Abstract
Cerebral ischemia induces neurogenesis including proliferation and differentiation of neural progenitor cells and migration of newly generated neuroblasts. microRNAs (miRNAs) are small non-coding RNAs that decrease gene expression through mRNA destabilization and/or translational repression. Emerging data indicate that miRNAs have a role in mediating processes of proliferation and differentiation of adult neural progenitor cells. This article reviews recent findings on miRNA profile changes in neural progenitor cells after cerebral infarction and the contributions of miRNAs to their ischemia-induced proliferation and differentiation. We highlight interactions between the miR-124 and the miR17-92 cluster and the Notch and Sonic hedgehog signaling pathways in mediating stroke-induced neurogenesis.
Keywords: miRNAs, Neural progenitor cells, Neurogenesis, Stroke
INTRODUCTION
Neural stem cells in the subventricular zone (SVZ) of the lateral ventricle and the subgranular zone of the dentate gyrus generate new neurons throughout the life of adult rodents (1-4). This neurogenesis has major impacts on neurological function (5-11). Focal cerebral ischemia/infarction is a major cause of disability (12-17). Preclinical and clinical studies demonstrate that stroke promotes neurogenesis in the adult brain (18-35), and in an experimental stroke model, blockage of newly generated neuroblasts exacerbates spontaneous neurological recovery (6). These findings suggest the possibility that amplifying neurogenesis may improve neurological outcomes following cerebral ischemia/infarction in humans (5-7, 10, 11).
microRNAs
microRNAs (miRNAs), a family of short noncoding RNA molecules of 20 to 25 nucleotides, play important roles in neural stem cells during brain development and are involved in physiologic function and in disease processes by decreasing gene expression through mRNA destabilization and/or translational repression (36). In mice, inhibition of miRNA biogenesis in neural stem cells during development by ablation of Dicer (an endoribonuclease that cleaves double-stranded RNA and pre-miRNA into short double-stranded RNA) results in reduction of neural stem cells, abnormal neuronal differentiation, and a thin cortical wall of the brain (37, 38). miRNAs also regulate neural stem cell function in the adult brain (39-46). The biological function of miRNAs in neurogenesis has recently been reviewed (39,47-51). Emerging data indicate that adult neural stem cells express miRNAs and that cerebral infarction substantially alters miRNA profiles in neural stem cells. In this review, we briefly describe neurogenesis and its potential involvement in ischemic brain repair and functional outcome, and then review evidence that miRNAs mediate processes cerebral infarct-induced neurogenesis.
Neurogenesis in Cerebral Ischemia
The SVZ of the lateral ventricle of adult rodents contains at least 3 types of cells: 1) neural stem cells, a subpopulation of glial fibrillary acidic protein-positive cells expressing nestin; 2) actively proliferating intermediate neural progenitor cells that express a Ascl1, a basic helix-loop-helix transcription factor; and 3) neuroblasts, which express doublecortin and polysialylated neural adhesion cell molecule (1-4). Under non-ischemic conditions, neural stem cells in the SVZ generate neuroblasts that travel the rostral migratory stream to the olfactory bulb where they differentiate into granule and periglomerular neurons (52-54). More than 30,000 neuroblasts are generated daily in the rodent SVZ (55, 56). Neuroblasts generated in the subgranular zone differentiate into dentate granule cells and integrate into the preexisting neuronal network (57).
Cerebral infarction increases neural stem cell proliferation that results in early expansion of the neural progenitor pool in the SVZ (58-60). Neural progenitor cells preferentially differentiate into neuroblasts (58-60); neuroblasts then migrate out of the SVZ to the ischemic cortex and striatum (Fig. 1) (61, 62). Studies in transgenic mice with inducible Cre recombinase under control of the Ascl1 or Nestin promoter indicate that newly arrived neuroblasts in the ischemic boundary regions exhibit phenotypes and electrophysiological characteristics of mature neurons (18, 19, 22, 23, 25, 33). This suggests that neuroblasts mature into resident neurons in the ischemic brain but that the effect of neuroblasts on the ischemic brain may extend beyond the replacement of damaged neurons. For example, neurogenesis is coupled with angiogenesis in the ischemic brain, i.e. ischemic neural progenitor cells promote endothelial cells to form capillary-like tubes (angiogenesis) by secreting vascular endothelial growth factor in vitro (63). Suppressing angiogenesis either with endostatin or with a neutralizing antibody against the angiopoietin receptor, Tie2, substantially attenuates migration of neuroblasts newly born in the SVZ to the ischemic region (11). Using transgenic mice that express herpes simplex virus thymidine kinase under the control of the promoter for doublecortin, Wang et al demonstrated that ablation of doublecortin-expressing neuroblasts substantially exacerbated ischemic damage and worsened neurological outcome (6). These data indicate that cerebral infarct-induced neurogenesis may affect stroke recovery.
