
- Fletcher M. Marshall K. Are solid surfaces of ecological significance to aquatic bacteria? Adv. Microb. Ecol. 1982;6:199. [Google Scholar]
- Florida Department of Environmental Protection. Domestic wastewater—Orlando Easterly Wetlands. 2011. www.dep.state.fl.us/water/wastewater/dom/oreastwet.htm. [Jul 22;2013 ]. www.dep.state.fl.us/water/wastewater/dom/oreastwet.htm
- Fortin D. Goulet R. Roy M. Seasonal cycling of Fe and S in a constructed wetland: The role of sulfate-reducing bacteria. Geomicrobiol. J. 2000;17:221. [Google Scholar]
- Francoeur S.N. Schaecher M. Neely R.K. Kuehn K.A. Periphytic photosynthetic stimulation of extracellular enzyme activity in aquatic microbial communities associated with decaying Typha litter. Microb. Ecol. 2006;52:662. doi: 10.1007/s00248-006-9084-2. [DOI] [PubMed] [Google Scholar]
- Fuchs V.J. Mihelcic J.R. Gierke J.S. Life cycle assessment of vertical and horizontal flow constructed wetlands for wastewater treatment considering nitrogen and carbon greenhouse gas emissions. Water Res. 2011;45:2073. doi: 10.1016/j.watres.2010.12.021. [DOI] [PubMed] [Google Scholar]
- Gagnon V. Chazarenc F. Comeau Y. Brisson J. Influence of macrophyte species on microbial density and activity in constructed wetlands. Water Sci. Technol. 2007;56:249. doi: 10.2166/wst.2007.510. [DOI] [PubMed] [Google Scholar]
- Gearheart R.A. The use of free surface constructed wetland as an alternative process treatment train to meet unrestricted water reclamation standards. Water Sci. Technol. 1999;40:375. [Google Scholar]
- Gerba C.P. Thurston J.A. Falabi J.A. Watt P.M. Karpiscak M.M. Optimization of artificial wetland design for removal of indicator microorganisms and pathogenic protozoa. Water Sci. Technol. 1999;40:363. [Google Scholar]
- Gerecke A.C. Canonica S. Müller S.R. Schärer M. Schwarzenbach R.P. Quantification of dissolved natural organic matter (DOM) mediated phototransformation of phenylurea herbicides in lakes. Environ. Sci. Technol. 2001;35:3915. doi: 10.1021/es010103x. [DOI] [PubMed] [Google Scholar]
- Gersberg R.M. Gearheart R.A. Ives M. Pathogen removal in constructed wetlands. In: Hammer D.A., editor. Constructed Wetlands for Wastewater Treatment; Municipal, Industrial and Agricultural. Chelsea, MI: Lewis; 1989. p. 431. [Google Scholar]
- Gersberg R.M. Lyon S.R. Brenner R. Elkins B.V. Fate of viruses in artificial wetlands. Appl. Environ. Microbiol. 1987;53:731. doi: 10.1128/aem.53.4.731-736.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianfreda L. Rao M.A. Potential of extra cellular enzymes in remediation of polluted soils. Enzyme Microb. Technol. 2004;35:339. [Google Scholar]
- Golet E.M. Xifra I. Siegrist H. Alder A.C. Giger W. Environmental exposure assessment of fluoroquinolone antibacterial agents from sewage to soil. Environ. Sci. Technol. 2003;37:3243. doi: 10.1021/es0264448. [DOI] [PubMed] [Google Scholar]
- Graczyk T.K. Conn D.C. Marcogliese D.M. Graczyk H.G. De Lafontaine Y. Accumulation of human waterborne parasites by zebra mussels (Dreissena polymorpha) and Asian freshwater clams (Corbicula fluminea) Parasitol. Res. 2003;89:107. doi: 10.1007/s00436-002-0729-x. [DOI] [PubMed] [Google Scholar]
- Graczyk T.K. Girouard A.S. Tamang L. Nappier S.P. Schwab K.J. Recovery, bioaccumulation, and inactivation of human waterborne pathogens by the Chesapeake Bay nonnative oyster, Crassostrea ariakensis. Appl. Environ. Microbiol. 2006;72:3390. doi: 10.1128/AEM.72.5.3390-3395.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graczyk T.K. Thompson R.C. Fayer R. Adams P. Morgan U.M. Lewis E.J. Giardia duodenalis cysts of genotype A recovered from clams in the Chesapeake Bay subestuary, Rhode River. Am. J. Trop. Med. Hyg. 1999;61:526. doi: 10.4269/ajtmh.1999.61.526. [DOI] [PubMed] [Google Scholar]
- Gray J.L. Sedlak D.L. The fate of estrogenic hormones in an engineered treatment wetland with dense macrophytes. Water Environ. Res. 2005;77:24. doi: 10.2175/106143005x41582. [DOI] [PubMed] [Google Scholar]
