Abstract
The harmful effects of lunar dust (LD) on directly exposed tissues are documented in the literature, whereas researchers are only recently beginning to consider its effects on indirectly exposed tissues. During inhalation, nano-/microsized particles are efficiently deposited in nasal, tracheobronchial, and alveolar regions and transported to the central nervous system. The neurotoxic potential of LD and martian dust (MD) has not yet been assessed.
Glutamate is the main excitatory neurotransmitter involved in most aspects of normal brain function, whereas disturbances in glutamate homeostasis contribute to the pathogenesis of major neurological disorders. The research was focused on the analysis of the effects of LD/MD simulants (JSC-1a/JSC, derived from volcanic ash) on the key characteristics of glutamatergic neurotransmission. The average size of LD and MD particles (even minor fractions) before and after sonication was determined by dynamic light scattering. With the use of radiolabeled l-[14C]glutamate, it was shown that there is an increase in l-[14C]glutamate binding to isolated rat brain nerve terminals (synaptosomes) in low [Na+] media and at low temperature in the presence of LD. MD caused significantly lesser changes under the same conditions, whereas nanoparticles of magnetite had no effect at all. Fluorimetric experiments with potential-sensitive dye rhodamine 6G and pH-sensitive dye acridine orange showed that the potential of the plasma membrane of the nerve terminals and acidification of synaptic vesicles were not altered by LD/MD (and nanoparticles of magnetite).
Thus, the unique effect of LD to increase glutamate binding to the nerve terminals was shown. This can have deleterious effects on extracellular glutamate homeostasis in the central nervous system and cause alterations in the ambient level of glutamate, which is extremely important for proper synaptic transmission. During a long-term mission, a combination of constant irritation due to dust particles, inflammation, stress, low gravity and microgravity, radiation, UV, and so on may consequently change the effects of the dust and aggravate neurological consequences. Key Words: Lunar dust simulant—Martian dust simulant—Volcanic ash—Glutamate binding—Membrane potential—Synaptic vesicle acidification—Glutamatergic neurotransmission—Rat brain nerve terminals. Astrobiology 13, 679–692.
1. Introduction
Manned extraterrestrial missions, which include extravehicular activities, require toxicity risk assessment of planetary dusts. It has been shown that lunar dust (LD) particles adhere to space suits and therefore are brought into spacecrafts (Wallace et al., 2009; Rehders et al., 2011). During several Apollo missions, irritation of the eyes, the respiratory system, and skin was a result of direct contact of LD with the human body. The formation, composition, and physical properties of LD (as well as martian dust, MD) have not been fully characterized with regard to human health (Linnarsson et al., 2012), although the harmful effects of LD on directly (primary) exposed tissues, that is, tissues that are subjected to direct contact with LD, have been documented in the literature (Lam et al., 2002a, 2002b; Rehders et al., 2011). The effects of LD or MD and other nano- and microsized particles on indirectly (secondary) exposed tissues, that is, extrapulmonary organs and tissues that are not subjected to direct interaction with these particles, are now coming under scrutiny (Oberdörster et al., 2004; Bourdon et al., 2012). Using TiO2 nano- and microsized particles of the same crystalline structure, Oberdörster et al. (1994) demonstrated in a 12-week inhalation experiment on rats a similar mass deposition of the two particle types in the lower respiratory tract.
In a mammalian organism, nanosized particles are efficiently deposited in nasal, tracheobronchial, and alveolar regions due to diffusion and, beside the redistribution between different organs, are transported along sensory axons of the olfactory nerve to the central nervous system (Mikawa et al., 2001; Qingnuan et al., 2002; Oberdörster et al., 2004; Wang et al., 2004; Kao et al., 2012). Oberdörster et al. (2004) showed that intranasally instilled nanosized particles can target the central nervous system. The most likely mechanism realizes through deposits of these particles on the olfactory mucosa of the nasopharyngeal region of the respiratory tract and their subsequent translocation via the olfactory nerve. In rats, approximately 20% of the nanosized particles deposited on the olfactory mucosa can move to the olfactory bulb of the brain, which could provide a portal for entry of nanosized particles into the central nervous system such that they would circumvent the blood-brain barrier (Oberdörster et al., 2004). TiO2 nanosized particles were found in the brain of exposed 6-week-old male mice (Takeda et al., 2009). Kreyling et al. (2012) suggested that chronic particle inhalation could trigger or modulate the autonomous nervous system or the release of soluble mediators into circulation and lead to adverse health effects. Besides the brain, it was shown that nanosized particles can be translocated to, and affect, the liver within 4–24 h post exposure (Oberdörster et al., 2002; Bourdon et al., 2012). On the cellular level, nanosized particles can be transported into the cells through endocytosis (Garred et al., 2001; Xia et al., 2008). In contrast, Geiser et al. (2005) suggested that in vitro uptake of nanosized particles into the cells does not occur by any of the expected endocytic processes but rather by diffusion or adhesive interactions. These particles cross cellular membranes by nonphagocytic mechanisms in the lungs and in cultured cells. Within cells they are not membrane bound and have direct access to intracellular proteins, organelles, and DNA that may greatly enhance their toxic potential (Geiser et al., 2005).
The neurotoxic effects of LD and MD have not yet been assessed. The duration of dust exposure during a long-term mission, especially in combination with other harmful factors for human physiology, such as low gravity and microgravity, radiation, UV, and so on, may consequently aggravate its harmful effects. It has been clearly demonstrated that exposure to LD as well as to nano- and microsized particles causes inflammation (Oberdörster et al., 1994; Chatterjee et al., 2010; Bourdon et al., 2012), which is known to alter blood-brain barrier permeability (Abbott, 2000). In contrast, the nanosized particles elicited a persistently high inflammatory reaction in the lungs of animals compared to the larger-sized microparticles (Oberdörster et al., 1994). Neurotoxic effects and estimation of neurotoxic risks for health from exposure to LD and MD can be assessed at various levels of nervous system organization. Our research was conducted at the neurochemical level, where an agent might alter the flow of ions across the cellular membranes and block uptake of the neurotransmitters in nerve terminals (according to Guidelines for Neurotoxicity Risk Assessment of the U.S. Environmental Protection Agency, 1998, based on paragraph 3, “Hazard Characterization: 3.1.2. Animal Studies; 3.1.2.3. Neurochemical Endpoints of Neurotoxicity; 3.1.3.4. In Vitro Data in Neurotoxicology”). In the experiments, we used simulants of LD (JSC-1a, Lunar Soil Simulant) and MD (JSC, Mars-1A) derived from volcanic airfall ash deposit by ORBITEC Orbital Technologies Corporation, Madison, Wisconsin. The main question we asked was how LD and MD can provoke the development of neurological consequences. We assessed, in vitro, the direct influence of LD and MD simulants on the key characteristics of glutamatergic neurotransmission. Glutamate is a key excitatory neurotransmitter in the mammalian central nervous system. Nerve signal transmission is initiated by depolarization of the plasma membrane of presynaptic nerve terminals followed by exocytotic release of the neurotransmitters into the synaptic cleft, which then interact with the postsynaptic receptors and activate the signaling pathways. Under normal physiological conditions, extracellular glutamate between episodes of exocytotic release is maintained at a low level, thereby preventing continual activation of glutamate receptors and protecting neurons from excitotoxic injury (Borisova et al., 2010b). The maintenance of a low extracellular glutamate concentration is accomplished by neurotransmitter uptake in neurons and glial cells that use high-affinity Na+-dependent glutamate transporters located in the plasma membrane. This is the only possibility to keep extracellular glutamate at a low level because the enzymes for glutamate degradation have not been found in the synaptic cleft. Glutamate transporters use Na+/K+ electrochemical gradients across the plasma membrane as a driving force. Changes in glutamate transporter activity are a typical feature of most known neurological and neurodegenerative disorders and occur during exposure to altered gravity conditions (Borisova et al., 2004; Borisova and Himmelreich, 2005; Borisova and Krisanova, 2008; Krisanova et al., 2009). Glutamate transporters are integral membrane proteins; thus their function is tightly associated with the plasma membrane and consequently can be modulated by changes in its properties. It was hypothesized that the plasma membrane of the cells may be affected by LD due to its wide reactive surfaces and porosity.
