Abstract
Objective:
Neuroprotective effect of naringenin against carbaryl toxicity was studied in mouse neuroblastoma cell line.
Materials and Methods:
Mouse neuroblastoma cells (Neuro 2A) obtained from National Center for Cell Sciences, Pune, India were either exposed to carbaryl or pre-treated with naringenin (a flavonoid prepared from grape fruit) before their exposure to carbaryl. Results were analyzed using MTT [3-4,5-Dimethylthiazol-2-yl)-2,5-diphenltetrazolium bromide] assay for cell viability, FACS (fluorescence assisted cell sorting) analysis for apoptotic and necrotic cell populations, DCFH-DA (2`,7`-dichlorofluorescin-diacetate) assay for Reactive Oxygen Species (ROS) visualization, JC-1 staining for determining mitochondrial membrane potential and real-time PCR for quantifying pro and anti-apoptotic gene expression.
Results:
Exposure to naringenin resulted in better survival of Neuro 2A cells which were subsequently subjected to carbaryl toxicity. Treatment with naringenin was found to reduce the oxidative stress by decreasing the ROS and was found to maintain the integrity of mitochondrial membrane potential. It was also found to downregulate pro-apoptotic genes (BAX and Caspase-3) while upregulating anti-apototic gene (Bcl2).
Conclusion:
The results of this pilot study underline the potential of naringenin in treating carbaryl induced neurotoxicity and further studies are warranted to establish the effect of naringenin in vivo conditions.
Keywords: Antioxidant, neuro 2A cells, neurotoxicity, oxidative stress
INTRODUCTION
Pesticides which were originally designed to target nervous system of insects can obviously also cause neurotoxicity to humans.[1,2,3,4] Carbaryl (1-naphthyl methylcarbamate) is an acetyl cholinesterase inhibitor classified under carbamates group of pesticides. In addition to its neurotoxicity, it was also declared as human carcinogen by International Agency for Research on Cancer, France and United States Environmental Protection Agency. Toxic effects of carbaryl were already documented. It was shown to inhibit neurite outgrowth by perturbing levels of NF-H and GAP43 in human as well as mouse neuroblastoma cells.[5,6,7] Carbaryl was found to cause heart malformation[8] and increase mortality rate during development of zebrafish.[9] Incidences of human exposures to carbaryl were not uncommon.[10]
In a pursuit to find strategy for negating carbaryl toxicity in humans, we stumbled upon the choice of naringenin. It is a natural flavonoid shown to have anti-inflammatory effect in lipopolysacharride/interferon-gamma stimulated glial cells.[11] Neuroprotective effect of naringenin was evident from both in vivo and in vitro studies. It was reported to offer neuroprotection in 6-hydroxy dopamine (6-OHDA) model of Parkinson disease[12] in animals and also in primary cultures of human mesencephalic neurons.[13,14] Ability of naringenin to cross blood-brain-barrier[15] attracted us to test its efficacy in treating carbaryl induced neurotoxicity.
In this study, an in vitro approach using Neuro 2A was used to evaluate the efficacy of naringenin which underline its potential in treating carbaryl-induced neurotoxicity.
MATERIALS AND METHODS
Cell line and reagents
Neuro 2A cells were obtained from National Centre for Cell Sciences, Pune, India. Minimum essential medium (MEM), antibiotics and antifungal additives (Amphotericin B, Gentamycin), GIBCO fetal bovine serum (FBS), Trizol reagent and JC-1 dye came from Invitrogen, USA. MTT (3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), Naringenin, Carbaryl, Bovine Serum Albumin (BSA), 2′,7′-Dichlorofluorescin diacetate (DCF-DA), TPP tissue culture plates and Trypsin/EDTA were purchased from Sigma Aldrich, USA. Materials required for qRT-PCR were procured from Qiagen, USA. All other chemicals were purchased from Sisco Research Laboratories, India.
Cell culture
Neuro 2A cells were routinely cultured in Eagle's minimum essential medium (MEM) supplemented with 10% FBS, gentamicin (50μg/ml) and amphotericin B (2.5μg/ml). Cells were cultured as monolayer in uncoated plastic dishes at 37°C under 5% CO2 and 95% air. Medium was changed every 3 days, and the cells were passed once they reached approximately 80% confluence. Trypsin (2.5%)/EDTA (0.38g/l) was used to dislodge the cells.
