Abstract
This study was undertaken to gain insights into the mechanism for Δ9-tetrahydrocannabinol (Δ9-THC)-mediated suppression of primary immunoglobulin M (IgM) responses in humans. An in vitro activation model, which employs cell surface-expressed CD40 ligand (CD40L) and recombinant cytokines [interleukin (IL)-2, IL-6, and IL-10], was used to differentiate human peripheral blood (HPB) naïve B cells into IgM secreting cells. Pretreatment with Δ9-THC significantly decreased the number of IgM secreting cells as determined by ELISPOT. The attenuation of IgM secretion by Δ9-THC involved, at least in part, the impairment of plasma cell differentiation as evidenced by suppression of immunoglobulin joining chain (IgJ) mRNA expression. The analysis at each of two different stages critically involved in plasma cell differentiation indicates that Δ9-THC impaired both the primary activation stage and proliferation of B cells. Interestingly, Δ9-THC selectively suppressed the surface expression of CD80, but not other measured B-cell activation markers (CD69, CD86, and ICAM1). Furthermore, pretreatment with Δ9-THC was accompanied by a robust decrease of STAT3 phosphorylation, whereas the phosphorylation of the p65 NFκB subunit was not affected. Collectively, these data provide new insights into the mechanisms for impaired B cell function by Δ9-THC.
Keywords: cannabinoids, T cell-dependent humoral immune response, immunoglobulin joining chain, STAT3, and CD80
Introduction
Marijuana is the most commonly used illegal drug in the United States for its psychoactive effects, but it is also becoming more commonly used for therapeutic purposes. In addition to increased use of marijuana for medical use in many states, MarinolΔ, the synthetic form of Δ9-THC, is a current, FDA-approved drug, that is indicated for chemotherapy-induced nausea and appetite stimulation in cancer and AIDS patients (Klein 2005). This raises concerns about: 1) the human health impact, especially because in vivo and in vitro studies suggest cannabinoids modulate the immune system [reviewed in (Croxford and Yamamura 2005)]; and 2) undesirable immunosuppressive side effect(s) of these drugs in patients whose immune system has already been compromised.
Δ9-THC has been demonstrated to modulate a variety of immune responses, of which the primary IgM antibody response against the T cell-dependent antigen, sheep erythrocytes (sRBC), is one of the most sensitive to suppression by cannabinoids (Kaminski et al. 1992; Schatz et al. 1993). Early studies suggested that cannabinoids primarily targeted accessory cells, such as the T cell, because Δ9-THC did not suppress IgM antibody forming cell responses induced by the T-cell independent antigen, dinitrophenyl-Ficoll, or the polyclonal B cell activator, lipopolysaccharide [LPS; (Schatz et al. 1993)]. However, advances in the ability to activate B cells and detect IgM production actually demonstrate that B cells are also affected by cannabinoids (Springs et al. 2008). Specifically, activation of B cells with irradiated CD40L-expressing L cells via the CD40L-CD40 interaction allows for assessment of CD40-dependent signaling in B cells in the absence of T cells (Ahmadi et al. 2008; Lu et al. 2009). Indeed, Δ9-THC suppressed IgM antibody production by CD40L-activated mouse B cells (Springs et al. 2008).
The CD40-CD40L interaction is critical for all stages involved in B cell to plasma cell differentiation, which results in antibody secretion (Bishop and Hostager 2001). Following initial contact with an antigen, B cells undergo clonal expansion, isotype switching, affinity maturation, and differentiation to plasma cells (or a subset of memory cells). The antibodies that are secreted initially are predominantly of the IgM isotype [reviewed in (Howard and Paul 1983)]. As IgM is secreted in its pentameric form, the IgJ polypeptide is necessary for polymerization of the secreted IgM and transcription of the IGJ gene occurs only in terminally differentiated plasma cells (Lamson and Koshland 1984; Niles et al. 1995). The differentiation process of B cells to plasma cells is tightly controlled by the dynamic expression of several transcription factors. For instance, the level of PAX5, a transcription factor that controls many B-cell characteristics, decreases, followed by the concomitant upregulation of Blimp1 (gene PRDM1), also known as the master regulator of plasmacytic differentiation [reviewed in (Alinikula and Lassila 2011)]. With the relatively recent finding that mouse B cells can be direct targets of suppression by Δ9-THC (Springs et al. 2008), the objective of the present study was to extend our investigation by evaluating whether Δ9-THC suppresses in vitro primary IgM antibody responses by human primary B cells and, if so, to elucidate critical events that are involved.
Materials and Methods
Reagents
Δ9-THC dissolved in 100% ethanol was provided by the National Institute on Drug Abuse (Bethesda, MD). Preliminary data demonstrated that human B cells are very sensitive to ethanol (data not shown). Therefore, for these studies, the ethanol was evaporated under nitrogen and the Δ9-THC was dissolved in 100% dimethyl sulfoxide (DMSO). Although the Δ9-THC concentrations used in this study range from 1–15 μM which are approximately 1.5–25 fold higher than peak plasma concentration of Δ9-THC found in marijuana smokers (Grotenhermen 2003), these concentrations are relevant for in vitro studies as previously discussed (Ngaotepprutaram et al. 2013). Unless otherwise noted, all other chemicals were obtained from Sigma-Aldrich (St Louis, MO).
Antibodies
Purified anti-human IgM antibody (clone Il/41) obtained from BD Biosciences (San Diego, CA) and Biotin-conjugated anti-human IgM antibody obtained from Sigma-Aldrich were used in ELISPOT assay. The following antibodies obtained from Biolegend (San Diego, CA) were used for staining surface expression of B cell activation markers; PE/Cy7 anti-human CD69 (clone FN50), PE/Cy5 anti-human CD80 (clone 2D10), PE anti-human CD86 (clone IT2.2), and APC anti-human CD54 (clone HCD54). The following antibodies used for staining of intracellular phosphorylated kinases; Alexa Fluor 647 Mouse Anti-STAT3 (pY705) (clone 4/P-STAT3) and Alexa Fluor 647 Mouse Anti-NFkB p65 (pS529) (clone K10–895.12.50) were obtained from BD Biosciences.
Preparation of CD40L-L cells
The stably transfected mouse fibroblast line expressing human CD40L (CD40L-L cells) was generous gifted from Dr. David Sherr (Boston University School of Public Health) and was prepared for in vitro IgM activation model as previously described (Lu et al. 2009). Briefly, CD40L-L cells were cultured in DMEM complete media [Dulbecco’s modified Eagle Medium (Gibco Invitrogen, Carlsbad, CA) supplemented with 10% bovine calf serum (Hyclone, Logan, UT), 50 μM 2-mercaptoethanol, 1X HT supplement (Gibco Invitrogen), and the antibiotics Penicillin (100 units/mL)/Streptomycin (100 μg/mL) (Gibco Invitrogen)] at 37°C in 5% CO2 3–4 days prior to irradiation. CD40L-L cells were trypsinized with 0.25% Trypsin-EDTA (Gibco Invitrogen) for 2–3 min at 37°C. After washing once with complete media, CD40L-L cells were resuspended in 500 μL complete media and X-ray-irradiated at 3500 Gy (XRAD-320, Precision X-Ray, North Branford, CT). The irradiated CD40L-L cells were washed once with complete media, seeded into 96-well plate at 1.5×103 cells/well, and incubated at 37°C in 5% CO2 1 day prior experimentation.