Our research was focused on the assessment of LD and MD simulant effects on (1) uptake of glutamate by rat brain nerve terminals via specific high-affinity Na+-dependent plasma membrane transporters using radiolabeled l-[14C]glutamate; (2) the membrane potential (Em) of the plasma membrane of nerve terminals using potential sensitive fluorescent dye rhodamine 6G; (3) acidification of synaptic vesicles in nerve terminals using pH-sensitive fluorescent dye acridine orange.
2. Materials and Methods
2.1. Isolation of rat brain nerve terminals (synaptosomes)
Wistar rats (males 100–120 g body weight from the vivarium of M.D. Strazhesko, Institute of Cardiology, Medical Academy of Sciences of Ukraine) were maintained in accordance with the European Guidelines and International Laws and Policies. Animals were kept in the animal facilities of the Palladin Institute of Biochemistry, National Academy of Sciences of Ukraine, Kiev. They were housed in a quiet, temperature-controlled room (22–23°C) and were provided with water and dry food pellets ad libitum. All procedures conformed to the guidelines of the Palladin Institute of Biochemistry. The cerebral hemispheres of decapitated animals were rapidly removed and homogenized in ice-cold 0.32 M sucrose, 5 mM HEPES-NaOH, pH 7.4, and 0.2 mM EDTA. The synaptosomes were prepared by differential and Ficoll-400 density gradient centrifugation of rat brain homogenate according to the method of Cotman (1974) with slight modifications. All manipulations were performed at 4°C. The synaptosomal suspensions were used in experiments for 2–4 h after isolation. The standard salt solution was oxygenated and contained (in mM): NaCl 126, KCl 5, MgCl2 2.0, NaH2PO4 1.0, HEPES 20; pH 7.4, and d-glucose 10. The Ca2+-supplemented medium contained 2 mM CaCl2. Protein concentration was measured as described by Larson et al. (1986).
2.2. Experiments with LD and MD simulants
Major elemental composition of LD simulant (JSC-1a, Lunar Soil Simulant) (in %): SiO2 (46.67), TiO2 (1.71), Al2O3 (15.79), Fe2O3 (12.5), FeO (8.17), MnO (0.19), MgO (9.39), CaO (9.9), Na2O (2.83), K2O (0.78), P2O5 (0.71). Composition of MD simulant (JSC Martian Soil Simulant) (in %): SiO2 (34.5), TiO2 (3), Al2O3 (18.5), Fe2O3 (19), FeO (2.5), MnO (0.2), MgO (2.5), CaO (5), Na2O (2), K2O (0.5), P2O5 (0.7). LD, MD, and magnetic nanoparticles were suspended in the standard salt solution with constant stirring and immediately used in dynamic light scattering, fluorimetry, and experiments with radiolabeled glutamate.
2.3. Glutamate uptake experiments
Uptake of l-[14C]glutamate by the synaptosomes was measured as follows: samples (125 μL of the suspension, 0.2 mg of protein/mL) were pre-incubated in standard salt solution at 37°C for 10 min. Uptake was initiated by the addition of 10 μM l-glutamate supplemented with 420 nM l-[14C]glutamate (0.1 μCi/mL), incubated during different time intervals (1, 2, 10 min) at 37°C and then rapidly sedimented in a microcentrifuge (20 s at 10,000g). For unspecific binding assessment, the above procedures were performed in Na+-free, NMDG-containing medium. l-[14C]glutamate uptake and binding was determined as a decrease in radioactivity in aliquots of the supernatant (100 μL) and an increase in radioactivity of the pellet (SDS-treated) measured by liquid scintillation counting with scintillation cocktail ACS (1.5 mL).
2.4. Measurements of acidification of synaptic vesicles in the synaptosomes
Acridine orange, a pH-sensitive fluorescent dye that is selectively accumulated by the acid compartments of the synaptosomes (synaptic vesicles), was used for monitoring synaptic vesicle acidification (Borisova et al., 2010a, 2011b; Tarasenko et al., 2010). Fluorescence changes were measured by a Hitachi MPF-4 spectrofluorimeter at excitation and emission wavelengths of 490 and 530 nm, respectively (slit bands 5 nm each). The reaction was started by addition of acridine orange (final concentration of 5 μM) to synaptosomal suspension (0.2 mg/mL of final protein concentration) that was preincubated in a stirred thermostatted cuvette at 30°C for 10 min. The equilibrium level of dye fluorescence was achieved for 15 min. Fluorescence (F) was defined as
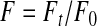 |
where F0 and Ft are the fluorescence intensities of acridine orange in the absence and presence of the synaptosomes, respectively. F0 was calculated by extrapolation of exponential decay function to t=0.
2.5. Measurement of synaptosomal plasma membrane potential (Em)
Membrane potential measurements were performed by using the potentiometric optical dye rhodamine 6G (0.5 μM) that bound with the plasma membrane. The suspension of the synaptosomes (∼0.15 mg/mL of final protein concentration) preincubated at 37°C for 10 min was added to a stirred thermostatted cuvette.
To estimate changes in the plasma membrane potential, the ratio (F) as an index of membrane potential was applied:
 |
where F0 and Ft are fluorescence intensities of rhodamine 6G in the absence and presence of the synaptosomes, respectively.
Rhodamine 6G fluorescence measurements were carried out with the Hitachi MPF-4 spectrofluorimeter at 528 nm (excitation) and 551 nm (emission) wavelengths (slit bands 5 nm each).
2.6. Assessment of binding of l-[14C]glutamate to LD and MD simulants
LD or MD simulant (2 mg/mL) in standard salt solution (200 μL) was incubated with l-[14C]glutamate (10 μM) for 10 min, filtered through Millipore filters (0.4 μm), and then washed with 3 mL of standard salt solution. In control experiments, probes were incubated and washed without LD or MD simulant.