- Gudimov A.V. Zooremediation, a new biotechnology solution for shoreline protection and cleanup. In Proceedings of the 25th Arctic and Marine Oil Spill Program (AMOP) Technical Seminar; Ottawa, Canada: Environment Canada; 2002. [Google Scholar]
- Gutknecht J.L.M. Goodman R.M. Balser T.C. Linking soil process and microbial ecology in freshwater wetland ecosystems. Plant Soil. 2006;289:17. [Google Scholar]
- Haag W.R. Hoigné J. Singlet oxygen in surface waters. 3. Photochemical formation and steady-state concentrations in various types of waters. Environ. Sci. Technol. 1986;20:341. doi: 10.1021/es00146a005. [DOI] [PubMed] [Google Scholar]
- Hahn M.W. Höfle M.G. Grazing of protozoa and its effect on populations of aquatic bacteria. FEMS Microbiol. Ecol. 2001;35:113. doi: 10.1111/j.1574-6941.2001.tb00794.x. [DOI] [PubMed] [Google Scholar]
- Haines K.C. The use of Corbicula as a clarifying agent in experimental tertiary sewage treatment process on St. Croix, US Virgin Islands. In Proc. 1st International Corbicula Symposium. Texas Christian University Research Foundation.1979. [Google Scholar]
- Hammer D.A. Knight R.L. Designing constructed wetlands for nitrogen removal. Water Sci. Technol. 1994;29:15. [Google Scholar]
- Harvey J.W. Saiers J.W. Newlin J.T. Solute transport and storage mechanisms in wetlands of the Everglades, south Florida. Water Resour. Res. 2005:41. [Google Scholar]
- Haven D.S. Morales-Alamo R. Aspects of biodeposition by oysters and other invertebrate filter feeders. Limnol. Oceanogr. 1966;11:487. [Google Scholar]
- Herskowitz J. Town of Listowel Artificial Marsh Project. Toronto, Canada: Ontario Ministry of the Environment; 1986. [Google Scholar]
- Hijosa-Valsero M. Matamoros V. Pedescoll A. Martín-Villacorta J. Bécares E. García J. Bayona J.M. Evaluation of primary treatment and loading regimes in the removal of pharmaceuticals and personal care products from urban wastewaters by subsurface-flow constructed wetlands. Int. J. Environ. Anal. Chem. 2011;91:632. [Google Scholar]
- Hijosa-Valsero M. Matamoros V. Sidrach-Cardona R. Martín-Villacorta J. Bécares E. Bayona J.M. Comprehensive assessment of the design configuration of constructed wetlands for the removal of pharmaceuticals and personal care products from urban wastewaters. Water Res. 2010;44:3669. doi: 10.1016/j.watres.2010.04.022. [DOI] [PubMed] [Google Scholar]
- Hill B.H. Elonen C.M. Jicha T.M. Cotter A.M. Trebitz A.S. Danz N.P. Sediment microbial enzyme activity as an indicator of nutrient limitation in Great Lakes coastal wetlands. Freshwater Biol. 2006;51:1670. [Google Scholar]
- Hill V.R. Sobsey M.D. Removal of Salmonella and microbial indicators in constructed wetlands treating swine wastewater. Water Sci. Technol. 2001;44:215. [PubMed] [Google Scholar]
- Horne A.J. Fleming-Singer M.S. Phytoremediation using constructed treatment wetlands: An overview. In: Fingerman M., editor; Nagabhushanam R., editor. Bioremediation of Aquatic and Terrestrial Ecosystems. Enfield, NH: Science Publishers; 2005. p. 329. [Google Scholar]
- Howell C.J. Crohn D.M. Omary M. Simulating nutrient cycling and removal through treatment wetlands in arid/semiarid environments. Ecol. Eng. 2005;25:25. [Google Scholar]
- Huang J. Mabury S.A. The role of carbonate radical in limiting the persistence of sulfur-containing chemicals in sunlit natural waters. Chemosphere. 2000;41:1775. doi: 10.1016/s0045-6535(00)00042-4. [DOI] [PubMed] [Google Scholar]
- Hull M.S. Chaurand P. Rose J. Auffan M. Bottero J.-Y. Jones J.C. Schultz I.R. Vikesland P.J. Filter-feeding bivalves store and biodepositcolloidally stable gold nanoparticles. Environ. Sci. Technol. 2011;45:6592. doi: 10.1021/es200809c. [DOI] [PubMed] [Google Scholar]
- Hussain S. Prasher S. Understanding the sorption of ionophoric pharmaceuticals in a treatment wetland. Wetlands. 2011;31:563. [Google Scholar]
- Ibekwe A.M. Lyon S.R. Leddy M. Jacobson-Meyers M. Impact of plant density and microbial composition on water quality from a free water surface constructed wetland. J. Appl. Microbiol. 2006;102:921. doi: 10.1111/j.1365-2672.2006.03181.x. [DOI] [PubMed] [Google Scholar]
