Abstract
Interferon-alpha (IFN-α) treatment frequently induces depression, potentially leading to early dose reductions or a shorter duration of treatment, which can adversely affect outcomes, including the quality of life. Defining relevant risk factors for IFN-α induced depression is essential in order to identify prophylactic treatment strategies. We examined whether a functional polymorphism (5-HTTLPR) in the gene encoding the serotonin transporter moderates IFN-α-induced depressive symptoms in 1,015 patients with chronic hepatitis C (CHC) receiving pegylated IFN-α and ribavirin. Depressive symptoms were assessed at 0, 12, and 20 weeks of treatment. Depressive symptoms increased during antiviral treatment. 5-HTTLPR genotype moderated IFN-α-induced depressive symptoms in both Non-Hispanic Caucasians (NHCs; p = 0.009) and Hispanics (p = 0.036), though the opposite risk allele was associated with depression in the two populations. 5-HTTLPR may moderate risk for the development of depressive symptoms during IFN-α-therapy for CHC in a population-specific manner.
Keywords: depression, hepatitis C, interferon-α, serotonin transporter promoter polymorphism, 5-HTTLPR
INTRODUCTION
Infection with the hepatitis C virus (HCV), which affects over 170 million people worldwide, (1–3), is a leading cause of chronic liver disease and the main indication for liver transplantation in the United States (3, 4). Currently, the most effective and available treatment for chronic HCV infection is the combination of pegylated interferon-alpha (IFN-α) and ribavirin. This combination therapy leads to a sustained virological response in over 40% of treated patients who complete 48 weeks of therapy (5–8). However, psychiatric side effects of IFN-α, including fatigue, sleep disturbances, irritability, appetite suppression and depressed mood, are common (9, 10). In particular, depressive symptoms that arise in 20–50% of patients receiving IFN-α may lead to early dose reductions or a shorter duration of treatment. Furthermore, depressed mood can occasionally lead to potentially serious complications such as suicide attempts or need for hospitalization (9, 10). These symptoms vary directly with the dosage of interferon administered and are typically manifested over the first 12–24 weeks of therapy (11–16). One factor that may increase the risk of developing depressive symptoms during cytokine therapy is a lifetime history of depression (17). The identification of other factors that increase vulnerability to the depressive effects of IFN-α-therapy may help to identify patients who are most in need of preventive strategies, including prophylactic antidepressant therapy.
Although the pathogenesis of IFN-α-induced depressive and other neuropsychiatric symptoms is poorly understood, hypotheses proposed to explain this effect include impairment of serotonergic function. Two studies (18, 19) have shown that cytokines known to be up-regulated by IFN-α serve to down-regulate serotonin synthesis by lowering the availability of the serotonin precursor tryptophan through activation of the tryptophan-metabolizing enzyme indoleamine-2,3-dioxygenase (IDO). IDO metabolizes tryptophan and when IDO is overactive it can lead to a reduction to plasma tryptophan (Russo et al. 2003), thereby reducing the brain serotonin concentration (Heyes et al. 1992).
The serotonin transporter protein (5-HTT) appears to play a key role in the biology of depression (for reviews see refs. 25 and 26) and is the major site of action of selective serotonin reuptake inhibitors (SSRIs), the most widely used class of antidepressant medications. Indeed, several SSRIs have been shown to be efficacious in treating the depression induced by IFN-α (11, 21–24). Consequently, we hypothesized that variation in SLC6A4, which maps to chromosome 17q11.2 and encodes the 5-HTT, could moderate IFN-α-induced depressive symptoms.
The promoter region of SLC6A4 is characterized by an insertion-deletion polymorphism (5-HTTLPR), which produces a short ("S") allele, with lower transcriptional efficiency than the long ("L") allele (27). A common A→G single nucleotide polymorphism (SNP) has been identified within the insertion-deletion polymorphism, making it, in effect, tri-allelic due to the existence of two forms of the L allele: LA and LG (28, 29). The LG and S alleles have comparable levels of 5-HTT expression and both are lower than that of LA (29). The frequency distribution of these alleles in the general US population varies by ethnic/racial group and was estimated in African-Americans (AAs) to be 24%, 25% and 51% and in Non-Hispanic Caucasians (NHCs) to be 14%, 36%, and 50%, respectively for the LG, S, and LA alleles (29–31). Published data are not available concerning the genotype frequency distribution in Hispanics. To simplify the nomenclature for this polymorphism, the LG and S alleles are referred to here as S’ and the LA allele as L’ (29).
The present study examined: 1) the association between the 5-HTTLPR polymorphism and a lifetime history of depression obtained using a computerized questionnaire and standardized diagnostic criteria, 2) the relationship between the 5-HTTLPR polymorphism and baseline depressive symptoms based on a self-administered symptom score, and 3) the role of 5-HTTLPR polymorphisms and the development of IFN-induced depression as defined by a self-administered symptom score during the first 20 weeks of IFN-α and ribavirin treatment in 1,015 subjects enrolled in the HALT-C trial.
MATERIALS AND METHODS
Overview of the HALT-C Trial
The HALT-C trial is a randomized, multi-center controlled study designed to determine whether continuing interferon treatment over several years suppresses HCV, prevents progression to cirrhosis and liver cancer, and reduces the need for liver transplantation (32, 33). Inclusion criteria for the trial included detectable serum HCV RNA, a liver biopsy within 12 months of enrollment demonstrating bridging fibrosis or cirrhosis, and lack of complete response to prior IFN (± ribavirin) treatment for at least 12 weeks (32, 33). Patients with any other co-existent liver disorder, a Child-Turcotte-Pugh score >6, or a history of variceal hemorrhage, ascites, or hepatic encephalopathy were excluded. Additional exclusion criteria included intolerance to IFN, reactivity to anti-HIV, active use of illicit injection drugs, ongoing excessive alcohol consumption, a suicide attempt or hospitalization for depression within the preceding 5 years, and a history of a severe or uncontrolled psychiatric condition within the preceding 6 months, as determined by the principal investigators of the clinical sites of the trial. The clinical judgment of these investigators was based on experience treating large numbers of patients with HCV infection. The trial was longitudinal, with blood samples and mood measures collected at baseline and at 12 and 20 weeks of therapy.
During the lead-in phase of the trial, all patients were treated with pegylated IFNα2a 180 mcg/week (Pegasys®, Roche Laboratories, Nutley, NJ) and ribavirin 1.0–1.2 g/day (Copegus®, Roche Laboratories, Nutley, NJ). The duration of therapy in the lead-in phase was either 24 or 48 weeks. If patients did not show a complete response to the treatment at 20 weeks (HCV RNA still detectable in serum), the lead-in treatment was stopped at 24 weeks, and they were invited to enter the randomized phase of the trial. If patients showed a full response at 20 weeks, the lead-in treatment was continued.
Study Sample
The HALT-C study and associated consent forms were approved by the National Institute of Diabetes and Digestive and Kidney Disease, and institutional review boards, General Clinical Research Centers, and other regulatory bodies within the participating centers. The study was conducted according to the principles of the Declaration of Helsinki regarding the proper procedures for human research. All subjects participating in this study signed individual informed consents for the HALT-C trial. The HALT-C trial is registered with NIH (NCT00006164).
