Abstract
It has been recently shown that various stress-inducing manipulations in isolated ventricular myocytes may lead to significant remodeling of t-tubules. Osmotic stress is one of the most common complications in various experimental and clinical settings. Therefore, this study was designed to determine the effects of a physiologically relevant type of osmotic stress, hypo-osmotic challenge, to the integrity of t-tubular system in mouse ventricular myocytes using two approaches: (1) electrophysiological measurements of membrane capacitance and inward rectifier (IK1) tail currents originating from K+ accumulation in t-tubules, and (2) confocal microscopy of fluorescent dextrans trapped in sealed t-tubules. Importantly, we found that removal of 0.6 Na (60% NaCl) hypo-osmotic solution, but not its application to myocytes, led to ~27% reduction in membrane capacitance, ~2.5-fold reduction in the amplitude of IK1 tail current and ~2-fold reduction in so-called IK1 ‘inactivation’ (due to depletion of t-tubular K+) at negative membrane potentials – all data being consistent with significant detubulation. Confocal imaging experiments also demonstrated that extracellularly applied dextrans become trapped in sealed t-tubules only upon removal of hypo-osmotic solutions, i.e. during shrinking phase, but not during initial swelling period. In light of these data, relevant previous studies, including those on EC coupling phenomena during hypo-osmotic stress, may need to be reinterpreted and the experimental design of future experiments should take into account the novel findings.
Keywords: cardiac myocytes, t-tubules, inward rectifier potassium channels
Introduction
Osmotic stress is a common complication during various experimental and clinically relevant conditions. Numerous studies over more than half a century showed that both hyper- and hypo-osmotic challenges to cardiac tissues and cells lead to a multitude of consequences which are largely (although not exclusively) detrimental to their electrical and contractile properties. The most detailed and mechanistic studies have begun with the introduction of enzymatically isolated cardiac myocytes and patch-clamp technique (for review (Vandenberg et al., 1996)), targeting, in particular, the central phenomenon of excitation-coupling. It has been shown that various types of osmotic shock lead to significant changes in many sarcolemmal currents, including Cl− currents (Sorota, 1992; Vandenberg et al., 1994), delayed rectifier current IKs and IKr (Rees et al., 1995), Ca2+ currents as well as currents generated by transporters (Whalley et al., 1991; Whalley et al., 1993) (for review (Cazorla et al., 1999)). Osmotic regulation of Ca2+ currents received the largest attention and studies have shown that effects of osmotic stress are quite complex and depend, in particular, on the type of cardiac cells (e.g. atrial vs ventricular). For example, it has been shown that during osmotically-induced swelling L Type of Ca2+ current increases in rabbit atrial cells (Matsuda et al., 1996) and decreases in rat ventricular myocytes (Brette et al., 2000), while swelling produces virtually no changes in guinea-pig ventricular myocytes (Groh et al., 1996). Detailed analysis of the time course of osmotic effects in rat and rabbit ventricular myocytes shows that the changes in the magnitude of L Type of Ca2+ current upon application of hypo-osmotic solution display biphasic behavior with initial increase followed by downregulation at later times (Li et al., 2002; Luo et al., 2010). Similar biphasic phenomena were also observed with other relevant parameters such as cell shortening (Li et al., 2002), APD and loading of sarcoplasmic reticulum (SR) (Brette et al., 2000).
It is clear that the changes in membrane stretch and water content (leading to dilution or concentration of intracellular milieu) in cardiac myocytes upon osmotic stress are the initial general events which lead to a multitude of complicated but specific consequences to distinct ion channels, transporters, intracellular organelles (e.g. SR) and integrative parameters such as AP and EC coupling. In ventricular myocytes, however, there is another general feature of paramount importance for the overall activity of the myocyte which may directly underlie and explain numerous osmotic phenomena – t-tubules. It has been long known that t-tubules in skeletal muscle can be removed (sealed) by strong hyper-osmotic shock with glycerol (Eisenberg & Eisenberg, 1968; Howell, 1969) or formamide (Argiro, 1981) with detubulation occurring upon withdrawal of osmolite.
The ‘formamide version’ of the detubulation method, which uses very high concentration of formamide (1.5 M), was introduced in the cardiac field by Kawai et al in 1999 (Kawai et al., 1999) and provided a great experimental tool for numerous t-tubular studies. However, the potential role of shock-induced detubulation in physiologically and pathophysiologically relevant conditions is essentially not known. To our knowledge, the only study where an attempt was made to look into the state of t-tubules during hypo-osmotic stress was that by Brette et al (2000) (Brette et al., 2000). The results were negative – swelling of rat ventricular myocytes in hypo-osmotic solution did not lead to detubulation. Importantly, however, the integrity of t-tubules in mentioned study was assessed only during swelling phase (i.e. application of hypo-osmotic solution).
In this study we demonstrate that despite previous unsuccessful attempts to elucidate the effects of physiologically relevant osmotic challenges on t-tubules their remodeling does occur. Specifically, we describe a novel and quite unexpected phenomenon of strong t-tubular remodeling which occurs nearly exclusively upon resolution (shrinking phase) but not during hypotonic stress itself (swelling phase). Experiments with isolated mouse ventricular myocytes show that washout of commonly used hypotonic solutions with osmolarity only ~25% less than normal leads to significant-to-dramatic t-tubular remodeling, comparable to detubulation produced by 1.5 M formamide. Since nearly all hypotonic challenges are eventually resolved, the potential physiological significance of this phenomenon is clear and will need to be addressed accordingly. The data also show that many relevant previous studies may need to be reinterpreted and the experimental design of future experiments should take into account the novel findings.
Methods
Animals
All experiments involving mice were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (8th Edition, Committee for the Update of the Guide for the Care and Use of Laboratory Animals, National Research Council; The National Academic Press, Washington, DC) and protocols approved by the veterinary staff of the University Committee on Use and Care of Animals (UCUCA) at the University of Michigan.
Solutions
Osmolarity was measured using Vapro Osmometer 5520 (Wescor, ELITechGroup, France) and presented as mean ± Standard Deviation (vs Standard Error (SE) in other places).
Tyrode (Tyr; modified) (mM)
137 NaCl, 5.4 KCl, 0.5 MgCl2, 0.3 CaCl2, 0.16 NaH2PO4, 3 NaHCO3, 5 HEPES, 5.5 Glucose, pH=7.35 with NaOH. 278.7 ± 3.5 mOsm/L. 10 mM glucose was used in patch-clamp experiments.
Solutions for isolation of ventricular myocytes (filtered using 0.22 μm filter) (mM)
A: 122 NaCl, 5.4 KCl, 4 MgCl2, 0.16 NaH2PO4, 3 NaHCO3, 15 HEPES, 10 Glucose, 0.1 μM EGTA, pH=7.35 with NaOH. 279.7 ± 6.4 mOsm/L. B: 50 ml A + 30 mg Collagenase (Type 2; Worthington Biochemical Corporation, Lakewood, NJ, USA). C: 180 ml A + 900 mg Bovine Serum Albumin + 250 mg Taurine. 288.7 ± 3.5 mOsm/L.