Isolation of HPB naïve B cells
Human leukocyte packs were obtained commercially from anonymous donors (Gulf Coast Regional Blood Center, Houston, TX). Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats by density gradient centrifugation using Ficoll-Paque Plus (GE Healthcare, Piscataway, NJ). HPB naïve B cells were isolated from human PBMCs by depletion of non-B cells and non-naïve B cells (negative selection) using MACS Naïve Human B Cells Isolation Kits following the manufacturer’s protocol (Miltenyl Biotec, Auburn, CA). HPB naïve B cells were maintained in RPMI complete media [RPMI medium (Gibco Invitrogen) supplemented with 10% heat inactivated bovine calf serum, and the antibiotics Penicillin (100 units/mL)/Streptomycin (100 μg/mL)] at 37°C in 5% CO2. The purity of isolated naïve B cells generally exceeded 92%.
In vitro CD40L-dependent polyclonal IgM antibody response
HPB naïve B cells (1×106 cells/mL) were pretreated with Δ9-THC or vehicle (0.02% DMSO) for 30 min prior to inducing the primary IgM response in vitro using CD40L-L cells plus cytokines as previously described (Lu et al. 2009). VH or Δ9-THC was present for the duration of the activation period without washing the cells following the 30-min pretreatment. Pretreated HPB naïve B cells (1.5×105 cells) were co-cultured with irradiated CD40L-L cells. The culture was supplemented with 10 U/mL of recombinant human IL-2 (Roche Applied Science, Indianapolis, IN, USA), 100 U/mL of recombinant human IL-6 (Roche Applied Science, Indianapolis, IN, USA), and 20 ng/mL of recombinant IL-10 (BioVision, Inc., Milpitas, CA). After 4 days of culture, B cells were transferred to a new 96-well plate without CD40L-L cells, and were cultured for an additional 3 days, after which the cells were harvested for IgM ELISPOT. Cell viability was determined using pronase as described previously (Schatz et al. 1993).
IgM ELISPOT
The number of IgM secreting cells were determined by IgM ELISPOT as previously described (Lu et al. 2009). Briefly, ELISPOT wells were coated with purified anti-human IgM antibody overnight at 4°C. The culture plates were washed with phosphate buffered saline (PBS) containing 0.1% Tween-20 and water. ELISPOT wells were then treated with blocking buffer containing 5% bovine serum albumin (BSA; Calbiochem, San Diego, CA) in PBS for 30 min at 37°C, to minimize nonspecific binding of immunoglobulins to the wells. Harvested cells were washed, diluted to the appropriate density, and incubated in the ELISPOT wells for 16–20 h at 37°C in 5% CO2. Cells were removed from the wells and the culture plates were washed with PBS containing 0.1% Tween-20 and water. For detection, Biotin-conjugated anti-human IgM antibody and streptavidin-horseradish peroxidase were added to the wells. The spots were developed with the aminoethylcarbazole staining kit. Data were collected and analyzed using the CTL ImmunoSpot system (Cellular Technology Ltd, Shaker Heights, OH).
Flow cytometry analysis
At the indicated time points, 0.5 to 1×106 cells were harvested. When necessary, dead cells were detected by staining with Live/Dead Fixable Dead Cell Stain Kit (near-infrared dye, Invitrogen). Briefly, cells were washed once with 1X Hank’s Balanced Salt Solution (HBSS, Gibco Invitrogen) following by incubation with near-infrared dye (Invitrogen) for 20 min at 4°C per manufacturer’s protocol. Surface Fc receptors were blocked by incubating with 20% human AB serum (Invitrogen) for 15 min at 4°C. The amount of antibodies used varied in staining for each specific antigen based on preliminary antibody titration and were typically pre-diluted in FACS buffer [1X HBSS containing 1% BSA and 0.1% sodium azide, pH 7.4–7.6] at appropriate amounts prior to addition to the cells. Staining for phosphorylated kinases was conducted on the same day and immediately followed by analysis with FACSCanto II (BD Biosciences). Data were analyzed using Kaluza (Bechman Coulter, Miami, FL) or FlowJo software (TreeStar, Ashland, OR).
Based on kinetic studies (data not shown), surface expression of ICAM1 was assessed by staining with APC anti-human CD54 on day 1 post activation; whereas surface expression of CD69, CD80, and CD86 were assessed by simultaneously staining with PE/Cy7 anti-human CD69, PE/Cy5 anti-human CD80, and PE anti-human CD86 on day 3 post activation. After 30 min incubation at 4°C in the dark, unbound antibodies were removed by washing once with FACS buffer. Cells were fixed with BD CytoFix™ Buffer (BD Biosciences) for 10 min at 4°C in the dark, followed by washing once with FACS buffer. Stained cells were then resuspended in FACS buffer and analyzed.
For staining of intracellular phosphorylated kinases, HPB naïve B cells were equilibrated at 37°C in 5% CO2 for 3 h to normalize baseline kinase activity. Cells were then pretreated with VH or Δ9-THC for 30 min, following by activation with recombinant CD40L (Enzo Life Sciences, Inc, Farmingdale, NY) plus IL-2, -6, and -10 for 10 min at 37°C in a water bath. Cells were fixed in 1.5% formaldehyde by directly diluting in cell culture from 32% stock (electron microscopy grade, Electron Microscopy Sciences, Hartfield, PA) for 10 min at 37°C followed by centrifugation at 600 x g for 6 min at 4°C. Cells were then permeabilized by drop-wise adding ice-cold 100% methanol while vortex mixing at medium speed and were stored in methanol at −80°C until ready to stain with indicated antibodies. Cells were washed 3 times with FACS buffer. Surface Fc receptors were blocked by incubating with 20% human AB serums (Invitrogen) at 4°C. The level of phosphorylated STAT3 (pSTAT3), and phosphorylated p65 (pp65) was assessed by simultaneously staining with Alexa Fluor 647 Mouse Anti-STAT3 and Alexa Fluor 647 Mouse Anti-NFκB p65 for 60 min at room temperature in the dark. Unbound antibodies were removed by washing twice with FACS buffer. Stained cells were then resuspended in FACS buffer and analyzed.
Proliferation assay
B cell proliferation studies were conducted as described previously (Lu et al. 2009). In brief, HPB naïve B cells were labeled by incubation with 5 μM of carboxyfluorescein succinimidyl ester (CFSE) (CellTrace Cell Proliferation Kits, Invitrogen) at 5×106 cells/mL following manufacturer’s protocols. The labeled cells were washed in complete medium, and then adjusted to the desired cell density prior to pretreatment with VH (0.02% DMSO) or Δ9-THC for 30 min. Cells were then co-cultured with irradiated CD40L-L cells plus IL-2, -6, and -10. After 4 days of culture, B cells were transferred to a new 96-well plate without CD40L-L cells, and were cultured for an additional 3 days. The cells were harvested on day 7 for flow cytometric analysis.
Real time polymerase chain reaction (RT-PCR)
Total RNA was isolated from activated human B cells (approximately 1×106 cells) using an RNAeasy Kit (Qiagen, Valencia, CA) at indicated time points. RNA was quantified using a Nanodrop 1000 (Thermo Scientific, Wilmington, DE). Total RNA was reverse-transcribed into cDNA using random primers with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). TaqManΔ Gene Expression Assay primers for target gene [human CD80 (Hs00175478_m1), human IGJ (Hs00376160_m1), human PAX5 (Hs00277134_m1), and human PDRM1 (Hs00153357_m1)] were purchased from Applied Biosystems. The relative mRNA levels of target genes were determined using ABI PRISMΔ 7900HT Sequence Detection System (Applied Biosystems). The fold-change value of relative mRNA levels of target genes were calculated using the ΔΔ-Ct method as described previously (Livak and Schmittgen 2001) and normalized to the endogenous reference, 18s rRNA.