2.7. Analysis of LD/MD simulants and the synaptosomes by dynamic light scattering
The size of particles of LD and MD simulants as well as the synaptosomes was measured by dynamic light scattering with a laser correlation spectrometer Zetasizer-3 (Malvern Instruments, UK), equipped with He-Ne laser LGN-111 (P=25 mW, λ=633 nm). The range of the instrument is from 1 nm to 50 microns. One milliliter of LD, MD simulant, or synaptosomal suspension was placed in a cylindrical quartz cuvette, 10 mm in diameter, which was injected into the laser correlation spectrometer under a constant temperature. Registration and statistical processing of laser scattered from water (n=1.33) suspensions of particles were performed repeatedly for 120 s at +22°C with scattering angle 90°. Data were processed with computer software service PCS-Size mode v1.61. The laser correlation spectrometer was equipped with multicomputing correlator type 7032 ce.
2.8. Electron microscopy of LD/MD simulants and the synaptosomes
Electron microscopy (H-600, Hitachi, Japan) of LD and MD simulants was performed by a negative staining method, and for the analysis of the synaptosomes an ultrathin sections method was used.
2.9. Statistical analysis
Results were expressed as mean±standard error of the mean of n independent experiments. The difference between two groups was compared by two-tailed Student t test. Differences were considered significant when P≤0.05.
2.10. Materials
JSC-1a Lunar Soil Simulant and JSC Martian Soil Simulant were purchased from ORBITEC, Orbital Technologies Corporation, Madison, Wisconsin, USA; EDTA, HEPES, NMDG, and analytical-grade salts were purchased from Sigma (USA). Ficoll 400, l-[14C]glutamate, aqueous counting scintillant (ACS) were from Amersham (UK). Acridine orange and rhodamine 6G were obtained from Molecular Probes (USA).
3. Results
3.1. Analysis of LD/MD simulants and the synaptosomes by dynamic light scattering
Analysis of the size of the particles of LD and MD simulants was performed by dynamic light scattering with the Zetasizer Nanosystem (Malvern Instruments) with a helium-neon laser. In the histograms, LD and MD simulants (concentration in the medium consisted of 2 mg/mL) show several heterogenic fractions. The average size of the particles in the suspension of LD and MD simulants in standard salt solution was evaluated based on five measurements, each for 1 min, and was equal to 2500±336 nm for LD simulant (Fig. 1 A) and 5300±1440 nm for MD simulant (Fig. 2A). In LD and MD simulants, we found minor fractions of nanoparticles with the size ∼50–60 nm (Figs. 1B, 2B). Both simulants were also analyzed by electron microscopy (Figs. 1E, 2E).
FIG. 1.

Dynamic light-scattering histograms: The analysis of the size of particles measured in the suspension of LD simulant (2 mg/mL) in a standard salt solution. Five measurements each during 1 min, sequentially 1—red, 2—blue, 3—green, 4—gray, 5—purple, are present in each histogram. (A) Suspension of LD simulant; (B) minor fraction of nanosized particles in LD simulant suspension; (C) suspension of LD simulant after treatment with ultrasound at 22 kHz for 1 min; (D) minor fraction of nanosized particles in sonicated LD simulant. The measurements were performed with a Zetasizer Nanosystem (Malvern Instruments) and a helium-neon laser. (E) Electron microscopy of LD simulant (method of negative staining). Color graphics available online at www.liebertonline.com/ast
FIG. 2.

Dynamic light-scattering histograms: The analysis of the size of particles measured in the suspension of MD simulant (2 mg/mL) in a standard salt solution. Five measurements each during 1 min, sequentially 1—red, 2—blue, 3—green, 4—gray, 5—purple, are present in each histogram. (A) Suspension of MD simulant; (B) minor fraction of nanosized particles in MD simulant suspension; (C) suspension of MD simulant after treatment with ultrasound at 22 kHz for 1 min; (D) minor fraction of nanosized particles in sonicated MD simulant. The measurements were performed with a Zetasizer Nanosystem (Malvern Instruments) and a helium-neon laser. (E) Electron microscopy of MD simulant (method of negative staining). Color graphics available online at www.liebertonline.com/ast
For more detailed analysis, LD and MD simulants were subjected to treatment with ultrasound at 22 kHz for 1 min. After sonication, the size of the particles of LD simulant was decreased more than twofold and consisted of 1110±67 nm (Figs. 1C, 3), whereas for MD simulant it was decreased by ∼16 % and equal to 4449±1030 nm (Figs. 2C, 3). Figures 1D and 2D show minor fractions of nanoparticles with the size ∼50–60 nm in sonicated LD and MD simulants.
FIG. 3.
The effect of ultrasound treatment on the diameter of particles of LD and MD simulants. Suspension of LD simulant (2 mg/mL) and MD simulant (2 mg/mL) in standard salt solution was subjected to the treatment with ultrasound at 22 kHz for 1 min, and the diameter of particles was measured immediately by dynamic light scattering with a Zetasizer Nanosystem (Malvern Instruments) and a helium-neon laser.
In the experiments, we used rat brain synaptosomes (see the Materials and Methods section), which retain all characteristics of intact nerve terminals, that is, the ability to maintain membrane potential and accomplish glutamate uptake, exocytosis, and so on. The analysis of the size of the synaptosomes was performed with the same instruments (Fig. 4A). The size of the synaptosomes was equal to 3.24±0.10 μm. Therefore, LD and MD simulants contained particles the size of which was comparable to, or lower than, the size of the nerve terminals, so they could interact with the synaptosomal plasma membrane and alter their molecular machinery.
FIG. 4.

(A) Dynamic light-scattering histograms: The analysis of the size of particles measured in the suspension of the synaptosomes (0.2 mg/mL) in standard salt solution. Three measurements each during 2 min sequentially 1—red, 2—blue, 3—green are present in the histogram. The measurements were performed with a Zetasizer Nanosystem (Malvern Instruments) and a helium-neon laser. (B) Electron microscopy of synaptosomes (method of ultrathin sections). Color graphics available online at www.liebertonline.com/ast
3.2. Assessments of direct binding of l-[14C]glutamate to LD and MD simulants
Before starting experiments on l-[14C]glutamate uptake by the synaptosomes, we analyzed whether LD and MD simulants per se were able to bind l-[14C]glutamate. This approach is of value because a decrease in apparent concentration of l-[14C]glutamate in the incubation media can result in an inaccuracy in the experiments with determination of the initial velocity of synaptosomal l-[14C]glutamate uptake. Taking into account our data on the average size of LD and MD simulants obtained by dynamic light scattering (Figs. 1–3), we used Millipore filters (0.45 μM) to separate LD and MD simulants. In this set of experiments, LD and MD simulants were incubated with 10 μM l-[14C]glutamate in standard salt solution for 10 min and then filtered through Millipore filters and washed with 3 mL of standard salt solution. It was revealed that the amount of radioactivity was the same in the control filters and filters in the presence of LD or MD simulant, so both simulants did not bind l-[14C]glutamate. Thus, the presence of LD and MD simulants did not change the concentration of l-[14C]glutamate in the incubation medium.