Pipette solution KINT (mM)
140 KCl, 0.5 EGTA, 10 HEPES, 5 K2ATP, pH=7.3 with KOH. 280.7 ± 2.3 mOsm/L. 0.2 CaCl2/2 EGTA was used in experiments in Fig. 7.
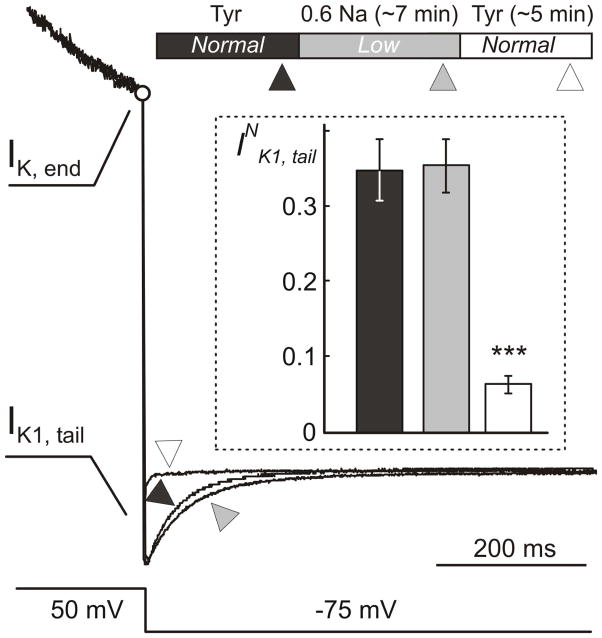
Figure 7. Time course of electrophysiological remodeling during hypo-osmotic stress.
Example of whole-cell currents in response to voltage protocol as in Fig. 6A recorded in the same myocyte at different times during hypo-osmotic stress and its resolution. (Top) The timing of the application of Tyrode (Tyr) and hypo-osmotic 0.6 Na solutions (Normal and Low osmolarity). Ionic currents were recorded within ~1–2 min before application of 0.6 Na solution (▲), ~1–2 min before (
 ) and ~3–6 min (△) after its removal. Current traces were normalized to each other at the end of depolarizing voltage step (IK, end; ○) in order to highlight the relative changes in IK1, tail currents. Fast capacitative transient upon repolarization is not shown. Insert: Quantification of the data shows that IK1, tail currents are not affected by the hypo-osmotic stress itself but are significantly reduced (by ~5 fold; p<0.001) shortly after resolution of the shock.
) and ~3–6 min (△) after its removal. Current traces were normalized to each other at the end of depolarizing voltage step (IK, end; ○) in order to highlight the relative changes in IK1, tail currents. Fast capacitative transient upon repolarization is not shown. Insert: Quantification of the data shows that IK1, tail currents are not affected by the hypo-osmotic stress itself but are significantly reduced (by ~5 fold; p<0.001) shortly after resolution of the shock.
PBS (standard Phosphate Buffer solution)
267.2 ± 2.5 mOsm/L. PBS_40 (PBS diluted by 40% with H20): 193.3 ± 2.9 mOsm/L. 10% PBS in Tyr solution: 277.8 ± 1.3 mOsm/L.
Osmotically modified solutions
0.6 Na: Tyr containing 60% NaCl (82.2 mM), 204.7 ± 4.2 mOsm/L. 9% PBS_40 in 0.6Na solution: 201.0 ± 2.7 mOsm/L. 0.8 Na: Tyr containing 80% NaCl (109.6 mM), 241.5 ± 3.7 mOsm/L. 0.6 Na/100 S: 0.6 Na containing 100 mM Sucrose, 284.9 ± 3.9 mOsm/L. 0.6 Na/200 S: 0.6 Na containing 200 mM Sucrose, 372.6 ± 3.0 mOsm/L.
Most experiments were performed using 0.6 Na solution where both NaCl concentration and osmolarity are changed. Stronger effects induced by this solution allow for easier quantification of the data as well as for revealing the modulating effect of NaCl in experiments involving 0.6 Na/100 S where only osmolarity is modified.
5 mg voltage-sensitive dye di-8-ANEPPS (Life Technologies, Carlsbad, CA, USA) was dissolved in 1 ml of DMSO and used as a stock (stored +4 °C). Working solution was prepared as 50:50 v/v mix of di-8-ANEPPS stock and 20% Pluronic acid (to facilitate the solubilization of di-8-ANEPPS).
Isolation of mouse ventricular cardiomyocytes
Ventricular myocytes were isolated from the hearts of adult (~2–6 month old) C57BL/6 mice of either sex anesthetized with Avertin (20 μl/g of working solution; intraperitoneal injection) using collagenase treatment. The Avertin stock solution was prepared as follows (McLerie & Lopatin, 2003): 10 g of tribromoethanol alcohol (Sigma-Aldrich, St. Louis, MO, USA) was mixed with 10 ml of tert-amyl alcohol (Aldrich, USA) and stored at −20 °C. Working solution of ~2.5% was prepared by mixing of 1 ml of Avertin stock with 39 ml of PBS and the addition of 1 mg/ml of heparin (Sigma, USA; 187 USP unit/mg). Briefly, hearts were perfused retrogradely (Langendorff perfusion) through aorta with appropriate solutions at a constant pressure of ~115 cm H2O and T=~28 °C using a water-jacketed supply tube. Atria were removed and AV valve surgically destroyed. Solution A was applied for ~5 min, followed by solution B. 44 μM Ca2+ was added ~2 min later, and the heart digested with solution recirculation for another 33 min. The lower part of the heart (~3/4) was cut off and the right ventricle outer wall removed. The left ventricle was opened, sliced into ~6–8 pieces, the tissue transferred to a 15 mL tube containing 10 ml of solution B and triturated at ~28 °C on a rocker for ~15 min. Myocytes suspension was filtered and procedure repeated with tissue chunks up to 5 times with increasing proportion of solution C. The myocytes were usually optimally isolated at steps 4 to 5. Myocytes were stored in respective solutions at room temperature (RT; 20–23 °C) and used in experiments within 1 to 5 h post isolation.