Pretreatment data on psychiatric disorders, longitudinal data on depressive symptoms, and a blood sample for genetic analysis were obtained from 1,042 individuals treated during the lead-in phase of the HALT-C Trial. Of this number, 27 patients were not classified into one of the three major population groups: Non-Hispanic Caucasian (NHC), African American (AA), and Hispanic. Consequently, the sample examined in the present study consisted of 1,015 individuals; nearly three-quarters were NHCs (n=776), with smaller groups of AAs (n=154) and Hispanics (n=85).
Baseline Assessment
Demographic and clinical characteristics including age, gender, years of education, occupation, and baseline psychotropic medication usage were recorded at baseline. Race/ethnicity was based on self-report. Lifetime psychiatric history was obtained using the self-administered, computerized version of the Composite International Diagnostic Interview (CIDI; (34) to categorize subjects as having experienced an anxiety disorder, depressive disorder, alcohol abuse or dependence, or drug abuse or dependence. A semi-quantitative estimate of lifetime alcohol consumption was obtained using an adaptation of the Lifetime Drinking History (35).
To measure depressive symptoms, the Beck Depression Inventory-II (BDI-II) (36), a 21–item, self-administered questionnaire, was administered at baseline and at 12 and 20 weeks of treatment. Each item is scaled from 0–3 and a total score is calculated by summing the responses to the individual items (range: 0–63). For analytical purposes, BDI-II scores were coded as no depression ≤ 10; minimal depression 11–14; mild depression 15–19; moderate depression 20–28; and severe depression ≥ 29 (36).
5-HTTLPR Genotyping
We genotyped the 5-HTTLPR tri-allelic insertionA/insertionG/deletion polymorphism using a two-stage TaqMan™ 5’nuclease allelic discrimination assay modified from that originally described by Hu et al. (29, 37). This method identifies the presence of the 14- vs. the 16-repeat variable number of tandem repeats [short (S) vs. long (L)], as well as two subtypes of the 16-repeat variant, LA, and LG, which are the products of the A→G SNP present at the sixth nucleotide of the first of two of the 23-bp repeat elements present in the 16-repeat L allele (28). Both the S and LG alleles have a 2-fold lower level of gene expression than the A allele variant (LA) (29).
Twenty-five µL PCR reactions contained 200 nM each of forward and reverse primers (5’ GCAACCTCCCAGCAACTCCCTGTA-3’ and 5’ GAGGTGCAGGGGGATGCTGGAA-3’), 1M Betaine, 1× ABI TaqMan Universal master mix (Applied Biosystems Inc., Foster City, CA), 25 ng genomic DNA, 120nM of an L allele specific Fam-labeled probe (6FAM-TGCAGCCCCCCCAGCATCTCCC-MGB) and 60 nM of a Vic-labeled internal control probe (VIC-TCCCCCCCTTCACCCCTCGCGGCATCC-MGB) whose target is present in the 5-HTTLPR region adjacent to the L-specific insertion, which served to distinguish the L vs. S insertion/deletion status. Samples were heated to 95°C for 10 minutes, followed by 40 thermal cycles of 98°C for 15 sec, followed by 62.5°C for 90 sec. The number of L alleles (0, 1, or 2) for each patient was identified by examination of scatter plots of endpoint Fam vs. Vic fluorescence levels captured using an ABI 7500 Sequence Detection System. A second TaqMan™ 5’nuclease allelic discrimination assay served to distinguish LA vs. LG alleles by using the same primers and amplification conditions as for the L vs. S allele assay but using LA vs. LG allele-specific probes, (6FAM-CCCCCCTGCACCCCCAGCATCCC-MGB and VIC-CCCCTGCACCCCCGGCATCCCC-MGB, respectively). We validated the closed-tube fluorescent assay of 5-HTTLPR L vs. S allele by comparing results obtained for 492 samples using this 5’nuclease TaqMan assay with those from a traditional 5-HTTLPR agarose gel-based PCR fragment length assay, with 100% agreement between methods. Additionally, we sequenced 8 samples for each of the genotypes (LA/LA, LA/LG and LG/LG) with 100% agreement between direct sequencing and the TaqMan LA vs. LG assay. We did not observe the G allele in samples from S allele homozygotes, consistent with the findings of Hu et al. (29).
Statistical Analysis
Prior to analysis, the distribution of data was examined to determine whether transformation was required to support the assumption of normality, so that parametric analytic methods could be used. Consistent with Hu et al. (29), genotypes were reclassified according to their level of expression as follows: LG/LG, LG/S, and S/S were designated as S’S’ (low expression levels), LA/S and LA/LG were designated as L’S’ (intermediate expression levels), and LA/LA was designated as L’L’ (high expression levels).
We examined the frequency and correlates of lifetime and current major depression and their association with 5-HTTLPR alleles. We also used the proc mixed procedure in SAS (38) to examine the relations between genotype (with the number of S’ alleles being a three-level variable: 0, 1, or 2) and scores on the BDI, with a lifetime depression diagnosis that was made using the CIDI (a two-level variable: present or absent) as a factor in the analysis. Using proc genmod with the binomial distribution and logit link function, we also modeled current antidepressant usage (as a dichotomous variable) as the dependent variable, since this would be expected to vary during the treatment trial. It should be noted that proc mixed and genmod, in contrast to repeated measures ANOVA, are robust to missing observations and to variation in the interval between repeated measures. The time points for these analyses were 0, 12, and 20 weeks, corresponding to the baseline and two follow-up time points during treatment with IFN-α and ribavirin. Because the trajectory of BDI scores was not linear, time was treated as a three-level class variable. In contrast, given the linear trajectory of antidepressant usage, time was treated as a continuous variable in the analysis of that outcome measure. Any significant interactions in these models were followed up with specified contrasts to decompose the interaction. The impact of sex was also examined in all analyses, but was not a significant factor and, therefore, was not retained in the final models.
RESULTS
Demographic and Clinical Characteristics of the Study Sample (Table 1)
Table 1.
