Abstract
Extracellular matrix remodeling is a continuous process that is critical to maintaining tissue homeostasis, and alterations in this process have been implicated in chronic diseases such as atherosclerosis, lung fibrosis, and emphysema. Collagen and elastin are subject to ascorbate-dependent hydroxylation. While this post-translational modification in collagen is critical for function, the role of hydroxylation of elastin is not well understood. A number of studies have indicated that ascorbate leads to reduced elastin synthesis. However, these studies were limited to analysis of cells grown under traditional 2D tissue culture conditions. To investigate this process we evaluated elastin and collagen synthesis in primary rat neonatal pulmonary fibroblasts in response to ascorbate treatment in traditional 2D culture and within 3D cross-linked gelatin matrices (Gelfoam). We observed little change in elastin or collagen biosynthesis in standard 2D cultures treated with ascorbate, yet observed a dramatic increase in elastin protein and mRNA levels in response to ascorbate in 3D cell-Gelfoam constructs. These data suggest that the cell-ECM architecture dictates pulmonary cell response to ascorbate, and that approaches aimed toward stimulating ECM repair or engineering functional cell-derived matrices should consider all aspects of the cellular environment.
Keywords: Vitamin C, Extracellular Matrix, Emphysema, Lung, Gelfoam
Introduction
The extracellular matrix (ECM) is the hydrated webbing comprised of proteins and polysaccharides that surrounds most cells in multicellular organisms. The chemical and mechanical properties of the ECM define tissue architecture and instruct cell function. Thus, tissue disruption during injury and disease is communicated to resident cells, in large part, through alterations in ECM (Hynes, 2009). Consequently, ECM remodeling is a continuous process that is critical to maintaining tissue homeostasis, and alterations in this process have been implicated in chronic diseases such as atherosclerosis, lung fibrosis, and emphysema (Arroyo and Iruela-Arispe, 2010). In chronic disease a self-perpetuating feed forward loop has been proposed where ECM dysfunction leads to improper remodeling and progressively compromised tissue function. For example, during the progression of emphysema uncontrolled proteolysis degrades ECM leading to mechanical failure and collapse of alveolar septal walls and airspace enlargement. As this process progresses, the dysfunctional ECM may signal the resident cells in an effort to stimulate tissue repair, yet the ECM produced is not properly organized or able to meet the demands of the tissue leading to continued failure and disease progression (Suki and Bates, 2008).
Collagen, elastin, and proteoglycans are three major components of the ECM. Collagen bears the majority of the tensile load, while the viscoelastic nature of elastin imparts compliance with its ability to stretch 2-3 times in length with nearly no loss of energy (Suki et al., 2005). There are at least 28 genetically distinct collagen types, while a single gene encodes elastin. Both collagen and elastin are subject to post-translational modifications that are required for function (Suki and Bates, 2008). Of particular importance to collagen stability is the enzymatic hydroxylation of proline and lysine residues that requires ascorbate (vitamin C) as a cofactor (Eyre et al., 1984). Elastin is also subjected to ascorbate-dependent hydroxylation yet the function of this modification is less well understood. In particular, a number of studies have indicated that ascorbate leads to enhanced collagen production and reduced elastin synthesis (Bergethon et al., 1989; Dunn and Franzblau, 1982; Leesa M. Barone, 1985). However, these studies were limited to analysis of cells grown under traditional 2-dimensional tissue culture conditions. Additional studies in animals have produced somewhat inconclusive results. Vitamin C deficiency in guinea pigs results in reduced elastin mRNA expression in blood vessels (Mahmoodian and Peterkofsky, 1999) and no change in elastin levels in skin (Barnes et al., 1969), while supplemental vitamin C reduced elastin deposition in neonatal rat aorta (Quaglino et al., 1991). Thus, it remains unclear how ascorbate-mediated modification ultimately influences elastin biosynthesis. It is likely that cell response to ascorbate will be dependent on cell type, tissue architecture and ECM organization.
Loss of elastin is a major contributing factor to the progression of emphysema and yet little is known about how ascorbate influences elastin biosynthesis in pulmonary cells. To investigate this process we evaluated elastin and collagen synthesis in primary rat neonatal pulmonary fibroblasts. We observed little change in elastin or collagen biosynthesis in standard 2D cultures treated with ascorbate. However, when we cultured these cells within 3D, cross-linked gelatin constructs (Gelfoam), we observed a dramatic increase in elastin deposition and expression of tropoelastin mRNA. These data suggest that the cell-ECM architecture dictates pulmonary cell response to ascorbate, and that approaches aimed toward stimulating ECM repair or engineering functional cell-derived matrices should consider all aspects of the cellular environment. Analysis of ECM remodeling within a 3D environment will provide insight into how cells in normal and diseased tissues respond to external stimuli and may be critical to the future development of treatments for ECM based diseases.