Cell viability assay
The cell viability assay was performed using MTT assay.[16] Cells grown in 96-well plates were exposed to different doses of carbaryl (2.5 to 10μM). In another set of experiment, cells were pre-treated with naringenin (5 to 100μM) for different time duration before they were subjected to carbaryl exposure. Viability was determined in all the cases through standard MTT assay. By keeping the viability of control cells (not treated with any of these) as 100%, survival of cells in experimental groups was expressed as percentage of cell viability.
Experimental design
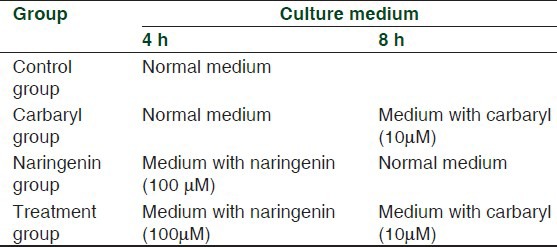
Based on cell viability studies, appropriate dosage for carbaryl and naringenin were selected for the experiments. The cell culture experiments were conducted as follows [Table 1].
Table 1.
Treatment of experimental groups
Thus, at the end of 12 h from the commencement of the experiments, cells were harvested for further analyses as given below.
Flow cytometric analysis
After washing the cells with phosphate buffered saline (PBS) on completion of experiment, FACS analysis of cell populations stained with Acridine Orange (AO) and Ethidium Bromide (EB) was carried out as per procedure described earlier.[17,18] Briefly, 0.1 mM of AO and 0.25mM of EB was used for staining followed by flow cytometric analysis using BD FacsVantage SE (National Centre for Ultra Fast Processes atUniversity of Madras, India). Emission of AO was detected at 525/20nm filter (FL1) and EB at 635/20nm (FL2). Signals were amplified logarithmically. Cells labeled either with Acridine Orange or Ethidium Bromide were used as controls in addition to the use of unlabelled cells as controls. The criteria for differentiating the cell population based on staining capacity was carried out as described by Kern and Kehrer[17] and Liegler et al.[19]
Analysis of intracellular ROS generation
Visualization of intracellular ROS generation was determined using the DCFH-DA assay.[15] The cells were photographed using a Nikon inverted microscope with fluorescent attachment using FITC filter set.
Determining mitochondrial membrane potential
Integrity of mitochondrial membrane potential was assessed using JC-1 staining procedure.[20] Protocol suggested by the manufacturer was adapted. Briefly, 200μM stock of JC-1 was prepared in DMSO from which culture medium with 2μM of JC-1 was prepared. In this working solution, cells were incubated for 30 min. At the end, cells were washed in PBS and were observed using a Nikon microscope with fluorescent attachment.
qRT-PCR estimation of gene expressions
Total cellular RNA was isolated using the Trizol reagent according to the manufacture's instruction. The RNA concentration and purity were determined spectrophotometrically. Total RNA (5ng) extracted from each sample was reverse transcribed to cDNA and real-time PCR was performed using an ABI 7000 (PE Applied-Biosystems) in the presence of SYBR-green for Bax, Bcl2 and Caspase-3. The optimization of the real time PCR reaction and the entire procedure was performed according to the manufacturer's Instructions. The fold changes were calculated using the 2 ΔΔCt method as previously described by Livak and Schmittgen.[21]
Statistical analysis
All data represent the mean of samples from six separately performed experiments. Results were expressed as mean and SEM/SD. The difference between groups was analyzed for significance using one-way ANOVA followed by Tukey's test. A value of P <0.05 was considered statistically significant.
RESULTS
Neuro 2A cells exposed to 2.5, 5, 7.5 and 10μM of carbaryl for different durations of 4, 8 and 12 h. By assuming the viability of control group as 100%, estimation using MTT assay showed progressive cell death with increasing dosages. Carbaryl at a dose of 10μM for 8 h resulted in 80% viability. Increasing the exposure duration further to 12 h did not result further decrease of viability. Therefore, the dosage of 10μM for 8 h was used in further experiments. Results are shown in Figure 1. For determining the effective dose for naringenin, cells were pre-treated with 5, 10, 25, 50 and 100μM of naringenin for 2 or 4 h before exposing them to 10μM of carbaryl for 8 h. Results of cell viability estimated through MTT assay are shown in Figure 2. Treating cells with 100μM of naringenin for 4 h was found to effectively prevent cell death caused by carbaryl at the given dose.
Figure 1.