Demographic, Clinical Characteristics and Genotypes of the Study Sample by Race/Ethnicity (N=1,015)
| NHC (n=776) |
AA (n=154) |
Hispanic (n=85) |
p-value | |
|---|---|---|---|---|
| Sex (%) | ||||
| Male | 75.4 | 58.4 | 71.8 | <0.001 |
| Female | 24.6 | 41.6 | 28.2 | |
| Age [yr; mean (SD)] | 49.6 (7.1) | 51.8 (6.6) | 49.5 (7.9) | 0.001 |
| Education (%) | ||||
| Did not complete high school | 7.7 | 11.2 | 27.7 | <0.001 |
| High school | 30.1 | 34.9 | 27.7 | |
| Some college | 34.1 | 38.2 | 25.3 | |
| College degree | 19.7 | 10.5 | 14.5 | |
| Graduate school | 8.4 | 5.3 | 4.8 | |
| Marital Status (% married) | 72.6 | 55.2 | 70.2 | <0.001 |
| Working Status (% employed) | 77.0 | 69.9 | 72.6 | 0.14 |
| Duration of HCV Infection [yr; mean (SD)] | 27.9 (7.7) | 28.9 (9.0) | 29.2 (9.1) | 0.20 |
| Baseline Laboratory Values [mean (SD)] | ||||
| Serum albumin (g/dL) | 3.92 (0.40) | 3.79 (0.38) | 3.76 (0.40) | <0.001 |
| Serum total bilirubin (mg/dL) | 0.80 (0.44) | 0.76 (0.37) | 0.81 (0.29) | 0.49 |
| Platelet count (per L × 103) | 168 (64.8) | 183 (65.2) | 152 (60.4) | 0.001 |
| Log HCV RNA | 6.44 (0.53) | 6.35 (0.54) | 6.25 (0.50) | 0.002 |
| Co-morbid Conditions (%) | ||||
| Anxiety Disorder (Lifetime) | 13.4 | 19.6 | 11.3 | 0.17 |
| Major Depression (Lifetime) | 13.7 | 16.1 | 16.9 | 0.66 |
| Alcohol Use Disorder (Lifetime) | 57.5 | 42.0 | 53.5 | 0.010 |
| Drug Use Disorder (Lifetime) | 43.1 | 42.9 | 40.8 | 0.94 |
| Cirrhosis | 37.2 | 34.4 | 57.6 | 0.001 |
| Diabetes mellitus | 13.1 | 33.8 | 20.0 | <0.001 |
| Systemic Arterial Hypertension | 30.2 | 59.1 | 25.9 | <0.001 |
| Genotype (%) | ||||
| L’L’ | 24.1 | 24.7 | 24.7 | 0.072 |
| L’S’ | 50.3 | 57.1 | 41.2 | |
| S’S’ | 25.6 | 18.2 | 34.1 |
NHC = Non-Hispanic Caucasian
P values compare results among the three groups
The sample was predominantly male (73%), moderately educated (over 90% with a high school degree and over 25% with a college degree), and mostly married (70%) and employed (76%). The mean age of the sample was 49.9 yr (SD=7.0), with a mean duration of chronic HCV infection of 28.2 years (SD=8.0). The most common co-occurring diseases were hepatic cirrhosis (39%), diabetes mellitus (23%), and systemic arterial hypertension (33%). Based on the CIDI, 13.6% of the sample met lifetime criteria for a major depressive disorder, with no difference in frequency by race or ethnicity (χ2(2)=0.99, p=.61). However, as shown in Table 1, NHCs, AAs, and Hispanics differed significantly on several demographic and clinical variables. In view of the pretreatment differences and genetic heterogeneity (see below), we conducted analyses involving genotype separately by population.
Genotypes of the Serotonin Transporter Promoter Polymorphism
The genotype distribution in all groups was consistent with Hardy-Weinberg equilibrium expectations (NHCs: χ2(2)=0.034, p=0.85; AAs: χ2(2)=3.09, p=0.079; Hispanics: χ2(2)=2.43, p=0.12). The frequency of the 5-HTTLPR S’ allele among NHCs, AAs, and Hispanics was 50.8%, 46.7%, and 54.7%, respectively. These differences did not reach statistical significance when examined as a three-level variable (χ2(4) = 8.35, p=0.072). However, given the imbalance in the size of the sub-samples, we conducted pair-wise comparisons, which showed a significantly greater frequency of the S’ allele among Hispanics than AAs (χ2(2) = 8.33, p=0.016). The frequency of the S’ allele did not differ significantly between NHCs and either AAs (χ2(2) = 3.84, p=0.14) or Hispanics (χ2(2) = 3.41, p=0.18). The genotype distribution among NHCs and AAs also did not differ significantly from the published frequencies for these racial/ethnic groups [χ2(2) =3.97, p=0.14 and χ2(2) = 5.11, p=0.078, respectively] (Hu et al. 2007, Roy et al. 2007). We did not find published allele frequencies for Hispanics.
Tables 2–4 show the pretreatment psychiatric and substance abuse histories for each genotype group by population. Among NHCs, although there was no difference between genotype groups in the percentage of subjects using any antidepressant (χ2(2)=2.24, p=.32), there was a significant difference by genotype group in the use of the SSRI subtype of antidepressants (χ2(2)=6.41, p=.041). Specifically, L’ homozygotes were least likely, and heterozygotes most likely to have been treated with an SSRI (Table 2). There were no associations between antidepressant use at baseline and genotype in either the AA or Hispanic groups (Tables 3 and 4, respectively).
Table 2.
Psychiatric and Substance Abuse History Among Non-Hispanic Caucasians (N=776)
| 5-HTTLPR Genotype | ||||
|---|---|---|---|---|
| L’L’ (n=187) |
L’S’ (n=391) |
S’S' (n=199) |
p-value | |
| Lifetime Anxiety Disorder (%) | 14.5 | 12.2 | 14.8 | 0.67 |
| Lifetime Major Depression (%) | 13.0 | 14.1 | 13.5 | 0.95 |
| Baseline Depressive Symptoms | ||||
| BDI score [mean, (SD)] | 7.01 (6.9) | 7.12 (7.7) | 8.18 (7.9) | 0.21 |
| Baseline Severity of Depression (%) | 74.9 | 78.1 | 71.2 | 0.24 |
| None (BDI<=10) | 12.3 | 8.5 | 10.1 | |
| Minimal (BDI=11–14) | 4.8 | 6.4 | 11.1 | |
| Mild (BDI=15–19) | 6.4 | 4.4 | 5.1 | |
| Moderate (BDI=20–29) | 1.6 | 2.6 | 2.5 | |
| Severe (BDI>=30) | ||||
| Lifetime Alcohol Use Disorder (%) | 53.4 | 57.2 | 61.3 | 0.41 |
| Lifetime Drug Use Disorder (%) | 47.3 | 42.4 | 40.6 | 0.50 |
| Medications at Baseline (% of patients) | ||||
| Anxiolytics | 11.2 | 14.4 | 15.6 | 0.44 |
| SSRI Antidepressants | 15.0 | 23.3 | 17.6 | 0.041 |
| Other Antidepressants | 13.4 | 10.8 | 17.1 | 0.097 |
Table 4.
Psychiatric and Substance Abuse History Among Hispanics (N=85)
| 5-HTTLPR Genotype | ||||
|---|---|---|---|---|
| L’L’ (n=21) |
L’S’ (n=35) |
S’S’ (n=29) |
p-value | |
| Lifetime Anxiety Disorder (%) | 12.5 | 12.9 | 8.3 | 0.85 |
| Lifetime Major Depression (%) | 25.0 | 16.1 | 12.5 | 0.58 |
| Baseline Depressive Symptoms | ||||
| Mean (SD) BDI score | 8.48 (7.7) | 8.14 (7.1) | 8.46 (7.5) | 0.98 |
| Baseline Severity of Depression (%) | 0.46 | |||
| None (BDI<=10) | 66.7 | 71.4 | 67.9 | |
| Minimal (BDI=11–14) | 19.0 | 8.6 | 7.1 | |
| Mild (BDI=15–19) | 9.5 | 8.6 | 14.3 | |
| Moderate (BDI=20–29) | 0.0 | 11.4 | 10.7 | |
| Severe (BDI>=30) | 4.8 | 0.0 | 0.0 | |
| Lifetime Alcohol Use Disorder (%) | 62.5 | 48.4 | 54.2 | 0.65 |
| Lifetime Drug Use Disorder (%) | 50.0 | 38.7 | 37.5 | 0.70 |
| Medications at Baseline (% of patients) | ||||
| Anxiolytics | 14.3 | 5.7 | 0.0 | 0.11 |
| SSRIs Antidepressants | 9.5 | 17.1 | 20.7 | 0.57 |
| Non-SSRIs Antidepressants | 14.3 | 17.1 | 17.2 | 0.95 |
Table 3.
