Senescence is an inevitable consequence of life. As a result of exposure to intrinsic- as well as extrinsic-aging factors, cellular aging is triggered in stem cells (SCs) by gradually accumulating DNA damage due to action of reactive oxygen species (ROS) and epigenetic changes.1 Thus, aging can be envisioned, at the cellular level, as a result of altered cell function in response to changes in DNA structure that directly affects proper gene expression. In addition, SCs also age by intrinsic mechanism encoded by phenomenon of telomere shortening.2
Several well-known risk factors, such as obesity, diabetes, and lack of physical activity that lead to atherosclerosis of the cardio-vascular system and impair the function of vital organs (e,g., heart, kidney, or brain), limit overall life span. It is obvious that all these risk factors somehow must ultimately have an impact on the basic units of tissue rejuvenation, which are SCs.1 They can directly affect SCs or damage the niches in which these cells reside and thereby impair SC self renewal and differentiation. On the other hand, these risk factors may also lead to enhanced turn-over/proliferation of SCs, resulting in premature exhaustion of these cells. The elucidation of these precise mechanisms will help to develop more efficient anti-aging strategies.
Evidence accumulates that increase in calorie uptake and insulin, insulin-like growth factor (Ins/Igf) level in peripheral blood (PB) accelerates aging.3–8 On the other hand calorie restriction and decrease in Ins/Igf signaling increases lifespan in worms, flies, and mammals. Furthermore, Insulin-like growth factor-1 (Igf1), Insulin-like growth factor-2 (Igf2) and Insulin (Ins) are described as stimulators of proliferation of normal and malignant hematopoietic stem progenitor cells (HSPCs).9 While Igf1 and Igf2 signal through tyrosine kinase receptor Igf1R, Ins together with Igf2 may activate classical insulin receptor (InsR) and all factors together may activate a hybrid Igf1R/InsR. In contrast Igf2R serves as a “decoy receptor” that prevents Igf2 from binding to Igf1R or InsR. While Ins is secreted after calorie uptake from pancreatic islands, Igf1 (known also as somatomedin-C) is secreted from liver into peripheral blood in growth hormone (GH) dependent manner.8
Interestingly, the expression of Igf2, Igf2R and Rasgrf1 (a small GTP exchange factor for Ras protein that is involved in Ins/Igf signaling) are regulated by changes in somatic imprinting. The imprinted genes play a crucial role in embryogenesis, fetal growth, the totipotential state of the zygote, and the pluripotency of developmentally early stem cells.10 The expression of imprinted genes is regulated by DNA methylation within differential methylated regions (DMRs), which are CpG-rich cis-elements within their gene loci.
Recently, our group demonstrated that adult tissues including bone marrow (BM) harbor a population of pluripotent Oct4+ SSEA-1+Sca-1+Lin−CD45− very small embryonic-like stem cells (VSELs), 11 and postulated that these pluripotent stem cells (PSCs) are deposited during early embryogenesis as a backup for long term repopulating hematopoietic stem cells (LT-HSC). In fact as recently demonstrated BM-derived VSELs could be specified into hematopoietic lineage in co-cultures over OP-9 stroma cell support.12 Furthermore, molecular analysis of VSELs revealed that their quiescence in adult BM and potentially premature depletion is controlled by epigenetic changes of some imprinted genes that regulate signaling of Ins/Igf (e.g., Igf2-H19 locus, Igf2R and Rasgrf1). 13 Accordingly, we observed that murine BM-sorted VSELs erase the paternally methylated imprints (e.g., DMRs at Igf2-H19 and Rasgrf1 loci); however, they hypermethylate the maternally methylated ones (e.g., DMRs at Igf2R).13 This epigenetic reprogramming of genomic imprinting negatively affects Ins/Igf signaling and maintains the quiescence of VSELs and protects them from premature aging and tumor formation.
Our initial studies performed on normal 4-week-old and 2-year-old mice revealed that the number of VSELs and their pluripotentiality decrease during ageing.14, 15 To support this, VSELs from old mice show lower expression of the pluripotentiality master-regulators such as Oct4, Nanog, Sox2, Klf4, and cMyc and, at the molecular level the Oct4 promoter in VSELs becomes hypermethylated with age and shows a closed chromatin structure. Furthermore, VSELs from old mice show the somatic type of methylation at both Igf2-H19 and Rasgrf1 loci, which suggests their increased sensitivity to Ins/Igf signaling.