Patch-clamp measurements
Ionic currents were recorded in whole-cell configuration of patch-clamp technique (Hamill et al., 1981) at RT essentially as described in (Cheng et al., 2011) with few minor modifications. In particular, resistance of patch pipettes (KIMBLE glass; no. 73813) varied from 2 to 4 MΩ when filled with KINT solution. In few experiments pipette tips were coated with a mixture of mineral oil and paraffin aimed to reduce stray capacitance of the pipette. Series resistance was always compensated leading to 2–3 MΩ access resistance. The data were filtered at 2 kHz. During patch-clamp measurements myocytes were continuously superfused with appropriate solutions using a flow chamber allowing for changes of solutions within few seconds. A 3 M KCl/agar salt bridge for grounding the bath is (always) used in order to minimize potential voltage offsets in experiments involving changes of extracellular solutions.
Membrane capacitance, CM, was measured using Clampex (Molecular Devices, USA) built-in algorithm employing mono-exponential fit to capacitative current in response to ΔVM= 5 mV depolarizing voltage step from a holding potential of −75 mV.
IK, end, inward rectifier tail currents, IK1, tail, and IK1 ‘inactivation’ (Fig. 6 A and C) were analyzed as described in (Cheng et al., 2011). Briefly, ionic currents were recorded in response to 400 ms voltage step to +50 mV followed by repolarization back to holding potential of −75 mV. IK, end is the current at the end of depolarizing step. IK1, tail current was fit using two-exponential function: , where A1 and A2 are the amplitudes and τ1 and τ2 are the corresponding time constants of exponential components, t is the time and C is the steady state current. Approximately ~7 ms of current traces was excluded from the fit to avoid contribution from capacitative currents and the amplitudes of individual exponential components (A1 and A2) were then recalculated to 0 time using measured time constants. Finally, the ratio was calculated.
Figure 6. Electrophysiological evidence for sealing of t-tubules in response to resolution of hypo-osmotic stress.
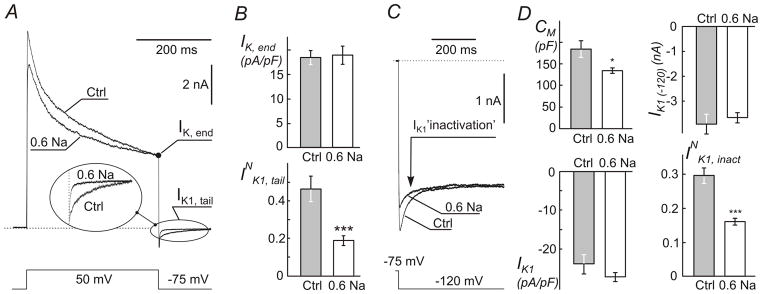
Myocytes were treated with 0.6 Na solution (as in Fig. 2) and whole-cell currents recorded with ~2 hours post shock. A, Representative current recordings in response to indicated voltage step show significant decrease in the amplitude of IK1 tail current (Insert) in. Individual myocytes with similar IK,end current were selected for presentation purpose. The amplitude of IK1 tail current depends only on IK,end, not the preceding part of the outward K+ current (Cheng et al., 2011). Dotted line represents zero current level. B, quantification of whole-cell IK, end and normalized IK1 tail currents (INK1, tail; see Methods). C, inactivation of IK1 current at far negative membrane potentials (originates from depletion of t-tubular K+ due to inward IK1 current) is significantly reduced in myocytes treated with 0.6 Na solution. IK1 currents were recorded in response to voltage step as indicated. Individual myocytes with similar steady-state IK1 current were selected for presentation purpose. D, quantification of changes in (top left) whole-cell membrane capacitance (CM), (top right) peak amplitude of whole-cell IK1 at −120 mV (IK1 (−120)), (bottom left) density of peak IK1 and (bottom right) normalized inactivation of IK1 (INK1, inact) (also see Methods).
IK1 was measured in response to a 400 ms voltage step from a holding potential −75 mV to −120 mV. The time course of IK1 ‘inactivation’, IK1, inact, was fit with two-exponential function and normalized current calculated as , where A1 and A2 are the amplitudes of the time-dependent component of IK1 ‘inactivation’ and IK1 (−120) is the (peak) magnitude of IK1 recalculated (Δt~8 ms) to time 0.
Formamide-induced detubulation of cardiac myocytes
Formamide-induced osmotic shock was implemented essentially as described in Kawai et al (Kawai et al., 1999) with the following modification: ‘C’ solution (see Solutions) was used for detubulation (i.e. washout of formamide) steps.
Osmotic shocks in dextran trapping experiments
High concentration of fluorescent dextran must be used in order to visualize and quantify rather small sealed t-tubules. Therefore, the following procedures were used. 3,000 MW (3K) tetramethylrhodamine (red) anionic dextran (Invitrogen, USA) was dissolved in PBS at concentration 10 mg/ml, part of the solution diluted by 40% with H2O (PBS_40; to better preserve the osmolarity of 0.6 Na solution when adding dextran) and both solutions stored at −20°C for future use.
‘Late’ application of dextran (Fig. 2; A2)
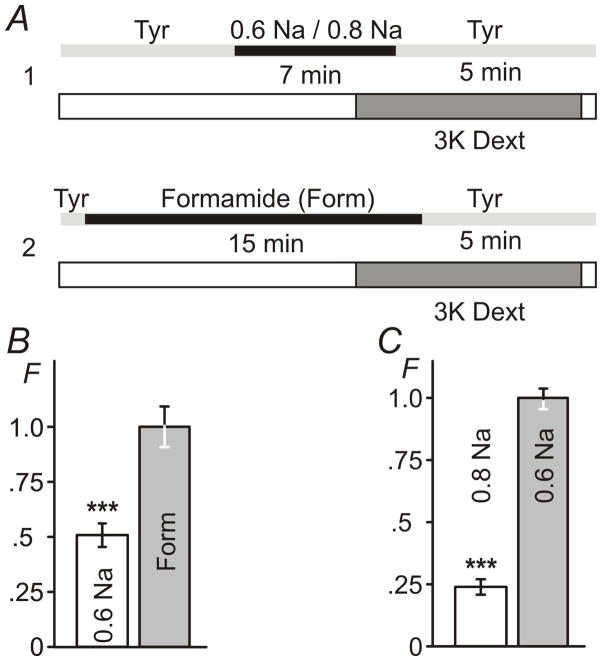
Figure 2. Sealing of t-tubules occurs exclusively during resolution of hypo-osmotic shock.