3.3. l-[14C]glutamate uptake by nerve terminals in the presence of LD and MD simulants
In synaptosomal preparations (Fig. 4), it was shown that the addition of LD simulant increased l-[14C]glutamate (10 μM) uptake for 1 min by ∼10% that was equal to 2.5±0.08 nmol min−1 mg−1 of protein in control synaptosomes and 2.79±0.08 nmol min−1 mg−1 of protein in the synaptosomes in the presence of LD simulant (P≤0.05, Student t test, n=6) (Fig. 5). In contrast, MD simulant added to the synaptosomes did not change significantly l-[14C]glutamate (10 μM) uptake for 1 min, which was equal to 2.59±0.08 nmol min−1 mg−1 of protein in the presence of MD simulant. Thus, LD and to a lesser degree MD simulants influenced l-[14C]glutamate uptake.
FIG. 5.
The time course of l-[14C]glutamate uptake by the synaptosomes in the control (solid line), in the presence of LD simulant (2 mg/mL) (dashed line) and MD simulant (2 mg/mL) (dotted line). Uptake was initiated by the addition of 10 μM l-glutamate supplemented with 420 nM l-[14C]glutamate (0.1 μCi/mL) to the synaptosomes (0.2 mg/mL of final protein concentration). The samples were incubated at 37°C for 1, 2, and 10 min and then rapidly centrifuged. l-[14C]glutamate radioactivity in the aliquots of the supernatant and pellet were determined as described in Materials and Methods. Data represent the mean±standard error of the mean of six independent experiments performed in triplicate.
As ferrum oxide is one of the main components of LD and MD simulants, we used synthetic nanoparticles of magnetite as an additional control. It was shown that synthetic nanoparticles did not increase the initial velocity of uptake of l-[14C]glutamate by nerve terminals. So the ability to alter glutamate uptake is a specific feature inherent to LD simulant only.
There are several main factors that can influence the initial velocity of synaptosomal uptake of l-[14C]glutamate: (i) changes in the membrane potential because it is a driving force of glutamate uptake; (ii) changes in the proton gradient of synaptic vesicles, which provides accumulation of glutamate by synaptic vesicles; or (iii) changes in the unspecific binding of l-[14C]glutamate to the synaptosomes.
3.4. The membrane potential of nerve terminals in the presence of LD and MD simulants
As a Na+/K+ electrochemical gradient across the plasma membrane is a driving force for transporter-mediated glutamate uptake, the changes in the membrane potential can alter the initial velocity of this process (Danbolt, 2001; Borisova et al., 2010b; Krisanova et al., 2012). As revealed, depolarization of the plasma membrane by 35 mM KCl led to a twofold decrease in the initial velocity of l-[14C]glutamate uptake (Kasatkina and Borisova, 2010).
Measurement of the plasma membrane potential Em was performed with the cationic potentiometric optical dye rhodamine 6G, which binds to negative charges of both plasma and mitochondrial membranes. As shown in Fig. 6, the addition of synaptosomal suspension to the medium that contained rhodamine 6G was accompanied by a partial decrease in the dye's fluorescence owing to its binding to the plasma membrane. Fst, the membrane potential index at the steady state level, was achieved for 3 min. The addition of LD or MD simulant did not cause an increase in the fluorescence signal reflecting the absence of depolarization of the plasma membrane of the nerve terminals. We also used synthesized nanoparticles of magnetite as an additional control, which did not reveal changes in the membrane potential of the nerve terminals in the presence of these nanoparticles.
FIG. 6.
The membrane potential of the synaptosomes in the presence of LD and MD simulants. The suspension of the synaptosomes (0.15 mg/mL of final protein concentration) preincubated at 30°C for 10 min was added to a stirred, thermostatted cuvette and then equilibrated with potential-sensitive dye rhodamine 6G (0.5 μM); when the steady level of the dye fluorescence was reached, LD simulant (2 mg/mL) or MD simulant (2 mg/mL) was added (arrow). Each trace is representative of four experimental data records performed with different synaptosomal preparations.
3.5. The proton gradient of synaptic vesicles in the presence of LD and MD simulants
After reaching the cytosol, glutamate is accumulated in synaptic vesicles by vesicular glutamate transporters, which use the proton electrochemical gradient as a driving force. Uptake of glutamate by plasma membrane transporters depends on the proton gradient of synaptic vesicles. We have shown that bafilomycin A1, specific inhibitor of V-ATPase, significantly decreased glutamate uptake by the synaptosomes (Borisova et al., 2011a). In these experiments we used a pH-sensitive fluorescent dye acridine orange to measure synaptic vesicle acidification as an important component of electrochemical proton gradient (ΔμH+).
As shown in Fig. 7, the addition of acridine orange to the synaptosomes was accompanied by partial quenching of the fluorescence signal due to dye accumulation in synaptic vesicles. After loading with acridine orange, LD and MD simulants were applied to the synaptosomes. We did not demonstrate significant changes in the fluorescence signal of acridine orange in response to the addition of LD and MD simulants. These data indicate that synaptic vesicles retain their proton gradient in the presence of both simulants.
FIG. 7.
The acidification of the synaptosomes in the presence of LD and MD simulants. The synaptosomes (0.15 mg/mL of final protein concentration) were incubated at 30°C for 10 min and then equilibrated with acridine orange (5 μM); when the steady level of the dye fluorescence was reached, LD simulant (2 mg/mL) or MD simulant (2 mg/mL) was added (arrow). Each trace is representative of four experimental data records performed with different synaptosomal preparations.
We concluded that neither changes in the membrane potential nor synaptic vesicle acidification cause an increase in the initial velocity of l-[14C]glutamate uptake in the presence of LD and MD simulants. In this set of the experiments, we also used synthesized nanoparticles of magnetite as an additional control, which revealed that these nanoparticles did not cause alterations in acidification of synaptic vesicles in the nerve terminals.
3.6. Binding of l-[14C]glutamate to nerve terminals in the presence of LD and MD simulants in low [Na+] media
As Na+/K+ gradient is a driving force for glutamate uptake by transporters, complete elimination of the extracellular Na+ is expected to inhibit transporter-mediated uptake of l-[14C]glutamate that allows us to measure the binding of l-[14C]glutamate to the plasma membrane of the synaptosomes. It should be noted that standard Na+-containing media is of 126 mM Na+ (see the Materials and Methods section). Using monovalent organic cations N-methyl-d-glucamine (NMDG) for almost complete replacement of extracellular sodium, we have shown that the binding of 10 μM l-[14C]glutamate to the synaptosomes in low [Na+] medium was increased in the presence of LD simulant in comparison with the control synaptosomes and consisted of 0.05±0.015 nmol/mg of protein in the control at 1 min time point after the addition of l-[14C]glutamate and 0.15±0.02 nmol/mg of protein - in the presence of LD simulant (P≤0.05, Student t test, n=6) (Fig. 8). In contrast, MD simulant did not cause significant increase in l-[14C]glutamate binding to the synaptosomes in low [Na+] media that consisted of 0.075±0.02 nmol/mg of protein in the presence of MD simulant (Fig. 8). We also used synthesized nanoparticles of magnetite as an additional control and did not reveal changes in l-[14C]glutamate binding to nerve terminals in low [Na+] media.