Figure 1.
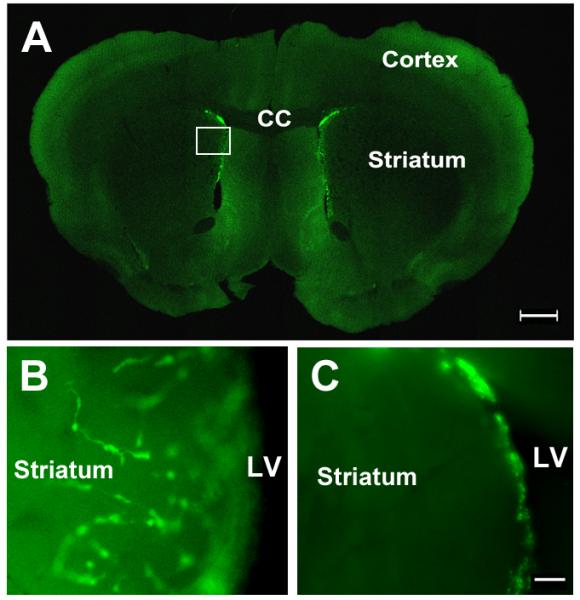
Time-lapse microscopic images of coronal brain slices obtained from doublecortin–green fluorescent protein (DCX-eGFP) transgenic mice. (A) Coronal section showing DCX-eGFP–expressing neuroblasts (green) in the subventricular zone (SVZ) of the lateral ventricle. (B) DCX-eGFP neuroblasts (green) emigrated laterally from the SVZ to the ischemic striatum in an ischemic brain slice. (C) DCX-eGFP neuroblasts (green) are localized in the SVZ in a non-ischemic brain slice. The boxed area in panel A indicates the location of panel B. CC, corpus callosum; LV, lateral ventricle. Bars: A = 1 mm; C = 50 μm.
miRNAs in Neural Progenitor Cells Following Cerebral Ischemia
miRNAs play important roles in the regulation of adult neurogenesis (64). For example, miR-124, the most abundant neuronal miRNA, is expressed by neural progenitor cells in the SVZ of adult rodent brains (40, 42). Attenuation of endogenous miR-124 in neural progenitor cells can abolish neuronal differentiation, whereas overexpression of miR-124 promoted neuronal differentiation in the mouse brain (40, 42). The SRY-box transcription factor Sox9 is a physiological target of miR124 (42). Another example is miR-9, which is also expressed by adult SVZ neural progenitor cells (65). miR-9 suppresses the expression of the orphan nuclear receptor TLX to negatively regulate neural stem cell proliferation and accelerate neural differentiation (65).
The biological functions of miRNAs in cerebral ischemia-induced neurogenesis have not been extensively studied. Using an miRNA array that detects mature miRNAs, Liu et al showed that cultured neural progenitor cells harvested from the SVZ of animals subjected to cerebral ischemia markedly altered miRNA expression profiles compared to neural progenitor cells from non-ischemic SVZ (64). Bioinformatics analysis of 18 and 21 miRNAs downregulated and upregulated by cerebral ischemia, respectively, revealed that these miRNAs selectively affect several signaling pathways including transforming growth factor β, Wnt, and Sonic hedgehog (Shh) signals, which are known to regulate neural stem cell function (66).
The Shh Signaling Pathway and miR17-92 Cluster in Ischemia-induced Neurogenesis
Shh is a member of the family of the hedgehog proteins known to exert important regulatory functions in patterning and growth in a large number of tissues during embryogenesis (67-69); it has an important role in regulating neural progenitor cell proliferation and differentiation in the brain (68, 69). Canonically, Shh binds to the transmembrane receptor protein, patched (ptc), which, in the absence of Shh, exerts an inhibitory effect on the 7 transmembrane receptor smoothened (Smo). Binding of Shh to ptc blocks the inhibitory effect of ptc on Smo (70-72). Once activated, Smo induces a complex series of intracellular reactions that target the Gli family of transcription factors (70, 72). Exogenous Shh increases neurogenesis in the SVZ, whereas inactivation of Shh signals depletes neural stem cells and intermediate neural progenitor cells in the SVZ (67, 70). The Shh pathway also mediates stroke-induced neurogenesis (73).