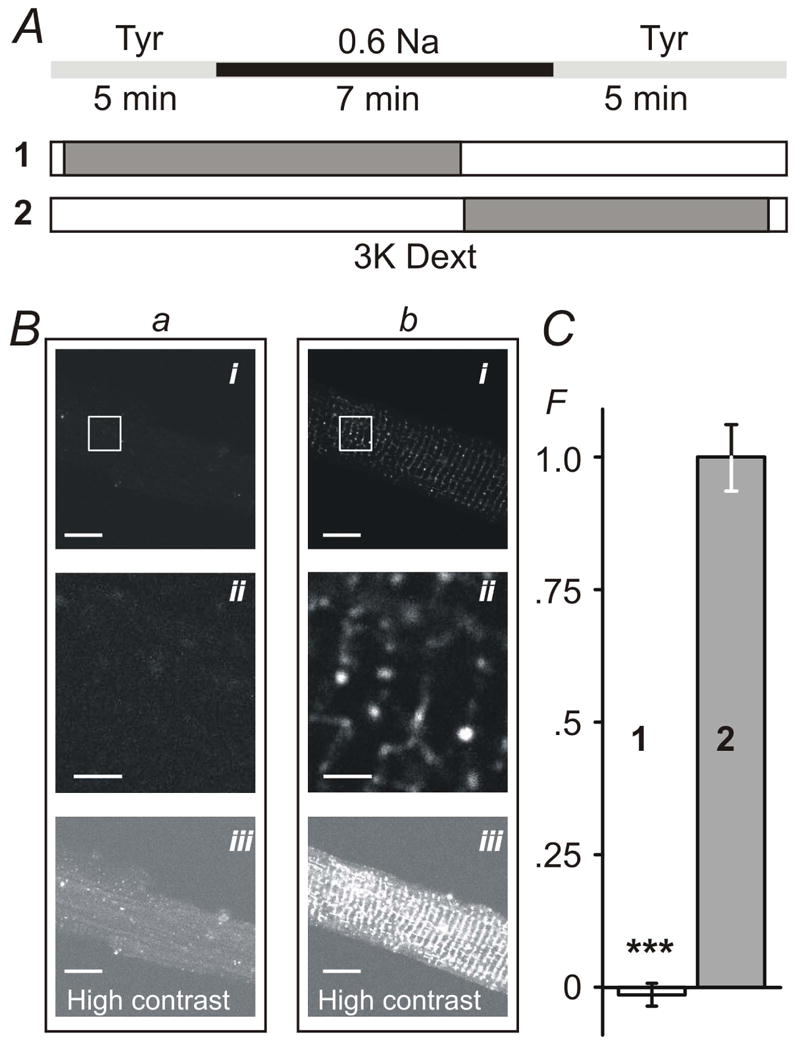
A, timing (±30 sec) of the application of 0.6 Na hypo-osmotic solution and 3K red fluorescent dextran (1 and 2). B, myocytes were imaged in dextran-free solution using confocal microscope (with identical settings) within 1–2 hours after hypo-osmotic shock. Bai and Bbi images correspond to protocols A1 and A2, respectively. Baii and Bbii images are magnified replicas of corresponding rectangular areas above. Baiii and Bbiii images are contrast-adjusted replicas of the Bai and Bbi images highlighting the overall magnitude of t-tubular sealing. C, the average intensity of intracellular fluorescence from within the outline of the myocytes (i.e., not including out-of-cell area; see Methods) was background-corrected using the average intracellular fluorescence in cells undergone complete protocol as in A2 but in the presence of Tyr solution instead of 0.6 Na solution. F – Relative fluorescence. Mean fluorescence in protocol A2 was set to 1. Bars in B: 10 μm for i and iii and 2 μm for ii. Note: small negative fluorescence in C reflects experimental error due to background correction.
A small aliquot (up to few hundred μl) of enzymatically digested myocytes was diluted in a large volume (usually 10 ml) of desired solution (e.g. 0.6 Na for hypo-osmotic swelling) and the timer started. Myocytes were then concentrated by centrifugation (2 minutes, 48 g; all other centrifugations were performed using same parameters), 10 μl of cell suspension transferred into separate tube and 1 μl of dextran stock (in PBS or PBS_40) added ~2 min before removing hypo-osmotic solution to allow dextran to fill accessible t-tubules. Normal (near isotonic) solution consisting of 90 μl of Tyr and 10 μl 3K dextran stock (in PBS_40) was added at 7 min in order to initiate myocytes shrinking. Addition of dextran dissolved in appropriate solution (PBS or PBS_40) does not lead to any significant changes in osmolarity of test solution. In order to remove most of extracellular dextran, cells were resuspended in ~10 ml of Tyr 5 min after the osmotic shock, concentrated by centrifugation to <~100 μl and resuspended again in ~0.5–1 ml of solution C.
‘Early’ application of dextran (Fig. 2; A1)
1 μl dextran stock was added to 10 μl of cell suspension just before osmotic shock. Hypo-osmotic solution consisted of 300 μl 0.6 Na and 30 μl of dextran stock. ~2 min before removing hypo-osmotic solution myocytes were resuspended in 10 ml of dextran-free 0.6 Na solution (in order to dilute dextran ~30 times) and concentrated by centrifugation. Exactly 7 min after the application of hypo-osmotic solution cells were resuspended in ~10 ml of normal Tyr in order to initiate cell shrinking. Finally, myocytes were concentrated by centrifugation and resuspended again in ~0.5–1 ml of solution C.
In experiments using low NaCl (e.g. 0.6 Na/100 S) solution as the initial control instead of normal Tyrode (Tyr) myocytes were preincubated in that solution for 5 min before applying the test solution.
All experiments were performed along with appropriate controls including all steps (dye mixing, centrifugations etc) but using normal Tyrode instead of osmotically modified solutions.
Confocal imaging and image analysis
Confocal imaging was performed using Olympus FV-500 microscope at the Microscopy and Image Analysis Laboratory (University of Michigan, Ann Arbor) using 60× oil immersion objective. Confocal images were visualized and analyzed using ImageJ software (http://imagej.nih.gov). In experiments using membrane labeling myocytes were exposed post-shock (i.e. after washout of test solutions) to di-8-ANEPPS (~0.5 μl working solution in 300 μl of cell suspension) for ~10–30 min at room temperature. In all experiments only fluorescence originating from within the outline of myocytes was analyzed. This was achieved by manually creating a region of interest contained within the border (but not including it) of the myocye and then calculating mean ‘intracellular’ fluorescence. This procedure eliminates any contribution of fluorescence from traces of extracellular dextran remaining after washout procedure and di-8-ANEPPS fluorescence originating from the outer sarcolemma (including highly convoluted intercalated disks). The data were corrected for background fluorescence measured in myocytes processed identical to that in test experiments but without application of osmotic shock (i.e. including the presence of 3K dextran and centrifugations at specific steps). Since we are only concerned with the total amount of fluorescence of trapped dextran (proportional to the volume of sealed t-tubules) and total amount of ‘intracellular’ fluorescence of di-8-ANEPPS (proportional to the surface area of accessible t-tubules) neither image deconvolution nor other sophisticated approaches (e.g. super-resolution microscopy techniques) useful for resolving the fine structure of t-tubules were necessary. We found that the use of ‘red’ dextran is superior to the ‘green’ version of the compound because of significantly lower background fluorescence of myocytes in the red part (vs green) of the spectra.