FIG. 8.
Binding of l-[14C]glutamate to the synaptosomes in low [Na+] (NMDG-containing) media in the control (the first in the triplet transparent columns) and in the presence of LD simulant (2 mg/mL) (the second in the triplet gray columns) and MD simulant (2 mg/mL) (the third in the triplet black columns). Samples (125 μL of the suspension, 0.2 mg of protein/mL) were pre-incubated in Na+-free, NMDG-containing salt solution at 37°C for 10 min. Binding was initiated by the addition of 10 μM l-glutamate supplemented with 420 nM l-[14C]glutamate (0.1 μCi/mL), incubated during different time intervals (1, 2, 10 min) at 37°C and then rapidly sedimented in a microcentrifuge (20 s at 10,000g). l-[14C]glutamate binding was measured as a decrease in radioactivity of the supernatant and an increase in radioactivity of the pellet by liquid scintillation counting with scintillation cocktail ACS (1.5 mL). *P≤0.05 as compared to control.
3.7. Binding of l-[14C]glutamate to nerve terminals in the presence of LD and MD simulants at low-temperature conditions
Many properties of biological membranes are particularly influenced by temperature, including membrane order (increasing temperatures fluidize membranes while decreasing temperatures order membranes), membrane phase transition, permeability, and thickness. As shown in Fig. 9, the binding of l-[14C]glutamate to the control synaptosomes and synaptosomes in the presence of LD simulant at low temperature (+4°C) was increased from 0.100±0.005 nmol/mg of protein in the control to 0.13±0.01 nmol/mg of protein in the presence of LD simulant at the 1 min time point after the addition of l-[14C]glutamate (10 μM). The analysis of the binding of l-[14C]glutamate to the synaptosomes in the presence of MD simulant and nanoparticles revealed that it was changed insignificantly as compared to the control. So our data on l-[14C]glutamate binding are in accordance with the above data obtained in low [Na+] media.
FIG. 9.
Binding of l-[14C]glutamate to the synaptosomes at low temperature (+4°C) in the control (the first in the triplet transparent columns) and in the presence of LD simulant (2 mg/mL) (the second in the triplet gray columns) and MD simulant (2 mg/mL) (the third in the triplet black columns). The samples (125 μL of the suspension, 0.2 mg of protein/mL) were pre-incubated at +4°C for 10 min. Binding was initiated by the addition of 10 μM l-glutamate supplemented with 420 nM l-[14C]glutamate (0.1 μCi/mL), incubated during different time intervals (1, 2, 10 min) at +4°C and then rapidly sedimented in a microcentrifuge (20 s at 10,000g). l-[14C]glutamate binding was measured as a decrease in radioactivity of the supernatant and an increase in radioactivity of the pellet by liquid scintillation counting with scintillation cocktail ACS (1.5 mL). *P≤0.05 as compared to control.
4. Discussion
The effects of LD on indirectly exposed tissues are almost completely unknown, whereas observations suggest that it can be deleterious to human physiology. Nanosized particles are not only efficiently deposited in nasal, tracheobronchial, and alveolar regions but also transported and redistributed between different organs (Mikawa et al., 2001; Qingnuan et al., 2002; Oberdörster et al., 2004; Wang et al., 2004; Bourdon et al., 2012). Kreyling et al. (2012) studied the biokinetics of inhaled biopersistent nano- and microsized particles to assess their toxicity and develop an understanding of their potential risks. When particles are inhaled, they do not necessarily remain at their sites of deposition in the respiratory tract. Instead they can undergo numerous transport processes within the various tissues of the lungs, including clearance from the lungs. The authors also observed particle redistribution from the epithelium toward, and within, the interstitium and lymph nodes of the lung, and particle translocation to blood circulation leading to subsequent accumulation in secondary organs (Kreyling et al., 2012). It was shown that inhaled nanosized particles can translocate across the blood-brain barrier in certain brain regions (Oberdörster et al., 2004); moreover, a fifth of them deposited on the olfactory mucosa can dislocate to the olfactory bulb. Exposure of mice to nanosized particles composed of TiO2, which is one of the main components of LD, was accompanied by transportation of these nanosized particles to the brain (Takeda et al., 2009). Nanosized particles can be transported through the plasma membrane into the cells by endocytosis (Garred et al., 2001; Xia et al., 2008) or by non-endocytotic mechanisms (Geiser et al., 2005). In this content, based on our data obtained with dynamic light scattering concerning the presence of minor nanoparticle-contained fraction in LD and MD simulants, it was suggested that endocytosis may be one of the mechanisms by which components of LD and MD simulants can reach nerve terminals.
In this study, the influence of LD and MD simulants on the key processes underlying proper synaptic transmission was assessed. The membrane potential of nerve terminals is extremely important for spontaneous and stimulated brain activity. Using potential sensitive fluorescent dye rhodamine 6G, we showed that the membrane potential of nerve terminals was not altered either by LD or by MD simulants (or by synthesized nanoparticles of magnetite) (Fig. 6). This fact also demonstrates that the integrity and energetic status of nerve terminals remained unchanged after the exposure to LD and MD simulants. The other parameter, which we analyzed by fluorimetry with pH-sensitive fluorescent dye acridine orange, was the acidification of synaptic vesicles. In nerve terminals, glutamate and other amino acid neurotransmitters are accumulated in synaptic vesicles, which release their content to the synaptic cleft in response to stimulation by means of exocytosis, that is, fusion of synaptic vesicles with the plasma membrane. Accumulation of the neurotransmitters into synaptic vesicles is accomplished by special vesicular glutamate transporters that use the electrochemical proton gradient across the vesicular membrane (created by vesicular ATPase) as a driving force. Acidification of synaptic vesicles (ΔpH) is an important component of the electrochemical proton gradient (ΔμH+). In the experiments, we revealed that LD and MD simulants (as well as nanoparticles of magnetite) did not change acidification of synaptic vesicles in nerve terminals. Unchanged membrane potential and synaptic vesicle acidification create background for proper exocytotic release of glutamate from nerve terminals under conditions of exposure to LD and MD simulants.
The influence of LD simulant on the binding of l-[14C]glutamate to nerve terminals was analyzed under conditions where Na+-dependent glutamate uptake was significantly inhibited. For these purposes, we used low [Na+] (NMDG-containing) media and low-temperature conditions. It was revealed that LD simulant caused a statistically significant increase in the binding of l-[14C]glutamate to nerve terminals in low [Na+] media (Fig. 8) and at a low temperature of +4°C (Fig. 9). In contrast, the application of MD simulant (or magnetic nanoparticles) did not change significantly l-[14C]glutamate binding to nerve terminals. Translating this data in vivo, an increase in glutamate binding may cause changes in the extracellular level of glutamate in the brain. Taking into account the abandon surface of nerve terminals in the brain, such changes can be enough to alter glutamate concentration in the interstitial fluid of the brain. From a physiological point of view, proper glutamate homeostasis in the brain, which is extremely important for proper synaptic transmission, may be disturbed.
