Abstract
Till date, NYU MASCIS (New York University, Multicenter Animal Spinal Cord Injury Study) impactor and Ohio State University electromagnetic spinal cord injury device impactor were under use for simulating an experimental spinal cord injury in rodents; functional recovery being assessed through Basso, Beattie and Bresnahan (BBB) scoring method which is an open field behavior based scoring system. Although, the cited impactors are state-of-art devices, affordability to scientists in developing and under developed countries is questionable. Since the acquisition of these impact devices are expensive, we designed a customized impact device based on the requirement, satisfying all the parameters to withstand a standard animal model for contusion type of spinal cord injury at the thoracic level without compromising the lesion reproducibility. Here, a spinal cord contusion is created using a blunt-force impactor in male Wistar rats. Our method gave consistent lesion effects as evaluated by behavior scoring methods. All the animals showed equal degree of performance in tests like narrow beam, inclined plane and horizontal ladder and in BBB scores (open field locomotor test). The aim of presenting our experience is to reinstate the fact that lack of affordability to get sophisticated instrumentation need not be a hurdle in the pursuit of science.
Keywords: CNS injury, method, laminectomy, thoracic level
INTRODUCTION
Allen in 1911[1] was the first person attempted to create a controlled experimental spinal cord injury. He used a simple yet irrefutable logic that when a known weight dropped from a constant height shall produce same impact force on all occasions. Based on this concept, he prepared a metal tube with pores. A rod of 10 g was inserted into the tube and can be stopped at various heights using a pin inserted into the pores on the tube at regular intervals. By aiming the tube over a surgically exposed spinal cord and by withdrawing the pin holding the rod, a reproducible impact force would be created when the rod get dropped on the spinal cord. For unknown reasons, Allen's model was adopted by researchers for a long period.
Later, sophisticated devices such as Ohio State University's electromagnetic spinal cord injury device and New York University's MASCIS impactor came into use.[2,3,4] While Ohio impactor use electromagnetic force to create contusion and concussion in the spinal cord of rats, NYU impactor use the Allen's method of dropping a 10 g rod from different heights with computer interface to monitor various parameters for quality control. Usage of these impactors requires intense training, extensive maintenance and sophisticated software which give more room to exclude the post-operative animals being used for the experiments. Once our goal of lesion reproducibility was attainable, it will be good enough to consider post-operative animals for further experiments. In this model, the elimination of post-operative animals will be minimal without compromising the quality and reproducibility, thereby serving the purpose of 3 R's (Reduction, Replacement and Refinement) for reducing the animal usage. Hence, in our model using customized impact device, aiming to use a simple aid to create experimental spinal cord injury in rats adopting the Allen's concept of dropping known weight from known height to induce contusive type of injury in spinal at the thoracic level shows how a simple modification perfected our attempt.
Customized instrument to create spinal cord injury
Adopting descriptions given by Allen, we prepared a metal tube having an inner diameter of 5 mm with holes drilled all along at regular intervals for the insertion of stopping pin. The metal tube was positioned over the exposed spinal cord with lower end just in contact with the spinal cord. A metal rod of 10 g weight 3 mm diameter was placed inside the tube which was stopped ahead of the spinal cord at 12.5-25 mm with the help of the pin. By withdrawing pin, the rod was allowed to drop on the spinal cord to create a contusive injury.
METHOD
However, this method was not yielding the required consistency due to the following reasons:
The tube has lost its linearity during the process of making holes all along and therefore, the rod was not sliding freely. This resulted in varying impact force
The vertebral column movement during impact nullify the force to varying extends.
To resolve these two problems, we later made two modifications as:
Instead making a long tube, a short tube of about 3-4 cm was taken with a wide diameter (about 8 mm). The impact rod was inserted through holes in the two lids which seal the tube. This entire setup was mounted to a perspex sheet in which holes were drilled at various heights. The impact rod can now be held at different heights using a pin passed through the holes in the perspex sheet which hold a small tag on the rod.
The vertebral column was held in a position using two customized clamps which pinch the spinous process cranical and caudal to the laminectomy site. Initially we used a pair of artery forceps, which was found to damage the spines and also holding vertebral column was difficult. Later, these clamps were fabricated in a local mechanical lathe.
The entire setup was mounted to a standard stereotaxic frame which now permitted the perfect orientation over the spinal cord using the adjustments available in the stereotaxic frame.
By these slight modifications, we could achieve our objective and a reproducible spinal cord injury could be created.
Validating our approach
By following standard procedures, under surgical anesthesia (ketamine 80 mg/kg + Xylazine 10 mg/kg) spinal cord in wistar rats were exposed at T10-T11 level by laminectomy with dura intact. Animals were mounted to the customized vertebral clamps and the impact rod was positioned over the spinal cord. Contusive injury was created by dropping the rod from different heights viz. 12.5 mm and 25 mm as shown in Figure 1.