Materials and Methods
Materials
The stable L-ascorbic acid analog (2-O-D-glucopyranosyl L-ascorbic acid) was purchased from Wako Chemicals (Richmond, VA). Compressed Gelfoam was obtained from Pharmacia and Upjohn Co., Division of Pfizer Inc (NY, NY). Primary polyclonal rat lung alpha-elastin antibody was obtained from Elastin Products (Owensville, MO). Secondary peroxidase-conjugated AffiniPure Donkey Anti-Goat IgG antibody was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). DAPI Vectastain and TO-PRO were from Life Technologies (Grand Island, NY). Compact Reaction Columns and 35 μm Compact Reaction Column Filters (upper) for three dimensional (3D) enzyme-linked immunosorbent assays (ELISAs) were obtained from USB Corporation (Cleveland, OH). Dulbecco’s Modified Eagle’s Medium (DMEM), phosphate buffered saline (PBS), penicillin/streptomycin, trypsin and nonessential amino acids, and Ethylenediaminetetraacetic acid (EDTA) were obtained from Life Technologies (Grand Island, NY). Fetal Bovine serum was purchased from Atlanta Biologicals (Lawrenceville, GA). Tris buffered saline (TBS), bovine serum albumin (BSA), TWEEN 20, guanidine thyiocyanate, guanidine hydrocholoride, and beta-mercaptoethanol were obtained from American Bioanalytical (Natick, MA). Formaldehyde (37%) was obtained from Fisher Scientific (Fair Lawn, NJ). Tetramethylbenzidine (TMB) 2-component microwell peroxidase substrate kit was purchased from KPL (Gaithersburg, MD). Optimax microtiter plate reader (Molecular Devices, Sunnyvale, CA) was used for absorption measurements. RNase-free DNase 1 was purchased from New England Biolabs (M0303S, Ipswich, MA). Rat elastin (RN01499782_m1), collagen 1 (RN00801649), and 18S rRNA endogenous control (4308329) Taqman gene expression assays were purchased from Applied Biosystems (Foster City, CA) for use with Applied Biosystems 7300 Real-Time PCR.
Isolation of Rat Neonatal Pulmonary Fibroblasts
Neonatal rat pulmonary fibroblasts (NNRLFs) were isolated from 2-3-day-old Sprague-Dawley rats (Foster et al., 1990). All studies with animals were conducted as approved by the Boston University Medical Campus Institutional Animal Care and Use Committee, and our animal facility is approved by the American Association for the Accreditation of Laboratory Animal Care. Lungs were removed and washed three times with PBS. Scissors were used to mince the tissue into small pieces (1-2mm3) that were then suspended (1 lung/ml) in 0.05% trypsin/0.53 mM EDTA solution. The mixture was shaken at 350 rpm as it incubated at 37°C for 20 minutes. A 100-μm Nucleopore filter was used to remove the digested tissue fragments. The resulting filtrate was centrifuged (300×g for 10 min) and the cell pellet was washed with DMEM, 5% fetal bovine serum (FBS) infused with a 1% penicillin/streptomycin antibiotic cocktail and 1% nonessential amino acids (NNRLF medium).
Lung fibroblast medium was used to resuspend the pellet. The resulting suspension was seeded into flasks and allowed to sit for 2 hours for adequate cell adhesion of fibroblasts before the media was changed. The media was replaced twice more that week with lung fibroblast medium. Trypsin was used to release the cells from the tissue-culture flasks. The cells were collected by centrifugation and resuspended in the lung fibroblast medium for 2-4 passages before being seeded onto plastic tissue culture dishes or Gelfoam matrix constructs.
Cell Seeding and maintenance
NNRLF were seeded onto plastic tissue culture dishes at a density of 30,000 cells/cm2. Gelfoam constructs were cut from a 12cm × 8cm × 0.3 cm sheet into 4 cm × 1 cm × 0.3 cm pieces the day prior to seeding. The constructs were fully submerged in NNRLF medium and were maintained in a tissue culture incubator at 37° C overnight. Constructs were placed onto 100 mm Petri dishes and were seeded with 250,000 cells by spot pipetting over the entire 4 cm × 1 cm surface. Samples were placed in the incubator for 2-3 hours to ensure adhesion had occurred. After the cell adhesion period, cell-Gelfoam constructs were placed in centrifuge tubes containing NNRLF medium, lids were left vented to allow gas exchange. Medium was changed 24 hours after seeding and two times weekly thereafter. L-ascorbic acid analog (10 mM) was added to half the samples at each feeding beginning on day 3 in culture. This concentration was selected based on previous studies that evaluated various concentrations of ascorbate (Welch et al., 1993) and compared the use of ascorbate with the analog used in the present study (Kumano et al., 1998).
ELISA Analysis in 2D Cultures
During days 7 through 28, NNRLF cells seeded onto 96 well tissue culture plates were washed with tris buffered saline (50 mM tris pH 7.6, 150 mM sodium chloride) (TBS) followed by a fixation step of 3.7% formaldehyde for 20 minutes and a final TBS wash. Cells were blocked in a 3% bovine serum albumin (BSA) in TBS overnight at 4°C. Samples were washed with TBS prior to adding 1 μg/mL RA-75 antibody in 3% BSA-TBS for 1.5 hours at room temperature. Samples were then washed with TBS and incubated with 0.4μg/mL peroxidase-conjugated donkey anti-goat IgG at room temperature followed by rinses with TBS containing 0.1% Tween-20 and with TBS. Finally, secondary antibody binding was detected with the TMB 2-component microwell peroxidase substrate kit and the reaction was stopped after 10 minutes by the addition of 1 N sulfuric acid. An Optimax microtiter plate reader was used to read the absorbance at 450 and 570 nm (background correction). Values were normalized to their controls.
3D Cell-Gelfoam ELISA Analysis
NNRLFs seeded in Gelfoam for various periods were fixed after washing with TBS by incubation in formaldehyde for 20 minutes and washing with TBS. In the experiment, no cell control sponges hydrated in media were used to access the level of background. The dry mass of the Gelfoam pieces was recorded after centrifuging (2000 rpm 30 sec) the pieces in compact reaction columns with 35 μm filter frits to remove excess TBS. The same protocol as above for the plastic samples was replicated except for differences in washes. To account for the physical characteristics of the cell-Gelfoam constructs the number of washes was reduced and their time was increased so that equilibration could be achieved without disrupting the cell-Gelfoam constructs. The initial blocking step was conducted for an hour, the first TBS wash was a second hour, the primary antibody incubation was 24 hours, the second TBS was done for one hour, the secondary antibody and the TBS 0.1% Tween-20 solution incubations were carried out for a half hour each, and the final TBS wash was an hour. The TMB peroxidase substrate reaction was stopped after 4.5 minutes. All incubations were done on a rocker at 4°C with the exception of the blocking step that occurred at 37°C. After the addition of each wash, the samples were vortexed. To remove each wash, a centrifugation step (2000 rpm 30 sec) was added. Samples were corrected for background with the no cell controls and normalized to their respective dry mass values. Further normalization was completed by dividing the corrected ascorbate treated sample values by the mean of the respective corrected control samples.