Our data are also of interest from the point of view of basic membranology. It is uncertain what type of interaction occurs between LD simulant and the plasma membrane of nerve terminals; however, it is characterized by unchanged integrity of the membrane and membrane potential but increased glutamate binding. It should be emphasized that this effect was specific for LD simulant; in contrast, MD simulant caused significantly lesser changes in glutamate binding to the synaptosomes, whereas nanoparticles of magnetite did not possess such properties. We suggested that this effect of LD simulant on the plasma membrane could be associated with some specific features of LD simulant in comparison with other particles. LD is composed of agglutinates with sharp, jagged edges that may be more potent during interaction with the membrane surface and change its features and trigger pathophysiological processes. Also, LD has a considerable amount of reactive surfaces, a high content of metallic iron, and a large surface area (porous). Particle size and surface area can play important roles in the response to inhaled particles, which is especially relevant for nanosized particles (Oberdörster, 1996). Using electron microscopy and a negative contrasting method, we showed that LD simulants contained microsized particles with sharp edges, whereas MD did not possess such properties (Figs. 1E, 2E).
It should be noted that our data are of interest from an ecological point of view. This is because LD and MD simulants used in the experiments are a volcanic airfall ash deposit of basaltic composition. Processing of the ash involves milling and sieving in order to achieve the desired grain size distribution. No chemical processing has been performed. So the data obtained in this study may be used for the prognosis of neurotoxic consequences from volcanic ash exposure.
The complexity involved in the study of LD and MD has to do with the fact that they are composed of a mixture of different particles, each of which can act on cells by their own mechanism. Because of heterogeneity of the simulants, it is difficult to determine precisely the dose dependence of dust effects. Studying inhaled particles, Oberdörster (1996) also emphasized that, although administered doses were customarily expressed in units of mass, this might not be the appropriate dose metric for a correlation with observed effects. The components of LD may be internalized with lipid fractions of the lung epithelium, which in turn may help ions overcome the blood-brain barrier. Data of Halatek et al. (2008) on early neurotoxic effects of inhalation exposure to airborne aluminum, manganese, or both show that the subclinical neurological symptoms and low phospholipid-binding Clara cell protein CC16 level can be associated with an internalization of aluminum ions by the lung epithelium, which can then penetrate the blood-brain barrier. The main components of LD (see the Materials and Methods section), for example, FeO, Fe2O3, MnO, are on one hand trace metals necessary for the growth and function of the brain and on the other neurotoxicants that have the potential to jeopardize public health (Borisova et al., 2011a). Recently, we showed that the heavy metal Mn, one of the components of LD and MD simulants, did not affect the binding of l-[14C]glutamate to nerve terminals, and within the range of the concentrations 50–500 μM did not change the initial velocity of Na+-dependent l-[14C]glutamate uptake by nerve terminals (Borisova et al., 2011a). Iron oxide causes cell death associated with membrane damage, whereas silica (another major component of LD) triggers an inflammation response without causing considerable cell death for both cancer and normal cells (Choi et al., 2009). So, the presence of potentially toxic metals in LD should be taken into consideration in neurotoxicity risk assessment. In our experiments, the observed effects of LD and MD simulants were not associated with the influence of metal ions. This is because LD and MD simulants contain metal oxides, aluminosilicates (see the Materials and Methods section), which are insoluble in the standard salt solution used in the experiments. Some of the metal ions, the components of LD and MD simulants, are even present in a high concentration in the standard salt solution.
In the lunar exosphere, the physical and chemical properties of LD can be modified by several specific factors (radiation, low gravity, UV, etc.) in comparison with ground-based surroundings. Unique features of actual LD, resulting from its formation by (micro)meteoroid impacts and its extended radiation exposure in the absence of oxygen and humidity, could lead to toxic effects significantly exceeding those of simulants made from Earth materials (Fubini and Fenoglio, 2007). Lunar samples acquired in situ exist in a pristine state that preserves the surface reactive chemical aspects thought to be present on the lunar surface (Linnarsson et al., 2012). Also, the region-to-region variability of LD argues that a full understanding of its chemical reactivity will require in situ analysis, on a region-to-region basis, to provide insight into the types of reactions that may occur when LD interacts with organic molecules on the surface of the Moon (Loftus et al., 2010).
Also, there are a considerable number of factors to consider with regard to in situ exposure, for example, constant irritation with dust particles, inflammation, stress, low gravity and microgravity, radiation, UV, and so on, all of which can affect the physiology of a mammalian organism and aggravate the harmful effects of the dusts. It has been clearly demonstrated that exposure to LD as well as nanosized particles causes an inflammation response (Chatterjee et al., 2010; Bourdon et al., 2012). The treatment of human alveolar macrophages with LD and MD simulants has shown a dose-dependent increase in cytotoxicity that causes preferential damage to the suppressor macrophage subpopulation and leads to a net increase in the ratio of activator (RFD1+) to suppressor (RFD1+7+) macrophages (Latch et al., 2008). A significant increase in the percentage of neutrophils was not observed with any dust-treated group at 4 h after the instillation but was observed after 24 h in all the dust-treated groups. This observation indicates that these dusts are not acutely toxic and the effects are gradual; it took some time for neutrophils to be recruited into the lung and accumulate significantly (Lam et al., 2002b). Lam et al. (2002a) concluded that the acute effects of these dusts in the lung indicate that LD is slightly more toxic than TiO2, and the effect of MD is comparable to that of quartz. These results were consistent with the subchronic histopathological findings, where the order of severity of lung toxicity was TiO2<LD<MD<quartz. Long-term missions will likely require an increase in the duration of exposure to planetary dust. Duration of the exposure to nano- and microsized particles plays a significant role that can result in a shift of exposure-dose-response relationships at the end of a subchronic study versus the end of a chronic study (Oberdörster et al., 1994). Lam et al. (2002b) showed that an increase in duration of LD and MD simulants' presence in the lung from 7 to 90 days transforms the acute inflammatory response to a chronic inflammatory lesion. Lung lesions in the LD and MD simulant groups were more severe with the ozone pretreatment. The effects of O3 and MD simulant coexposure appear to be more than additive (Lam et al., 2002b). Morimoto et al. (2010) reviewed the effect of LD on humans in combination with microgravity of the Moon. In microgravity, (1) the deposition of particles of less than 1 μm in diameter in the human lung was not decreased; (2) the functions of macrophages, including phagocytosis, were suppressed; (3) pulmonary inflammation was changed (Morimoto et al., 2010). In a micro-/hypogravity environment, the risk of inhalation of dust is increased due to reduced gravity-induced sedimentation. Inhaled particles tend to deposit more peripherally and thus may be retained in the lungs for longer periods in reduced gravity (Darquenne and Prisk, 2008; Peterson et al., 2008). Inhalation of particles of varying size may affect the respiratory and cardiovascular systems in deleterious ways and lead to airway inflammation and increased respiratory and cardiovascular morbidity (Frampton et al., 2006). Several signal mechanisms have been proposed to be involved in inflammatory response and dust-associated cellular toxicity. A concentration-response study of LD simulant was undertaken on the murine macrophage cell line RAW 264.7. Results demonstrate that LD simulant in the concentrations of 50–2000 μg/mL induced the enhanced expression of inducible nitric oxide synthase (Chatterjee et al., 2010).