- Imfeld G. Braeckevelt M. Kuschk P. Richnow H.H. Monitoring and assessing processes of organic chemicals removal in constructed wetlands. Chemosphere. 2009;74:349. doi: 10.1016/j.chemosphere.2008.09.062. [DOI] [PubMed] [Google Scholar]
- Ishii S. Yan T. Vu H. Hansen D.L. Hicks R.E. Sadowsky M.J. Factors controlling long-term survival and growth of naturalized Escherichia coli populations in temperate field soils. Microb. Environ. 2010;25:8. doi: 10.1264/jsme2.me09172. [DOI] [PubMed] [Google Scholar]
- Jagger J. Solar-UV Actions on Living Cells. New York: Praeger; 1985. [Google Scholar]
- Jasper J.T. Sedlak D.L. Phototransformation of wastewater-derived trace organic contaminants in open-water unit process treatment wetlands. Environ. Sci. Technol. 2013 2013 Mar 7; doi: 10.1021/es304334w. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Jiang C. Li S.-X. Luo F.-F. Jin K. Wang Q. Hao Z.-Y. Wu L.-L. Zhao G.-C. Ma G.-F. Shen P.-H. Tang X.-L. Wu B. Biochemical characterization of two novel β-glucosidase genes by metagenome expression cloning. Bioresour. Technol. 2011;102:3272. doi: 10.1016/j.biortech.2010.09.114. [DOI] [PubMed] [Google Scholar]
- Kadlec R.H. Detention and mixing in free water wetlands. Ecol. Eng. 1994;3:345. [Google Scholar]
- Kadlec R.H. The inadequacy of first-order treatment wetland models. Ecol. Eng. 2000;15:105. [Google Scholar]
- Kadlec R.H. Constructed marshes for nitrate removal. Crit. Rev. Environ. Sci. Technol. 2012;42:934. [Google Scholar]
- Kadlec R.H. Wallace S. Treatment Wetlands. 2nd. Boca Raton: CRC Press; 2009. [Google Scholar]
- Karpiscak M.M. Sanchez L.R. Freitas R.J. Gerba C.P. Removal of bacterial indicators and pathogens from dairy wastewater by a multi-component treatment system. Water Sci. Technol. 2001;44:813. [PubMed] [Google Scholar]
- Kasanavia T. Vu D. Sabatini D.A. Fluorescent dye and media properties affecting sorption and tracer selection. Ground Water. 1999;37:376. [Google Scholar]
- Keefe S.H. Barber L.B. Runkel R.L. Ryan J.N. McKnight D.M. Wass R.D. Conservative and reactive solute transport in constructed wetlands. Water Resour. Res. 2004;40:W01201. [Google Scholar]
- Kjellin J. Worman A. Johansson H. Lindahl A. Controlling factors for water residence time and flow patterns in Ekeby treatment wetland, Sweden. Adv. Water Resour. 2007;30:838. [Google Scholar]
- Knight R.L. Effuent distribution and basin design for enhanced pollutant assimilation by freshwater wetlands. In: Reddy K.R., editor; Smith W.H., editor. Aquatic Plants for Water Treatment and Resource Recovery. Orlando, FL: Magnolia Publishing Company; 1987. p. 913. [Google Scholar]
- Kohn T. Nelson K.L. Sunlight-mediated inactivation of MS2 coliphage via exogenous singlet oxygen produced by sensitizers in natural waters. Environ. Sci. Technol. 2007;41:192. doi: 10.1021/es061716i. [DOI] [PubMed] [Google Scholar]
- Kolpin D.W. Furlong E.T. Meyer M.T. Thurman E.M. Zaugg S.D. Barber L.B. Buxton H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: A national reconnaissance. Environ. Sci. Technol. 2002;36:1202. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- Kruger E.L. Coats J.R. Zhu B.E. Relative mobilities of atrazine, five atrazine degradates, metolachlor, and simazine in soils of Iowa. Environ. Toxicol. Chem. 1996;15:691. [Google Scholar]
- Kryger J. Riisgård H.U. Filtration rate capacities in 6 species of European freshwater bivalves. Oecologia. 1988;77:34. doi: 10.1007/BF00380921. [DOI] [PubMed] [Google Scholar]
- Ksoll W.B. Ishii S. Sadowsky M.J. Hicks R.E. Presence and sources of fecal coliform bacteria in epilithic periphyton communities of Lake Superior. Appl. Environ. Microbiol. 2007;73:3771. doi: 10.1128/AEM.02654-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyambadde J. Kansiime F. Gumaelius L. Dalhammar G. A comparative study of Cyperus papyrus and Miscanthidium violaceum-based constructed wetlands for wastewater treatment in a tropical climate. Water Res. 2004;38:475. doi: 10.1016/j.watres.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Lam M.W. Mabury S.A. Photodegradation of the pharmaceuticals atorvastatin, carbamazepine, levofloxacin, and sulfamethoxazole in natural waters. Aquat. Sci. 2005;67:177. [Google Scholar]