Real Time PCR Analysis
Total RNA purification was completed on days 14 and 21 from cells cultured on Gelfoam and tissue culture plates. Gelfoam samples were gently agitated in PBS baths to remove Gelfoam debris and excess media. Following washing, excess fluid was drained off the sponges and they were cut into small pieces with scissors. A previously described protocol involving the use of guanidinium thiocyanate, guanidinium hydochloride, acetic acid, and cold ethanol was used to extract the total RNA from the cut Gelfoam pieces (Chirgwin et al., 1979). One step of the RNA purification protocol was modified for Gelfoam total RNA extraction. Before homogenization of the sample, Gelfoam sponges were centrifuged (16,000 × g for 10 minutes) and the supernatant was utilized for the remaining portion of the protocol. Cells on tissue culture plastic underwent the traditional protocol.
The samples were incubated with RNase-free DNase I and an RNase inhibitor to remove any genomic DNA. The resulting purified RNA was annealed with random hexamer and oligo-dt primers allowing MuLV reverse transcription to cDNA to begin. No reverse transcriptase was used in the negative controls. To carry out RT-PCR analysis, an Applied Biosystems 7300 Real-Time PCR system was utilized. ABI TaqMan gene expression assays for rat elastin and collagen 1A1 was used as target probes and eukaryotic 18 S rRNA was used as an endogenous control. Standard cycling parameters of 50 °C for 10 minutes, 95°C for 2 minutes, and 40 cycles of 95°C for 15 seconds and 60°C for 1 minute were completed. The -ΔΔCT data analysis method was utilized normalizing treated samples to the mean of their respective control sample.
Gelfoam Histology
Cell-Gelfoam constructs for confocal analysis were washed three times with gentle shaking for 10 minutes in sterile PBS at room temperature and fixed in 4% paraformaldehyde in PBS overnight at 4°C with gentle shaking. The constructs were then rinsed three more times in sterile PBS as above and stored in sterile PBS at 4°C. A piece of the construct was cut from the center. The construct piece was washed three times in sterile PBS as above and cell nuclei stained with TO-PRO (25ng/ml) in PBS for five minutes with gently shaking. The construct was rinsed three times in sterile PBS, mounted and viewed using a Zeiss 200M LSM510 confocal microscope. A z-section stack of images was collected from the top down into the construct to visualize the distribution of cells throughout the matrix.
Cell-Gelfoam constructs for histology were fixed and a piece was cut from the center as described above. The cell-Gelfoam piece was dehydrated and embedded in paraffin. Five-micron sections were cut and mounted on glass slides. The sections were then treated to remove the paraffin and rehydrated with deionized water. The elastin matrix was stained with Verhoeff’s elastin stain without the Van Gieson counterstain (Accustain, Sigma Aldrich, St. Louis, MO) according to the provided protocol. For immunohistochemistry, sections were rehydrated with deionized water and then PBS. The sections were blocked at room temperature for one hour in a humidified chamber in 3% BSA in PBS and then incubated overnight at 4°C in primary antibody diluted in 3%BSA in PBS in a humidified chamber. For elastin, goat polyclonal antibody RA-75 (Elastin Products, Owensville, Mo.) was used at 1:500 dilution. For collagen, rabbit polyclonal antibody to Type 1 collagen 600-401-103s (Rockland Immunochemicals Inc., Gilbertsville, PA) was used at a 1:200 dilution. The slides were washed once at room temperature for fifteen minutes with gently rocking in PBS with 0.1% Triton X-100 (PBST) and once with 0.1% BSA in phosphate buffered saline with tween-20 (PBST) in the same conditions. The slides were then incubated at room temperature in the humidified chamber in the dark with conjugated (Cy3 or FITC) secondary antibody (1:1000) in 3%BSA in PBS for an hour. The slides were then washed for 10 minutes at room temperature in the dark with PBST twice, and then once with PBS for 10 minutes and mounted for viewing. Images were collected on a Nikon Eclipse TE 200 microscope with SPOT imaging camera.
Results
Ascorbate does not alter elastin and collagen production in pulmonary fibroblasts cultured in 2D
Pulmonary fibroblasts are considered a principal source of ECM in the lung. Approaches aimed at treating diseases associated with deficient ECM, such as emphysema, often target these cells for regulation. Neonatal pulmonary fibroblasts produce an elastin-rich ECM in vitro (Campagnone et al., 1987), so we utilized this cell system to explore the possible influence of ascorbate on elastin production. NNRLFs were cultured on standard 2D tissue culture plastic surfaces in the presence and absence of ascorbate for 28 days and elastin deposition measured using a quantitative ELISA (Fig 1A). Consistent with previous studies, these cells deposited elastin by day 7 in culture and continued to accumulate elastin over the 4 week course of the experiment. However, the presence of ascorbate had only a small effect on elastin levels. To more fully evaluate the influence of ascorbate on ECM production, we measured elastin and collagen (type I) protein and mRNA levels at day 14 and day 21 in culture (Fig 1B and C). At both time points ascorbate treatment caused a slight decrease in both elastin and collagen levels in the cultures, and had no effect on mRNA levels for each protein. The ability of ascorbate to show both a slight increase (Fig 1A) and decrease (Fig 1B) in elastin deposition indicates that its effect is below the level of inter-experimental variation in these long-term studies. Thus, we conclude that ascorbate does not meaningfully modify elastin levels in 2D cultures of NNRLFs.
Figure 1. Elastin and collagen gene expression in pulmonary fibroblasts in 2D culture.