The miR17-92 cluster comprises a cluster of 6 miRNAs (miR-17, miR-18a, miR-19a, miR-20a, miR-19b-1, and miR-92a-1) on chromosome 13 that is transcribed as a single polycistronic unit (74). During development, the miR17-92 cluster regulates neural progenitor cell proliferation and oligodendrogenesis (75, 76). Using a conditional knockout of the miR17-92 cluster in 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase)-expressing oligodendrocytes, Budde et al showed that ablation of the miR17-92 cluster substantially reduced oligodendrocyte progenitor cell proliferation and survival in post-natal mice (76). The clinical relevance of the miR17-92 cluster has recently been suggested in patients with the autosomal dominant Feingold syndrome in which there is a germline deletion of the miR17-92 cluster that is associated with microcephaly and skeletal abnormalities (77). The miR17-92 cluster is upregulated miRNA in SVZ neural progenitor cells after cerebral ischemia (64, 78). Overexpression of the miR17-92 cluster enhances stroke-induced progenitor cell proliferation, whereas attenuation of endogenous miR-18a and miR-19a suppresses this neural progenitor cell proliferation. Cerebral ischemia substantially reduces individual members of 2 miR17-92 cluster analogs (i.e. miR106a-363 and miR106b-25 [78]), but the biological function of these clusters in adult neurogenesis has not been studied. The positive effect of the miR17-92 cluster on neurogenesis may be partially attributed to suppression of phosphatase and tensin homolog deleted on chromosome 10 (i.e. PTEN), a protein that negatively regulates embryonic neural stem cell proliferation and survival (79-85).
Emerging data also indicate that the Shh pathway is closely associated with expression of the miR17-92 cluster, i.e. the Shh signaling pathway interacts with the miR-17-92 cluster to contribute to neural progenitor cell proliferation (75, 86). In cultured neural progenitor cells, attenuation of endogenous Shh and addition of exogenous Shh downregulate and upregulate the miR17-92 cluster expression, respectively (78). Intraventricular administration of exogenous Shh to animals with cerebral ischemia further upregulated miR17-92 cluster expression in SVZ neural progenitor cells, whereas blockage of the Shh pathway suppressed ischemia-upregulated miR17-92 cluster expression and ischemia-increased neural progenitor cell proliferation (78).
The Shh pathway likely regulates miR17-92 cluster expression in SVZ neural progenitor cells in ischemic brain via Myc, one of the most potent oncogenic agents (87). Myc is a downstream target of Shh and the miR17-92 cluster is a direct transcriptional target of c-Myc (88, 89). Cerebral ischemia enhances the binding of c-Myc to the promoter region of the miR17-92 cluster in neural progenitor cells (78). Thus, the miR17-92 cluster likely mediates processes of Shh-induced neural progenitor cell proliferation (Fig. 2A).
Figure 2.
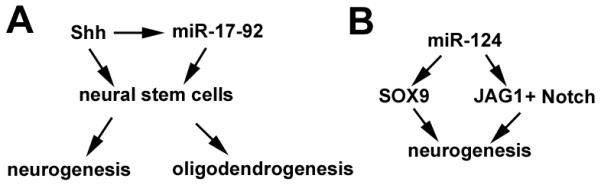
(A) Schematic representation of miR-17-9 cluster and the sonic hedgehog (Shh) signaling pathways in mediating processes of neurogenesis and oligodendrogenesis. (B) The miR-124 and Notch signaling pathways involved in neurogenesis.
The Notch Signaling Pathway and miR-124 in Cerebral Ischemia-induced Neurogenesis
Notch receptors are transmembrane proteins activated by Delta and Jagged ligands (90, 91). On activation, the Notch internal cellular domain is cleaved by presenilin-1 and the γ-secretase enzyme complex is translocated into the nucleus, leading to activation of transcription factors (91). The Notch signaling pathway plays a pivotal role in maintaining the embryonic neural stem cell pool and promoting gliogenesis (92). In the ischemic brain, activation of the Notch pathway increases neural progenitor cell proliferation, whereas blockage of the pathway abolishes stroke-increased progenitor cell proliferation (93). In non-ischemic brain, miR-124a in neural progenitor cells regulates neuronal differentiation by targeting SOX9 (42). Cerebral ischemia substantially reduces miR-124a expression in SVZ neural progenitor cells, which is inversely associated with upregulation of Jagged-1 (JAG1), one of the target genes of miR-124a (64). Introduction of miR-124a dramatically inhibits stroke-increased neural progenitor cell proliferation and promotes the neuronal differentiation of the progenitor cells by targeting JAG1, but not SOX9 (64). These data suggest that miR-124a mediates adult neurogenesis either by targeting SOX9 or the Notch signaling pathway under non-ischemic and ischemic conditions (Fig. 2B).