Based on this we became interested in a role of prolonged Ins/Igf signaling in homeostasis of pool of BM-residing VSELs and VSELs-derived LT-HSC. To address better a role of Ins/Igf signaling on a pool of VSELs and HSCs we performed previously experiments in Laron dwarf mice. Due to a deficiency of GH receptor (GHR), these animals display a severe reduction in the Igf1 plasma level and do not display increase in GH-mediated Igf-1 plasma level in response to caloric uptake. Interestingly, these animals live 30–40% longer than their normal littermates.3 Therefore, we measured the number of VSELs in BM of 20 month old murine Laron dwarfs (GHR−/−) and corresponding normal heterozygous littermates (GHR+/−) by FACS. Interestingly, we noticed that the number of VSELs in the BM of plasma Igf1-deficient Laron dwarfs is maintained at a 3–4-fold higher level than normal GHR+/− littermates during aging.15 Our molecular analysis studies have additionally demonstrated that the Oct4 promoter in these animals shows also a higher level of demethylation. We also observed that Laron dwarf mice have in BM i) a ~3-fold increase in the number of Sca-1+c-kit+lineage− (SKL) hematopoietic stem cells and ii) up to 4-fold higher number of clonogenic progenitors. 15 Of note, more recently we even found more pronounced differences in number of VSELs and HSPCs between 26 month old Laron dwarf mice and their littermates (manuscript in preparation).
To extend these studies, we employed in the current work “a reverse model” that is transgenic mice that overexpress bovine growth hormone (bGH) and thus in contrast to Laron dwarf mice display chronic elevation of Igf1 in PB. As already reported, due to increased Igf1 level these mice are living much shorter than normal littermates (up to 1 year only) and develop frequently malignancies.16 These animals are also significantly larger than normal controls and display several symptoms of acromegaly.
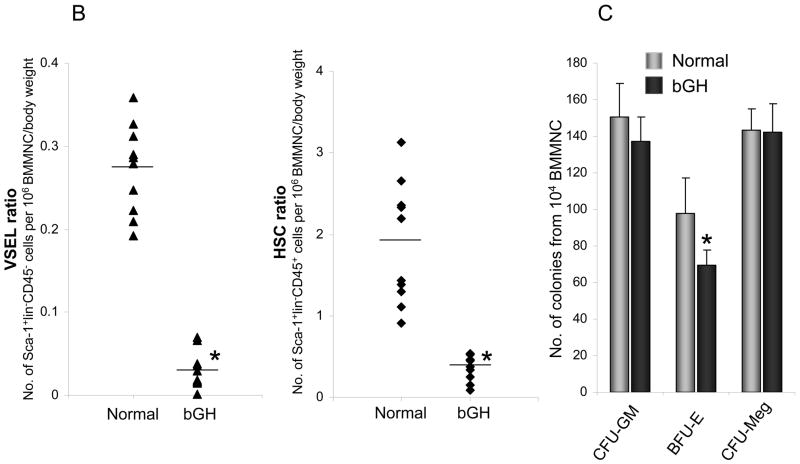
In the current study 6 month old phosphoenol pyruvate carboxykinase bovine growth hormone (PEPCK-bGH) transgenic males and control normal animals were employed and we noticed that bGH transgenic mice are slightly anemic as compared to controls (Table I). The low hematocrit level (Ht) and hemoglobin (Hb) concentration (Figure 1 A) in combination with normal number of erythrocytes is indicative for a presence of microcytic anemia in these animals. Transgenic mice had also slightly elevated platelet counts (Table I). More importantly, we noticed severe reduction of Sca-1+Lin−CD45− VSELs and Sca-1+Lin−CD45+ HSCs ratio in BM of bGH transgenic mice (Figure 1 B and C). However, at the same time we did not observe differences in number of clonogeneic progenitors (CFU-GM, CFU-Meg) except slight decrease in number of BFU-E, suggesting that defect in hematopoietic compartment was more profound at level of most primitive HSCs than hematopoietic progenitor cells (Figure 1 C), but this reduced number of HSCs still was able to maintain normal number of clonogeneic progenitors.