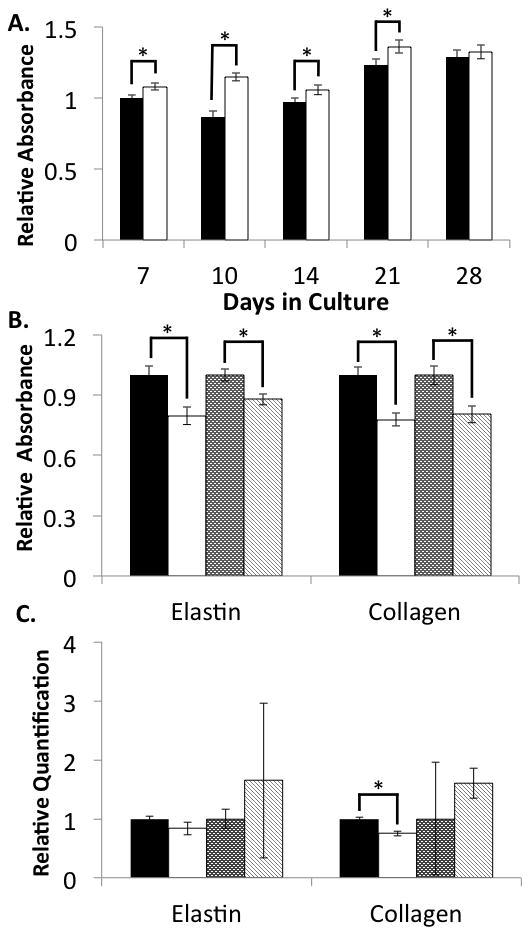
NNRLFs were cultured on tissue culture plastic for the indicated time in the presence and absence ascorbate (10 mM). Cell/ECM samples were fixed and ELISAs conducted with anti-elastin or anti-collagen antibodies as described in “Materials and Methods” (A and B). For relative mRNA quantitation (C) cells were extracted and analyzed by qPCR. (A) Elastin levels were determined in control (filled bars) ascorbate treated (open bars) NNRLFs and normalized to the non-treated control sample at day 7 (average of 10 samples ±SEM). The small differences at days 7, 10, 14, and 21 were statistically significant (p < 0.05; 2 sided t-test). There was no significant difference at day 28. (B) Elastin and collagen protein levels were measured after 14 days without (black bars) and with (white bars) ascorbate, and 21 days without (dark stippled bars) and with (light cross-hatched bars) ascorbate. Data at each time point were normalized to the untreated control sample (average of 5 samples ±SEM; p < 0.05 for ascorbate versus control). (C) Elastin and collagen mRNA levels were measured by qPCR at days 14 and 21 in control and ascorbate treated cultures (bar code described above for panel (B)). Data at each time point were normalized to the untreated control sample (average of 4 samples ±SEM; p = NS for ascorbate versus control for all but the day 14 collagen sample).
NNRLFs Grow and Produce ECM in 3D Gelfoam Matrices
The lack of an effect of ascorbate on ECM protein production by NNRLFs may reflect the artificial nature of the 2D monolayer culture arrangement used. Pulmonary fibroblasts in vivo are not organized into 2D monolayers, instead they are distributed within the 3D interstitial ECM of the lung. Thus, it is possible that ECM production by these cells is influenced by the architecture of the substrate and the differences in intercellular interactions that can form based on the geometry of the culture substrate. To address these possibilities we cultured NNRLFs in a 3D cross-linked gelatin scaffold (Gelfoam) that has previously been used in tissue engineering applications (Andrade et al., 2007; Centra et al., 1992; Nathan et al., 1995). NNRLFs were seeded into Gelfoam sponges, grew and reached a steady state after 10 days (Fig 2A). Cell-Gelfoam constructs were fixed, sectioned, and analyzed by histology at day 14 (Fig 2B). We observed clusters of cells and associated fibrous material, presumably cell-derived ECM, arranged within the Gelfoam pores. In some regions we noted that cell clusters appeared to bridge Gelfoam fibers causing reduced pore size and increased cellularity, potentially through the ability of the cells to physically contract the Gelfoam locally. We also examined whole-mount cell-Gelfoam constructs by confocal laser scanning microscopy to evaluate the distribution of the cells throughout the matrix after staining the nuclei with DAPI (Fig 2C), and noted that the cells were well distributed throughout the thickness of the Gelfoam with clusters of cells observed at various depths.
Figure 2. Pulmonary fibroblasts grow within Gelfoam matrices.
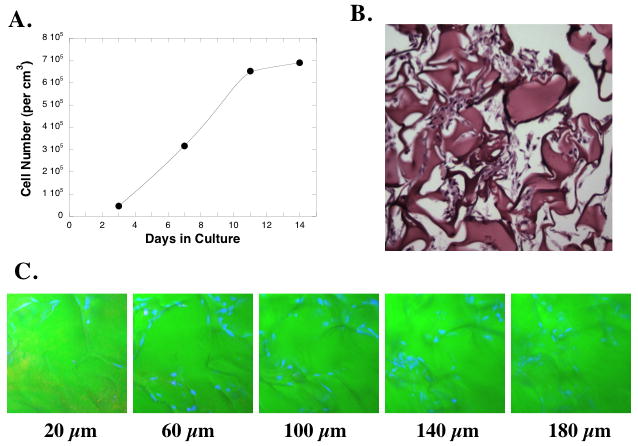
(A) NNRLFs were cultured in Gelfoam sponges for the indicated time and cell number was determined after solubilization of the Gelfoam. (B) Histological specimen on NNRLFs in Gelfoam after 14 days in culture. (C) Confocal microscopic images of NNRLFs within Gelfoam sponges. NNRLF-Gelfoam constructs were fixed and cell nuclei stained with TO-PRO (blue) and a z-stack was collected at 20 μm intervals from the top to the bottom of the construct (total width of construct ~3 mm). Samples are shown for the indicated depth.