Other Signaling Pathways and miRNAs
Other signaling pathways such as Wnt and bone morphogenic protein (BMP) also regulate neurogenesis in the adult brain (94, 95). Overexpression of BMP7 in ependymal cells inhibits neural progenitor cell proliferation and neuroblast production, demonstrating that BMPs potently inhibit neurogenesis (94). On the other hand, the Wnt pathway promotes neurogenesis in the dentate gyrus (95). Cerebral ischemia changes expression of Wnt and BMP family genes in SVZ neural progenitor cells of adult rodents (96, 97). Let-7b and miR-9 regulate adult neurogenesis by controlling the balance between the proliferation and differentiation of neural stem cells through the TLX nuclear receptor, which forms a feedback regulatory loop in the mouse (65, 98). Cerebral ischemia substantially downregulates Let-7 and miR-964. Wnt/β-catenin signaling represses Let 7 in tumor cells, while miR-92 regulates BMP signals (99, 100). However, it remains to be determined whether the coupling of stroke-altered miRNAs with the signaling pathways mediates proliferation and differentiation of neural progenitor cells in stroke-induced neurogenesis.
Future Perspectives
Individual signaling pathways likely affect a cluster of miRNAs that subsequently trigger or repress other pathways. The intertwined interaction of miRNAs and signaling pathways in regulating neural stem cell functions has provided new insights into how miRNAs function during adult neurogenesis under normal and ischemic conditions. Ultimately, further identification of miRNA function in neural stem cells in the adult brain may provide a novel avenue for development of miRNA-based therapies for treating diseases of the CNS. Additional studies are warranted to examine whether the preclinical findings in rodents described in this review can be translated into clinical applications in humans.
ACKNOWLEDGMENTS
The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
This work was supported by National Institutes of Health Grants RO1 AG037506 (MC), and RO1 NS075156 (ZGZ) and AHA Scientist Development Grant 10SDG2790012 (XL).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Alvarez-Buylla A, Herrera DG, Wichterle H. The subventricular zone: Source of neuronal precursors for brain repair. Prog Brain Res. 2000;127:1–11. doi: 10.1016/s0079-6123(00)27002-7. [DOI] [PubMed] [Google Scholar]
- 2.Gage FH, Ray J, Fisher LJ. Isolation, characterization, and use of stem cells from the cns. Annu Rev Neurosci. 1995;18:159–92. doi: 10.1146/annurev.ne.18.030195.001111. [DOI] [PubMed] [Google Scholar]
- 3.Kirschenbaum B, Doetsch F, Lois C, et al. Adult subventricular zone neuronal precursors continue to proliferate and migrate in the absence of the olfactory bulb. J Neurosci. 1999;19:2171–80. doi: 10.1523/JNEUROSCI.19-06-02171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luskin MB, Zigova T, Soteres BJ, et al. Neuronal progenitor cells derived from the anterior subventricular zone of the neonatal rat forebrain continue to proliferate in vitro and express a neuronal phenotype. Mol Cell Neurosci. 1997;8:351–66. doi: 10.1006/mcne.1996.0592. [DOI] [PubMed] [Google Scholar]
- 5.Jin K, Wang X, Xie L, et al. Transgenic ablation of doublecortin-expressing cells suppresses adult neurogenesis and worsens stroke outcome in mice. Proc Natl Acad Sci U S A. 2010;107:7993–8. doi: 10.1073/pnas.1000154107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Mao X, Xie L, et al. Conditional depletion of neurogenesis inhibits long-term recovery after experimental stroke in mice. PLoS One. 2012;7:e38932. doi: 10.1371/journal.pone.0038932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun F, Wang X, Mao X, et al. Ablation of neurogenesis attenuates recovery of motor function after focal cerebral ischemia in middle-aged mice. PLoS One. 2012;7:e46326. doi: 10.1371/journal.pone.0046326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellenchi GC, Volpicelli F, Piscopo V, et al. Adult neural stem cells: An endogenous tool to repair brain injury? Journal of neurochemistry. 2013;124:159–67. doi: 10.1111/jnc.12084. [DOI] [PubMed] [Google Scholar]
- 9.Raber J, Fan Y, Matsumori Y, et al. Irradiation attenuates neurogenesis and exacerbates ischemia-induced deficits. Ann Neurol. 2004;55:381–9. doi: 10.1002/ana.10853. [DOI] [PubMed] [Google Scholar]
- 10.Zhang RL, Zhang ZG, Chopp M. Neurogenesis in the adult ischemic brain: Generation, migration, survival, and restorative therapy. Neuroscientist. 2005;11:408–16. doi: 10.1177/1073858405278865. [DOI] [PubMed] [Google Scholar]
- 11.Ohab JJ, Fleming S, Blesch A, et al. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–16. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zivin JA. Acute stroke therapy with tissue plasminogen activator (tpa) since it was approved by the u.S. Food and drug administration (fda) Ann Neurol. 2009;66:6–10. doi: 10.1002/ana.21750. [DOI] [PubMed] [Google Scholar]