Table I.
Peripheral blood parameters in male 6 month old normal and bGH-transgenic mice (n=10).
| Normal controls | bGH-transgenic mice | |
|---|---|---|
| White Blood Cells (103/μl) | 7.84+/−1.97 | 7.40+/−1.81 |
| Neutrophils (103/μl) | 1.95+/−0.94 | 1.81+/−0.54 |
| Lymphocytes (103/μl) | 5.18+/−2.09 | 4.78+/−1.26 |
| Red Blood Cells (106/μl) | 8.66+/−0.42 | 8.49+/−1.09 |
| Platelets (103/μl) | 1046+/−167 | 1369+/−198 * |
| Hemoglobin (g/dL) | 11.53+/−0.44 | 9.34+/−0.99 ** |
| Hematocrit (%) | 43.70+/−1.49 | 35.24+/−5.15 * |
p < 0.001,
p < 0.0001.
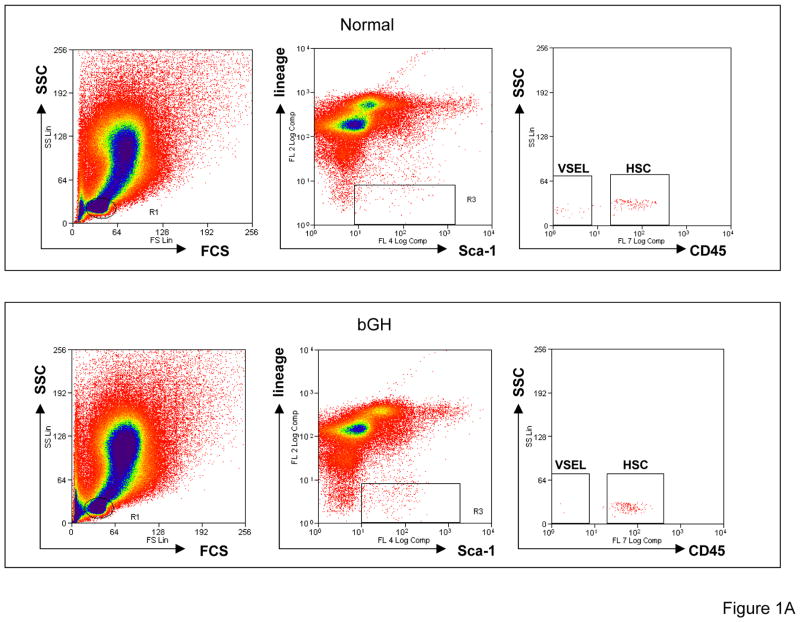
Figure 1. Increased number of VSELs and HSPCs in BM of bGH mice.
(A) Sorting of Sca-1+Lin−CD45− (VSELs) and Sca-1+Lin−CD45+ (HSCs) from mBM- derived MNCs by employing MoFlo High-Performance Cell Sorter. BMMNCs from region R1 are analyzed for Sca-1 and Lin expression. Sca-1+Lin− population which is included in region R2 is subsequently analyzed based on CD45 antigen expression and CD45− and CD45+ subpopulations visualized on dot plot, i.e., Sca-1+Lin−CD45− (VSELs) and Sca-1+Lin−CD45+ (HSCs). (B) The ratio for Sca-1+Lin−CD45− (VSELs) and Sca-1+Lin−CD45+ (HSCs) was evaluated as the number of events per 1×106 BMMNC/body weight by employing LSR II FACS analyzer. Analysis of Variance (ANOVA) with Bonferroni’s Multiple Comparison Test *p<0.00001 as compared to normal counterparts. (C) The number of clonogenic BFU-E, CFU-GM and CFU-Meg from BM MNC isolated from 6 months old normal and bGH transgenic mice. Analysis of Variance (ANOVA) with Bonferroni’s Multiple Comparison Test *p<0.001 as compared to normal counterparts.