Statistical analyses
GraphPad Prism 4.00 (Graphpad Software, San Diego, CA) was used for all statistical analysis. Data acquired as percentage of control from either ELISPOT or flow cytometry were transformed into log scale before performing statistical analysis. In the case of mRNA data, the transformed fold-change values were used in statistical analysis. For comparisons among treatment groups, one-way ANOVA was used. Dunnett’s post-hoc test was used to test for significance between treatment groups and control. Outliers were eliminated using a Grubb’s test. A value of p < 0.05 was considered significant.
Results
1. Δ9-THC attenuated CD40L plus cytokine-induced primary IgM responses in HPB B cells
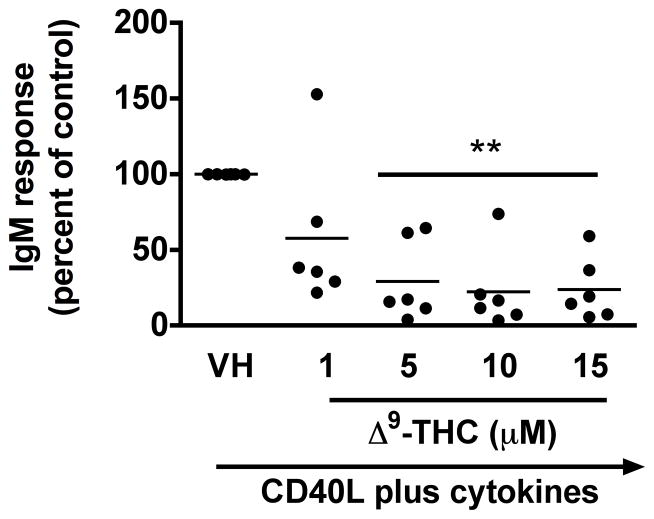
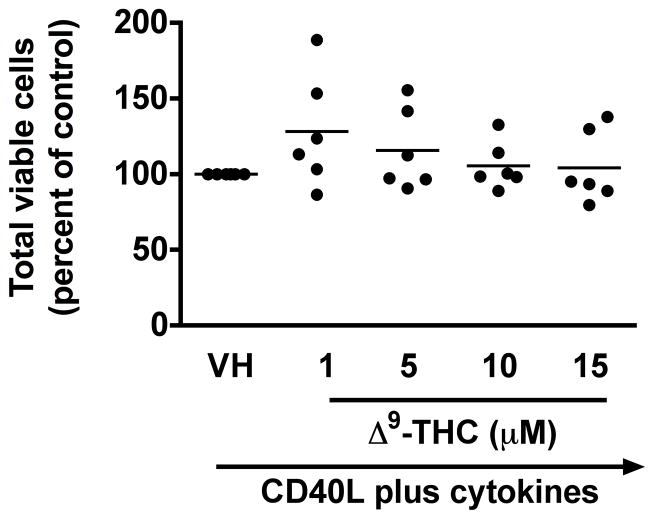
Previously, we demonstrated a marked suppression of the in vitro T cell-dependent IgM antibody response by Δ9-THC in the mouse, which was due, in part, to a direct effect on B cells (Springs et al. 2008). Our laboratory also established a similar method of human B cell activation (Lu et al. 2009) and therefore, the effect of Δ9-THC on the primary antibody response in human B cells was investigated. The effect of Δ9-THC on IgM responses was assessed using B cells from 6 donors and the results are presented as a percentage of control (VH-treated group). Pretreatment with Δ9-THC significantly decreased the percentage of IgM secreting cells induced by CD40L plus cytokines (Figure 1a), but not the total number of viable cells (Figure 1b). These results suggest that Δ9-THC-mediated suppression of the IgM response by HPB B cells involves perturbation of differentiation of naïve B cells into IgM antibody secreting cells rather than through direct cytotoxicity.
Fig. 1. Effect of Δ9-THC on CD40L plus cytokine-induced IgM secreting cell response in humans.
HPB naïve B cells were pretreated with Δ9-THC at indicated concentrations or VH (0.02% DMSO) and then activated with CD40L plus cytokines. IgM secreting cells were enumerated by ELISPOT on day 7. The total viable cells were counted by Z1 Coulter Counter following pronase treatment. Data were normalized to the VH-treated group and presented as percent of control with at least three replicates per groups. (a) Scatter plot represents the number of IgM secreting cells. The mean ± SEM between human subjects from VH group was 1368 ± 189.6 spots/106 viable cells. (b) Scatter plot represents the total viable cells. The mean ± SEM between human subjects from VH group was 47404±189.6 viable cells. ** p < 0.01 as compared to VH-treated group. Each dot represents data from one individual donor.
2. Δ9-THC suppressed CD40L plus cytokine-induced surface expression of CD80, but not CD69, CD86, or ICAM1 in HPB B cells
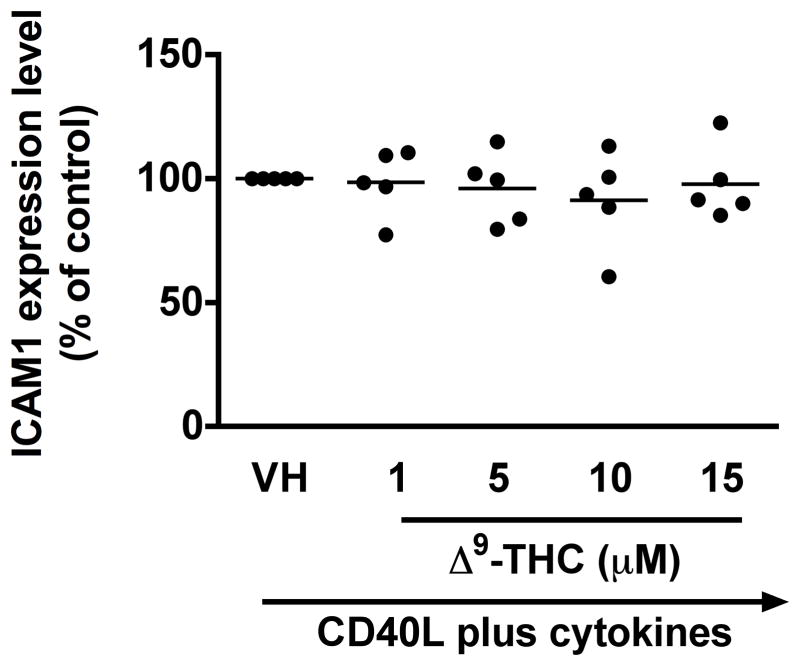
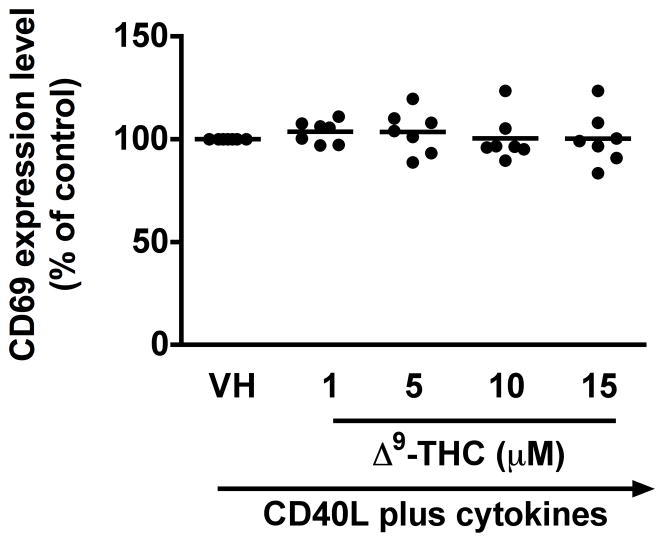
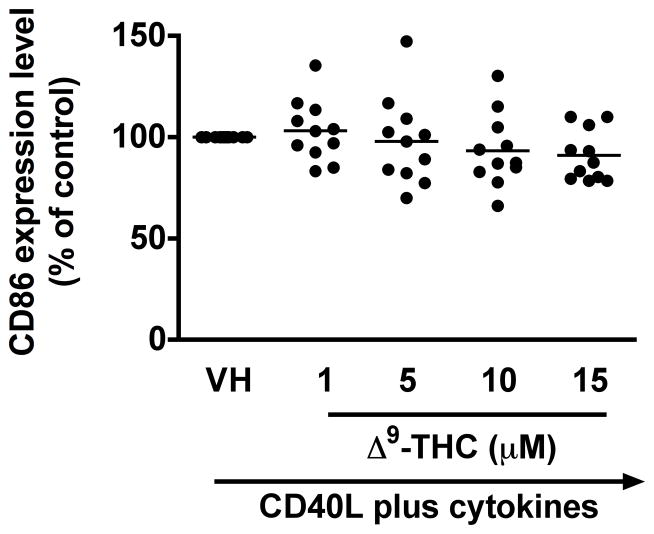
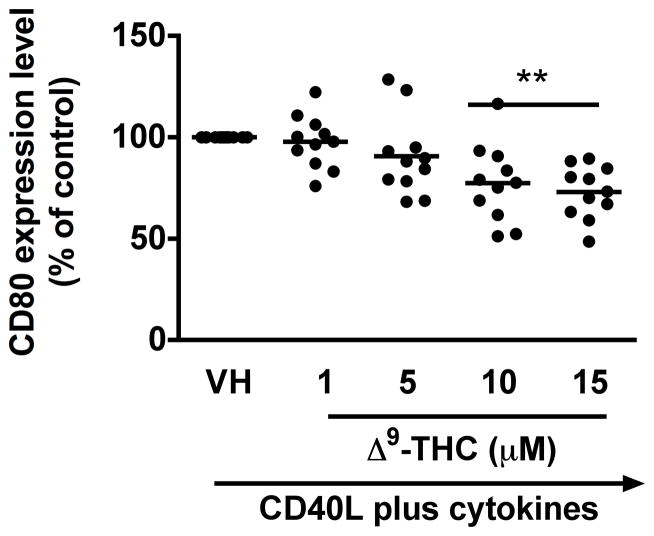
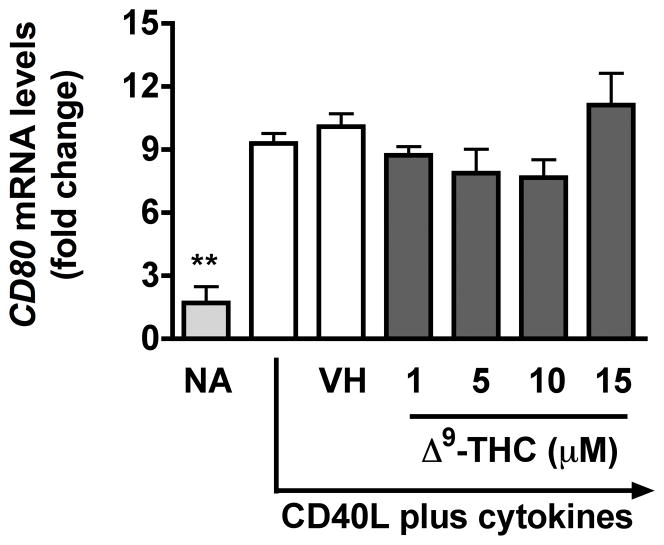
To identify which stages of B cell differentiation are modulated by Δ9-THC, we first examined whether Δ9-THC affects B cell activation by assessing surface expression of CD80, CD86, CD69, and ICAM1 on activated B cells by flow cytometry. Kinetic studies showed that surface expression of ICAM1 peaked on day 1, whereas the peak expression of surface CD80 was on day 3 post activation (data not shown). The magnitude of surface expression for both CD69 and CD86 by HPB B cells was highest on day 4 post activation (Lu et al. 2009). With the limited number of HPB naïve B cells isolated