To determine if the NNRLFs were able to deposit elastin and collagen within the constructs we used species-specific antibodies to detect rat cell-derived elastin and collagen by immunofluorescence within the porcine-derived gelfoam (Fig 3). The collagen and elastin antibodies showed labeling in localized regions distinct from the main Gelfoam struts, indicating that these ECM proteins are being deposited within the cell-Gelfoam constructs. Thus, we evaluated the ability of ascorbate to modulate ECM protein deposition in these constructs by ELISA (Fig 4) and immunofluorescence (Fig 5). Elastin deposition in NNRFL-Gelfoam constructs was observed after 7 days of culture and nearly doubled by 28 days. In the presence of ascorbate a significant increase in elastin deposition was observed at all time points with maximal levels observed after 21 days (Fig 4A). We further analyzed the level of elastin and type I collagen protein and mRNA in constructs at days 14 and 21 (Fig 4B and C). Slightly increased levels of elastin and collagen protein in ascorbate treated constructs were observed at day 14, whereas at day 21 an ~4.5-fold increase in elastin and a nearly 2-fold increase in collagen were observed. The dramatic increase in protein levels in the ascorbate treated constructs at day 21 was correlated with increased steady-state levels of mRNA for elastin (8.8-fold) and collagen (3.6-fold). At day 14 mRNA levels were slightly increased (1.5-fold) for elastin and slightly decreased for collagen. Immunofluorescence analysis of day 21 samples was consistent with the ELISA data showing increased levels of collagen and elastin in the ascorbate treated constructs (Fig 5).
Figure 3. Pulmonary fibroblasts deposit elastin and collagen within Gelfoam matrices.
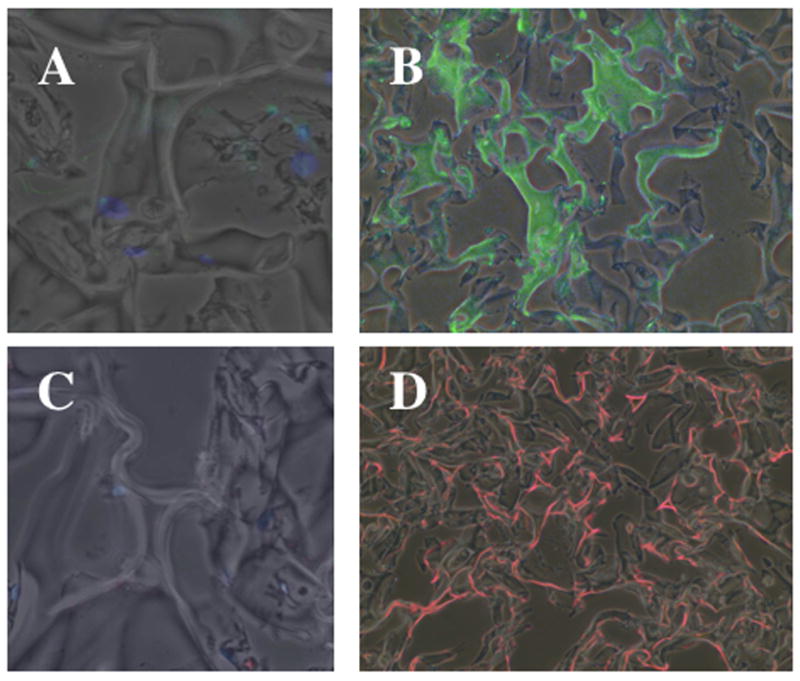
NNRLFs were cultured in Gelfoam sponges for 14 days, fixed, sectioned and incubated without (A and C) and with primary antibodies to elastin (B), and type I collagen (D), followed by incubation with FITC-linked (A and B, green) or Cy3-linked (C and D, red) secondary antibodies. Secondary antibody alone control samples were visualized using a 20x objective in order to be able to observe the low background fluorescence. The primary antibody samples were observed with a 10x objective so that the distribution of elastin and collagen deposition could be visualized throughout a larger region of the cell-Gelfoam constructs.
Figure 4. Elastin and collagen expression in pulmonary fibroblasts within 3D Gelfoam matrices.
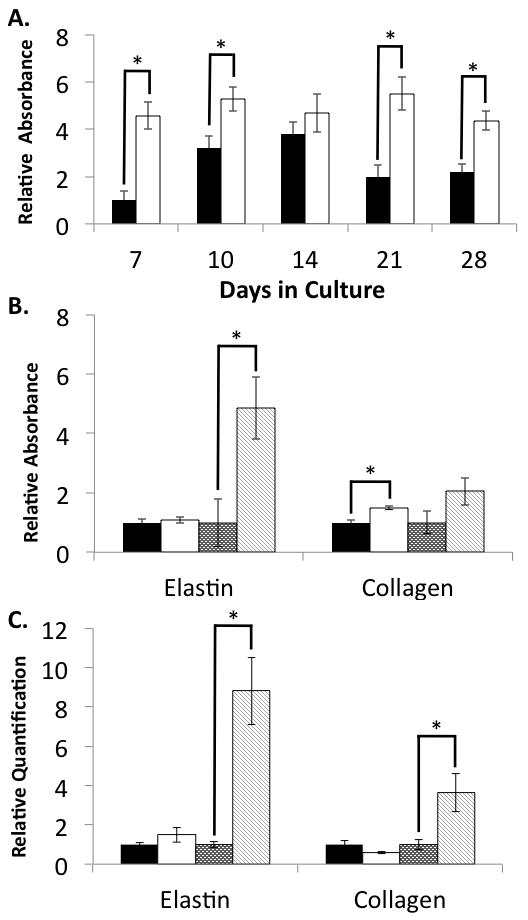
NNRLFs were cultured Gelfoam sponges for the indicated time in the presence and absence ascorbate (10 mM). NNRLF-Gelfoam samples were fixed and ELISAs conducted with anti-elastin or anti-collagen antibodies as described in “Materials and Methods” (A and B). For relative mRNA quantitation (C) cells were extracted and analyzed by qPCR. (A) Elastin levels were determined in control (filled bars) ascorbate treated (open bars) NNRLF-Gelfoam constructs and normalized to the non-treated control sample at day 7 (average of 4 samples ±SEM; p < 0.05 for ascorbate versus control for all but the day 14 time point). (B) Elastin and collagen protein levels within NNRLF-Gelfoam constructs were measured by ELISA after 14 days without (black bars) and with (white bars) ascorbate, and 21 days without (dark stippled bars) and with (light cross-hatched bars) ascorbate. Data at each time point were normalized to the untreated control sample (average of 10 samples ±SEM; p < 0.05 for day 21 elastin ascorbate versus control and for collagen at day 14). (C) Elastin and collagen mRNA levels within the NNRLF-Gelfoam constructs were measured at days 14 (N = 5) and 21 (N = 6) in control and ascorbate treated cultures (bar code described above for panel (B)). Data at each time point were normalized to the untreated control sample (average ±SEM; p < 0.05 for elastin and collagen ascorbate versus control at day 21).