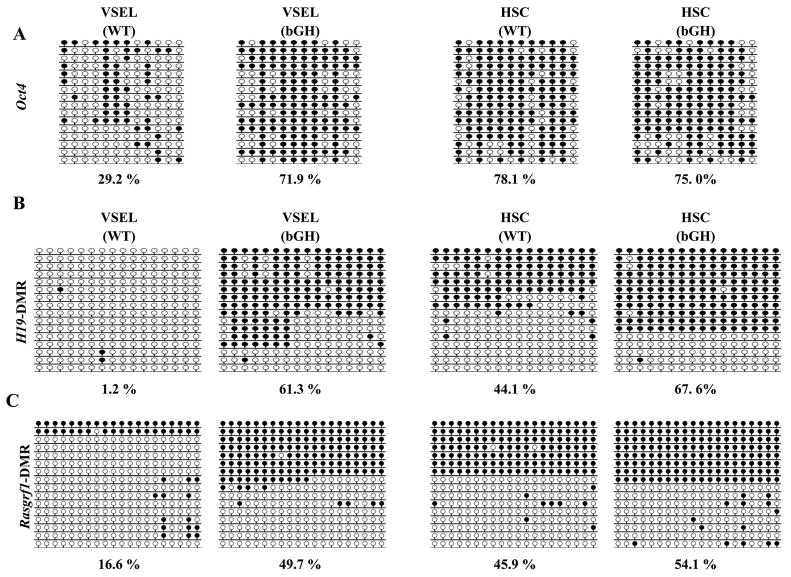
In parallel we performed molecular analysis of VSELs isolated from bGH transgenic and normal control mice. Figure 2 A demonstrates that Oct-4 promoter in 6 month old bGH transgenic mice-derived VSELs is hypermethylated (~72%) to the level similar as seen in HSCs. In contrast Oct-4 promoter in VSELs isolated from 6 month old normal mice still shows significant degree of hypomethylation (~30%). This suggests that VSELs in BM of bGH transgenic mice significantly lost their pluripotential character. At the same time DMR which regulates expression of H19-Igf2 locus that is hypomethylated in normal mice-derived VSELs (~1%) become hypermethylated in VSELs isolated from BM of 6 month old bGH transgenic mice. This indicates loss of imprinting and its reversal to somatic pattern and enhanced autocrine expression of Igf2 in these cells during aging (Figure 2 B). Similarly, DMR region for RasGrf1 locus, that encodes GTP-exchange factor for H-Ras involved in Ins/Igf signaling, that remains hypomethylated in VSELs from normal mice, become methylated in VSELs isolated from BM of bGH transgenic animals what again indicates restoration of somatic imprint at this locus (Figure 2 C). These epigenetic changes in toto lead to de-repression of imprinted genes that keep VSELs quiescent. Therefore, an enhanced Ins/Igf signaling in VSELs from bGH transgenic mice in combination with elevated plasma Igf1 level in these animals leads to premature depletion of these cells.
Figure 2. Change of Oct-4 promoter methylation and genomic imprinting in VSELs from 6 month old male normal and bGH transgenic mice.
(A) Bisulfite-sequencing results of DNA methylation of the Oct4 promoter in VSELs isolated from 6 month old normal and bGH transgenic mice (bGH). Methylated and unmethylated CpG sites are shown in filled and open circles, respectively. The numbers indicated below bisulfite-sequencing profiles presents the percentage of methylated CpG sites. Bisulfite sequencing results of DNA methylation of DMR for H19-Igf2 (B) and Rasgrf1 (C) loci in VSELs isolated from 6 month old normal and b-GH transgenic mice (bGH). Methylated and unmethylated CpG sites are shown in filled and open circles, respectively. The numbers indicated below bisulfite-sequencing profiles presents the percentage of methylated CpG sites.
Overall, since the plasma Igf1 level is regulated in mice by caloric uptake and in humans by caloric uptake providing that protein uptake is also low, these data shed new light on caloric restriction, senescence, and the size of hematopoietic stem cell compartment. Based on our findings, we propose that chronically elevated levels of Igf1, resulting, for example, from high caloric uptake, may lead to premature depletion of the stem cell pool, including VSELs and HSCs, and thus be responsible for premature aging in mice.