Statistics
Data are presented as a mean ± Standard Error (SE) except for the data on osmolarity (above) where Standard Deviation is used. All data used in image analysis were corrected for the background fluorescence. Statistical significance was calculated using a two-tail t-test with the exception of the data in Fig. 7 where paired t-test was used. Throughout the manuscript asterisks *, ** and ***, represent p<0.05, <0.01 and <0.001, respectively. All data, with the exception of largely introductory and confirmatory data in Fig 1, are from at least two heart preparations with multiple (9 to 50; exact numbers indicated in the text) myocytes used in each experimental setting.
Figure 1. Effects of hypo-osmotic challenge on dimensions of ventricular myocytes.
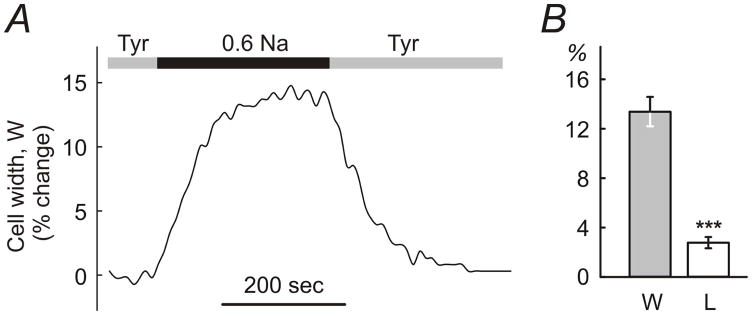
A, application of 0.6 Na hypo-osmotic solution to ventricular myocytes leads to fast and significant changes in their width. Dimensions of myocytes return to normal upon re-application of control solution (Tyrode; Tyr). B, the changes in the width (W) of the myocytes are ~5-fold larger than changes in their length (L). The change in dimensions is expressed in % relative to the resting values in Tyr solution.
Results
Effects of hypo-osmotic stress on the cells dimensions
Fig. 1A shows a representative example of an experiment in which a myocyte was first superfused in a flow chamber with Tyrode solution (Tyr), in order to wash out storage solution (C), then hypo-osmotic solution 0.6 Na applied and myocytes then returned back to Tyr solution. The dimensions of the myocytes stabilized within ~3–4 minutes in hypo-osmotic solution and returned back to normal also within ~3–4 minutes upon resolution of stress. The speed of the change of solutions in the chamber, more specifically the speed of change around the individual cells, is likely one of the contributing factors to the kinetics of the swelling which potentially may occur faster with faster speeds of perfusion (this was not investigated further). For practical purposes, however, in order to be sure that the swelling of myocytes in 0.6 Na and 0.8 Na reached the steady-state, 7 minute exposure was used (vs 15 min exposure to formamide as in Kawai et al (Kawai et al., 1999)). Consistent with the previous studies, the myocytes responded to hypo-osmotic challenge primarily by changes in the cell width rather than length (Roos, 1986; Boyett et al., 1991; Brette et al., 2000) (Fig. 1B). The change in width of the myocytes was 7nearly 5-fold larger than the change in their length: 13.3 ± 1.2 % and 2.7 ± 0.5 % (n=10; p<0.001) relative to initial values for width and length, respectively. Myocyte dimensions returned back to normal within ~1% precision upon resolution of stress. In contrast to some studies (Brette et al., 2000) application of hypo-osmotic solutions was not accompanied by any visual deterioration of myocytes such as membrane blebbing.
Resolution of hypo-osmotic stress but not the stress itself leads to sealing of t-tubules
Fig. 2 summarizes the central finding of this study. The effects of hypo-osmotic stress on the integrity of t-tubular system were assessed using fluorescent dextrans (Brette et al., 2002). Ventricular myocytes were first exposed to 0.6 Na solution, as indicated in Fig. 2A, with extracellular 3K red dextran present during different phases of the stress (protocols 1 and 2; Fig. 2A), and confocal images taken within ~2 hours post-shock in normal Tyrode solution to assess the presence of dextran (present only for ~5 min after the resolution of shock) potentially trapped in sealed or highly constricted t-tubules. If extracellular dextran was present before and during swelling phase caused by application of 0.6 Na solution, but not at or during cells shrinking (Fig. 2A), confocal imaging of myocytes after the recovery from shock did not reveal any significant intracellular fluorescence (Fig. 2B; a). However, when dextran was present in the 0.6 Na solution just before and during shrinking phase (Fig. 2A; protocol 2) significant ‘intracellular’ fluorescence could then be easily observed (Fig. 2B; b). The only plausible explanation for the results is that dextran was trapped in sealed t-tubules at the time of or shortly after the washout of 0.6 Na solution. The magnitude of ‘intracellular’ fluorescence of dextran observed in the whole cell is seemingly quite small (Fig. 2B; bi) as it may be perceived by looking at the images. However, it should be taken into account that t-tubules are relatively sparse and thin (in large degree are point-type objects) and their volume relative to that that of the whole cell is also quite small, less than few percent (Stewart & Page, 1978; Soeller & Cannell, 1999). In fact, in contrast to this potential illusion some pixels in the images are saturated due to intense fluorescence of dextran trapped in sealed t-tubules. Zooming into the image (Fig. 2B; bii) or increasing its contrast (Fig. 2B; biii) highlights the effect. Specifically, the density of 3K dextran fluorescence (amount of fluorescence per unit area within the myocyte borders; arbitrary units but identical imaging conditions; see Methods for details) were as follows: intracellular background fluorescence (Control) 4.0 ± 0.1, ‘early’ application of 3K dextran as in protocol 1 (Fig. 2A) 3.9 ± 0.1, and ‘late’ application of dextran as in protocol 2 (Fig. 2A) 6.0 ± 0.5 (n=24, 24 and 24 for the above conditions, respectively; p<0.001). For presentation purposes in Fig. 2C the data were first corrected for background fluorescence by subtracting fluorescence measured in Control myocytes which were treated identically as test myocytes (i.e. including all steps such as centrifugations) in the presence of extracellular dextran but using iso-osmotic solutions instead of 0.6 Na hypo-osmotic solution. The data were further normalized to that in protocol 2 (Fig. 2A). Importantly, quantification procedure of the fluorescence (see Methods) is robust as it does not require any detailed knowledge of the fine structure of t-tubules (which is of no concern in this study), and the data show dramatic effects of the removal of hypo-osmotic shock on the amount of trapped dextran suggesting significant t-tubular remodeling (sealing of t-tubules).