from each donor, the effect of Δ9-THC was investigated on day 1 and/or day 3 post activation. Pretreatment with Δ9-THC did not affect the upregulation of CD86 (Fig 2a and 2b), ICAM1 or CD69 (data not shown), but significantly suppressed the upregulation of surface CD80 (Fig 2c and 2d). As the induction of surface CD80 by CD40 ligation in B cells is primarily regulated at the level of transcription (Ishikawa et al. 2002; Niu et al. 2003), we next investigated whether Δ9-THC affected the levels of CD80 mRNA by RT-PCR. Pretreatment with Δ9-THC did not affect CD80 mRNA expression induced by CD40 plus cytokines in activated HPB B cells (Fig 3). These data demonstrate that Δ9-THC impaired CD40L plus cytokine-induced B-cell activation, as evidenced by an attenuation of surface CD80.
Fig. 2. Effect of Δ9-THC on surface expression of activation markers in activated HPB B cells.
HPB naïve B cells were pretreated with Δ9-THC at indicated concentrations or VH (0.02% DMSO) and then activated with CD40L plus cytokines. Surface expression of B-cell activation markers were determined by flow cytometric analysis. (a) Scatter plot represents MFI of CD86 gated on viable cells. Data were normalized to the VH-treated group and presented as percent of control. The mean ± SEM between human subjects from VH group was 34.9 ± 10.5. (b) A representative dot plot of surface CD86 expression. Numbers in parentheses represents the percentage of CD86+. (c) Scatter plot represents MFI of CD80 gated on viable cells. Data were normalized to the VH-treated group and presented as percent of control. The mean ± SEM between human subjects from VH group was 30.8 ± 4.5. (d) A representative dot plot of surface CD80 expression. Numbers in parentheses represent the percentage of CD80+. The unstimulated HPB B cells express each surface marker with the average less than 3%. ** p < 0.01 as compared to VH-treated group. Each dot represents data from one individual donor.
Fig. 3. Effect of Δ9-THC on CD80 mRNA levels in activated HPB B cells.
HPB naïve B cells were pretreated with Δ9-THC at indicated concentrations or VH (0.02% DMSO) and then activated with CD40L plus cytokines. CD80 mRNA levels were determined by real-time PCR on day 2. The fold difference of CD80 mRNA molecules relative to nonactivated resting cells (naïve, NA) was normalized using the endogenous reference, 18s rRNA. Results are representative of three different donors with four replicates per treatment group.
3. Δ9-THC impaired CD40L plus cytokine-induced proliferation of HPB B cells
The clonal expansion of activated B cells is another critical step during plasma cell differentiation [reviewed in (Shapiro-Shelef and Calame 2005)]. We therefore examined the effect of Δ9-THC on CD40L plus cytokine-induced proliferation of activated B cells by flow cytometry on day 7. HPB naïve B cells were labeled with CFSE dye before pretreatment with VH or Δ9-THC. As shown in Figure 4, stimulation with CD40L plus cytokines induced B cells to undergo multiple divisions, as revealed by the dilution of CFSE signal. In general, Δ9-THC did not affect the number of cell divisions, but rather significantly decreased the number of cells that progressed to the latest round of division, thereby retaining B cells in earlier cell divisions (Table 1). These results suggest that attenuation of B-cell proliferation plays a role in Δ9-THC-mediated suppression of CD40 plus cytokine-induced IgM response by HPB B cells.
Fig. 4. Δ9-THC impaired CD40L plus cytokine-induced proliferation of HPB B cells.