Figure 5. Ascorbate treatment enhances elastin and collagen deposition by pulmonary fibroblast within 3D Gelfoam constructs.
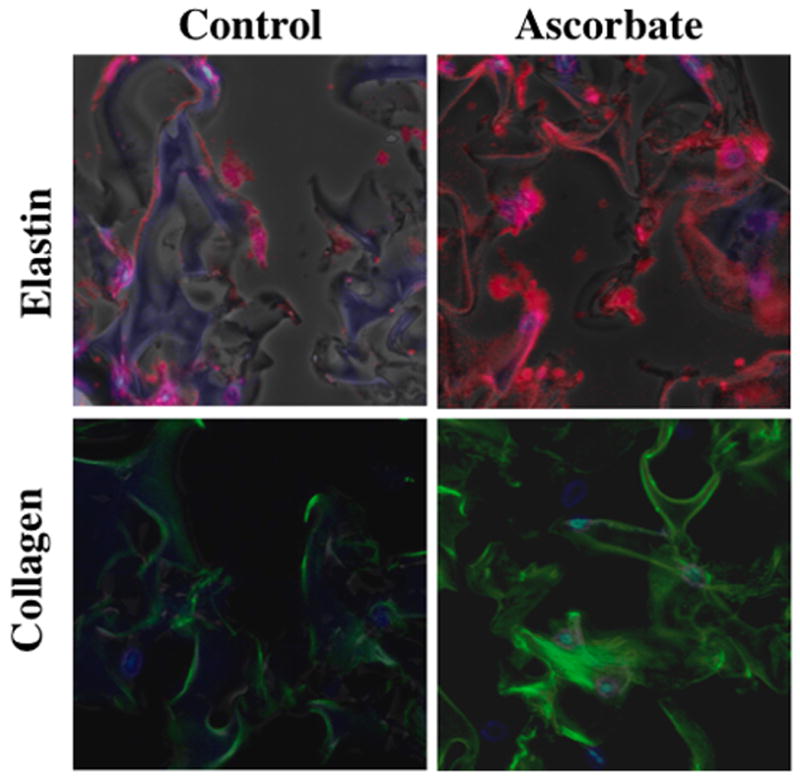
NNRLFs were cultured Gelfoam sponges in the presence (B and D) and absence (A and C) of ascorbate (10 mM) for 21 days. Cell-Gelfoam constructs were fixed, sectioned and stained with DAPI to visualize cell nuclei (blue) and with antibodies to elastin (A and B; red; 1:100 dilution) or type I collagen (C and D; green; 1:100 dilution). Images were collected using a 20x objective. Fluorescence exposure levels were kept constant for samples treated with and without ascorbate.
Discussion
The regulation of ECM protein synthesis and deposition is complex and has been studied extensively (Tsang et al., 2010). However, to date, most of this work has involved the analysis of cells grown in traditional 2D monolayer cultures. In addition, the role of ascorbate in modulating ECM production has been an area of interest, with most studies focused on how ascorbate modulates collagen deposition (Davidson et al., 1997; Eyre et al., 1984; Geesin et al., 1988). While elastin is also subject to ascorbate-dependent hydroxylation, the role that this post-translational modification plays in elastin deposition or stability remains unknown. In the present study we evaluated the effects of ascorbate on NNRLFs cultured on 2D tissue-culture plastic and within 3D Gelfoam sponges and noted dramatic distinctions between the two systems. Whereas ascorbate had little detectable influence on ECM protein production in 2D, it dramatically enhanced elastin and collagen deposition within cell-Gelfoam constructs. These data indicate that substrate architecture and composition may dictate ECM production. While the mechanistic processes underlying the differences in response to ascorbate observed under the two culture conditions remain unknown, these findings suggest that cell orientation, shape and cell-cell contacts may be important factors controlling ECM homeostasis and repair. It is also possible that the mechanical stiffness of the substrate may dictate cell synthesis as has been shown in previous studies (Engler et al., 2006; Sazonova et al., 2011). These findings may have practical implications for engineering ECM-rich tissue replacements or therapies that aim to enhance ECM protein production.