sychiatric and Substance Abuse History Among African Americans (N=154)
| 5-HTTLPR Genotype | ||||
|---|---|---|---|---|
| L’L’ (n=38) |
L’S’ (n=87) |
S’S’ (n=28) |
p-value | |
| Lifetime Anxiety Disorder (%) | 21.4 | 20.6 | 14.3 | 0.79 |
| Lifetime Major Depression (%) | 17.9 | 19.0 | 4.8 | 0.29 |
| Baseline Depressive Symptoms | ||||
| BDI score [mean, (SD)] | 8.29 (7.1) | 7.89 (6.9) | 5.96 (4.7) | 0.33 |
| Baseline Severity of Depression (%) | 73.7 | 72.7 | 82.1 | 0.38 |
| None (BDI<=10) | 13.2 | 8.0 | 10.7 | |
| Minimal (BDI=11–14) | 0.0 | 9.1 | 7.1 | |
| Mild (BDI=15–19) | 13.2 | 10.2 | 0.0 | |
| Moderate (BDI=20–29) | 0.0 | 0.0 | 0.0 | |
| Severe (BDI>=30) | ||||
| Lifetime Alcohol Use Disorder (%) | 42.9 | 41.3 | 42.9 | 0.99 |
| Lifetime Drug Use Disorder (%) | 50.0 | 36.5 | 52.4 | 0.30 |
| Medications at Baseline (% of patients) | ||||
| Anxiolytics | 10.5 | 3.4 | 3.6 | 0.23 |
| SSRIs Antidepressants | 15.8 | 15.9 | 7.1 | 0.49 |
| Non-SSRIs Antidepressants | 10.5 | 14.8 | 0.0 | 0.094 |
Effects of IFN-α Treatment on Depressive Symptoms
Using a score of ≥11 on the BDI as indicative of current depression, about one-quarter of the sample (25.3%) was depressed at baseline, which did not differ by ethnic or racial group (χ2(2)=1.75, p=0.42). More than 60% of the sample reported increased BDI scores during treatment, with the frequency of IFN-α-induced depression differing by population. After 12 weeks of antiviral therapy, NHCs showed a significantly higher rate (39%) of current depression than AAs (29%; χ2(1)=5.26, p=0.022) or Hispanics (28%; χ 2(1)=4.24, p=0.040). At 20 weeks, however, the prevalence of current depression decreased slightly from 12 weeks in both NHCs (37%) and AAs (28%), but increased slightly in Hispanic patients (31%), such that the differences between populations were no longer statistically significant (NHCs vs. AAs: χ2(1)=3.76, p=0.052; NHCs vs. Hispanics: χ2(1)=0.92, p=0.34). Differences over time that were seen in the prevalence of current depression do not appear to be explicable on the basis of differential attrition, since patients with elevated BDI scores were not significantly more likely to leave treatment prematurely than those with lower BDI scores among NHCs (χ2(1)=3.23, p=0.072), AAs (Fisher’s exact test: p=0.29), or Hispanics (Fisher’s exact test: p=0.10). It should also be noted that there was no significant difference at week 12 (p=0.49) or week 20 (p=0.91) in the discontinuation rate among the three genotype groups.
Effects of 5-HTTLPR Genotype and Lifetime Depression Diagnosis on Depressive Symptoms as a Function of Antiviral Treatment
Non-Hispanic Caucasians
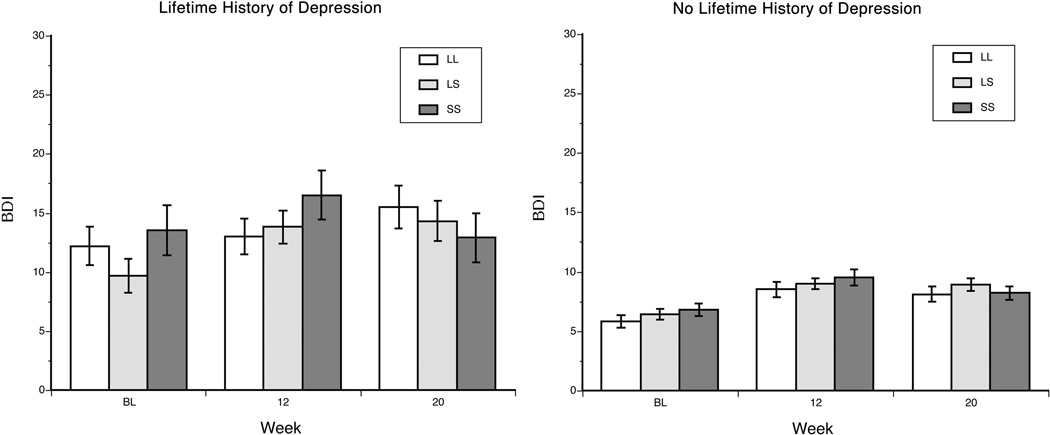
Among NHCs, during the 20 weeks of the study, individuals with a lifetime diagnosis of major depression had higher mean depressive symptom scores [mean baseline BDI score=13.1 (SD=8.9)] than those without such a diagnosis [mean baseline BDI score=8.0 (SD=7.0)] [F(1,591)=16.99, p<0.001]. In addition, depression scores increased significantly [F(2,1097)=14.57, p<0.001] following the initiation and maintenance of IFN-α treatment [mean BDI scores: 7.4 (SD=7.4), 10.1 (SD=7.9), and 9.9 (SD=8.0) at baseline, week 12, and week 20, respectively]. There was a trend for an interaction of lifetime depression diagnosis by time [F(2,1097)=2.53, p=0.081], reflecting a marginally greater increase in depressive symptoms during IFN-α treatment in patients with a lifetime depression diagnosis. A significant three-way interaction of lifetime depression diagnosis × 5-HTTLPR genotype × time was also evident [F(2,1097)=3.00, p=0.050]. Further analysis of the interaction revealed that the effects were limited to the subgroup with a lifetime diagnosis of major depression. In this subgroup, although BDI scores increased equally across genotype groups from baseline to week 12, S’ homozygotes showed a decrease, heterozygotes showed no change, and L’ homozygotes showed a modest increase in depressive symptoms from weeks 12 to 20 [F(1,1097)=5.89, p=0.015].
African Americans
Among AAs, individuals with a lifetime diagnosis of major depression had significantly higher mean baseline BDI scores than those without such a diagnosis [11.6 (SD=6.6) and 8.0 (SD=7.0), respectively] [F(1,108)=5.33, p=0.023]. In this group, there were no significant interactions of lifetime depression, genotype, or time, although the interaction of lifetime depression diagnosis × 5-HTTLPR genotype × time showed a trend towards statistical significance [F(2,200)=2.17, p=0.12].