- 13.del Zoppo GJ. Inflammation and the neurovascular unit in the setting of focal cerebral ischemia. Neuroscience. 2009;158:972–82. doi: 10.1016/j.neuroscience.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armstead WM, Ganguly K, Kiessling JW, et al. Signaling, delivery and age as emerging issues in the benefit/risk ratio outcome of tpa for treatment of cns ischemic disorders. J Neurochem. 2010;113:303–12. doi: 10.1111/j.1471-4159.2010.06613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher M, Bastan B. Treating acute ischemic stroke. Curr Opin Drug Discov Devel. 2008;11:626–32. [PubMed] [Google Scholar]
- 16.Chavez JC, Hurko O, Barone FC, et al. Pharmacologic interventions for stroke: Looking beyond the thrombolysis time window into the penumbra with biomarkers, not a stopwatch. Stroke. 2009;40:e558–63. doi: 10.1161/STROKEAHA.109.559914. [DOI] [PubMed] [Google Scholar]
- 17.Ginsberg MD. Current status of neuroprotection for cerebral ischemia: Synoptic overview. Stroke. 2009;40:S111–4. doi: 10.1161/STROKEAHA.108.528877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang RL, Zhang ZG, Zhang L, et al. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- 19.Jin K, Minami M, Lan JQ, et al. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001;98:4710–5. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshimura S, Takagi Y, Harada J, et al. Fgf-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc Natl Acad Sci U S A. 2001;98:5874–9. doi: 10.1073/pnas.101034998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tonchev AB, Yamashima T, Zhao L, et al. Proliferation of neural and neuronal progenitors after global brain ischemia in young adult macaque monkeys. Mol Cell Neurosci. 2003;23:292–301. doi: 10.1016/s1044-7431(03)00058-7. [DOI] [PubMed] [Google Scholar]
- 22.Parent JM, Vexler ZS, Gong C, et al. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–13. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- 23.Arvidsson A, Collin T, Kirik D, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–70. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Solway K, Messing RO, et al. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18:7768–78. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang R, Zhang Z, Wang L, et al. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24:441–8. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Iwai M, Sato K, Omori N, et al. Three steps of neural stem cells development in gerbil dentate gyrus after transient ischemia. J Cereb Blood Flow Metab. 2002;22:411–9. doi: 10.1097/00004647-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Iwai M, Sato K, Kamada H, et al. Temporal profile of stem cell division, migration, and differentiation from subventricular zone to olfactory bulb after transient forebrain ischemia in gerbils. J Cereb Blood Flow Metab. 2003;23:331–41. doi: 10.1097/01.WCB.0000050060.57184.E7. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt W, Reymann KG. Proliferating cells differentiate into neurons in the hippocampal ca1 region of gerbils after global cerebral ischemia. Neurosci Lett. 2002;334:153–6. doi: 10.1016/s0304-3940(02)01072-8. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka R, Yamashiro K, Mochizuki H, et al. Neurogenesis after transient global ischemia in the adult hippocampus visualized by improved retroviral vector. Stroke. 2004;35:1454–9. doi: 10.1161/01.STR.0000126480.40967.b3. [DOI] [PubMed] [Google Scholar]
- 30.Kee NJ, Preston E, Wojtowicz JM. Enhanced neurogenesis after transient global ischemia in the dentate gyrus of the rat. Exp Brain Res. 2001;136:313–20. doi: 10.1007/s002210000591. [DOI] [PubMed] [Google Scholar]
- 31.Yagita Y, Kitagawa K, Ohtsuki T, et al. Neurogenesis by progenitor cells in the ischemic adult rat hippocampus. Stroke. 2001;32:1890–6. doi: 10.1161/01.str.32.8.1890. [DOI] [PubMed] [Google Scholar]
- 32.Zhu DY, Liu SH, Sun HS, et al. Expression of inducible nitric oxide synthase after focal cerebral ischemia stimulates neurogenesis in the adult rodent dentate gyrus. J Neurosci. 2003;23:223–9. doi: 10.1523/JNEUROSCI.23-01-00223.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thored P, Arvidsson A, Cacci E, et al. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–47. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- 34.Yamashita T, Ninomiya M, Hernandez Acosta P, et al. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–36. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macas J, Nern C, Plate KH, et al. Increased generation of neuronal progenitors after ischemic injury in the aged adult human forebrain. J Neurosci. 2006;26:13114–9. doi: 10.1523/JNEUROSCI.4667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammond SM, Bernstein E, Beach D, et al. An rna-directed nuclease mediates post-transcriptional gene silencing in drosophila cells. Nature. 2000;404:293–6. doi: 10.1038/35005107. [DOI] [PubMed] [Google Scholar]