The extent of hypo-osmotic t-tubular sealing
At this juncture, however, the above data do not provide enough information on the extent of hypo-osmotic detubulation relative, in particular, to that produced by formamide treatment (Kawai et al., 1999). The data in Fig. 3 compare the effects of formamide and various hypo-osmotic treatments on changes in the volume of sealed t-tubules. First, Fig. 3B shows that the relative amount of 3K dextran trapped in t-tubules is reduced from 1.00 ± 0.09 during treatment with 1.5 M formamide to about 0.51 ± 0.05, or by 50%, after application of 0.6 Na solution (n=26, 27, 27 for control, 0.6 Na and formamide conditions, respectively; p<0.001). This is quite a remarkable level of detubulation considering a relatively small change in osmolarity in 0.6 Na solution (~80 mOsm reduction relative to normal Tyr solution) compared to that in 1.5 M formamide (membrane permeable agent though) containing solution (estimated osmolarity >1400 mosmol; unpublished). The data in Fig. 3C also show that hypo-osmotic detubulation, as one may expect, is a graded process with intermediate (but yet significant) level of 3K dextran trapping observed in less hypo-osmotic solutions such as 0.8 Na. Specifically, after treatment with 0.8 Na solution the trapping of 3K dextran is reduced to 0.24 ± 0.03, or to ~25%, relative to 1.00 ± 0.04 after exposure to 0. 6 Na solution (n=41, 50, 47 for control, 0.6 Na and 0.8 Na conditions, respectively; p<0.001).
Figure 3. The extent of hypo-osmotic t-tubular sealing.
A, timing of the application of various hypo-osmotic (A1) and hyper-osmotic formamide containing (A2) solutions using ‘late’ application of 3K fluorescent dextran (see Methods). B, relative amount of trapped 3K dextran after stress with 0.6 Na solution is ~50% of that observed after formamide treatment. C, relative amount of trapped 3K dextran after stress with 0.8 Na solution is ~25% of that of after treatment with 0.6 Na solution. F - Relative fluorescence. Mean fluorescence in B for Formamide treatment and in C for treatment with 0.6 Na solution was set to 1.
Fig. 4 shows the effects of hypo-osmotic detubulation employing a more ‘classical’ approach for assessing detubulation using membrane specific dye (Kawai et al., 1999; Cheng et al., 2011). Ventricular myocytes were shocked with 0.6 Na hypo-osmotic and 1.5 M formamide hyper-osmotic solutions as described earlier (see Fig. 2 and Fig. 3 along with corresponding text) and accessible sarcolemmal membrane labeled with di-8-ANEPPS dye. Both types of treatment led to significant reduction of t-tubular labeling compared to that in control myocytes indicating strong, yet quantitatively different, detubulation. Specifically, after the treatment with 0.6 Na solution t-tubular fluorescence of di-8-ANEPPS was reduced to 43 ± 2.4 % of that observed in control myocytes (or, alternatively, ~57% of t-tubular membrane compartment was inaccessible). Consistent with the data in Fig. 3B detubulation by formamide was more effective with t-tubular labeling reduced to 24 ± 1.5 % of that in control myocytes (or, ~76% detubulation). With regard to quantitative comparisons of experiments employing membrane specific and water soluble dyes, it is important to mention the principal difference between the use of dextran and di-8ANEPPS: the former reports the volume of sealed t-tubules while the latter reports the surface area of accessible membrane.
Figure 4. The effect of hypo- and hyper-osmotic detubulation observed with membrane specific dye di-8-ANEPPS.
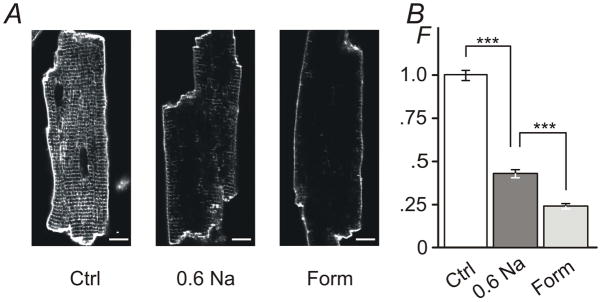
A, Ventricular myocytes were exposed to 0.6 Na hypo-osmotic or 1.5 M formamide hyper-osmotic solutions for 7 min and 15 min, respectively, returned to normal Tyr solution, labeled with di-8-ANEPPS dye along with control cells (Ctrl; not subjected to osmotic stress) and imaged using confocal microscopy. Ctrl myocytes display strong intracellular fluorescence originating from t-tubular membranes. In contrast, both types of osmotic shock lead to significant reduction of ‘intracellular’ labeling consistent with detubulation. Bar: 10 μm. B, Quantification of ‘intracellular’ fluorescence (not including the outer sarcolemma or intercalated disks; see Methods). The amount of t-tubular fluorescence is reduced to ~43% and to ~24% of that in control myocytes after treatment with 0.6 Na and 1.5 M formamide solutions, respectively. F -Relative fluorescence. Mean fluorescence for Ctrl was set to 1.
The effects of NaCl, osmolarity and cell shrinking on t-tubular sealing
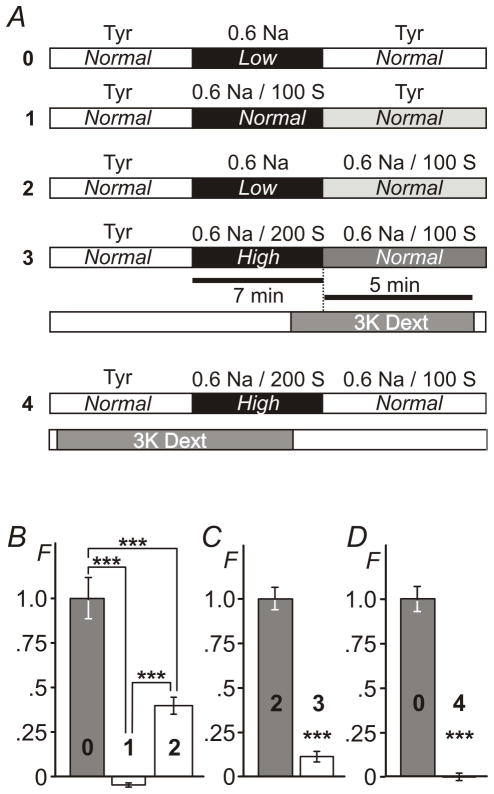
It seemed reasonable to suggest that the effects of hypo-osmotic shock with 0.6 Na solution may actually be due to changes in the concentration of NaCl rather than changes in osmolarity. Specifically, washout of 0.6 Na solution (the period of time when detubulation occurs) is associated with return to both normal NaCl and normal osmolarity (Fig. 5A; protocol 0). Therefore, we first performed experiments where osmolarity was kept normal throughout the procedure using 100 mM sucrose in 0.6 Na solution (0.6 Na/100 S solution) and therefore the concentration of NaCl was the only variable (Fig. 5A; protocol 1). No measurable trapping of 3K dextran was observed (Fig. 5B): −0.049 ± 0.001 compared to that in protocol 0 1.00 ± 0.01 (n=20, 20 and 20 for background control, protocol 0 and protocol 1, respectively; p<0.001). The data strongly suggest that the change in osmolarity is the primary reason for detubulation upon treatment with hypo-osmotic 0.6 Na solution.