Figure 1.
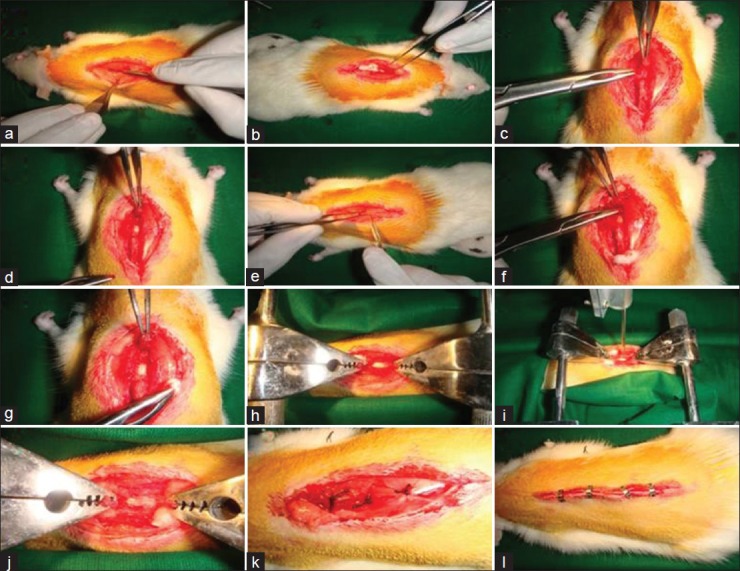
Laminectomy and contusive spinal cord injury at T10-T12 level (under Ketamine [80 mg/Kg] and xylazine [10 mg/kg]). Contusion created using customized impact device for aneathetized control and experimental animals which were grouped as per study design. Surgery was performed under highly sterilized condition with extreme post-operative care. (a - e) Vertebrae exposed; (f - h) Laminectomy; (i - j) Injury created with clamp; (k - l) Injury site was sutured and closed.
Validation on table
Proper injury creation was ensured by discoloration of the spinal cord due to internal hemorrhage and by the presence of twitches in hind limbs and/or tail. Voiding of urine due to relaxation of sphincter was also observed in some animals.
Validation during acute and chronic stages
BBB (Basso, Beattie and Bresnahan) scores of zero/one upto 3 days following lesion was taken as an indication of successful lesion. BBB scores[5] of the animals were recorded thereafter at weekly intervals up to 10 weeks. Once the animals showed sufficient grade of recovery in BBB scale, i.e., above 8-10, they were also tested in other methods such as narrow beam walking test, inclined plane balancing test and horizontal ladder walking test as per methods published earlier.
RESULTS
Our method gave consistent lesion effects as evaluated by behavior scoring methods. All the animals showed equal degree of performance in tests like narrow beam, inclined plane and horizontal ladder. BBB scores were found not only to be consistent within our group of animals but also found to be comparable with BBB scores published by other groups including those who have used sophisticated impactors.
CONCLUSION
We do not consider histology as a reliable method to evaluate lesion consistency as quantification of histological changes such as cavity formation, axonal degenerations, demyelinations etc., were extremely difficult. Also, we believe histological changes need not be exactly the same even after same degree of lesion probably due to the individual variations. Although we did not intend to replace professional impact devices to create spinal cord injury, we wish to propose that using a cost-effective approach like ours can help in pursuing spinal cord injury research provided the consistency of the lesion is established using suitable behavior methods.
ACKNOWLEDGEMENTS
The authors were gratefully acknowledges the guidance given by Dr. V. Sankar, Department of Anatomy, University of Madras, India and also acknowledges the financial support received from Lady Tata Memorial Trust as senior research fellow.
Footnotes
Source of Support: Lady Tata Memorial Trust as senior research fellow
Conflict of Interest: None declared.
REFERENCES
- 1.Anderson TE. A controlled pneumatic technique for experimental spinal cord contusion. J Neurosci Methods. 1982;6:327–33. doi: 10.1016/0165-0270(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 2.Young W. Spinal cord contusion models. Prog Brain Res. 2002;137:231–55. doi: 10.1016/s0079-6123(02)37019-5. [DOI] [PubMed] [Google Scholar]
- 3.Jakeman LB, Guan Z, Wei P, Ponnappan R, Dzwonczyk R, Popovich PG, et al. Traumatic spinal cord injury produced by controlled contusion in mouse. J Neurotrauma. 2000;17:299–319. doi: 10.1089/neu.2000.17.299. [DOI] [PubMed] [Google Scholar]
- 4.Stokes BT, Jakeman LB. Experimental modelling of human spinal cord injury: A model that crosses the species barrier and mimics the spectrum of human cytopathology. Spinal Cord. 2002;40:101–9. doi: 10.1038/sj.sc.3101254. [DOI] [PubMed] [Google Scholar]
- 5.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–56. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]