In this study, we found that LD simulant caused an increase in glutamate binding to the nerve terminal that could alter extracellular glutamate homeostasis in the brain, which is extremely important for proper synaptic transmission, whereas the membrane potential and acidification of synaptic vesicles were not changed in nerve terminals. A combination of constant irritation with dust particles in a long-term mission, inflammation, stress, and other harmful factors for human physiology, such as low gravity and microgravity, radiation, UV, and so on, may consequently change the effects of dust on the central nervous system and aggravate neurological consequences. From a public health point of view, the investigations of LD and MD simulant effects may open new modes of disease prevention through the elimination or reduction of neurotoxic risks.
Acknowledgments
This work was supported by a grant in the frame of the Program on Scientific Space Research of NAS of Ukraine 2012–2016.
We would like to thank Dr. O. Chunikhin for the excellent technical assistance and help in dynamic light-scattering experiments and Dr. V. Chernyshov and T. Kurchenko for electron microscopy. We appreciate Dr. I. Trikash and V. Gumenuk for help with fluorimetry.
Author Disclosure Statement
The authors declare no actual or potential conflict of interest.
Abbreviations
ACS, aqueous counting scintillant; LD, lunar dust; MD, martian dust; NMDG, N-methyl-d-glucamine.
References
- Abbott N.J. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol. 2000;20:131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisova T. Himmelreich N. Centrifuge-induced hypergravity: [3H]GABA and l-[14C]glutamate uptake, exocytosis and efflux mediated by high-affinity, sodium-dependent transporters. Adv Space Res. 2005;36:1340–1345. [Google Scholar]
- Borisova T. Krisanova N. Presynaptic transporter-mediated release of glutamate evoked by the protonophore FCCP increases under altered gravity conditions. Adv Space Res. 2008;42:1971–1979. [Google Scholar]
- Borisova T. Krisanova N. Himmelreich N. Exposure of animals to artificial gravity conditions leads to the alteration of the glutamate release from rat cerebral hemispheres nerve terminals. Adv Space Res. 2004;33:1362–1367. doi: 10.1016/j.asr.2003.09.039. [DOI] [PubMed] [Google Scholar]
- Borisova T. Sivko R. Borysov A. Krisanova N. Diverse presynaptic mechanisms underlying methyl-beta-cyclodextrin–mediated changes in glutamate transport. Cell Mol Neurobiol. 2010a;30:1013–1023. doi: 10.1007/s10571-010-9532-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisova T. Krisanova N. Sivko R. Borysov A. Cholesterol depletion attenuates tonic release but increases the ambient level of glutamate in rat brain synaptosomes. Neurochem Int. 2010b;56:466–478. doi: 10.1016/j.neuint.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Borisova T. Krisanova N. Sivko R. Kasatkina L. Borysov A. Griffin S. Wireman M. Presynaptic malfunction: the neurotoxic effects of cadmium and lead on the proton gradient of synaptic vesicles and glutamate transport. Neurochem Int. 2011a;59:272–279. doi: 10.1016/j.neuint.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Borisova T. Kasatkina L. Ostapchenko L. The proton gradient of secretory granules and glutamate transport in blood platelets during cholesterol depletion of the plasma membrane by methyl-beta-cyclodextrin. Neurochem Int. 2011b;59:965–975. doi: 10.1016/j.neuint.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Bourdon J.A. Saber A.T. Jacobsen N.R. Jensen K.A. Madsen A.M. Lamson J.S. Wallin H. Møller P. Loft S. Yauk C.L. Vogel U.B. Carbon black nanoparticle instillation induces sustained inflammation and genotoxicity in mouse lung and liver. Part Fibre Toxicol. 2012;9 doi: 10.1186/1743-8977-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. Wang A. Lera M. Bhattacharya S. Lunar soil simulant uptake produces a concentration-dependent increase in inducible nitric oxide synthase expression in murine RAW 264.7 macrophage cells. J Toxicol Environ Health A. 2010;73:623–636. doi: 10.1080/15287390903578182. [DOI] [PubMed] [Google Scholar]
- Choi S.J. Oh J.M. Choy J.H. Toxicological effects of inorganic nanoparticles on human lung cancer A549 cells. J Inorg Biochem. 2009;103:463–471. doi: 10.1016/j.jinorgbio.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Cotman C.W. Isolation of synaptosomal and synaptic plasma membrane fractions. Methods Enzymol. 1974;31:445–452. doi: 10.1016/0076-6879(74)31050-6. [DOI] [PubMed] [Google Scholar]
- Danbolt N.C. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Darquenne C. Prisk G. Deposition of inhaled particles in the human lung is more peripheral in lunar than in normal gravity. Eur J Appl Physiol. 2008;103:687–695. doi: 10.1007/s00421-008-0766-y. [DOI] [PubMed] [Google Scholar]
- Frampton M.W. Stewart J.C. Oberdörster G. Morrow P.E. Chalupa D. Pietropaoli A.P. Frasier L.M. Speers D.M. Cox C. Huang L.S. Utell M.J. Inhalation of ultrafine particles alters blood leukocyte expression of adhesion molecules in humans. Environ Health Perspect. 2006;114:51–58. doi: 10.1289/ehp.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fubini B. Fenoglio I. Toxic potential of mineral dusts. Elements. 2007;3:407–414. [Google Scholar]
- Garred Ø. Rodal S.K. van Deurs B. Sandvig K. Reconstitution of clathrin-independent endocytosis at the apical domain of permeabilized MDCK II cells: requirement for a Rho-family GTPase. Traffic. 2001;2:26–36. doi: 10.1034/j.1600-0854.2001.020105.x. [DOI] [PubMed] [Google Scholar]
- Geiser M. Rothen-Rutishauser B. Kapp N. Schürch S. Kreyling W. Schulz H. Semmler M. Im Hof V. Heyder J. Gehr P. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ Health Perspect. 2005;113:1555–1560. doi: 10.1289/ehp.8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halatek T. Sinczuk-Walczak H. Rydzynski K. Early neurotoxic effects of inhalation exposure to aluminum and/or manganese assessed by serum levels of phospholipid-binding Clara cells protein. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2008;43:118–124. doi: 10.1080/10934520701781178. [DOI] [PubMed] [Google Scholar]