Figure 5. Effects of NaCl, osmolarity and cell shrinking on t-tubular sealing.
A, timing of the application of various modified solutions (A0, A1, A2 and A3) using ‘late’ and ‘early’ (A4) application of 3K fluorescent dextran. B, comparing the outcomes of experiments using protocol 0 and protocol 1 shows that osmotic stress rather than a change in the concentration of NaCl is responsible for sealing of t-tubules. However, the extent of t-tubular sealing is modulated by the concentration of extracellular NaCl as can be concluded by comparing the outcomes of experiments using protocol 0 and protocol 2. C, resolution of hyper-osmotic stress equivalent to that produced by 100 mM sucrose (protocol 3) has little effect of t- tubular sealing. D, Cell shrinking early in response to the application of hyper-osmotic solution (protocol 4) does not cause sealing of t-tubules as well. F - Relative fluorescence. Mean fluorescence for protocol 0 in B and D and protocol 2 in C was set to 1. Note: small negative fluorescence in B reflects experimental error due to background correction.
However, not contradictory to the above, NaCl does play significant role in regulating osmotically-induced detubulation. Specifically, as shown in Fig. 5B equal hypo-osmotic stress leads to different level of detubulation at different concentrations of NaCl. When removal of hypo-osmotic stress occurs at reduced concentration of NaCl (0.6 Na; protocol 2) the amount of trapped 3K dextran is reduced to 0.40 ± 0.05 (n=20, 20 and 20 myocytes for background control, protocol 0 and protocol 2, respectively; p<0.001) of that observed in the presence of normal concentration of NaCl (protocol 0).
Since in the above experiments initial transition to test solutions proceeded from normal Tyrode it is possible that some or all effects observed above might be due to the effect of reduction in NaCl rather than decrease in osmolarity. However, no difference in the amount of trapped dextran (‘late’ application; as in Fig. 5; protocols 0–3) was observed whether cells were transitioned from Tyr (normal osmolarity/normal NaCl) to 0.6 Na or from 0.6 Na/100 S (normal osmolarity/low NaCl) to 0.6 Na (data not shown).
We have also performed a ‘reverse osmosis’ experiment in order to test whether the resolution of hyper-osmotic stress of a similar magnitude (equal to that provided by 100 mM sucrose) leads to similar level of detubulation as hypo-osmotic challenge (Fig. 5A; protocols 2 vs 3). Importantly, in these experiments concentration of NaCl was kept unchanged during swelling and shrinking phases. The data show that the effect is highly asymmetric (Fig. 5C). Specifically, the amount of trapped dextran after hyper-osmotic challenge with 0.6 Na/200 S solution (protocol 3) was only ~11% of that observed during hypo-osmotic challenge (protocol 2): 0.11 ± 0.03 vs 1.00 ± 0.06 (n=22, 24 and 22 myocytes for background control, protocol 2 and protocol 3, respectively; p<0.001).
Similar to the effects of NaCl in the initial control solution discussed above, virtually no dextran trapping was observed in experiments using hypertonic solutions (as in Fig. 5 A; protocol 3) whether cells were transitioned from Tyr to Tyr/100 S or from 0.6 Na/100 S to 0.6 Na/200 S (data not shown).
One common feature of all above experiments where dextran trapping was observed during ‘late’ application of dextran is the shrinking of the cells. Therefore, it remains possible that a lack of significant dextran trapping upon washout of hyper-osmotic 0.6 Na/200 S solution in ‘late’ type of experiments (Fig. 5. C) is because t-tubules were already sealed during preceding swelling period and were no longer accessible to extracellular molecules. In order to test this hypothesis we turned back to ‘early’ type of experiments as in Fig. 2 (protocol A1) to check whether extracellular dextran is trapped upon application of 0.6 Na/200 S solution. The results were negative. No measurable trapping of extracellular dextran was observed after application of 0.6 Na/200 S solution hypertonic solution when compared to that produced by washout of 0.6 Na hypotonic solution (Fig. 5. D): 1.00 ± 0.07 vs 0.00 ± 0.01 (n=15, p<0.001), for hypo- and hyper-osmotic challenges, respectively.
Electrophysiological evidence of hypo-osmotic detubulation
There are at least three essentially independent but intrinsically linked electrophysiological measures of detubulation in ventricular myocytes: (1) change in the membrane capacitance, (2) change in so-called IK1 tail currents at near resting membrane potential, and (3) change in IK1 ‘inactivation’ at hyperpolarized membrane potentials (Cheng et al., 2011).
In the first approach, membrane capacitance, CM, was measured in the whole-cell configuration of patch-clamp technique using built-in Clampex utility (see Methods). Ventricular myocytes were first treated with 0.6 Na hypo-osmotic solution and CM was then measured within ~2 hours post shock in normal Tyr solution, concurrently with measurements of ionic currents (below). The data show (Fig. 6D) that hypo-osmotic shock leads to nearly 27% (p<0.01) reduction in CM, from 183.5 ± 19.5 pF (n=9) to 134.5 ± 6.7 pF (n=14) in Ctrl and treated myocytes, respectively, consistent with significant detubulation.
The second approach is based on a well-established experimental finding that large outward potassium currents flowing into the highly restricted t-tubular space lead to significant accumulation of local K+. The latter in turn can be assessed electrophysiologically my measuring inward potassium currents flowing through t-tubular IK1 channels upon membrane repolarization to near the reversal potential corresponding to that at normal extracellular concentration of K+ (Yasui et al., 1993; Clark et al., 2001; Cheng et al., 2011). Fig. 6 highlights the major features of the phenomena and the effects of detubulation induced by hypo-osmotic shock on various components of outward and inward K+ currents, including so-called IK1 ‘inactivation’. Experimentally, t-tubular accumulation of K+ was induced by long lasting outward K+ currents in response to a voltage step from holding potential of −75 mV to +50 mV which activates various voltage-dependent K+ channels (Fig. 6A). Importantly, we have recently shown (Cheng et al., 2011) that the magnitude of t-tubular accumulation of K+ depends essentially only on the magnitude of the K+ current at the end of the voltage step (400 ms), and therefore, the relevant data are normalized to that current (IK, end). With this in mind, for presentation purpose Fig. 6A shows recordings from Ctrl and treated myocytes having IK, end of similar magnitude. Inspection of currents upon repolarization back to −75 mV shows that IK1, tail currents are significantly reduced in the myocyte treated with 0.6 Na hypo-osmotic solution (Fig. 6A; insert), suggesting significant detubulation. Interestingly, hypo-osmotic shock with 0.6 Na solution did not affect the density of IK, end itself (Fig. 6B; top): 18.5 ± 1.4 pA/pF (n=9) and 19.0 ± 1.9 pA/pF (n=14) in Ctrl and treated myocytes, respectively. Normalization of the amplitude of inward IK1, tail currents by IK, end (which underlies K+ accumulation in t-tubules) takes care of cell-to-cell variation of this current and the data provide an additional proof of significant detubulation (INK1, tail; Fig. 6B; bottom). Specifically, resolution of hypo-osmotic shock induced by 0.6 Na solution leads to a nearly 2.5-fold reduction of normalized IK1, tail current (p<0.001) from 0.46 ± 0.07 (n=9) to 0.19 ± 0.03 (n=14).