- 37.De Pietri Tonelli D, Pulvers JN, et al. Mirnas are essential for survival and differentiation of newborn neurons but not for expansion of neural progenitors during early neurogenesis in the mouse embryonic neocortex. Development. 2008;135:3911–21. doi: 10.1242/dev.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawase-Koga Y, Otaegi G, Sun T. Different timings of dicer deletion affect neurogenesis and gliogenesis in the developing mouse central nervous system. Dev Dyn. 2009;238:2800–12. doi: 10.1002/dvdy.22109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawahara H, Imai T, Okano H. Micrornas in neural stem cells and neurogenesis. Front Neurosci. 2012;6:30. doi: 10.3389/fnins.2012.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akerblom M, Sachdeva R, Barde I, et al. Microrna-124 is a subventricular zone neuronal fate determinant. J Neurosci. 2012;32:8879–89. doi: 10.1523/JNEUROSCI.0558-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brett JO, Renault VM, Rafalski VA, et al. The microrna cluster mir-106b~25 regulates adult neural stem/progenitor cell proliferation and neuronal differentiation. Aging. 2011;3:108–24. doi: 10.18632/aging.100285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng LC, Pastrana E, Tavazoie M, et al. Mir-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu C, Teng ZQ, McQuate AL, et al. An epigenetic feedback regulatory loop involving microrna-195 and mbd1 governs neural stem cell differentiation. PLoS One. 2013;8:e51436. doi: 10.1371/journal.pone.0051436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu C, Teng ZQ, Santistevan NJ, et al. Epigenetic regulation of mir-184 by mbd1 governs neural stem cell proliferation and differentiation. Cell Stem Cell. 2010;6:433–44. doi: 10.1016/j.stem.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magill ST, Cambronne XA, Luikart BW, et al. Microrna-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proc Natl Acad Sci U S A. 2010;107:20382–7. doi: 10.1073/pnas.1015691107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szulwach KE, Li X, Smrt RD, et al. Cross talk between microrna and epigenetic regulation in adult neurogenesis. J Cell Biol. 2010;189:127–41. doi: 10.1083/jcb.200908151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akerblom M, Sachdeva R, Jakobsson J. Functional studies of micrornas in neural stem cells: Problems and perspectives. Front Neurosci. 2012;6:14. doi: 10.3389/fnins.2012.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jobe EM, McQuate AL, Zhao X. Crosstalk among epigenetic pathways regulates neurogenesis. Front Neurosci. 2012;6:59. doi: 10.3389/fnins.2012.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lang MF, Shi Y. Dynamic roles of micrornas in neurogenesis. Front Neurosci. 2012;6:71. doi: 10.3389/fnins.2012.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu C, Zhao X. Micrornas in adult and embryonic neurogenesis. Neuromolecular Med. 2009;11:141–52. doi: 10.1007/s12017-009-8077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi Y, Zhao X, Hsieh J, et al. Microrna regulation of neural stem cells and neurogenesis. J Neurosci. 2010;30:14931–6. doi: 10.1523/JNEUROSCI.4280-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morshead CM, Reynolds BA, Craig CG, et al. Neural stem cells in the adult mammalian forebrain: A relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–82. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 53.Luskin MB. Neuroblasts of the postnatal mammalian forebrain: Their phenotype and fate. J Neurobiol. 1998;36:221–33. [PubMed] [Google Scholar]
- 54.Garcia-Verdugo JM, Doetsch F, Wichterle H, et al. Architecture and cell types of the adult subventricular zone: In search of the stem cells. J Neurobiol. 1998;36:234–48. doi: 10.1002/(sici)1097-4695(199808)36:2<234::aid-neu10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 55.Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–93. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- 56.Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–93. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- 57.Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: From precursors to network and physiology. Physiol Rev. 2005;85:523–69. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- 58.Smith CM, Luskin MB. Cell cycle length of olfactory bulb neuronal progenitors in the rostral migratory stream. Dev Dyn. 1998;213:220–7. doi: 10.1002/(SICI)1097-0177(199810)213:2<220::AID-AJA7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 59.Schultze B, Korr H. Cell kinetic studies of different cell types in the developing and adult brain of the rat and the mouse: A review. Cell Tissue Kinet. 1981;14:309–25. doi: 10.1111/j.1365-2184.1981.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 60.Zhang RL, Zhang ZG, Lu M, et al. Reduction of the cell cycle length by decreasing g(1) phase and cell cycle reentry expand neuronal progenitor cells in the subventricular zone of adult rat after stroke. J Cereb Blood Flow Metab. 2006;26:857–63. doi: 10.1038/sj.jcbfm.9600237. [DOI] [PubMed] [Google Scholar]