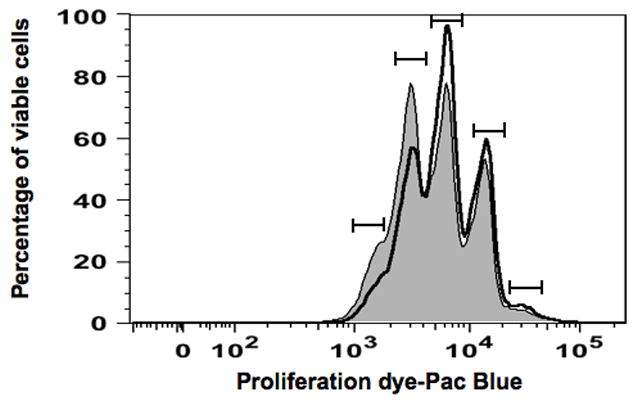
HPB naïve B cells were labeled with the proliferation dye prior to pretreatment with Δ9-THC at indicated concentrations or VH (0.02% DMSO) and then activated with CD40L plus cytokines. The concatenated data from three replicates per treatment group were used. Histogram plot represents proliferation profile compared between groups treated with either VH (shaded) or 15 μM Δ9-THC (open). Each peak defined by (
 ) represents a separate cell division, with cell division 0 (non-proliferating cells) on the right side of the histogram and cell division 4 (rapidly proliferating cells) on the left side of the histogram. Results are representative of five different donors. Data from the entire THC concentration response study are provided in Table 1.
) represents a separate cell division, with cell division 0 (non-proliferating cells) on the right side of the histogram and cell division 4 (rapidly proliferating cells) on the left side of the histogram. Results are representative of five different donors. Data from the entire THC concentration response study are provided in Table 1.
Table 1.
Δ9-THC impairs CD40L plus cytokine-induced proliferation of HPB B cells.
| Groups | CD4PL | VH | Δ-THC (μM) | |||
|---|---|---|---|---|---|---|
| Cell division at day 7 | 1 | 5 | 10 | 15 | ||
| 0 | 1.87±0.11 | 1.92±0.09 | 2.24±0.15 | 2.41±0.14 | 2.40±0.07 | 2.63±0.21* |
| 1 | 15.63±0.85 | 15.03 ±0.41 | 15.33±0.35 | 17.00±0.83 | 17.97±0.33 | 17.77±1.27 |
| 2 | 15.43±0.62 | 12.33±0.58 | 13.53±0.67 | 14.60±0.64 | 16.00±0.34** | 15.33±0.99* |
| 3 | 26.53±0.66 | 24.30±0.75 | 26.57±0.60 | 28.90±0.55** | 30.87±0.47** | 30.07±0.43** |
| 4 | 24.30±0.36 | 24.73±0.26 | 23.80±0.32 | 21.17±0.19** | 20.00±0.64** | 19.83±0.68** |
Summary of effects of various concentrations of THC on B cell proliferation (only VH and 15 μM THC are depicted in Figure 4 for simplicity). Cell division 0 represents non-proliferating cells, whereas cell division 4 represents rapidly proliferating cells. Numbers are the percentage of viable cells ± SEM at each cell division.
p < 0.05,
p < 0.01 as compared to VH-treated group within a cell division. Data are representative of 5 donors with three replicates per treatment group.
4. Δ9-THC suppressed CD40L plus cytokine-induced mRNA expression of IGJ in HPB B cells
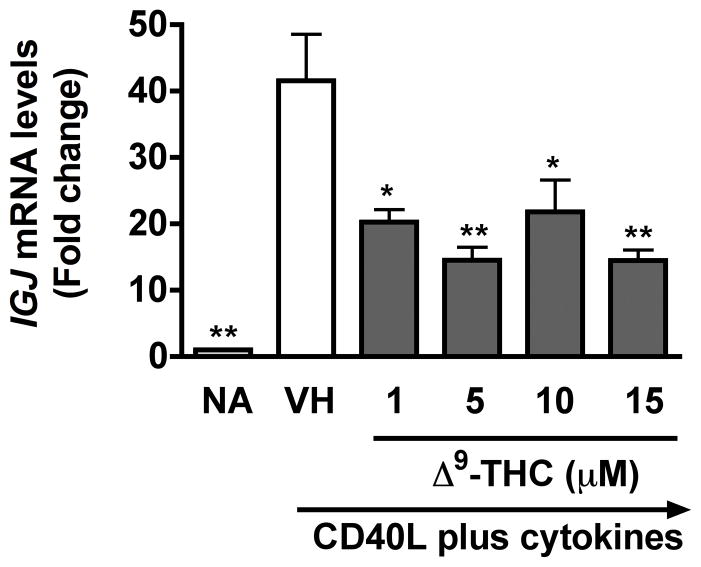
To investigate whether Δ9-THC perturbs the transcriptional regulatory network of terminally differentiated plasma cells, the mRNA expression of genes critical in plasmacytic differentiation including, but not limited to, PRDM1 (encodes Blimp-1), PAX5, and IGJ was assessed by RT-PCR on day 6 post activation, which was the peak time of induction (Lu et al. 2011). Pretreatment with Δ9-THC did not affect the upregulation of PRDM1 mRNA levels or the downregulation of PAX5 mRNA levels (data not shown), but significantly suppressed the upregulation of IGJ mRNA levels in activated HPB B cells (Fig. 5). These results demonstrate that attenuation of IgM responses by Δ9-THC involved impairment of IGJ mRNA expression.
Fig. 5. Δ9-THC suppressed CD40L plus cytokine-induced IGJ mRNA levels in activated HPB B cells.
HPB naïve B cells were pretreated with Δ9-THC at indicated concentrations or VH (0.02% DMSO) and then activated with CD40L plus cytokines. IGJ mRNA levels were determined by real-time PCR on day 6. The fold difference of IGJ mRNA expression relative to nonactivated resting cells (naïve, NA) was normalized using the endogenous reference, 18s rRNA. * p < 0.05, ** p <0.01 as compared to VH-treated group. Results are representative of three different donors with four replicates per treatment group.