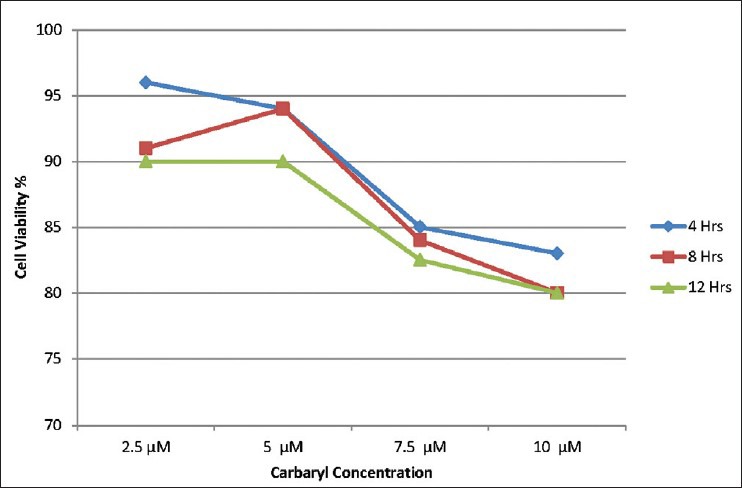
Dose-time dependent effect of carbaryl toxicity on cell viability in neuro 2A cells
Figure 2.
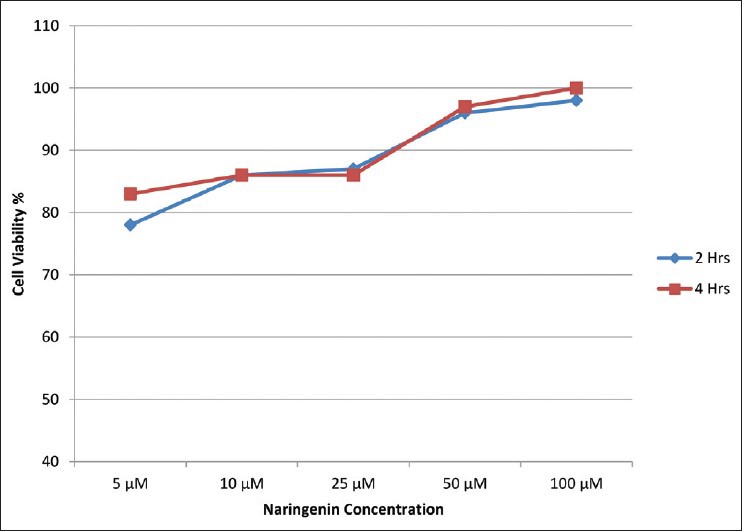
Effect of naringenin on cell viability over 10 μM carbaryl induced neurotoxicity for 8 hours in neuro 2A cells
For ROS analysis, JC-1 staining and qRT-PCR estimation of gene expression, the dosages of carbaryl and naringenin standardized as given above were used. The phase contrast microscopic images of the various groups of cells at the end of the experimental period (12 h) are illustrated in Figure 3.
Figure 3.
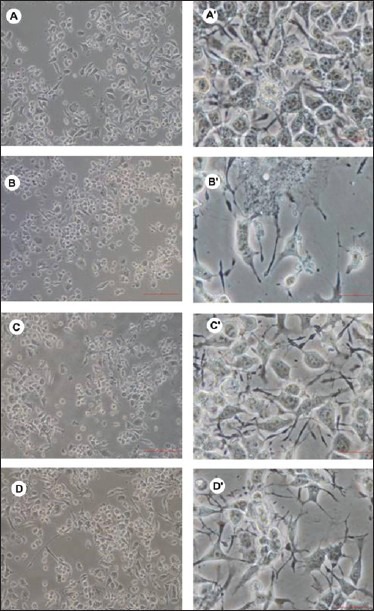
Phase contrast images of different groups of cells
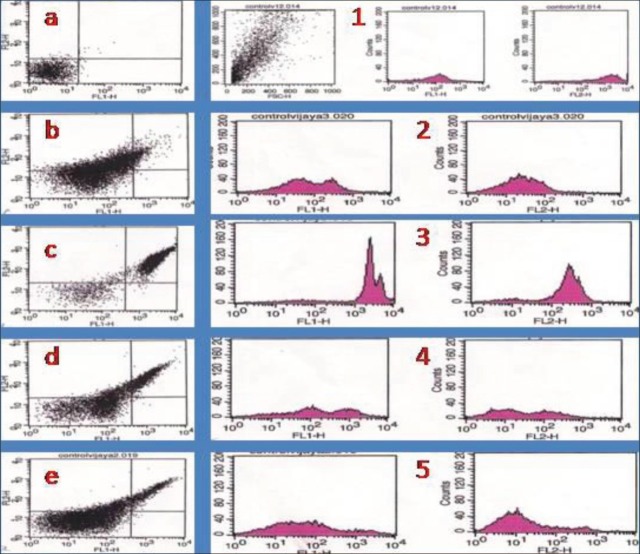
Flow cytometric analysis of AO-EB stained cells of different groups indicated a sharp increase in both apoptotic and necrotic cell populations in carbaryl group. Treatment with naringen in prior to carbaryl exposure (Treatment group) had shown significantly reduced cell death. However, unlike MTT results which showed near normal cell viability, FACS analysis showed presence of apoptotic and necrotic cells presence in treatment group as shown in Figure 4.