- 61.Zhang RL, Chopp M, Gregg SR, et al. Patterns and dynamics of subventricular zone neuroblast migration in the ischemic striatum of the adult mouse. J Cereb Blood Flow Metab. 2009;29:1240–50. doi: 10.1038/jcbfm.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jin K, Sun Y, Xie L, et al. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci. 2003;24:171–89. doi: 10.1016/s1044-7431(03)00159-3. [DOI] [PubMed] [Google Scholar]
- 63.Teng H, Zhang ZG, Wang L, et al. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J Cereb Blood Flow Metab. 2008;28:764–71. doi: 10.1038/sj.jcbfm.9600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu XS, Chopp M, Zhang RL, et al. Microrna profiling in subventricular zone after stroke: Mir-124a regulates proliferation of neural progenitor cells through notch signaling pathway. PLoS One. 2011;6:e23461. doi: 10.1371/journal.pone.0023461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao C, Sun G, Li S, et al. A feedback regulatory loop involving microrna-9 and nuclear receptor tlx in neural stem cell fate determination. Nat Struct Mol Biol. 2009;16:365–71. doi: 10.1038/nsmb.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu XS, Zhang ZG, Zhang RL, et al. Stroke induces gene profile changes associated with neurogenesis and angiogenesis in adult subventricular zone progenitor cells. J Cereb Blood Flow Metab. 2007;27:564–74. doi: 10.1038/sj.jcbfm.9600371. [DOI] [PubMed] [Google Scholar]
- 67.Lai K, Kaspar BK, Gage FH, et al. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6:21–7. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- 68.Marti E, Bovolenta P. Sonic hedgehog in cns development: One signal, multiple outputs. Trends Neurosci. 2002;25:89–96. doi: 10.1016/s0166-2236(02)02062-3. [DOI] [PubMed] [Google Scholar]
- 69.Wang L, Zhang ZG, Gregg SR, et al. The sonic hedgehog pathway mediates carbamylated erythropoietin-enhanced proliferation and differentiation of adult neural progenitor cells. J Biol Chem. 2007;282:32462–70. doi: 10.1074/jbc.M706880200. [DOI] [PubMed] [Google Scholar]
- 70.Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to sonic hedgehog. Nature. 2005;437:894–7. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- 71.Ingham PW, McMahon AP. Hedgehog signaling in animal development: Paradigms and principles. Genes Dev. 2001;15:3059–87. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 72.Ruiz i Altaba A, Sanchez P, et al. Gli and hedgehog in cancer: Tumours, embryos and stem cells. Nat Rev Cancer. 2002;2:361–72. doi: 10.1038/nrc796. [DOI] [PubMed] [Google Scholar]
- 73.Sims JR, Lee SW, Topalkara K, et al. Sonic hedgehog regulates ischemia/hypoxia-induced neural progenitor proliferation. Stroke. 2009;40:3618–26. doi: 10.1161/STROKEAHA.109.561951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xiao C, Srinivasan L, Calado DP, et al. Lymphoproliferative disease and autoimmunity in mice with increased mir-17-92 expression in lymphocytes. Nat Immunol. 2008;9:405–14. doi: 10.1038/ni1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Uziel T, Karginov FV, Xie S, et al. The mir-17~92 cluster collaborates with the sonic hedgehog pathway in medulloblastoma. Proc Natl Acad Sci U S A. 2009;106:2812–7. doi: 10.1073/pnas.0809579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Budde H, Schmitt S, Fitzner D, et al. Control of oligodendroglial cell number by the mir-17-92 cluster. Development. 2010;137:2127–32. doi: 10.1242/dev.050633. [DOI] [PubMed] [Google Scholar]
- 77.de Pontual L, Yao E, Callier P, et al. Germline deletion of the mir-17 approximately 92 cluster causes skeletal and growth defects in humans. Nat Genet. 2011;43:1026–30. doi: 10.1038/ng.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu XS, Chopp M, Wang XL, et al. Microrna-17/92 cluster mediates the proliferation and survival of neural progenitor cells after stroke. J Biol Chem. 2013;288:12478–88. doi: 10.1074/jbc.M112.449025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gregorian C, Nakashima J, Le Belle J, et al. Pten deletion in adult neural stem/progenitor cells enhances constitutive neurogenesis. J Neurosci. 2009;29:1874–86. doi: 10.1523/JNEUROSCI.3095-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Groszer M, Erickson R, Scripture-Adams DD, et al. Pten negatively regulates neural stem cell self-renewal by modulating g0-g1 cell cycle entry. Proc Natl Acad Sci U S A. 2006;103:111–16. doi: 10.1073/pnas.0509939103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Groszer M, Erickson R, Scripture-Adams DD, et al. Negative regulation of neural stem/progenitor cell proliferation by the pten tumor suppressor gene in vivo. Science. 2001;294:2186–9. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- 82.Li L, Liu F, Ross AH. Pten regulation of neural development and cns stem cells. J Cell Biochem. 2003;88:24–8. doi: 10.1002/jcb.10312. [DOI] [PubMed] [Google Scholar]