- Lam M.W. Tantuco K. Mabury S.A. PhotoFate: A new approach in accounting for the contribution of indirect photolysis of pesticides and pharmaceuticals in surface waters. Environ. Sci. Technol. 2003;37:899. doi: 10.1021/es025902+. [DOI] [PubMed] [Google Scholar]
- Latch D.E. Stender B.L. Packer J.L. Arnold W.A. McNeill K. Photochemical fate of pharmaceuticals in the environment: Cimetidine and ranitidine. Environ. Sci. Technol. 2003;37:3342. doi: 10.1021/es0340782. [DOI] [PubMed] [Google Scholar]
- Li X.-N. Song H.-L. Li W. Lu X.-W. Nishimura O. An integrated ecological floating-bed employing plant, freshwater clam and biofilm carrier for purification of eutrophic water. Ecol. Eng. 2010;36:382. [Google Scholar]
- Lightbody A.F. Avener M.E. Nepf H.M. Observations of short-circuiting flow paths within a free-surface wetland in Augusta, Georgia, USA. Limnol. Oceanogr. 2008;53:1040. [Google Scholar]
- Lightbody A.F. Nepf H.M. Bays J.S. Mixing in deep zones within constructed treatment wetlands. Ecol. Eng. 2007;29:209. [Google Scholar]
- Lightbody A.F. Nepf H.M. Bays J.S. Modeling the hydraulic effect of transverse deep zones on the performance of short-circuiting constructed treatment wetlands. Ecol. Eng. 2009;35:754. [Google Scholar]
- Lim M.-H. Snyder S.A. Sedlak D.L. Use of biodegradable dissolved organic carbon (BDOC) to assess the potential for transformation of wastewater-derived contaminants in surface waters. Water Res. 2008;42:2943. doi: 10.1016/j.watres.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Lin A.Y.-C. Debroux J.-F. Cunningham J.A. Reinhard M. Comparison of rhodamine WT and bromide in the determination of hydraulic characteristics of constructed wetlands. Ecol. Eng. 2003;20:75. [Google Scholar]
- Lin A.Y.-C. Reinhard M. Photodegradation of common environmental pharmaceuticals and estrogens in river water. Environ. Toxicol. Chem. 2005;24:1303. doi: 10.1897/04-236r.1. [DOI] [PubMed] [Google Scholar]
- Llorens E. Matamoros V. Domingo V. Bayona J.M. García J. Water quality improvement in a full-scale tertiary constructed wetland: Effects on conventional and specific organic contaminants. Sci. Total Environ. 2009;407:2517. doi: 10.1016/j.scitotenv.2008.12.042. [DOI] [PubMed] [Google Scholar]
- Lu J. Huang Q. Mao L. Removal of acetaminophen using enzyme-mediated oxidative coupling processes: I. Reaction rates and pathways. Environ. Sci. Technol. 2009;43:7062. doi: 10.1021/es9002422. [DOI] [PubMed] [Google Scholar]
- MacIntyre M.E. Warner B.G. Slawson R.M. Escherichia coli control in a surface flow treatment wetland. J. Water Health. 2006;4:211. [PubMed] [Google Scholar]
- Malaviya P. Singh A. Constructed wetlands for management of urban stormwater runoff. Crit. Rev. Environ. Sci. Technol. 2012;42:2153. [Google Scholar]
- Mandi L. Bouhoum K. Ouazzani N. Application of constructed wetlands for domestic wastewater treatment in an arid climate. Water Sci. Technol. 1998;38:379. [Google Scholar]
- Mandi L. Houhoum B. Asmama S. Schwartzbrod J. Wastewater treatment by reed beds. Water Res. 1996;30:2009. [Google Scholar]
- Martinez C.J. Wise W.R. Hydraulic analysis of Orlando easterly wetland. J. Environ. Eng. 2003;129:553. [Google Scholar]
- Matamoros V. Arias C. Brix H. Bayona J.M. Removal of pharmaceuticals and personal care products (PPCPs) from urban wastewater in a pilot vertical flow constructed wetland and a sand filter. Environ. Sci. Technol. 2007;41:8171. doi: 10.1021/es071594+. [DOI] [PubMed] [Google Scholar]
- Matamoros V. Arias C. Brix H. Bayona J.M. Preliminary screening of small-scale domestic wastewater treatment systems for removal of pharmaceutical and personal care products. Water Res. 2009;43:55. doi: 10.1016/j.watres.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Matamoros V. Bayona J.M. Elimination of pharmaceuticals and personal care products in subsurface flow constructed wetlands. Environ. Sci. Technol. 2006;40:5811. doi: 10.1021/es0607741. [DOI] [PubMed] [Google Scholar]