Other studies have demonstrated that the cellular environment is critical to elastin production. For instance, Hong et al examined vascular smooth muscle cells on various types of three dimensional scaffolds and showed varying collagen I and tropoelastin mRNA production based on the type of matrix (Hong and Stegemann, 2008). Additional studies with vascular smooth muscle cells have established a more contractile phenotype and increased elastin synthesis in three dimensional scaffolds and with biochemical modulators such as TGF-β (Lin et al., 2011; Stegemann et al., 2005). Similarly, TGF-β induces elastin synthesis in 2D cultures of neonatal rat lung fibroblasts (Eickelberg et al., 1999; Kuang et al., 2007). All of these results indicate that cells remodel their extracellular matrices in response to the particular environmental conditions. It is possible that similar alterations in phenotype are occurring in the fibroblast cultures under the two conditions compared in this study. The shape and function of fibroblasts has been shown to change in three dimensional cultures with various mechanical inputs (Grinnell, 2003). Our findings suggest that the fibroblasts are activated in the three dimension cultures, potentially analogous to the transition of fibroblasts to myofibroblast that is accompanied by increased collagen production (Luchsinger et al., 2011). Myofibroblasts are involved in extracellular matrix production during development and in disease states that are associated with excessive ECM production such as emphysema or lung fibrosis (Rishikof et al., 2006). Phenotypic alteration may make the cells more sensitized to ascorbic acid-stimulation of matrix production. Various studies have demonstrated that ascorbate is not just an antioxidant and a co-factor for hydroxyproline formation, but is also involved in transcription and post transcriptional modifications (Arrigoni and De Tullio, 2002).
Cells sense and respond to their environment based on the collective effects of the various chemical and physical extracellular cues. While most studies looking at extracellular matrix remodeling in response to ascorbate showed either no effect or decreased elastin production in vascular smooth muscle cells, we observed a marked increase in elastin production by pulmonary fibroblasts grown within three dimensional Gelfoam sponges. These findings suggest that the cellular response to biochemical regulatory factors can be mediated by the geometry of the ECM. However, it is important to note that other differences between the two cell substrates, such as stiffness, may underlie the differences in response to ascorbate. A number of tissue engineering approaches have been proposed based on seeding cells into biodegradable 3D scaffolds to establish tissue-like constructs. The concept behind these approaches is to use the initial structure of the scaffold to guide the deposition of cell-derived ECM, but equally important may be the cellular response to extracellular stimuli such as ascorbate, hormones and growth factors. Increased understanding of how to control extracellular matrix remodeling within 3D substrates may lead to the development of better models to understand the pathophysiology of diseases such as emphysema and possible treatments through tissue engineering approaches.
Acknowledgments
We thank Dr. Helen M. Nugent at Pervasis Therapeutics, Inc. for helpful advice on working with Gelfoam sponges. K.E.D. was supported in part by a Boston Women’s Council Scholarship and is a recipient of an American Medical Association Foundation Seed Grant. This work was supported in part by National Institutes of Health Grant HL088572 to M.A.N.
Contract Grant Number R01 HL088572
Footnotes
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrade CF, Wong AP, Waddell TK, Keshavjee S, Liu M. Cell-based tissue engineering for lung regeneration. Am J Physiol Lung Cell Mol Physiol. 2007;292(2):L510–518. doi: 10.1152/ajplung.00175.2006. [DOI] [PubMed] [Google Scholar]
- Arrigoni O, De Tullio MC. Ascorbic acid: much more than just an antioxidant. Biochimica et Biophysica Acta (BBA) - General Subjects. 2002;1569(1-3):1–9. doi: 10.1016/s0304-4165(01)00235-5. [DOI] [PubMed] [Google Scholar]
- Arroyo AG, Iruela-Arispe ML. Extracellular matrix, inflammation, and the angiogenic response. Cardiovascular Research. 2010;86(2):226–235. doi: 10.1093/cvr/cvq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes MJ, Constable BJ, Kodicek E. Studies in vivo on the biosynthesis of collagen and elastin in ascorbic acid-deficient guinea pigs. The Biochemical journal. 1969;113(2):387–397. doi: 10.1042/bj1130387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergethon PR, Mogayzel PJ, Franzblau C. Effect of the reducing environment on the accumulation of elastin and collagen in cultured smooth-muscle cells. Journal of Biochemistry. 1989;1(258):279–284. doi: 10.1042/bj2580279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnone R, Regan J, Rich CB, Miller M, Keene DR, Sakai L, Foster JA. Pulmonary fibroblasts: a model system for studying elastin synthesis. Lab Invest. 1987;56(2):224–230. [PubMed] [Google Scholar]
- Centra M, Ratych RE, Cao GL, Li J, Williams E, Taylor RM, Rosen GM. Culture of bovine pulmonary artery endothelial cells on Gelfoam blocks. Faseb J. 1992;6(12):3117–3121. doi: 10.1096/fasebj.6.12.1521742. [DOI] [PubMed] [Google Scholar]
- Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Davidson JM, LuValle PA, Zoia O, Quaglino D, Jr, Giro M. Ascorbate Differentially Regulates Elastin and Collagen Biosynthesis in Vascular Smooth Muscle Cells and Skin Fibroblasts by Pretranslational Mechanisms. J Biol Chem. 1997;272(1):345–352. doi: 10.1074/jbc.272.1.345. [DOI] [PubMed] [Google Scholar]