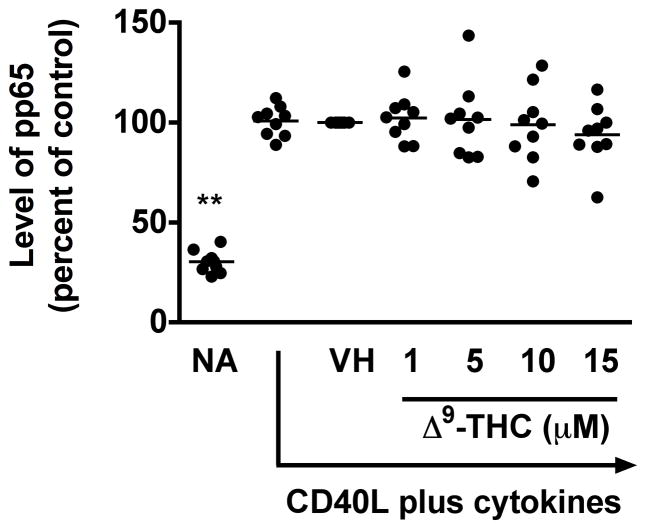
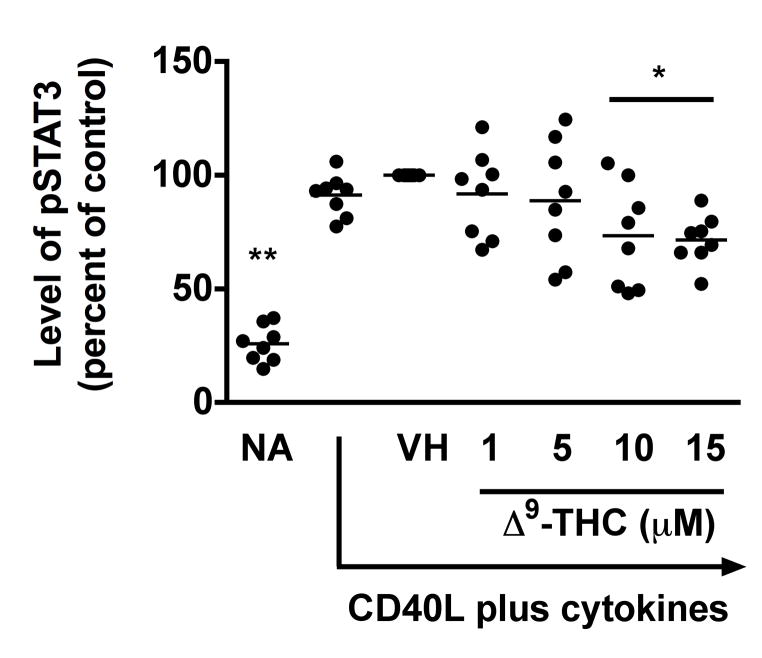
5. Δ9-THC suppressed CD40L plus cytokine-induced phosphorylation of STAT3, but not p65 NFκB, in HPB B cells
The mechanism by which normal human B cells respond to external signals is not entirely clear, but suppression of NFκB signaling has a profound effect on CD40-mediated B-cell proliferation and differentiation (Lu et al. 2007). In addition, STAT signaling is involved in cell survival, proliferation and differentiation [reviewed in (Chen et al. 2012)]. Specifically, STAT3 induced by IL-6 promotes both B cell proliferation and differentiation into plasma cells (Faris et al. 1997). In light of the above, we further investigated the effect of Δ9-THC on the immediate activation of p65 NFκB and STAT3. Due to the dynamic changes in phosphorylation status, we selected an early time point after activation to increase sensitivity of detection, and therefore, the phosphorylation status of p65 NFκB and STAT3 in activated HPB B cells was assessed by flow cytometry at 10 min post activation. In these studies, soluble recombinant human CD40L in combination with IL-2, -6, and -10 was utilized to promote rapid activation of the signaling pathways of interest. Stimulation of HPB naïve B cells with recombinant CD40L plus cytokines for 10 min induced phosphorylation of p65 and STAT3 (Fig 6a–6c). Pretreatment with Δ9-THC did not alter the level of phosphorylated p65 (pp65) (Fig 6a), but significantly suppressed the level of phosphorylated STAT3 (pSTAT3) in a concentration-dependent manner (Fig 6b and 6c). These results suggest that STAT3, or signaling events upstream of STAT3, are targeted by Δ9-THC.
Fig. 6. Effect of Δ9-THC on the phosphorylation of p65 NFκB and STAT3 in activated HPB B cells.
HPB naïve B cells were pretreated with Δ9-THC at indicated concentrations or VH (0.02% DMSO) and then activated with CD40L plus cytokines. Cells were fixed with 1.5% paraformaldehyde and permeabilized with ice-cold 100% methanol. The intracellular expression of pSTAT3 and pp65 NFκB was assessed by phosflow cytometry. (a) Scatter plot represents MFI of pp65 NFκB. Data were normalized to the VH-treated group and presented as percent of control. The mean ± SEM between human subjects from VH group was 2.1 ± 0.2. (b) Scatter plot represents the level of pSTAT3. Data were normalized to the VH-treated group and presented as percent of control. The mean ± SEM between human subjects from VH group was 1.3 ± 0.2. (c) A representative dot plot of pSTAT3 level. Numbers in parentheses represent the percentage of pSTAT3+. ** p < 0.01 as compared to VH-treated group. Each dot represents data from one individual donor.
Discussion
In this study, we demonstrate that Δ9-THC significantly attenuated the primary antibody response by HPB B cells activated with CD40L plus cytokines. Our results are consistent with a previous study investigating mouse splenic B cells in which treatment with Δ9-THC decreased the number of IgM secreting cells induced by CD40L plus cytokines (Springs et al. 2008). These results clearly demonstrate that the B cells are directly impaired by Δ9-THC in the suppression of IgM antibody responses induced by CD40L plus cytokines in both mouse and humans.
Not only terminally differentiated plasma cells, but also activated B cells can secrete IgM (Lamson and Koshland 1984). Thus, the decrease of IgM secreting cells by Δ9-THC suggests that Δ9-THC perturbs the differentiation of naïve B cells into plasma cells and/or the activation of naïve B cells. It is noteworthy that CP55940, a synthetic cannabinoid receptor agonist, promotes antibody class switching to IgE by splenic mouse B cells when cultured in the presence of IL-4 (Agudelo et al. 2008). Thus, while it is possible that Δ9-THC reduces IgM through promotion of isotype switching, this is unlikely to happen here as our activation model was optimized to produce IgM (i.e., in the absence of the class-switch promoting cytokine IL-4), but not other subtypes (Lu et al. 2009).
Here we demonstrate that Δ9-THC selectively altered the initial activation of HPB B cells as evidenced by significant suppression of surface CD80 expression induced by CD40L plus cytokines. However, the expression of other B cell surface molecules, CD69, CD86, or ICAM1, were not affected by treatment with Δ9-THC. These aforementioned molecules play critical roles in T cell-B cell interactions, especially CD80 and CD86 [reviewed in (Greenwald et al. 2005)]. Both CD80 and CD86 serve as ligands for CD28 or CTLA4 expressed on activated CD4+ T cells, thereby increasing or decreasing the T cell activation signal, respectively (Collins et al. 2002). Therefore, the attenuation of surface CD80 expression on activated B cells by Δ9-THC could play an important role in the initial phase of plasma cell differentiation by disrupting the antigen presentation capacity of B cells. A recent study suggested that CD80 plays an important role in the formation and/or maintenance of long-live plasma cells, in which the antibody production from long-lived plasma cells, but not plasmablasts, was impaired in CD80-deficient mice (Good-Jacobson et al. 2012). Therefore, it is possible that the selective inhibitory effect of Δ9-THC on the upregulation of CD80 might impair the production of high-affinity long-lived plasma cells and memory B cells. The exact mechanism(s) by which Δ9-THC mediates suppression of surface CD80 expression is still unknown and warrants further investigation, but our observation that Δ9-THC did not affect the level of CD80 mRNA suggests that Δ9-THC regulates the expression of surface CD80 at the post-transcriptional level. One possibility is Δ9-THC increases internalization and degradation of surface CD80. Although regulation of CD80 degradation is not understood, the ubiquitin-dependent degradation of surface CD86 has been shown to involve ubiquitin ligase membrane-associated RING-CH-1 (MARCH1) (Corcoran et al.). While overexpression of MARCH1 did not affect the surface expression of CD80 (Corcoran et al.), the possibility that Δ9-THC may facilitate the internalization and/or degradation of CD80 by altering a yet-unknown ubiquitin ligase cannot be ruled out.
Our finding that Δ9-THC impaired the ability of activated B cells to divide correlates well with the suppressive effects observed on the generation of IgM secreting cells since differentiation into Ig-secreting cells is also linked to cell division (Hodgkin et al. 1996; Tangye et al. 2003). The anti-proliferative effects as well as suppression of the IgM response by Δ9-THC cannot be attributed to cell viability. Although Δ9-THC and other cannabinoids have been shown to induce apoptosis in immune cells [reviewed in (Rieder et al. 2009)], in the present study there was no evidence of cell loss. Next we investigated whether Δ9-THC impaired the transcriptional regulatory network involved in plasmacytic differentiation and demonstrated that Δ9-THC significantly suppressed IGJ mRNA expression, but did not alter the mRNA level of PRDM1, “the master regulator” of plasma cell differentiation, or PAX5, one of critical transcription factors in maintaining B cell identity [reviewed in (Alinikula and Lassila 2011)]. Taken together, these results suggest that suppression by Δ9-THC of the primary IgM response in HBP B cells is, at least in part, mediated through the impairment of IgJ expression.