- Matamoros V. Bayona J.M. Behavior of emerging pollutants in constructed wetlands. In: Barceló D., editor; Petrovic M., editor. Emerging Contaminants from Industrial and Municipal Waste. Berlin/Heidelberg: Springer; 2008. p. 199. [Google Scholar]
- Matamoros V. García J. Bayona J.M. Behavior of selected pharmaceuticals in subsurface flow constructed wetlands: A pilot-scale study. Environ. Sci. Technol. 2005;39:5449. doi: 10.1021/es050022r. [DOI] [PubMed] [Google Scholar]
- Matamoros V. García J. Bayona J.M. Organic micropollutant removal in a full-scale surface flow constructed wetland fed with secondary effluent. Water Res. 2008;42:653. doi: 10.1016/j.watres.2007.08.016. [DOI] [PubMed] [Google Scholar]
- McIvor A.L. Freshwater Mussels as Biofilters [Doctoral thesis] United Kingdom: Pembroke College; 2004. [Google Scholar]
- Meltz Steinberg K. Levin B.R. Grazing protozoa and the evolution of the Escherichia coli O157. Proc. Biol. Sci. 2007;274:1921. doi: 10.1098/rspb.2007.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelssohn I.A. Kleiss B.A. Wakeley J.S. Factors controlling the formation of oxidized root channels. Wetlands. 1995;15:37. [Google Scholar]
- Miller P.L. Chin Y.-P. Photoinduced degradation of carbaryl in a wetland surface water. J. Agric. Food Chem. 2002;50:6758. doi: 10.1021/jf025545m. [DOI] [PubMed] [Google Scholar]
- Miller P.L. Chin Y.-P. Indirect photolysis promoted by natural and engineered wetland water constituents: Processes leading to alachlor degradation. Environ. Sci. Technol. 2005;39:4454. doi: 10.1021/es049111e. [DOI] [PubMed] [Google Scholar]
- Mitsch W.J. Gosselink J.G. Wetlands. 4th. New York: John Wiley & Sons, Inc.; 2007. [Google Scholar]
- Møhlenberg F. Riisgård H.U. Filtration rate, using a new indirect technique, in thirteen species of suspension-feeding bivalves. Mar. Biol. 1979;54:143. [Google Scholar]
- Moore M.T. Schulz R. Cooper C.M. Smith S., Jr. Rodgers J.H., Jr. Mitigation of chlorpyrifos runoff using constructed wetlands. Chemosphere. 2002;46:827. doi: 10.1016/s0045-6535(01)00189-8. [DOI] [PubMed] [Google Scholar]
- Münch C. Neu T. Kuschk P. Röske I. The root surface as the definitive detail for microbial transformation processes in constructed wetlands—A biofilm characteristic. Water Sci. Technol. 2007;56:271. doi: 10.2166/wst.2007.527. [DOI] [PubMed] [Google Scholar]
- Nappier S.P. Graczyk T.K. Schwab K.J. Bioaccumulation, retention, and depuration of enteric viruses by Crassostrea virginica and Crassostrea ariakensis oysters. Appl. Environ. Microbiol. 2008;74:6825. doi: 10.1128/AEM.01000-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. National Oceanic and Atmospheric Administration (NOAA) Woods Hole, MA: National Oceanic and Atmospheric Administration; 2011. [Mar 7;2012 ]. Ribbed mussels offer possible natural way to improve urban water quality. [Google Scholar]
- Orange County Water District. Prado Wetlands. 2013. www.ocwd.com/ Environment/PradoWetlands.aspx">http://www.ocwd.com/">www.ocwd.com/ Environment/PradoWetlands.aspx. [Jul 22;2013 ]. www.ocwd.com/ Environment/PradoWetlands.aspx">http://www.ocwd.com/">www.ocwd.com/ Environment/PradoWetlands.aspx
- O'Rourke S. Drouillard K.G. Haffner G.D. Determination of laboratory and field elimination rates of polychlorinated biphenyls (PCBs) in the freshwater mussel, Elliptio complanata. Arch. Environ. Contam. Toxicol. 2004;47:74. doi: 10.1007/s00244-004-2295-y. [DOI] [PubMed] [Google Scholar]
- Park N. Vanderford B.J. Snyder S.A. Sarp S. Kim S.D. Cho J. Effective controls of micropollutants included in wastewater effluent using constructed wetlands under anoxic condition. Ecol. Eng. 2009;35:418. [Google Scholar]
- Persson J. The hydraulic performance of ponds of various layouts. Urban Water. 2000;2:243. [Google Scholar]
- Peven C.S. Uhler A.D. Querzoli F.J. Caged mussels and semipermeable membrane devices as indicators of organic contaminant uptake in dorchester and duxbury bays. Environ. Toxicol. Chem. 1996;15:144. [Google Scholar]