Further studies are needed to link the effect of chronic high Igf1 signaling in VSELs with the enhanced frequency of cancer in bGH tarnsgenic mice. Since many human malignancies are activated by Igf1 and Igf2 signaling (e.g., due to loss of heterozygosity or loss of imprinting at the Igf2-H19 locus), we hypothesize that excessive activation of VSELs in an Ins/Igf-dependent manner could promote malignant transformation of these cells. 17
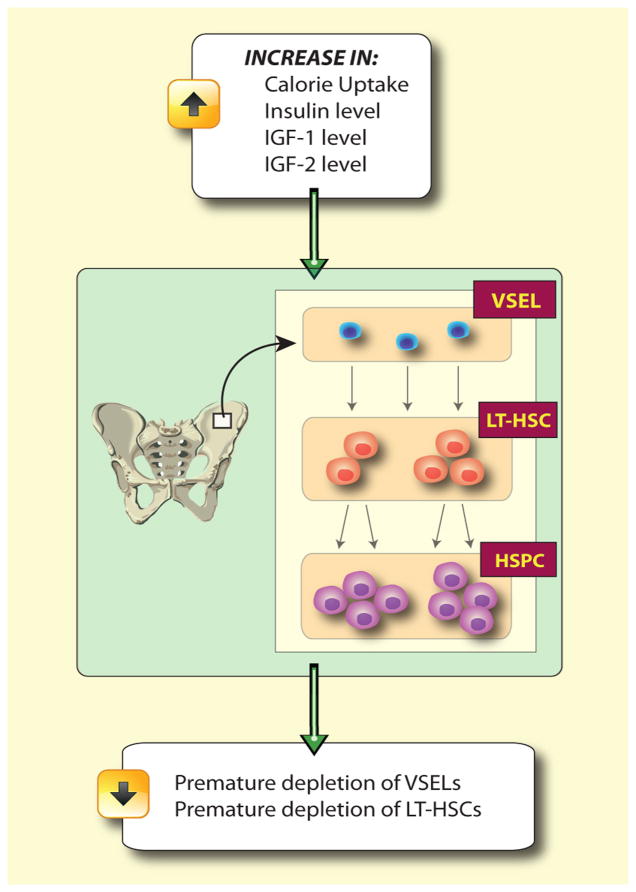
Based on our previously published data Laron dwarf mice with low circulating levels of Igf1 and current observations in bGH transgenic mice with high circulating levels of Igf1, we postulate novel linkages between the Igf1 level, aging, and the stem cell compartment (Figure 3). According to our hypothesis, early in development a population of VSELs would be deposited in developing organs as a backup for tissue-committed stem cells that plays a role in rejuvenation of tissues and organ regeneration after damage. These cells seem to be protected from uncontrolled proliferation and age-related depletion by changes in imprinted genes that regulate Ins/Igf signaling. We hypothesize that the pool of VSELs residing in adult tissues including BM is regulated by the circulating Igf1 level. An increase in Igf1 level (e.g., bGH transgenic animals, chronic high caloric uptake) would accelerate in an Ins/Igf-dependent manner an age-dependent depletion of the pool of VSELs and their potential to rejuvenate tissues (e.g., in BM to supply LT-HSCs). By contrast, a low Igf1 level (e.g., Laron dwarf mutants, caloric restriction) would have an opposite and protective effect on these cells leading to longevity.
Figure 3. Hypothesis of depletion of Oct-4+ epiblast-derived VSELs in adult bone marrow by chronic Ins/Igf signaling in bGH transgenic mcie.
Epiblast-derived VSELs are deposited in developing tissues including bone marrow as a backup population of SCs for production of tissue committed stem cells. We envision that in bone marrow VSELs are a backup population for long term repopulating hematopoietic stem cells (LT-HSCs) that give rise to hematopoietic stem/progenitor cells (HSPCs). Prolonged signaling by Insulin, Igf1, and Igf2 (e.g., due to high caloric uptake or as result of GH overexpression in bGH transgenic mice) may lead to premature depletion of these cells. A decrease in the number of VSELs in bone marrow will directly affect a pool of LT-HSC and finally over time also HSPCs.
Thus, these data further support our hypothesis that relates aging, longevity, insulin factor signaling, and high caloric uptake to the abundance and function of pluripotent VSELs deposited in adult tissues. A decrease in the number of these cells will affect pools of tissue committed stem cel (e.g., HSCs in BM) and have an impact on tissue rejuvenation and life span. Strategies to attenuate Ins/Igf stimulation of VSELs may lead to improve longevity.