- Kao Y.Y. Cheng T.J. Yang D.M. Wang C.T. Chiung Y.M. Liu P.S. Demonstration of an olfactory bulb–brain translocation pathway for ZnO nanoparticles in rodent cells in vitro and in vivo. J Mol Neurosci. 2012;48:464–471. doi: 10.1007/s12031-012-9756-y. [DOI] [PubMed] [Google Scholar]
- Kasatkina L. Borisova T. Impaired Na+-dependent glutamate uptake in platelets during depolarization of their plasma membrane. Neurochem Int. 2010;56:711–719. doi: 10.1016/j.neuint.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Kreyling W.G. Semmler-Behnke M. Takenaka S. Moller W. Differences in the biokinetics of inhaled nano- versus micrometer-sized particles. Acc Chem Res. 2012;46:714–722. doi: 10.1021/ar300043r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisanova N. Trikash I. Borisova T. Synaptopathy under conditions of altered gravity: changes in synaptic vesicle fusion and glutamate release. Neurochem Int. 2009;55:724–731. doi: 10.1016/j.neuint.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Krisanova N. Sivko R. Kasatkina L. Borisova T. Neuroprotection by lowering cholesterol: a decrease in membrane cholesterol content reduces transporter-mediated glutamate release from brain nerve terminals. Biochim Biophys Acta. 2012;1822:1553–1561. doi: 10.1016/j.bbadis.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Lam C.W. James J.T. Latch J.N. Hamilton R.F., Jr. Holian A. Pulmonary toxicity of simulated lunar and martian dusts in mice: II. Biomarkers of acute responses after intratracheal instillation. Inhal Toxicol. 2002a;14:917–928. doi: 10.1080/08958370290084692. [DOI] [PubMed] [Google Scholar]
- Lam C.W. James J.T. McCluskey R. Cowper S. Balis J. Muro-Cacho C. Pulmonary toxicity of simulated lunar and martian dusts in mice: I. Histopathology 7 and 90 days after intratracheal instillation. Inhal Toxicol. 2002b;14:901–916. doi: 10.1080/08958370290084683. [DOI] [PubMed] [Google Scholar]
- Larson E. Howlett B. Jagendorf A. Artificial reductant enhancement of the Lowry method for protein determination. Anal Biochem. 1986;155:243–248. doi: 10.1016/0003-2697(86)90432-x. [DOI] [PubMed] [Google Scholar]
- Latch J.N. Hamilton R.F., Jr. Holian A. James J.T. Lam C.W. Toxicity of lunar and martian dust simulants to alveolar macrophages isolated from human volunteers. Inhal Toxicol. 2008;20:157–165. doi: 10.1080/08958370701821219. [DOI] [PubMed] [Google Scholar]
- Linnarsson D. Carpenter J. Fubini B. Gerde P. Karlsson L.L. Loftus D.J. Prisk G.K. Staufer U. Tranfield E.M. van Westrenen W. Toxicity of lunar dust. Planet Space Sci. 2012;74:57–71. [Google Scholar]
- Loftus D.J. Rask J.C. McCrossin C.G. Tranfield E.M. The chemical reactivity of lunar dust: from toxicity to astrobiology physics and astronomy. Earth Moon Planets. 2010;107:95–105. [Google Scholar]
- Mikawa M. Kato H. Okumura M. Narazaki M. Kanazawa Y. Miwa N. Shinohara H. Paramagnetic water-soluble metallofullerenes having the highest relaxivity for MRI contrast agents. Bioconjug Chem. 2001;12:510–514. doi: 10.1021/bc000136m. [DOI] [PubMed] [Google Scholar]
- Morimoto Y. Miki T. Higashi T. Horie S. Tanaka K. Mukai C. Effect of lunar dust on humans: lunar dust: regolith. Nihon Eiseigaku Zasshi. 2010;65:479–485. doi: 10.1265/jjh.65.479. [DOI] [PubMed] [Google Scholar]
- Oberdörster G. Significance of particle parameters in the evaluation of exposure-dose-response relationships of inhaled particles. Inhal Toxicol. 1996;8(Supplement):73–89. [PubMed] [Google Scholar]
- Oberdörster G. Ferin J. Lehnert B. Correlation between particle size, in vivo particle persistence, and lung injury. Environ Health Perspect. 1994;102(Supplement 5):173–179. doi: 10.1289/ehp.102-1567252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G. Sharp Z. Atudorei V. Elder A. Gelein R. Lunts A. Kreyling W. Cox C. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health. 2002;65:1531–1543. doi: 10.1080/00984100290071658. [DOI] [PubMed] [Google Scholar]
- Oberdörster G. Sharp Z. Atudorei V. Elder A. Gelein R. Kreyling W. Cox C. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- Peterson J.B. Prisk G.K. Darquenne C. Aerosol deposition in the human lung periphery is increased by reduced-density gas breathing. J Aerosol Med Pulm Drug Deliv. 2008;21:159–168. doi: 10.1089/jamp.2007.0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qingnuan L. Yan X. Xiaodong Z. Ruili L. Quiqui D. Xiaoguang S. Shaoliang C. Wenxin L. Preparation of (99m)Tc-C(60)(OH)(x) and its biodistribution studies. Nucl Med Biol. 2002;29:707–710. doi: 10.1016/s0969-8051(02)00313-x. [DOI] [PubMed] [Google Scholar]
- Rehders M. Grosshäuser B.B. Smarandache A. Sadhukhan A. Mirastschijski U. Kempf J. Dünne M. Slenzka K. Brix K. Effects of lunar and Mars dust simulants on HaCaT keratinocytes and CHO-K1 fibroblasts. Adv Space Res. 2011;47:1200–1213. [Google Scholar]
- Takeda K. Suzuki K.I. Ishihara A. Kubo-Irie M. Fujimoto R. Tabata M. Oshio S. Nihei Y. Ihara T. Sugamata M. Nanoparticles transferred from pregnant mice to their offspring can damage the genital and cranial nerve systems. J Health Sci. 2009;55:95–102. [Google Scholar]
- Tarasenko A.S. Sivko R.V. Krisanova N.V. Himmelreich N.H. Borisova T.A. Cholesterol depletion from the plasma membrane impairs proton and glutamate storage in synaptic vesicles of nerve terminals. J Mol Neurosci. 2010;41:358–367. doi: 10.1007/s12031-010-9351-z. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. Guidelines for Neurotoxicity Risk Assessment. U.S. Environmental Protection Agency; Washington, DC: 1998. EPA/630/R-95/001F. [Google Scholar]
- Wallace W.T. Tayler L.A. Liu Y. Cooper B.L. McKay D.S. Chen B. Jeevarajan A.S. Lunar dust and lunar simulant activation and monitoring. Meteorit Planet Sci. 2009;44:961–970. [Google Scholar]
- Wang H. Wang J. Deng X. Sun H. Shi Z. Gu Z. Liu Y. Zhao Y. Biodistribution of carbon single-wall carbon nanotubes in mice. J Nanosci Nanotechnol. 2004;4:1019–1024. doi: 10.1166/jnn.2004.146. [DOI] [PubMed] [Google Scholar]
- Xia T. Kovochich M. Liong M. Zink J.I. Nel A.E. Cationic polystyrene nanosphere toxicity depends on cell-specific endocytic and mitochondrial injury pathways. ACS Nano. 2008;2:85–96. doi: 10.1021/nn700256c. [DOI] [PubMed] [Google Scholar]