- Pollard P.C. Bacterial activity in plant (Schoenoplectus validus) biofilms of constructed wetlands. Water Res. 2010;44:5939. doi: 10.1016/j.watres.2010.07.047. [DOI] [PubMed] [Google Scholar]
- Porter A.J. Microbial Community Function in Freshwater Wetland Soils: Using Extracellular Enzyme Analysis to Study the Effect of Moisture and Vegetation [Masters thesis] Richmond, VA: Virginia Commonwealth University; 2011. [Google Scholar]
- Proakis E. Pathogen removal in constructed wetlands focusing on biological predation and marine recreational water quality. Proc. Water Environ. Fed. 2003:31–40. 310. [Google Scholar]
- Pruell R.J. Lake J.L. Davis W.R. Quinn J.G. Uptake and depuration of organic contaminants by blue mussels (Mytilusedulis) exposed to environmentally contaminated sediment. Mar. Biol. 1986;91:497. [Google Scholar]
- Quiñónez-Díaz M.J. Karpiscak M.M. Ellman E.D. Gerba C.P. Removal of pathogenic and indicator microorganisms by a constructed wetland receiving untreated domestic wastewater. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2001;36:1311. doi: 10.1081/ese-100104880. [DOI] [PubMed] [Google Scholar]
- Reddy K.R. D'Angelo E.M. Biogeochemical indicators to evaluate pollutant removal efficiency in constructed wetlands. Water Sci. Technol. 1997;35:1. [Google Scholar]
- Reed S.H. Crites R.W. Middlebrooks E.J. Natural Systems for Waste Management and Treatment. 2nd. New York: McGraw-Hill; 1995. [Google Scholar]
- Reichenberger S. Bach M. Skitschak A. Frede H.-G. Mitigation strategies to reduce pesticide inputs into ground- and surface water and their effectiveness; A review. Sci. Total Environ. 2007;384:1. doi: 10.1016/j.scitotenv.2007.04.046. [DOI] [PubMed] [Google Scholar]
- Reinhold D.M. Vishwanathan S. Park J.J. Oh D. Michael Saunders F. Assessment of plant-driven removal of emerging organic pollutants by duckweed. Chemosphere. 2010;80:687. doi: 10.1016/j.chemosphere.2010.05.045. [DOI] [PubMed] [Google Scholar]
- Reinoso R. Torres L.A. Bécares E. Efficiency of natural systems for removal of bacteria and pathogenic parasites from wastewater. Sci. Total Environ. 2008;395:80. doi: 10.1016/j.scitotenv.2008.02.039. [DOI] [PubMed] [Google Scholar]
- Reyes-Contreras C. Matamoros V. Ruiz I. Soto M. Bayona J.M. Evaluation of PPCPs removal in a combined anaerobic digester-constructed wetland pilot plant treating urban wastewater. Chemosphere. 2011;84:1200. doi: 10.1016/j.chemosphere.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Rier S.T. Kuehn K.A. Francoeur S.N. Algal regulation of extracellular enzyme activity in stream microbial communities associated with inert substrata and detritus. J. N. Am. Benthol. Soc. 2007;26:439. [Google Scholar]
- Riisgård H.U. On measurement of filtration rates in bivalves—The stony road to reliable data: Review and interpretation. Mar. Ecol. Prog. Ser. 2001;211:275. [Google Scholar]
- Romani A.M. Guasch H. Muñoz I. Ruana J. Vilalta E. Schwartz T. Emtiazi F. Sabater S. Biofilm structure and function and possible implications for riverine DOC dynamics. Microb. Ecol. 2003;47:316. doi: 10.1007/s00248-003-2019-2. [DOI] [PubMed] [Google Scholar]
- Ryan C.C. Tan D.T. Arnold W.A. Direct and indirect photolysis of sulfamethoxazole and trimethoprim in wastewater treatment plant effluent. Water Res. 2011;45:1280. doi: 10.1016/j.watres.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Schwarzenbach R.P. Gschwend P.M. Imboden D.M. Environmental Organic Chemistry. 2nd. Hoboken, NJ: John Wiley & Sons, Inc.; 2003. [Google Scholar]
- Sengupta M.E. Thamsborg S.M. Andersen T.J. Olsen A. Dalsgaard A. Sedimentation of helminth eggs in water. Water Res. 2011;45:4651. doi: 10.1016/j.watres.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Shackle V.J. Freeman C. Reynolds B. Carbon supply and the regulation of enzyme activity in constructed wetlands. Soil Biol. Biochem. 2000;32:1935. [Google Scholar]
- Shilton A. Harrison J. Guidelines for the Hydraulic Design of Waste Stabilisation Ponds Website. Palmerston North, New Zealand: Massey University; 2003. [Google Scholar]
- Shpigel M. Gasith A. Kimmel E. A biomechanical filter for treating fish-pond effluents. Aquaculture. 1997;152:103. [Google Scholar]