- Dunn DM, Franzblau C. Effects of Ascorbate on Insoluble Elastin Accumlulation and Cross-Link Formation in Rabbit Pulmonary Artery Smooth Muscle Cultures. Biochemistry. 1982;21(18):4195–4202. doi: 10.1021/bi00261a001. [DOI] [PubMed] [Google Scholar]
- Eickelberg O, Köhler E, Reichenberger F, Bertschin S, Woodtli T, Erne P, Perruchoud AP, Roth M. Extracellular matrix deposition by primary human lung fibroblasts in response to TGF-β1 and TGF-β3. American Journal of Physiology - Lung Cellular and Molecular Physiology. 1999;276(5):L814–L824. doi: 10.1152/ajplung.1999.276.5.L814. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Eyre DR, Paz MA, Gallop PM. Cross-Linking in Collagen and Elastin. Annual Review of Biochemistry. 1984;53(1):717–748. doi: 10.1146/annurev.bi.53.070184.003441. [DOI] [PubMed] [Google Scholar]
- Foster JA, Rich CB, Miller MF. Pulmonary fibroblasts: an in vitro model of emphysema. Regulation of elastin gene expression. J Biol Chem. 1990;265(26):15544–15549. [PubMed] [Google Scholar]
- Geesin JC, Darr D, Kaufman R, Murad S, Pinnell SR. Ascorbic Acid Specifically Increases Type I and Type III Procollagen Messenger RNA Levels in Human Skin Fibroblasts. J Investig Dermatol. 1988;90(4):420–424. doi: 10.1111/1523-1747.ep12460849. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends in Cell Biology. 2003;13(5):264–269. doi: 10.1016/s0962-8924(03)00057-6. [DOI] [PubMed] [Google Scholar]
- Hong H, Stegemann JP. 2D and 3D collagen and fibrin biopolymers promote specific ECM and integrin gene expression by vascular smooth muscle cells. Journal of biomaterials science Polymer edition. 2008;19(10):1279. doi: 10.1163/156856208786052380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. The Extracellular Matrix: Not Just Pretty Fibrils. Science. 2009;326(5957):1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang P-P, Zhang X-H, Rich CB, Foster JA, Subramanian M, Goldstein RH. Activation of elastin transcription by transforming growth factor-β in human lung fibroblasts. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2007;292(4):L944–L952. doi: 10.1152/ajplung.00184.2006. [DOI] [PubMed] [Google Scholar]
- Kumano Y, Sakamoto T, Egawa M, Tanaka M, Yamamoto I. Enhancing effect of 2-O-alpha-D-glucopyranosyl-L-ascorbic acid, a stable ascorbic acid derivative, on collagen synthesis. Biological & pharmaceutical bulletin. 1998;21(7):662–666. doi: 10.1248/bpb.21.662. [DOI] [PubMed] [Google Scholar]
- Barone Leesa M, F B, Chipman Stewart D, Toselli Paul, Oakes Barry W, Franzblau Carl. Alteration of the extracellular matrix of smooth muscle cells by ascorbate treatment. Biochimica et Biophysica Acta. 1985;(840):245–254. doi: 10.1016/0304-4165(85)90125-4. [DOI] [PubMed] [Google Scholar]
- Lin S, Sandig M, Mequanint K. Three-dimensional topography of synthetic scaffolds induces elastin synthesis by human coronary artery smooth muscle cells. Tissue Eng Part A. 2011;17(11-12):1561–1571. doi: 10.1089/ten.TEA.2010.0593. [DOI] [PubMed] [Google Scholar]
- Luchsinger LL, Patenaude CA, Smith BD, Layne MD. Myocardin-related transcription factor-A complexes activate type I collagen expression in lung fibroblasts. The Journal of biological chemistry. 2011;286(51):44116–44125. doi: 10.1074/jbc.M111.276931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoodian F, Peterkofsky B. Vitamin C deficiency in guinea pigs differentially affects the expression of type IV collagen, laminin, and elastin in blood vessels. The Journal of nutrition. 1999;129(1):83–91. doi: 10.1093/jn/129.1.83. [DOI] [PubMed] [Google Scholar]
- Nathan A, Nugent MA, Edelman ER. Tissue engineered perivascular endothelial cell implants regulate vascular injury. Proc Natl Acad Sci U S A. 1995;92(18):8130–8134. doi: 10.1073/pnas.92.18.8130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaglino D, Fornieri C, Botti B, Davidson JM, Pasquali-Ronchetti I. Opposing effects of ascorbate on collagen and elastin deposition in the neonatal rat aorta. European journal of cell biology. 1991;54(1):18–26. [PubMed] [Google Scholar]
- Rishikof D, Lucey E, Kuang P-P, Snider G, Goldstein R. Induction of the myofibroblast phenotype following elastolytic injury to mouse lung. Histochemistry and Cell Biology. 2006;125(5):527–534. doi: 10.1007/s00418-005-0109-6. [DOI] [PubMed] [Google Scholar]
- Sazonova OV, Lee KL, Isenberg BC, Rich CB, Nugent MA, Wong JY. Cell-cell interactions mediate the response of vascular smooth muscle cells to substrate stiffness. Biophysical journal. 2011;101(3):622–630. doi: 10.1016/j.bpj.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegemann JP, Hong H, Nerem RM. Mechanical, biochemical, and extracellular matrix effects on vascular smooth muscle cell phenotype. Journal of Applied Physiology. 2005;98(6):2321–2327. doi: 10.1152/japplphysiol.01114.2004. [DOI] [PubMed] [Google Scholar]
- Suki B, Bates JH. Extracellular matrix mechanics in lung parenchymal diseases. Respir Physiol Neurobiol. 2008;163(1-3):33–43. doi: 10.1016/j.resp.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suki B, Ito S, Stamenovic D, Lutchen KR, Ingenito EP. Biomechanics of the lung parenchyma: critical roles of collagen and mechanical forces. Journal of Applied Physiology. 2005;98(5):1892–1899. doi: 10.1152/japplphysiol.01087.2004. [DOI] [PubMed] [Google Scholar]
- Tsang KY, Cheung MC, Chan D, Cheah KS. The developmental roles of the extracellular matrix: beyond structure to regulation. Cell and tissue research. 2010;339(1):93–110. doi: 10.1007/s00441-009-0893-8. [DOI] [PubMed] [Google Scholar]
- Welch RW, Bergsten P, Butler JD, Levine M. Ascorbic acid accumulation and transport in human fibroblasts. The Biochemical journal. 1993;294(Pt 2):505–510. doi: 10.1042/bj2940505. [DOI] [PMC free article] [PubMed] [Google Scholar]