Hispanics
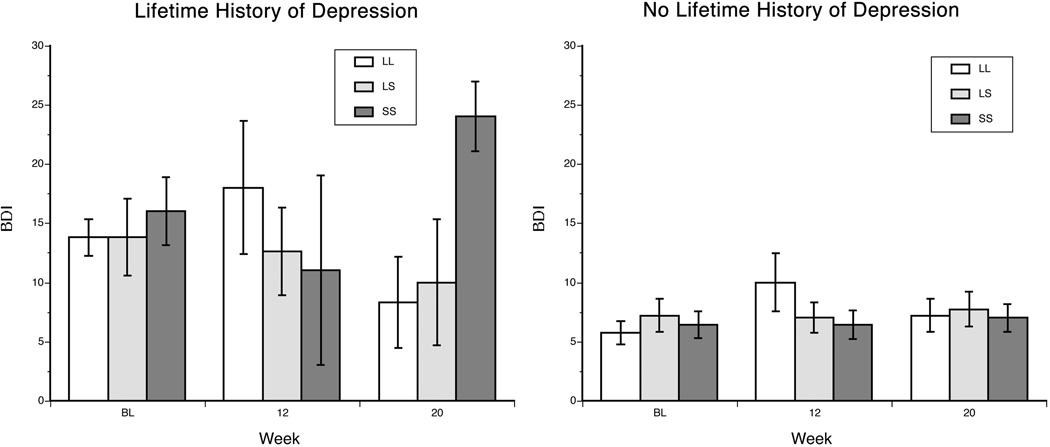
Among Hispanics, there was a trend for a main effect of lifetime major depression on BDI scores [F(1,68)=3.45, p=0.068]. There was also a trend for an interaction of lifetime major depression diagnosis × time [F(2,126)=2.80, p=0.065], such that patients with the diagnosis showed a greater increase in BDI at weeks 12 and 20 (i.e., during IFN-α treatment) than those who were never depressed. In this population, there was also a significant three-way interaction of depression × 5-HTTLPR genotype × time [F(2,126)=4.33, p=0.015]. Further analysis revealed that the effects were present only in the subgroup with a lifetime history of major depression. In this subgroup, there were no significant changes in BDI score from baseline to 12 weeks as a function of genotype. However, from weeks 12 to 20, S’ homozygotes showed a large increase, heterozygotes showed a slight decrease and L’ homozygotes showed a large decrease in depression scores (Figure 2).
Figure 2.
Estimated mean (SEM) BDI score by 5-HTTLPR genotype, stratified by lifetime diagnosis of major depression in the Hispanic sample. BDI = Beck Depression Inventory, L’L’ = L’ allele homozygotes, L’S’= heterozygotes, and S’S’= S’ allele homozygotes. BL = at baseline, prior to IFN therapy
Antidepressant Usage as a Function of Time and Lifetime Depression Diagnosis
Table 5 shows the percentage of subjects in each of the three population groups who were receiving antidepressant therapy at each time point, by history of lifetime depression. The frequency of use of antidepressants was high at baseline, especially in those with a history of depression, and it rose significantly over time (i.e., with antiviral treatment; Z = 3.81, p = 0.0001) and as a function of a lifetime diagnosis of depression (Z=3.49, p=0.0005). However, these factors did not interact significantly (Z=1.1, p=0.26), nor did the effects differ by population group [χ2(2)=0.35, p=0.84].
Table 5.
Antidepressant Usage (% of Patients) By Ethnic/Racial Group, Diagnosis of Lifetime Depression, and Time on Interferon Therapy
| Lifetime Depression | No Lifetime Depression | |
|---|---|---|
| Non-Hispanic Caucasian | ||
| Baseline | 65.9 | 29.0 |
| Week 12 | 76.6 | 47.4 |
| Week 20 | 82.2 | 52.0 |
| African-American | ||
| Baseline | 55.6 | 17.0 |
| Week 12 | 66.7 | 25.6 |
| Week 20 | 70.6 | 28.7 |
| Hispanic | ||
| Baseline | 83.3 | 18.3 |
| Week 12 | 90.9 | 38.3 |
| Week 20 | 100 | 43.1 |
DISCUSSION
In this study, we examined changes in IFN-α-induced depressive symptom levels in patients treated with pegylated IFN-α for HCV infection as a function of both a lifetime diagnosis of depression and a functional polymorphism (5-HTTLPR) in the gene encoding the serotonin transporter. Consistent with other studies (10, 11, 15, 39, 40), we found that over 60% of the sample experienced a worsening of their depressive symptoms following the initiation of antiviral therapy. Also, consistent with the increase in depressive symptoms, the percentage of patients receiving antidepressant medication increased over time. In addition, we found that the likelihood of developing IFN-α-induced depression differed by population, at least during the initial 12 weeks of treatment. During this time, NHCs showed higher rates of treatment-associated depressive symptoms than either AAs or Hispanics. To our knowledge, this is the first study comparing the prevalence of IFN-α-induced depression across racial/ethnic groups. It should be noted that the population differences with respect to the increase in depressive symptoms could not be attributed to a population difference in BDI score at baseline, attrition rates, or the rates of antidepressant use.
The observation that a lifetime diagnosis of depression was associated with a greater likelihood of antidepressant treatment during antiviral therapy is consistent with the findings of Capuron and Miller (17). These investigators found that patients exhibiting sub-syndromal levels of depression prior to cytokine therapy were more likely to develop pronounced depressive illness in response to treatment with the cytokine.
Interestingly, the populations differed with respect to the moderating effect of 5-HTTLPR genotype on risk of depressive symptoms during antiviral treatment. There was no effect of this polymorphism in AA patients, which was the smallest sub-sample. Although in both NHCs and Hispanics, the 5-HTTLPR polymorphism increased the risk of developing IFN-induced depressive symptoms, the effects were opposite in direction in the two groups. In the NHC sample, L’ allele homozygotes showed greater depressive symptoms during IFN-α therapy than did S’ allele carriers. In contrast, among Hispanics, although L’ allele homozygotes initially showed a worsening of mood symptoms compared to S’ allele carriers, subsequently, S’ allele homozygotes reported much higher depression symptom scores. One possible explanation for the difference between NHCs and Hispanics is the small number of Hispanics (only about 20% as many as NHCs) on which the findings are based, with disproportionate effects contributed by a few individuals. Alternatively, there may be real population differences in the moderating role of this polymorphism. Further research is warranted to address this question. Given what appears to be a complex interaction of 5-HTTLPR genotype with IFN-α-induced depression, statistical power is a key consideration for subsequent studies to take into consideration.
Statistical power considerations may help to explain findings reported recently by Kraus et al. (41). These investigators studied the 5-HTTLPR polymorphism in 139 German patients with HCV during treatment with IFN-α and ribavirin. They found no effect of that polymorphism on treatment-induced depressive symptoms, though a polymorphism in HTR1A, which encodes the 5-HT1A receptor was a significant predictor. Recently, Bull et al. (2008) reported a small protective effect of variation in SLC6A4 in 98 Caucasian patients treated with IFN-α, with the LL genotype being associated with a less marked increase in depressive symptoms. In this study, a polymorphism in the gene encoding IL-6 showed a more robust effect. In addition to the fact that the sample studied by these investigators was comparatively small, they did not genotype the SNP that has been identified within the insertion-deletion polymorphism studied here (i.e., the tri-allelic polymorphism), which limits the comparability of the two studies. Further study of the tri-allelic polymorphism in the promoter region of the gene encoding 5-HTT appears warranted.
The mechanism underlying IFN-α-induced mood disorders remains poorly understood. There are a number of pathways by which IFN-α may cause neuropsychiatric complications, one of which is the depletion of tryptophan (14, 24, 42), which results in reduced serotonin function. Capuron et al. (42) tested this hypothesis in 26 patients with malignant melanoma who were randomly assigned to receive the SSRI antidepressant paroxetine or placebo, beginning 2 weeks before and continuing for 12 weeks after initiation of IFN-α therapy. Paroxetine reduced depressive symptoms despite significant IFN-α-induced increases in plasma kynurenine and neopterin concentrations, as well as in the kynurenine/tryptophan ratio (42), consistent with findings previously described in patients with chronic HCV infection who were treated with IFN-α (15). These findings suggest that reduced tryptophan availability plays a role in IFN-α-induced depressive symptoms, and that paroxetine, although not altering the increased concentrations of kynurenine or neopterin, attenuates the behavioral consequences of IFN-α-mediated tryptophan depletion (42).