- Silverman H. Achberger E.C. Lynn J.W. Dietz T.H. Filtration and utilization of laboratory-cultured bacteria by Dreissena polymorpha, Corbicula fluminea, and Carunculina texasensis. Biol. Bull. 1995;189:308. doi: 10.2307/1542148. [DOI] [PubMed] [Google Scholar]
- Sinsabaugh R.L. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol. Biochem. 2010;42:391. [Google Scholar]
- Sirivedhin T. Gray K.A. Factors affecting denitrification rates in experimental wetlands. Ecol. Eng. 2006;26:167. [Google Scholar]
- Snyder S.A. Westerhoff P. Yoon Y. Sedlak D.L. Pharmaceuticals, personal care products, and endocrine disruptors in water: Implications for the water industry. Environ. Eng. Sci. 2003;20:449. [Google Scholar]
- Song H.-L. Yang X.-L. Nakano K. Nomura M. Nishimura O. Li X.-N. Elimination of estrogens and estrogenic activity from sewage treatment works effluents in subsurface and surface flow constructed wetlands. Int. J. Environ. Anal. Chem. 2011;91:600. [Google Scholar]
- Song Z. Sun Q. Yu M. Zhou Y. Kong X. Zhao Y. Seasonal variation and correlation of Escherichia coli and Salmonellae in a full-scale constructed wetland for wastewater treatment in China, presented at the 4th International Conference on Bioinformatics and Biomedical Engineering (iCBBE).2010. [Google Scholar]
- Song Z.W. Wu L. Yang G. Xu M. Wen S.P. Indicator microorganisms and pathogens removal function performed by copepods in constructed wetlands. Bull. Environ. Contam. Toxicol. 2008;81:459. doi: 10.1007/s00128-008-9527-1. [DOI] [PubMed] [Google Scholar]
- Stott R. May E. Matsushita E. Warren A. Protozoan predation as a mechanism for the removal of Cryptosporidium oocysts from wastewaters in constructed wetlands. Water Sci. Technol. 2001;44:191. [PubMed] [Google Scholar]
- Stott R. Tanner C.C. Influence of biofilm on removal of surrogate faecal microbes in a constructed wetland and maturation pond. Water Sci. Technol. 2005;51:315. [PubMed] [Google Scholar]
- Stuer-Lauridsen F. Birkved M. Hansen L.P. HoltenLützhøft H.-C. Halling-Sørensen B. Environmental risk assessment of human pharmaceuticals in Denmark after normal therapeutic use. Chemosphere. 2000;40:783. doi: 10.1016/s0045-6535(99)00453-1. [DOI] [PubMed] [Google Scholar]
- Suárez S. Carballa M. Omil F. Lema J. How are pharmaceutical and personal care products (PPCPs) removed from urban wastewaters? Rev. Environ. Sci. Biotechnol. 2008;7:125. [Google Scholar]
- Tarrant Regional Water District. Wetlands—Overview. 2013. www.trwd.com/wetlands. [Jul 22;2013 ]. www.trwd.com/wetlands
- Ternes T.A. Herrmann N. Bonerz M. Knacker T. Siegrist H. Joss A. A rapid method to measure the solid–water distribution coefficient (Kd) for pharmaceuticals and musk fragrances in sewage sludge. Water Res. 2004a;38:4075. doi: 10.1016/j.watres.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Ternes T.A. Joss A. Siegrist H. Peer reviewed: Scrutinizing pharmaceuticals and personal care products in wastewater treatment. Environ. Sci. Technol. 2004b;38:392A. doi: 10.1021/es040639t. [DOI] [PubMed] [Google Scholar]
- Thomas V. McDonnell G. Denyer S.P. Maillard J. Free-living amoebae and their intracellular pathogenic microorganisms. FEMS Microbiol. Rev. 2010;34:231. doi: 10.1111/j.1574-6976.2009.00190.x. [DOI] [PubMed] [Google Scholar]
- Thullen J.S. Sartoris J.J. Nelson S.M. Managing vegetation in surface-flow wastewater-treatment wetlands for optimal treatment performance. Ecol. Eng. 2005;25:583. [Google Scholar]
- Tolls J. Sorption of veterinary pharmaceuticals in soils: A review. Environ. Sci. Technol. 2001;35:3397. doi: 10.1021/es0003021. [DOI] [PubMed] [Google Scholar]
- Tront J.M. Reinhold D.M. Bragg A.W. Saunders F.M. Uptake of halogenated phenols by aquatic plants. J. Environ. Eng. 2007;133:955. [Google Scholar]
- Trout J.M. Walsh E.J. Fayer R. Rotifers ingest giardia cysts. J. Parisitol. 2002;88:1038. doi: 10.1645/0022-3395(2002)088[1038:RIGC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Truu M. Juhanson J. Truu J. Microbial biomass, activity and community composition in constructed wetlands. Sci. Total Environ. 2009;407:3958. doi: 10.1016/j.scitotenv.2008.11.036. [DOI] [PubMed] [Google Scholar]