Figure 4.
Flow cytometric analysis of cell death
Fluorescent microscopic analysis of cells of different groups using DCFH-DA staining showed high fluorescence in carbaryl group which was indicative of ROS level. Pre-treatment with naringenin can be seen to effectively reduce the ROS level in the cells which were subsequently exposed to carbaryl. This was evident from the lack of fluorescence in treatment group as shown in Figure 5. Fluorescent microscopic analysis of JC-1 staining showed green fluorescence indicative of loss of mitochondrial membrane potential; treatment with naringenin was found to preserve mitochondrial membrane potential evident from the red fluorescence seen in treatment group. Results of JC-1 staining were given as Figure 6.
Figure 5.

Analysis of reactive oxygen species using DCFH-DA dye. (a) control group; (b) Carberyl group; (c) Naringenin group; (d) Treatment group
Figure 6.
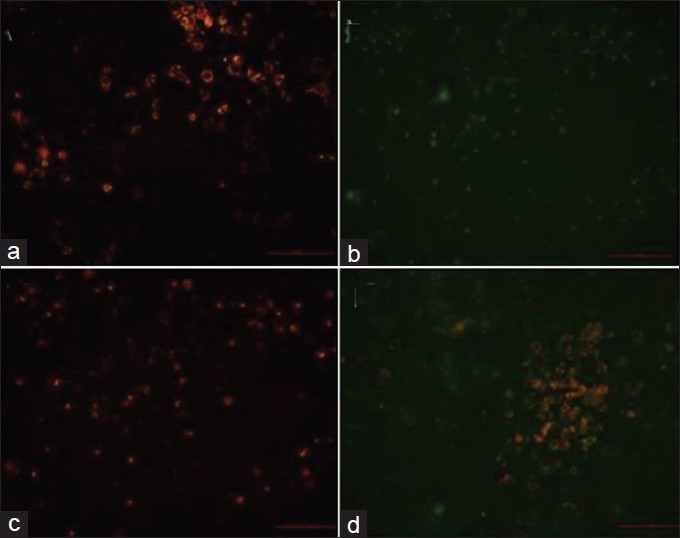
Analysis of mitochondrial membrane potential using JC-1 staining. (a) control group; (b) Carberyl group; (c) Naringenin group; (d) Treatment group
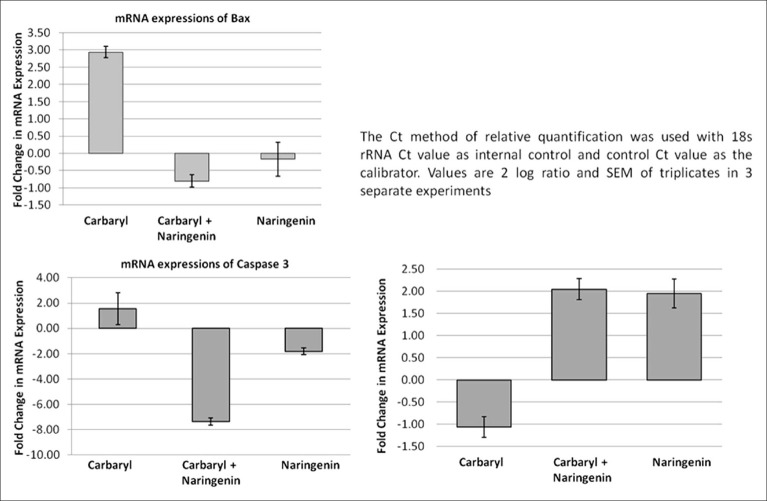
The mRNA expression studies showed increased expression of pro-apototic genes in carbaryl treated cells which were effectively attenuated in naringenin treatment. Pro-apoptotic gene Bax showed 3 fold increases due to carbaryl treatment. Similarly, caspase 3 the final executor of cell death which was upregulated in carbaryl group was downregulated strongly by naringenin. Predictably, anti-apototic gene Bcl2 expression was downregulated by carbaryl which was effectively countered by naringenin treatment. All these changes/differences were statistically significant. Thus qRT-PCR study clearly showed induction of apoptosis by carbaryl and the ability of the naringenin to reverse these changes [Figure 7].