Acknowledgments
This work was supported by NIH R01 DK074720, KBN grant (N N401 024536), EU structural funds, Innovative Economy Operational Program POIG.01.01.01-00-109/09-01 and the Henry M. and Stella M. Hoenig Endowment to MZR, by NIH P20RR018733 from the National Center for Research Resources to MK, NIH P01 AG031736 to AB and NIH AG032290 to MM.
Footnotes
Conflict Of Interest Statement
The authors declare no conflict of interests.
Reference List
- 1.Shin DM, Kucia M, Ratajczak MZ. Nuclear and Chromatin Reorganization during Cell Senescence and Aging - A Mini-Review. Gerontology. 2010 Feb 4; doi: 10.1159/000281882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flores I, Blasco MA. The role of telomeres and telomerase in stem cell aging. FEBS Lett. 2010;584 :3826–3830. doi: 10.1016/j.febslet.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 3.Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141:2608–2613. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- 4.Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144:3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- 5.Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008;7:681–687. doi: 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartke A. Can growth hormone (GH) accelerate aging? Evidence from GH-transgenic mice. Neuroendocrinology. 2003;78:210–216. doi: 10.1159/000073704. [DOI] [PubMed] [Google Scholar]
- 7.Ikeno Y, Hubbard GB, Lee S, Cortez LA, Lew CM, Webb CR, Berryman DE, List EO, Kopchick JJ, Bartke A. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2009;64:522–529. doi: 10.1093/gerona/glp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell SJ, Kahn CR. Endocrine regulation of ageing. Nat Rev Mol Cell Biol. 2007;8:681–691. doi: 10.1038/nrm2234. [DOI] [PubMed] [Google Scholar]
- 9.Ratajczak J, Zhang Q, Pertusini E, Wojczyk BS, Wasik MA, Ratajczak MZ. The role of insulin (INS) and insulin-like growth factor-I (IGF-I) in regulating human erythropoiesis. Studies in vitro under serum-free conditions--comparison to other cytokines and growth factors. Leukemia. 1998;12:371–381. doi: 10.1038/sj.leu.2400927. [DOI] [PubMed] [Google Scholar]
- 10.Reik W, Walter J. Genomic imprinting: parental influence on the genome. Nat Rev Genet. 2001;2:21–32. doi: 10.1038/35047554. [DOI] [PubMed] [Google Scholar]
- 11.Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, et al. A population of very small embryonic-like (VSEL) CXCR4+SSEA-1+Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857–869. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- 12.Ratajczak J, Wysoczynski M, Zuba-Surma E, Wan W, Kucia M, Yoder MC, et al. Adult murine bone marrow-derived very small embryoniclike Stem cells (vsels) differentiate into the hematopoietic Lineage after co-culture over op9 stromal cells. Exp Hematol. 2011;39:225–237. doi: 10.1016/j.exphem.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin DM, Zuba-Surma EK, Wu W, Ratajczak J, Wysoczynski M, Ratajczak MZ, et al. Novel epigenetic mechanisms that control pluripotency and quiescence of adult bone marrow-derived Oct4+ very small embryonic-like stem cells. Leukemia. 2009;23:2042–2051. doi: 10.1038/leu.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratajczak MZ, Shin DM, Ratajczak J, Kucia M, Bartke A. A novel insight into aging: are there pluripotent very small embryonic-like stem cells (VSELs) in adult tissues overtime depleted in an Igf-1-dependent manner? Aging. 2010;2:875–883. doi: 10.18632/aging.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ratajczak J, Shin DM, Wan W, Liu R, Masternak MM, Piotrowska K, Wiszniewska B, Kucia M, Bartke A, Ratajczak MZ. Higher number of stem cells in the bone marrow of circulating low Igf-1 level Laron dwarf mice-novel view on Igf-1, stem cells and aging. Leukemia. 2011 doi: 10.1038/leu.2010.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westbrook R, Bonkowski MS, Strader AD, Bartke A. Alterations in oxygen consumption, respiratory quotient, and heat production in long-lived GHRKO and Ames dwarf mice, and short-lived bGH transgenic mice. J Gerontol A Biol Sci Med Sci. 2009;64:443–451. doi: 10.1093/gerona/gln075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447:433–440. doi: 10.1038/nature05919. [DOI] [PubMed] [Google Scholar]