Additionally, interferon treatment may modulate 5-HTT activity levels. In human, placental choriocarcinoma cells, a 3-hour treatment with IFN-α or IFN-γ increased levels of 5-HTT mRNA, an effect that was blocked by actinomycin D, an inhibitor of transcription (43). Treatment with IFN-α or IFN-γ for 3–6 h, but not for 30 min, also increased 5-HTT uptake activity. These data are consistent with the hypothesis that IFN-induced psychiatric effects may be modulated by regulation of 5-HTT transcription. Treatment with IFN-α in humans increased 5-HTT expression, protein level and serotonin uptake, suggesting that increases in 5-HTT in circulating blood lymphocytes after IFN-α therapy may decrease levels of circulating serotonin, thereby causing depressive symptoms (44).
Pre-treatment with an SSRI is a potentially useful strategy to prevent IFN-α-induced depressive symptoms (45). In addition to its moderating effects on IFN-α-induced depressive symptoms, several pharmacogenetic studies have shown that the 5-HTTLPR polymorphism moderates the response to SSRI antidepressants. Individuals with the LL genotype show an earlier and more robust response to SSRI treatment of major depression than is observed in individuals with the LS or SS genotype (46–51). Pollock et al. (49) found that elderly depressed L homozygotes had a greater response to paroxetine than individuals with the LS or SS genotype, an effect that was absent in patients treated with nortriptyline, suggesting that the differential response based on 5-HTTLPR genotype is unique to SSRI antidepressants.
Although depressive symptoms and other adverse effects limit treatment of HCV infection with IFN-α, Loftis et al. (52) showed that the rate of response to IFN-α treatment was significantly higher in patients who developed IFN-α-induced depression than in those who did not. This suggests that IFN-α-induced depression may be a predictor of a positive response to IFN-α therapy, or an indicator of optimal dosing or of greater susceptibility to biological responses to IFN-α. The dataset examined in our report included data only from the lead-in phase of the HALT-C trial, with information on treatment response limited to an examination of viral clearance by week 20. Unlike Loftis et al. (52) we did not observe an association between a change in treatment emergent depression and viral response during the 20-week study period. Subjects who achieved a viral response by week 20 did not have a greater increase in BDI during treatment (data not shown). Similiarly, subjects with a clinically significant increase in BDI (i.e., an increase ≥ 11 points) after the first 12 weeks of treatment were no more likely to have a negative HCV viral serum test result at week 20 than the other subjects for whom these data were obtained.
The data presented here support a moderating role of the serotonin transporter promoter polymorphism of 5-HTTLPR in IFN-α-induced depressive symptoms, although the pattern of findings is complex. The small size of the AA and Hispanic sub-samples limits confidence in the findings for those groups. The use of self-reported race/ethnicity, rather than ancestry informative markers (53) may have resulted in some incorrect assignments to population group. Further, the HALT-C trial was not designed to evaluate the genetic moderator hypothesis and patients with a history of suicide attempt or hospitalization for depression within the preceding five years were excluded. Thus, it is likely to have underestimated the level of IFN-α-induced depressive symptoms and could have underestimated the moderating role of the 5-HTTLPR polymorphism in those symptoms. Because antidepressant treatment and other factors directly relevant to the hypotheses were not controlled, these may also have confounded the results. The fact that homozygous subjects’ showed a greater likelihood of having been treated with an antidepressant may represent a chance finding. A prospective study in a larger, less select sample is needed to determine the validity of these findings.
If confirmed, these results could have important clinical implications, in that it could be possible to identify patients who are at increased risk to develop depressive symptoms with this antiviral therapy. This may be important both for the therapeutic response to IFN-α therapy (52) and patients’ capacity to tolerate the medication. Improved accuracy of risk assessment may enhance the potential therapeutic effects of IFN-α therapy without subjecting individuals who are not predisposed to depression to unnecessary antidepressant prophylaxis, which carries with it unwanted adverse effects and costs.
Figure 1.
Estimated mean (SEM) BDI score by 5-HTTLPR genotype, stratified by lifetime diagnosis of major depression in the non-Hispanic Caucasian sample. BDI = Beck Depression Inventory, L’L’ = L’ allele homozygotes, L’S’= heterozygotes, and S’S’= S’ allele homozygotes. BL = at baseline, prior to IFN therapy
Acknowledgment
This study was supported by the National Institute of Diabetes & Digestive & Kidney Diseases (contract numbers are listed below). Additional support was provided by the National Institute of Allergy and Infectious Diseases, the National Cancer Institute, the National Center for Minority Health and Health Disparities, the National Center for Research Resources (grant numbers are listed below), the National Institute on Alcohol Abuse and Alcoholism grant K24 AA13736, and by Hoffmann-La Roche, Inc. through a Cooperative Research and Development Agreement (CRADA) with the National Institutes of Health.
In addition to the authors of this manuscript, the following individuals were instrumental in the planning, conduct and/or care of patients enrolled in this study at each of the participating institutions as follows:
University of Massachusetts Medical Center, Worcester, MA: (Contract N01-DK-9-2326) Gyongyi Szabo, MD, Maureen Cormier, RN, Donna Giansiracusa, RN
University of Connecticut Health Center, Farmington, CT: (Grant M01RR-06192) Michelle Kelley, RN, ANP
Saint Louis University School of Medicine, St Louis, MO: (Contract N01-DK-9-2324) Adrian M. Di Bisceglie, MD, Bruce Bacon, MD, Brent Neuschwander-Tetri, MD, Debra King, RN
Massachusetts General Hospital, Boston, MA: (Contract N01-DK-9-2319, Grant M01RR-01066), Jules L. Dienstag, MD, Andrea E. Reid, MD, Raymond T. Chung, MD, Wallis A Molchen
University of Colorado School of Medicine, Denver, CO: (Contract N01-DK-9-2327, Grant M01RR-00051) Gregory T. Everson, MD, Jennifer DeSanto, RN, Carol McKinley, RN
University of California - Irvine, Irvine, CA: (Contract N01-DK-9-2320, Grant M01RR-00827) Timothy R. Morgan, MD, John C. Hoefs, MD, Choon Park, RN
University of Texas Southwestern Medical Center, Dallas, TX: (Contract N01-DK-9-2321, Grant M01RR-00633) William M. Lee, MD, Janel Shelton, Nicole Crowder, LVN, Rivka Elbein, RN, BSN
University of Southern California, Los Angeles, CA: (Contract N01-DK-9-2325, Grant M01RR-00043) Karen L. Lindsay, MD, MMM, Carol B. Jones, RN, Susan L. Milstein, RN
University of Michigan Medical Center, Ann Arbor, MI: (Contract N01-DK-9-2323, Grant M01RR-00042) Anna S.F. Lok, MD, Pamela A. Richtmyer, LPN, CCRC
Virginia Commonwealth University Health System, Richmond, VA: (Contract N01-DK-9-2322, Grant M01RR-00065) Mitchell L. Shiffman, MD, Charlotte Hofmann, RN, Paula Smith, RN
National Institute of Diabetes and Digestive and Kidney Diseases, Liver Disease Branch, Bethesda, MD: Marc G. Ghany, MD, T. Jake Liang, MD, Yoon Park, RN, Elenita Rivera, RN, Vanessa Haynes-Williams, RN
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD: James E. Everhart, MD, Leonard B. Seeff, MD, Patricia R. Robuck, PhD, Jay H. Hoofnagle, MD, Elizabeth C. Wright, PhD
University of Washington, Seattle, WA: (Contract N01-DK-9-2318), Chihiro Morishima, MD, David R. Gretch, MD, Minjun Chung, BS, ASCP
New England Research Institutes, Watertown, MA: (Contract N01-DK-9-2328) Latha Padmanabhan, MS, Teresa M. Curto, MPH, Linda J. Massey
Armed Forces Institute of Pathology, Washington, DC: Zachary D Goodman, MD
Data and Safety Monitoring Board Members: (Chair) Gary L. Davis, MD, Guadalupe Garcia-Tsao, MD, Michael Kutner, PhD, Stanley M. Lemon, MD, Robert P. Perrillo, MD
Footnotes
Financial disclosures
Financial relationships of the authors with Hoffmann-La Roche, Inc., are as follows: R.K. Sterling is a consultant, on the speaker's bureau, and receives research support; R.J. Fontana is on the speaker’s bureau. Authors with no financial relationships related to this project are: A. Pierucci-Lagha, J. Covault, H.L. Bonkovsky, R. Feinn, C. Abreu, and H.R. Kranzler.