- Verrengia Guerrero N.R. Taylor M.G. Davies N.A. Lawrence M.A.M. Edwards P.A. Simkiss K. Wider E.A. Evidence of differences in the biotransformation of organic contaminants in three species of freshwater invertebrates. Environ. Pollut. 2002;117:523. doi: 10.1016/s0269-7491(01)00132-4. [DOI] [PubMed] [Google Scholar]
- Vilchez-Vargas R. Junca H. Pieper D.H. Metabolic networks, microbial ecology and ‘OMICS' technologies. Environ. Microbiol. 2010;12:3089. doi: 10.1111/j.1462-2920.2010.02340.x. [DOI] [PubMed] [Google Scholar]
- Vymazal J. Removal of enteric bacteria in constructed treatment wetlands with emergent macrophytes: A review. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2005;40:1355. doi: 10.1081/ese-200055851. [DOI] [PubMed] [Google Scholar]
- Vymazal J. Removal of nutrients in various types of constructed wetlands. Sci. Total Environ. 2007;380:48. doi: 10.1016/j.scitotenv.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Vymazal J. The use constructed wetlands with horizontal sub-surface flow for various types of wastewater. Ecol. Eng. 2009;35:1. [Google Scholar]
- Vymazal J. Constructed wetlands for wastewater treatment: five decades of experience. Environ. Sci. Technol. 2010;45:61. doi: 10.1021/es101403q. [DOI] [PubMed] [Google Scholar]
- Waltman E.L. Venables B.J. Waller W.T. Triclosan in a North Texas wastewater treatment plant and the influent and effluent of an experimental constructed wetland. Environ. Toxicol. Chem. 2006;25:367. doi: 10.1897/05-112r.1. [DOI] [PubMed] [Google Scholar]
- Wang N. Mitsch W.J. A detailed ecosystem model of phosphorus dynamics in created riparian wetlands. Ecol. Model. 2000;126:101. [Google Scholar]
- Wetzel R.G. Periphyton of Freshwater Ecosystems. The Hague, The Netherlands: Dr. W. Junk Publishers; 1983. [Google Scholar]
- Wick A. Fink G. Joss A. Siegrist H. Ternes T.A. Fate of beta blockers and psycho-active drugs in conventional wastewater treatment. Water Res. 2009;43:1060. doi: 10.1016/j.watres.2008.11.031. [DOI] [PubMed] [Google Scholar]
- Winter J.E. A review on the knowledge of suspension-feeding in lamellibranchiate bivalves, with special reference to artificial aquaculture systems. Aquaculture. 1978;13:1. [Google Scholar]
- Wörman A. Kronnäs V. Effect of pond shape and vegetation heterogeneity on flow and treatment performance of constructed wetlands. J. Hydrol. 2005;301:123. [Google Scholar]
- Wright A.L. Reddy K.R. Phosphorous loading effects on extracellular enzyme activity in everglades wetland soils. Soil Sci. Soc. Am. J. 2001;65:588. [Google Scholar]
- Writer J.H. Ryan J.N. Barber L.B. Role of biofilms in sorptive removal of steroidal hormones and 4-nonylphenol compounds from streams. Environ. Sci. Technol. 2011;45:7275. doi: 10.1021/es2008038. [DOI] [PubMed] [Google Scholar]
- Wu Y. He J. Yang L. Evaluating adsorption and biodegradation mechanisms during the removal of microcystin-RR by periphyton. Environ. Sci. Technol. 2010;44:6319. doi: 10.1021/es903761y. [DOI] [PubMed] [Google Scholar]
- Yamamoto H. Nakamura Y. Moriguchi S. Nakamura Y. Honda Y. Tamura I. Hirata Y. Hayashi A. Sekizawa J. Persistence and partitioning of eight selected pharmaceuticals in the aquatic environment: Laboratory photolysis, biodegradation, and sorption experiments. Water Res. 2009;43:351. doi: 10.1016/j.watres.2008.10.039. [DOI] [PubMed] [Google Scholar]
- Zepp R.G. Hoigné J. Bader H. Nitrate-induced photooxidation of trace organic chemicals in water. Environ. Sci. Technol. 1987;21:443. doi: 10.1021/es00159a004. [DOI] [PubMed] [Google Scholar]
- Zepp R.G. Wolfe N.L. Baughman G.L. Hollis R.C. Singlet oxygen in natural waters. Nature. 1977;267:421. [Google Scholar]
- Zhang D.Q. Gersberg R.M. Hua T. Zhu J. Tuan N.A. Tan S.K. Pharmaceutical removal in tropical subsurface flow constructed wetlands at varying hydraulic loading rates. Chemosphere. 2012;87:273. doi: 10.1016/j.chemosphere.2011.12.067. [DOI] [PubMed] [Google Scholar]