Figure 7.
QPCR analysis of mRNA expression of bax, caspase-2 and Bcl2 on the role of naringenin under carbaryl toxicity in neuro 2A cells
DISCUSSION
Environmental pollution affecting human has become the order of the day. Pesticides used in agriculture easily find their way into human through food chain as well as directly from environment. These chemicals induce toxicity by producing pro-oxidants in cells.[22] As stated earlier, carbaryl toxicity in human can occur and therefore evolving strategies to counter its effects would be highly beneficial. Although, effects of carbaryl toxicity were reported earlier,[8,9] mechanism for its neurotoxicity has not been fully deciphered.
Results of the present study confirm the oxidative injury caused by carbaryl in neurons as evident from bright fluorescence in DCFH-DA assay which is directly proportional to the levels of ROS. JC-1 staining showed a clear red to green shift in fluorescence which is the hall mark of loss of mitochondrial membrane potential; loss of which would release pro-apoptotic factors. qRT-PCR analysis of mRNA expression clearly established the progress of apoptosis in the cells exposed to carbaryl. FACS analysis of AO-EB preparation had more apoptotic or dead cells due to carbaryl exposure. Thus carbaryl exposure leads to severe oxidative stress to the cells eventually resulting in their death which was an expected result.
The main stay of this work was to test the efficacy of naringenin in treating carbaryl toxicity. Results of the present study provided overwhelming support to our hypothesis as pre-treatment with naringenin was found to counter the effects of carbaryl in all aspects. It effectively reduced the ROS level induced by carbaryl and maintained the mitochondrial membrane potential. naringenin treatment upregulated anti-apototic gene Bcl-2 and downregulated pro-apoptotic genes such as Bax and Caspase-3. Increased cell survival due to naringenin treatment was evident from MTT as well as FACS analysis.
The results of this pilot study were encouraging and further in vivo studies are required to establish the efficacy of naringenin in humans.
ACKNOWLEDGMENT
Vijaya Prakash KM was supported by Senior Research Fellowship from Lady Tata Memorial Trust. Kirubhanand C and Felicia Mary M were supported by DST-INSPIRE fellowship. This study was partly funded through UICIC program of University of Madras.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Moser VC, Padilla S, Simmons JE, Haber LT, Hertzberg RC. Impact of chemical proportions on the acute neurotoxicity of a mixture of seven carbamates in preweanling and adult rats. Toxicol Sci. 2012;129:126–34. doi: 10.1093/toxsci/kfs190. [DOI] [PubMed] [Google Scholar]
- 2.Hargreaves AJ. Neurodegenerations induced by organophosphorous compounds. Adv Exp Med Biol. 2012;724:189–204. doi: 10.1007/978-1-4614-0653-2_15. [DOI] [PubMed] [Google Scholar]
- 3.Banks CN, Lein PJ. A review of experimental evidence linking neurotoxic organophosphorus compounds and inflammation. Neurotoxicology. 2012;33:575–84. doi: 10.1016/j.neuro.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lein PJ, Bonner MR, Farahat FM, Olson JR, Rohlman DS, Fenske RA, et al. Experimental strategy for translational studies of organophosphorus pesticide neurotoxicity based on real-world occupational exposures to chlorpyrifos. Neurotoxicology. 2012;33:660–8. doi: 10.1016/j.neuro.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sachana M, Flaskos J, Alexaki E, Hargreaves AJ. Inhibition of neurite outgrowth in N2a cells by leptophos and carbaryl: Effects on neurofilament heavy chain, GAP-43 and HSP-70. Toxicol In Vitro. 2003;17:115–20. doi: 10.1016/s0887-2333(02)00121-2. [DOI] [PubMed] [Google Scholar]
- 6.Flaskos J, Fowler MJ, Teurtrie C, Hargreaves AJ. The effects of carbaryl and trichlorphon on differentiating mouse N2a neuroblastoma cells. Toxicol Lett. 1999;110:79–84. doi: 10.1016/s0378-4274(99)00142-3. [DOI] [PubMed] [Google Scholar]