REFERENCES
- 1.Alter MJ. Epidemiology of hepatitis C in the West. Semin Liver Dis. 1995;15:5–14. doi: 10.1055/s-2007-1007259. [DOI] [PubMed] [Google Scholar]
- 2.Alter MJ. Epidemiology of hepatitis C. Hepatology. 1997;26:62S–65S. doi: 10.1002/hep.510260711. [DOI] [PubMed] [Google Scholar]
- 3.Davis GL. Current treatment for chronic hepatitis C. Rev Gastroenterol Disord. 2001;1:59–72. [PubMed] [Google Scholar]
- 4.Alter MJ, Mast EE. The epidemiology of viral hepatitis in the United States. Gastroenterol Clin North Am. 1994;23:437–455. [PubMed] [Google Scholar]
- 5.Poynard T, Leroy V, Cohard M, Thevenot T, Mathurin P, Opolon P, Zarski JP. Meta-analysis of interferon randomized trials in the treatment of viral hepatitis C: effects of dose and duration. Hepatology. 1996;24:778–789. doi: 10.1002/hep.510240405. [DOI] [PubMed] [Google Scholar]
- 6.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 7.Zeuzem S, Feinman SV, Rasenack J, Heathcote EJ, Lai MY, Gane E, O'Grady J, Reichen J, Diago M, Lin A, Hoffman J, Brunda MJ. Peginterferon alfa-2a in patients with chronic hepatitis C. N Engl J Med. 2000;343:1666–1672. doi: 10.1056/NEJM200012073432301. [DOI] [PubMed] [Google Scholar]
- 8.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, Haussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 9.Zdilar D, Franco-Bronson K, Buchler N, Locala JA, Younossi ZM. Hepatitis C, interferon alfa, and depression. Hepatology. 2000;31:1207–1211. doi: 10.1053/jhep.2000.7880. [DOI] [PubMed] [Google Scholar]
- 10.Castera L, Zigante F, Bastie A, Buffet C, Dhumeaux D, Hardy P. Incidence of interferon alfa-induced depression in patients with chronic hepatitis C. Hepatology. 2002;35:978–979. doi: 10.1053/jhep.2002.32104. [DOI] [PubMed] [Google Scholar]
- 11.Hauser P, Khosla J, Aurora H, Laurin J, Kling MA, Hill J, Gulati M, Thornton AJ, Schultz RL, Valentine AD, Meyers CA, Howell CD. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7:942–947. doi: 10.1038/sj.mp.4001119. [DOI] [PubMed] [Google Scholar]
- 12.Dieperink E, Ho SB, Tetrick L, Thuras P, Dua K, Willenbring ML. Suicidal ideation during interferon-alpha2b and ribavirin treatment of patients with chronic hepatitis C. Gen Hosp Psychiatry. 2004;26:237–240. doi: 10.1016/j.genhosppsych.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Kraus MR, Wilms K. Interferon alpha. Effect, indications, therapy monitoring and side-effects. Internist (Berl) 2000;41:1399–1404. [PubMed] [Google Scholar]
- 14.Maes M, Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, Almerighi C, Meltzer H. Treatment with interferon-alpha (IFN alpha) of hepatitis C patients induces lower serum dipeptidyl peptidase IV activity, which is related to IFN alpha-induced depressive and anxiety symptoms and immune activation. Mol Psychiatry. 2001;6:475–480. doi: 10.1038/sj.mp.4000872. [DOI] [PubMed] [Google Scholar]
- 15.Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, Almerighi C, Verkerk R, Meltzer H, Maes M. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J Clin Psychopharmacol. 2002;22:86–90. doi: 10.1097/00004714-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Miyaoka H, Otsubo T, Kamijima K, Ishii M, Onuki M, Mitamura K. Depression from interferon therapy in patients with hepatitis C. Am J Psychiatry. 1999;156:1120. doi: 10.1176/ajp.156.7.1120. [DOI] [PubMed] [Google Scholar]
- 17.Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry. 2004;56:819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Dang Y, Dale WE, Brown OR. Comparative effects of oxygen on indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase of the kynurenine pathway. Free Radic Biol Med. 2000;28:615–624. doi: 10.1016/s0891-5849(99)00272-5. [DOI] [PubMed] [Google Scholar]
- 19.Sakash JB, Byrne GI, Lichtman A, Libby P. Cytokines induce indoleamine 2,3-dioxygenase expression in human atheroma-asociated cells: implications for persistent Chlamydophila pneumoniae infection. Infect Immun. 2002;70:3959–3961. doi: 10.1128/IAI.70.7.3959-3961.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002;7:468–473. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- 21.Levenson JL, Fallon HJ. Fluoxetine treatment of depression caused by interferon-alpha. Am J Gastroenterol. 1993;88:760–761. [PubMed] [Google Scholar]
- 22.Schramm TM, Lawford BR, Macdonald GA, Cooksley WG. Sertraline treatment of interferon-alfa-induced depressive disorder. Med J Aust. 2000;173:359–361. doi: 10.5694/j.1326-5377.2000.tb125687.x. [DOI] [PubMed] [Google Scholar]
- 23.Kraus MR, Schafer A, Faller H, Csef H, Scheurlen M. Paroxetine for the treatment of interferon-alpha-induced depression in chronic hepatitis C. Aliment Pharmacol Ther. 2002;16:1091–1099. doi: 10.1046/j.1365-2036.2002.01265.x. [DOI] [PubMed] [Google Scholar]
- 24.Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 25.Mann JJ, Huang YY, Underwood MD, Kassir SA, Oppenheim S, Kelly TM, Dwork AJ, Arango V. A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry. 2000;57:729–738. doi: 10.1001/archpsyc.57.8.729. [DOI] [PubMed] [Google Scholar]
- 26.Lotrich FE, Pollock BG. Meta-analysis of serotonin transporter polymorphisms and affective disorders. Psychiatr Genet. 2004;14:121–129. doi: 10.1097/00041444-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura M, Ueno S, Sano A, Tanabe H. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Mol Psychiatry. 2000;5:32–38. doi: 10.1038/sj.mp.4000698. [DOI] [PubMed] [Google Scholar]
- 29.Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL, Goldman D. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roy A, Hu XZ, Janal MN, Goldman D. Interaction between childhood trauma and serotonin transporter gene variation in suicide. Neuropsychopharmacology. 2007;32:2046–2052. doi: 10.1038/sj.npp.1301331. [DOI] [PubMed] [Google Scholar]
- 31.Hu XZ, Rush AJ, Charney D, Wilson AF, Sorant AJ, Papanicolaou GJ, Fava M, Trivedi MH, Wisniewski SR, Laje G, Paddock S, McMahon FJ, Manji H, Lipsky RH. Association between a functional serotonin transporter promoter polymorphism and citalopram treatment in adult outpatients with major depression. Arch Gen Psychiatry. 2007;64:783–792. doi: 10.1001/archpsyc.64.7.783. [DOI] [PubMed] [Google Scholar]