Δ9-THC treatment also attenuated the phosphorylation of STAT3, but not p65 NFκB, in response to recombinant CD40L and cytokines. It is well established that CD40 signals mainly through NFκB, whereas cytokines signal mainly through STAT pathways; in particular IL-10 induces phosphorylation of STAT3 (Lafarge et al. 2011). STAT3 is necessary for the generation of plasma cells by promoting B cell survival in vitro (Levy and Brouet 1994; Otero et al. 2006) and increasing the upregulation of Blimp1 (Diehl et al. 2008). Further, mutations of STAT3 were associated with decreased induction of Blimp1 in response to recombinant CD40L plus IL-10 or IL-21 (Avery et al. 2010). Taken together, this finding suggests that suppression of IgM responses by Δ9-THC is mediated, at least in part, through attenuation of STAT3 activation in activated HPB cells.
The inability of Δ9-THC to modulate the phosphorylation of p65 NFκB in HPB B cells is in contrast to previous studies from our laboratory demonstrating that cannabinol, another immunomodulatory plant-derived cannabinoid, primarily suppressed the DNA binding activity of p65 and c-Rel in mouse T cells (Herring and Kaminski 1999). Similarly, Δ9-THC also suppressed the DNA-binding activity of NFκB in activated human T cells (Ngaotepprutaram et al. 2013). These findings suggest that cannabinoids exhibit stimulation- and/or cell type-specific inhibition of NFκB. It is noteworthy that NFκB is one of the critical transcription factors involved in the regulation of genes encoding CD69, CD80, CD86, or ICAM1 (Craxton et al. 1998; Lopez-Cabrera et al. 1995; Zhao et al. 1996; Zou and Hu 2005). Therefore, the lack of an effect of Δ9-THC on CD40-mediated phosphorylation of p65 NFκB supports our findings that Δ9-THC did not affect cell surface expression of CD86, CD69 or ICAM1 or gene transcription of CD80.
Collectively, this present study demonstrates that suppression by Δ9-THC of the IgM response by human primary B cells is mediated, at least in part, through impairment of plasmacytic differentiation as evidenced by significant suppression of IGJ gene and the activation of STAT3. In addition, our results suggest that perturbation of B cell proliferation by Δ9-THC may play an important role in the generation of IgM secreting cells. Finally, we provided the first conclusive evidence that Δ9-THC selectively suppresses surface expression of CD80 in activated human B cells. Taken together, these critical observations are important for two reasons. First, understanding the mechanism(s) by which Δ9-THC suppresses B cell function provides additional information about the suppressive effect of Δ9-THC on the immune response, which is important in weighing the risk to benefit ratio of marijuana use in immunocompromised patients. Second, the novel identification that Δ9-THC selectively suppresses surface CD80 expression and STAT3 phosphorylation in activated HPB B cells might allow for the development of effective and safer therapeutic strategies in the treatment of diseases mediated by excessive CD80 activation, such as Minimal Change Disease, the most common nephrotic syndrome in children [reviewed in (Ishimoto et al. 2011)] or diffuse large B cell lymphoma, which has constitutive activation of STAT3 (Ding et al. 2008). In conclusion, these data provide new insights into the mechanisms for impaired B cell function by Δ9-THC.
Acknowledgments
We thank Dr. David Sherr at Boston University School of Public Health for generously providing human CD40L-expressing mouse fibroblast. We also thank Ashwini Phadnis-Moghe for helpful discussion and Mrs. Kimberly Hambleton for assistance in submitting the manuscript. This work was supported in part by National Institute of Health grant RO1 DA07908 to N.E.K., and Royal Thai Government Scholarship to T.N.
Abbreviations
- CD40L
CD40 ligand
- CFSE
carboxyfluorescein succinimidyl ester
- Δ9-THC
Δ9-tetrahydrocannabinol
- HPB
human peripheral blood
- IgM
immunoglobulin M
- ICAM
intercellular adhesion molecule
- IL
interleukin
- NA
naive
- STAT
signal transducer and activator of transcription
- VH
vehicle
Footnotes
Conflicts of interest
The authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agudelo M, Newton C, Widen R, Sherwood T, Nong L, Friedman H, Klein TW. Cannabinoid receptor 2 (CB2) mediates immunoglobulin class switching from IgM to IgE in cultures of murine-purified B lymphocytes. J Neuroimmune Pharmacol. 2008;3:35–42. doi: 10.1007/s11481-007-9088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi T, Flies A, Efebera Y, Sherr DH. CD40 Ligand-activated, antigen-specific B cells are comparable to mature dendritic cells in presenting protein antigens and major histocompatibility complex class I- and class II-binding peptides. Immunology. 2008;124:129–140. doi: 10.1111/j.1365-2567.2007.02749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alinikula J, Lassila O. Gene interaction network regulates plasma cell differentiation. Scand J Immunol. 2011;73:512–519. doi: 10.1111/j.1365-3083.2011.02556.x. [DOI] [PubMed] [Google Scholar]
- Avery DT, Deenick EK, Ma CS, Suryani S, Simpson N, Chew GY, Chan TD, Palendira U, Bustamante J, Boisson-Dupuis S, Choo S, Bleasel KE, Peake J, King C, French MA, Engelhard D, Al-Hajjar S, Al-Muhsen S, Magdorf K, Roesler J, Arkwright PD, Hissaria P, Riminton DS, Wong M, Brink R, Fulcher DA, Casanova JL, Cook MC, Tangye SG. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J Exp Med. 2010;207:155–171. doi: 10.1084/jem.20091706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GA, Hostager BS. B lymphocyte activation by contact-mediated interactions with T lymphocytes. Curr Opin Immunol. 2001;13:278–285. doi: 10.1016/s0952-7915(00)00216-8. [DOI] [PubMed] [Google Scholar]
- Chen E, Staudt LM, Green AR. Janus kinase deregulation in leukemia and lymphoma. Immunity. 2012;36:529–541. doi: 10.1016/j.immuni.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AV, Brodie DW, Gilbert RJ, Iaboni A, Manso-Sancho R, Walse B, Stuart DI, van der Merwe PA, Davis SJ. The interaction properties of costimulatory molecules revisited. Immunity. 2002;17:201–210. doi: 10.1016/s1074-7613(02)00362-x. [DOI] [PubMed] [Google Scholar]
- Corcoran K, Jabbour M, Bhagwandin C, Deymier MJ, Theisen DL, Lybarger L. Ubiquitin-mediated regulation of CD86 protein expression by the ubiquitin ligase membrane-associated RING-CH-1 (MARCH1) J Biol Chem. 286:37168–37180. doi: 10.1074/jbc.M110.204040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craxton A, Shu G, Graves JD, Saklatvala J, Krebs EG, Clark EA. p38 MAPK is required for CD40-induced gene expression and proliferation in B lymphocytes. J Immunol. 1998;161:3225–3236. [PubMed] [Google Scholar]