- 7.Chang PA, Wu YJ, Li W, Leng XF. Effect of carbamate esters on neurite outgrowth in differentiating human SK-N-SH neuroblastoma cells. ChemBiol Interact. 2006;159:65–72. doi: 10.1016/j.cbi.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Lin CC, Hui MN, Cheng SH. Toxicity and cardiac effects of carbaryl in early developing zebrafish (Daniorerio) embryos. Toxicol Appl Pharmacol. 2007;222:159–68. doi: 10.1016/j.taap.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Todd NE, Van Leeuwen M. Effects of Sevin (carbaryl insecticide) on early life stages of zebrafish (Daniorerio) Ecotoxicol Environ Saf. 2002;53:267–72. doi: 10.1006/eesa.2002.2231. [DOI] [PubMed] [Google Scholar]
- 10.Branch RA, Jacqz E. Subacute neurotoxicity following long-term exposure to Carbaryl. Am J Med. 1986;80:741–5. doi: 10.1016/0002-9343(86)90837-5. [DOI] [PubMed] [Google Scholar]
- 11.Vafeiadou K, Vauzour D, Lee HY, Rodriguez-Mateos A, Williams RJ, Spencer JP. The citrus flavanonenaringenin inhibits inflammatory signalling in glial cells and protects against neuroinflammatory injury. Arch BiochemBiophys. 2009;484:100–9. doi: 10.1016/j.abb.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Zbarsky V, Datla KP, Parkar S, Rai DK, Aruoma OI, Dexter DT. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson's disease. Free Radic Res. 2005;39:1119–25. doi: 10.1080/10715760500233113. [DOI] [PubMed] [Google Scholar]
- 13.Braidy N, Grant R, Adams S, Guillemin GJ. Neuroprotective effects of naturally occurring polyphenols on quinolinic acid-induced excitotoxicity in human neurons. FEBS J. 2010;277:368–82. doi: 10.1111/j.1742-4658.2009.07487.x. [DOI] [PubMed] [Google Scholar]
- 14.Mercer LD, Kelly BL, Horne MK, Beart PM. Dietary polyphenols protect dopamine neurons from oxidative insults and apoptosis: Investigations in primary rat mesencephalic cultures. Biochem Pharmacol. 2005;69:339–45. doi: 10.1016/j.bcp.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Youdim KA, Qaiser MZ, Begley DJ, Rice-Evans CA, Abbott NJ. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic Biol Med. 2004;36:592–604. doi: 10.1016/j.freeradbiomed.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 16.Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, et al. Feasibility of drug screening with panels of human tumor cell lines using a microculturetetrazolium assay. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- 17.Kern JC, Kehrer JP. Acrolein-induced cell death: Acaspase-influenced decision between apoptosis and oncosis/necrosis. ChemBiol Interact. 2002;139:79–95. doi: 10.1016/s0009-2797(01)00295-2. [DOI] [PubMed] [Google Scholar]
- 18.Liegler TJ, Hyun W, Yen TS, Stites DP. Detection and quantification of live, apoptotic, and necrotic human peripheral lymphocytes by single-laser flow cytometry. Clin Diagn Lab Immunol. 1995;2:369–76. doi: 10.1128/cdli.2.3.369-376.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Joseph JA. Quantifying cellular oxidative stress B by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999;27:612–6. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 20.StJohn JC, Amaral A, Bowles E, Oliveira JF, Lloyd R, Freitas M, et al. The analysis of mitochondria and mitochondrial DNA in human embryonic stem cells. Methods Mol Biol. 2006;331:347–74. doi: 10.1385/1-59745-046-4:347. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta DeltaC (T) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Tellez-Bañuelos MC, Santerre A, Casas-Solis J, Bravo-Cuellar A, Zaitseva G. Oxidative stress in macrophages from spleen of Nile tilapia (Oreochromisniloticus) exposed to sublethal concentration of endosulfan. Fish Shellfish Immunol. 2009;27:105–11. doi: 10.1016/j.fsi.2008.11.002. [DOI] [PubMed] [Google Scholar]