- 32.Lee WM, Dienstag JL, Lindsay KL, Lok AS, Bonkovsky HL, Shiffman ML, Everson GT, Di Bisceglie AM, Morgan TR, Ghany MG, Morishima C, Wright EC, Everhart JE. Evolution of the HALT-C Trial: pegylated interferon as maintenance therapy for chronic hepatitis C in previous interferon nonresponders. Control Clin Trials. 2004;25:472–492. doi: 10.1016/j.cct.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Shiffman ML, Di Bisceglie AM, Lindsay KL, Morishima C, Wright EC, Everson GT, Lok AS, Morgan TR, Bonkovsky HL, Lee WM, Dienstag JL, Ghany MG, Goodman ZD, Everhart JE. Peginterferon alfa-2a and ribavirin in patients with chronic hepatitis C who have failed prior treatment. Gastroenterology. 2004;126:1015–1023. doi: 10.1053/j.gastro.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Wittchen HU. Reliability and validity studies of the WHO--Composite International Diagnostic Interview (CIDI): a critical review. J Psychiatr Res. 1994;28:57–84. doi: 10.1016/0022-3956(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 35.Skinner HA, Sheu WJ. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J Stud Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- 36.Beck A, Steer R, Brown G. Manual for the Beck Depression Inventory. 2nd ed. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 37.Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- 38.Littell R, Milliken G, Stroup W, Wolfinger R. SAS System for Mixed Models. Cary, NC: SAS Institute Inc; 1996. [Google Scholar]
- 39.Dieperink E, Willenbring M, Ho SB. Neuropsychiatric symptoms associated with hepatitis C and interferon alpha: A review. Am J Psychiatry. 2000;157:867–876. doi: 10.1176/appi.ajp.157.6.867. [DOI] [PubMed] [Google Scholar]
- 40.Dieperink E, Ho SB, Thuras P, Willenbring ML. A prospective study of neuropsychiatric symptoms associated with interferon-alpha-2b and ribavirin therapy for patients with chronic hepatitis C. Psychosomatics. 2003;44:104–112. doi: 10.1176/appi.psy.44.2.104. [DOI] [PubMed] [Google Scholar]
- 41.Kraus MR, Al-Taie O, Schafer A, Pfersdorff M, Lesch KP, Scheurlen M. Serotonin-1A receptor gene HTR1A variation predicts interferon-induced depression in chronic hepatitis C. Gastroenterology. 2007;132:1279–1286. doi: 10.1053/j.gastro.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 42.Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, Miller AH. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol Psychiatry. 2003;54:906–914. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 43.Morikawa O, Sakai N, Obara H, Saito N. Effects of interferon-alpha, interferon-gamma and cAMP on the transcriptional regulation of the serotonin transporter. Eur J Pharmacol. 1998;349:317–324. doi: 10.1016/s0014-2999(98)00187-3. [DOI] [PubMed] [Google Scholar]
- 44.Tsao CW, Lin YS, Cheng JT, Chang WW, Chen CL, Wu SR, Fan CW, Lo HY. Serotonin transporter mRNA expression is decreased by lamivudine and ribavirin and increased by interferon in immune cells. Scand J Immunol. 2006;63:106–115. doi: 10.1111/j.1365-3083.2005.01715.x. [DOI] [PubMed] [Google Scholar]
- 45.Kraus MR, Schafer A, Al-Taie O, Scheurlen M. Prophylactic SSRI during interferon alpha re-therapy in patients with chronic hepatitis C and a history of interferon-induced depression. J Viral Hepat. 2005;12:96–100. doi: 10.1111/j.1365-2893.2005.00554.x. [DOI] [PubMed] [Google Scholar]
- 46.Smeraldi E, Zanardi R, Benedetti F, Di Bella D, Perez J, Catalano M. Polymorphism within the promoter of the serotonin transporter gene and antidepressant efficacy of fluvoxamine. Mol Psychiatry. 1998;3:508–511. doi: 10.1038/sj.mp.4000425. [DOI] [PubMed] [Google Scholar]
- 47.Benedetti F, Serretti A, Colombo C, Campori E, Barbini B, di Bella D, Smeraldi E. Influence of a functional polymorphism within the promoter of the serotonin transporter gene on the effects of total sleep deprivation in bipolar depression. Am J Psychiatry. 1999;156:1450–1452. doi: 10.1176/ajp.156.9.1450. [DOI] [PubMed] [Google Scholar]
- 48.Zanardi R, Benedetti F, Di Bella D, Catalano M, Smeraldi E. Efficacy of paroxetine in depression is influenced by a functional polymorphism within the promoter of the serotonin transporter gene. J Clin Psychopharmacol. 2000;20:105–107. doi: 10.1097/00004714-200002000-00021. [DOI] [PubMed] [Google Scholar]
- 49.Pollock BG, Ferrell RE, Mulsant BH, Mazumdar S, Miller M, Sweet RA, Davis S, Kirshner MA, Houck PR, Stack JA, Reynolds CF, Kupfer DJ. Allelic variation in the serotonin transporter promoter affects onset of paroxetine treatment response in late-life depression. Neuropsychopharmacology. 2000;23:587–590. doi: 10.1016/S0893-133X(00)00132-9. [DOI] [PubMed] [Google Scholar]
- 50.Yu YW, Tsai SJ, Chen TJ, Lin CH, Hong CJ. Association study of the serotonin transporter promoter polymorphism and symptomatology and antidepressant response in major depressive disorders. Mol Psychiatry. 2002;7:1115–1119. doi: 10.1038/sj.mp.4001141. [DOI] [PubMed] [Google Scholar]
- 51.Rausch JL, Johnson ME, Fei YJ, Li JQ, Shendarkar N, Hobby HM, Ganapathy V, Leibach FH. Initial conditions of serotonin transporter kinetics and genotype: influence on SSRI treatment trial outcome. Biol Psychiatry. 2002;51:723–732. doi: 10.1016/s0006-3223(01)01283-5. [DOI] [PubMed] [Google Scholar]
- 52.Loftis JM, Socherman RE, Howell CD, Whitehead AJ, Hill JA, Dominitz JA, Hauser P. Association of interferon-alpha-induced depression and improved treatment response in patients with hepatitis C. Neurosci Lett. 2004;365:87–91. doi: 10.1016/j.neulet.2004.04.058. [DOI] [PubMed] [Google Scholar]
- 53.Yang BZ, Zhao H, Kranzler HR, Gelernter J. Practical population group assignment with selected informative markers: Characteristics and properties of Bayesian clustering via STRUCTURE. Genet Epidemiol. 2005;28:302–312. doi: 10.1002/gepi.20070. [DOI] [PubMed] [Google Scholar]