- Croxford JL, Yamamura T. Cannabinoids and the immune system: potential for the treatment of inflammatory diseases? J Neuroimmunol. 2005;166:3–18. doi: 10.1016/j.jneuroim.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Diehl SA, Schmidlin H, Nagasawa M, van Haren SD, Kwakkenbos MJ, Yasuda E, Beaumont T, Scheeren FA, Spits H. STAT3-mediated up-regulation of BLIMP1 Is coordinated with BCL6 down-regulation to control human plasma cell differentiation. J Immunol. 2008;180:4805–4815. doi: 10.4049/jimmunol.180.7.4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding BB, Yu JJ, Yu RY, Mendez LM, Shaknovich R, Zhang Y, Cattoretti G, Ye BH. Constitutively activated STAT3 promotes cell proliferation and survival in the activated B-cell subtype of diffuse large B-cell lymphomas. Blood. 2008;111:1515–1523. doi: 10.1182/blood-2007-04-087734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faris M, Kokot N, Stahl N, Nel AE. Involvement of Stat3 in interleukin-6- induced IgM production in a human B-cell line. Immunology. 1997;90:350–357. doi: 10.1111/j.1365-2567.1997.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good-Jacobson KL, Song E, Anderson S, Sharpe AH, Shlomchik MJ. CD80 expression on B cells regulates murine T follicular helper development, germinal center B cell survival, and plasma cell generation. J Immunol. 2012;188:4217–4225. doi: 10.4049/jimmunol.1102885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42:327–360. doi: 10.2165/00003088-200342040-00003. [DOI] [PubMed] [Google Scholar]
- Herring AC, Kaminski NE. Cannabinol-mediated inhibition of nuclear factor-kappaB, cAMP response element-binding protein, and interleukin-2 secretion by activated thymocytes. J Pharmacol Exp Ther. 1999;291:1156–1163. [PubMed] [Google Scholar]
- Hodgkin PD, Lee JH, Lyons AB. B cell differentiation and isotype switching is related to division cycle number. J Exp Med. 1996;184:277–281. doi: 10.1084/jem.184.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M, Paul WE. Regulation of B-cell growth and differentiation by soluble factors. Annu Rev Immunol. 1983;1:307–333. doi: 10.1146/annurev.iy.01.040183.001515. [DOI] [PubMed] [Google Scholar]
- Ishikawa F, Nakano H, Seo A, Okada Y, Torihata H, Tanaka Y, Uchida T, Miyake H, Kakiuchi T. Irradiation up-regulates CD80 expression through induction of tumour necrosis factor-alpha and CD40 ligand expression on B lymphoma cells. Immunology. 2002;106:354–362. doi: 10.1046/j.1365-2567.2002.01434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto T, Shimada M, Araya CE, Huskey J, Garin EH, Johnson RJ. Minimal change disease: a CD80 podocytopathy? Semin Nephrol. 2011;31:320–325. doi: 10.1016/j.semnephrol.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Kaminski NE, Abood ME, Kessler FK, Martin BR, Schatz AR. Identification of a functionally relevant cannabinoid receptor on mouse spleen cells that is involved in cannabinoid-mediated immune modulation. Mol Pharmacol. 1992;42:736–742. [PMC free article] [PubMed] [Google Scholar]
- Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol. 2005;5:400–411. doi: 10.1038/nri1602. [DOI] [PubMed] [Google Scholar]
- Lafarge S, Hamzeh-Cognasse H, Richard Y, Pozzetto B, Cogne M, Cognasse F, Garraud O. Complexes between nuclear factor-kappaB p65 and signal transducer and activator of transcription 3 are key actors in inducing activation-induced cytidine deaminase expression and immunoglobulin A production in CD40L plus interleukin-10-treated human blood B cells. Clin Exp Immunol. 2011;166:171–183. doi: 10.1111/j.1365-2249.2011.04465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamson G, Koshland ME. Changes in J chain and mu chain RNA expression as a function of B cell differentiation. J Exp Med. 1984;160:877–892. doi: 10.1084/jem.160.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy Y, Brouet JC. Interleukin-10 prevents spontaneous death of germinal center B cells by induction of the bcl-2 protein. J Clin Invest. 1994;93:424–428. doi: 10.1172/JCI116977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lopez-Cabrera M, Munoz E, Blazquez MV, Ursa MA, Santis AG, Sanchez-Madrid F. Transcriptional regulation of the gene encoding the human C-type lectin leukocyte receptor AIM/CD69 and functional characterization of its tumor necrosis factor-alpha-responsive elements. J Biol Chem. 1995;270:21545–21551. doi: 10.1074/jbc.270.37.21545. [DOI] [PubMed] [Google Scholar]
- Lu H, Crawford RB, Kaplan BL, Kaminski NE. 2,3,7,8- Tetrachlorodibenzo-p-dioxin-mediated disruption of the CD40 ligand-induced activation of primary human B cells. Toxicol Appl Pharmacol. 2011;255:251–260. doi: 10.1016/j.taap.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Crawford RB, North CM, Kaplan BL, Kaminski NE. Establishment of an immunoglobulin m antibody-forming cell response model for characterizing immunotoxicity in primary human B cells. Toxicol Sci. 2009;112:363–373. doi: 10.1093/toxsci/kfp224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LF, Ahonen CL, Lind EF, Raman VS, Cook WJ, Lin LL, Noelle RJ. The in vivo function of a noncanonical TRAF2-binding domain in the C-terminus of CD40 in driving B-cell growth and differentiation. Blood. 2007;110:193–200. doi: 10.1182/blood-2006-07-038414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngaotepprutaram T, Kaplan BL, Kaminski NE. Impaired NFAT and NFκB Activation are Involved in Suppression of CD40 Ligand Expression by Δ 9-Tetrahydrocannabinol in Human CD4+ T cells. Toxicol Appl Pharmacol. 2013 doi: 10.1016/j.taap.2013.08.023. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niles MJ, Matsuuchi L, Koshland ME. Polymer IgM assembly and secretion in lymphoid and nonlymphoid cell lines: evidence that J chain is required for pentamer IgM synthesis. Proc Natl Acad Sci U S A. 1995;92:2884–2888. doi: 10.1073/pnas.92.7.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu H, Cattoretti G, Dalla-Favera R. BCL6 controls the expression of the B7–1/CD80 costimulatory receptor in germinal center B cells. J Exp Med. 2003;198:211–221. doi: 10.1084/jem.20021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero DC, Poli V, David M, Rickert RC. Cutting edge: inherent and acquired resistance to radiation-induced apoptosis in B cells: a pivotal role for STAT3. J Immunol. 2006;177:6593–6597. doi: 10.4049/jimmunol.177.10.6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder SA, Chauhan A, Singh U, Nagarkatti M, Nagarkatti P. Cannabinoid-induced apoptosis in immune cells as a pathway to immunosuppression. Immunobiology. 2009;215:598–605. doi: 10.1016/j.imbio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz AR, Koh WS, Kaminski NE. Delta 9-tetrahydrocannabinol selectively inhibits T-cell dependent humoral immune responses through direct inhibition of accessory T-cell function. Immunopharmacology. 1993;26:129–137. doi: 10.1016/0162-3109(93)90005-b. [DOI] [PubMed] [Google Scholar]
- Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol. 2005;5:230–242. doi: 10.1038/nri1572. [DOI] [PubMed] [Google Scholar]
- Springs AE, Karmaus PW, Crawford RB, Kaplan BL, Kaminski NE. Effects of targeted deletion of cannabinoid receptors CB1 and CB2 on immune competence and sensitivity to immune modulation by Delta9-tetrahydrocannabinol. J Leukoc Biol. 2008;84:1574–1584. doi: 10.1189/jlb.0508282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangye SG, Avery DT, Hodgkin PD. A division-linked mechanism for the rapid generation of Ig-secreting cells from human memory B cells. J Immunol. 2003;170:261–269. doi: 10.4049/jimmunol.170.1.261. [DOI] [PubMed] [Google Scholar]
- Zhao J, Freeman GJ, Gray GS, Nadler LM, Glimcher LH. A cell type-specific enhancer in the human B7.1 gene regulated by NF-kappaB. J Exp Med. 1996;183:777–789. doi: 10.1084/jem.183.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou GM, Hu WY. LIGHT regulates CD86 expression on dendritic cells through NF-kappaB, but not JNK/AP-1 signal transduction pathway. J Cell Physiol. 2005;205:437–443. doi: 10.1002/jcp.20420. [DOI] [PubMed] [Google Scholar]