- 83.Li L, Liu F, Salmonsen RA, et al. Pten in neural precursor cells: Regulation of migration, apoptosis, and proliferation. Mol Cell Neurosci. 2002;20:21–9. doi: 10.1006/mcne.2002.1115. [DOI] [PubMed] [Google Scholar]
- 84.Otaegi G, Yusta-Boyo MJ, Vergano-Vera E, et al. Modulation of the pi 3-kinase-akt signalling pathway by igf-i and pten regulates the differentiation of neural stem/precursor cells. J Cell Sci. 2006;119:2739–48. doi: 10.1242/jcs.03012. [DOI] [PubMed] [Google Scholar]
- 85.Zheng H, Ying H, Yan H, et al. P53 and pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature. 2008;455:1129–33. doi: 10.1038/nature07443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Northcott PA, Fernandez LA, Hagan JP, et al. The mir-17/92 polycistron is up-regulated in sonic hedgehog-driven medulloblastomas and induced by n-myc in sonic hedgehog-treated cerebellar neural precursors. Cancer Res. 2009;69:3249–55. doi: 10.1158/0008-5472.CAN-08-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hermeking H. The myc oncogene as a cancer drug target. Curr Cancer Drug Targets. 2003;3:163–75. doi: 10.2174/1568009033481949. [DOI] [PubMed] [Google Scholar]
- 88.Hatton BA, Knoepfler PS, Kenney AM, et al. N-myc is an essential downstream effector of shh signaling during both normal and neoplastic cerebellar growth. Cancer Res. 2006;66:8655–61. doi: 10.1158/0008-5472.CAN-06-1621. [DOI] [PubMed] [Google Scholar]
- 89.O’Donnell KA, Wentzel EA, Zeller KI, et al. C-myc-regulated micrornas modulate e2f1 expression. Nature. 2005;435:839–43. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 90.Jones PMG, Healy L, Brown J, et al. Stromal expression of jagged 1 promotes colony formation by fetal hematopoietic progenitor cells. Blood. 1998;92:1505–11. [PubMed] [Google Scholar]
- 91.Fischer A, Gessler M. Delta-notch--and then? Protein interactions and proposed modes of repression by hes and hey bhlh factors. Nucleic Acids Res. 2007;35:4583–96. doi: 10.1093/nar/gkm477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gaiano N, Fishell G. The role of notch in promoting glial and neural stem cell fates. Annu Rev Neurosci. 2002;25:471–90. doi: 10.1146/annurev.neuro.25.030702.130823. [DOI] [PubMed] [Google Scholar]
- 93.Wang L, Chopp M, Zhang RL, et al. The notch pathway mediates expansion of a progenitor pool and neuronal differentiation in adult neural progenitor cells after stroke. Neuroscience. 2009;158:1356–63. doi: 10.1016/j.neuroscience.2008.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lim DA, Tramontin AD, Trevejo JM, et al. Noggin antagonizes bmp signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–26. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 95.Lie DC, Colamarino SA, Song HJ, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–5. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 96.Liu XS, Zhang ZG, Zhang RL, et al. Comparison of in vivo and in vitro gene expression profiles in subventricular zone neural progenitor cells from the adult mouse after middle cerebral artery occlusion. Neuroscience. 2007;146:1053–61. doi: 10.1016/j.neuroscience.2007.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morris DC, Zhang ZG, Wang Y, et al. Wnt expression in the adult rat subventricular zone after stroke. Neurosci Lett. 2007;418:170–4. doi: 10.1016/j.neulet.2007.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao C, Sun G, Ye P, et al. Microrna let-7d regulates the tlx/microrna-9 cascade to control neural cell fate and neurogenesis. Sci Rep. 2013;3:1329. doi: 10.1038/srep01329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cai WY, Wei TZ, Luo QC, et al. Wnt/beta-catenin pathway represses let-7 micrornas expression via transactivation of lin28 to augment breast cancer stem cell expansion. J Cell Sci. 2013 doi: 10.1242/jcs.123810. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 100.Dews M, Fox JL, Hultine S, et al. The myc-mir-17~92 axis blunts tgf{beta} signaling and production of multiple tgf{beta}-dependent antiangiogenic factors. Cancer Res. 2010;70:8233–46. doi: 10.1158/0008-5472.CAN-10-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]