The third electrophysiological approach is based on a similar phenomenon of restricted diffusion of K+ in t-tubules. In ventricular myocytes large inward IK1 currents flowing during membrane hyperpolarization beyond EK reversal potential lead to depletion of t-tubular K+ which can be observed as time-dependent decline of IK1 current (called ‘inactivation’ in this case) (Cheng et al., 2011). Fig. 6C shows recordings of IK1 currents in selected Ctrl and treated myocytes chosen to have similar steady state currents in order to highlight the effect of hypo-osmotic shock with 0.6 Na solution on IK1 ‘inactivation’. The data clearly show significant reduction in the amplitude of the time dependent component of inward IK1 current (‘inactivation’) upon treatment of the myocytes with 0.6 Na solution. Quantified data presented in Fig. 6D (bottom-right) point to significant detubulation as well. Specifically, the relative amplitude of IK1 ‘inactivation’ (normalized to the peak IK1 recalculated to time zero; IK1 (−120)) was reduced by ~2 fold (p<0.001) from 0.30 ± 0.02 (n=8) in Ctrl myocytes to 0.16 ± 0.01 (n=13) in treated myocytes.
Surprisingly, despite significant loss of membrane capacitance (CM; Fig. 6D) the peak amplitude of the whole-cell IK1 (IK1 (-120); Fig. 6D) was essentially unaffected by hypo-osmotic shock: −3.9 ± 0.4 nA (n=8) and −3.7 ± 0.2 nA (n=13) in Ctrl and treated myocytes, respectively. As a consequence of the above, the density of IK1 was somewhat increased (however, not statistically significant; p=0.18) after the treatment with hypo-osmotic 0.6 Na solution suggesting potential upregulation of IK1 channels remaining in the outer sarcolemma after t-tubule sealing (this was not investigated further). Importantly, normalization of ‘inactivating’ currents takes care of changes in the activity of IK1 channels and strongly highlights a hidden stress-induced remodeling of t-tubular system.
It may be argued that the changes in CM and ionic currents are the result of slow recovery (recordings were performed within ~2 hours) of myocytes after the shock. Therefore, we have also performed experiments where ventricular myocytes were under the whole-cell voltage clamp during both application and removal of hypo-osmotic shock. Fig. 7 shows an example of an experiment where recordings of IK1, tail current (as in Fig. 6A) were taken just before the application hypo-osmotic solution, just before its removal and shortly after the resolution of the shock. For presentation purpose the outward currents were normalized to each other at the end of depolarization step. The example shows that normalized IK1, tail current is essentially not affected by application of 0.6 Na hypo-osmotic solution but is significantly reduced shortly after its washout. Quantitatively, the average normalized IK1, tail currents before and during swelling were indistinguishable, 0.35 ± 0.04 vs 0.35 ± 0.04, respectively, but were reduced by more than 5 fold to 0.064 ± 0.01 (n=10; p<0.001) right after the shock (Fig. 7; Insert).
Changes in membrane capacitance CM measured during the same experiments were also consistent with sealing of t-tubules right upon resolution of hypo-osmotic stress. The average data were 157 ± 10, 156 ± 11 and 122 ± 6 pF (n=9), before the application hypo-osmotic solution, before and after its removal, respectively. The data show a loss of ~17% of CM (p<0.001) upon resolution of the hypo-osmotic shock.
Discussion
Numerous studies have investigated the effects of hypo-osmotic stress on various ion currents in ventricular cardiac myocytes. Besides Ca2+, Cl− and some K+ currents mentioned in the Introduction, swelling significantly affects transient outward current Ito (Wang et al., 2005), ATP-dependent K+ current (Priebe & Beuckelmann, 1998) and Na currents as well (Hu et al., 2009). Despite a wealth of data, the exact mechanisms underlying the observed effects remain in a large degree unknown. Two obvious general mechanisms include a simple dilution of intracellular milieu and stretch of the membrane during swelling phase. Both dilution and stretch, however, go away upon return to normal osmolarity. Less general mechanisms would involve activation of intracellular signaling systems leading, for example, to changes in channel phosphorylation (Missan et al., 2006; Missan et al., 2008).
In all previous studies it was silently assumed that channels located in different membrane compartments of ventricular myocytes, including t-tubules and intercalated disks, respond similarly to osmotic stress. This is surely a very reasonable approximation, especially assuming a lack of approaches to separate the currents originating from different membrane compartments. However, even if one would suggest that ion channels respond similarly to hypo-osmotic stress there still exists another scenario of events in which those membrane compartments themselves may be affected. The latter, in turn, would lead to a hypothesis that at least some of the observed effects of hypo-osmotic stress may be explained by remodeling of t-tubules which are known to harbor various ion channels and transporters (Brette & Orchard, 2007; Chase & Orchard, 2011). Dilation or constriction of t-tubules may, for example, significantly affect the flow of ions through t-tubular lumen with predictable consequences on t-tubular membrane potential and ionic homeostasis. One of the well-studied phenomena in this area is t-tubular accumulation of K+ due to the flow of outward K+ currents through voltage-dependent K+ channels (Clark et al., 2001). The latter effect is observed in normal freshly isolated ventricular myocytes and is a hallmark of intact t-tubules. One of the electrophysiological markers of t-tubular accumulation is a so-called IK1 tail current (Fig. 6; (Yasui et al., 1993; Clark et al., 2001; Cheng et al., 2011)). This current is not observed in ventricular myocytes detubulated using hyper-osmotic shock with 1.5 M formamide Cheng et al., 2011). Formamide-induced detubulation leads to significant changes in whole-cell ionic currents depending on specific ion channel type. For example, it has been shown in experiment employing formamide that about 80% of L-type Ca2+ channels reside in t-tubules (Brette et al., 2004). Clearly, osmotic stress of lesser strengths (and different type, i.e. hypo-osmotic stress) may lead to varying degrees of t-tubular remodeling, likely including partial and/or even reversible constriction of t-tubular lumen.
Unfortunately, to our knowledge there is only one study where the involvement of t-tubular remodeling in response to commonly used hypo-osmotic stresses was considered and tested. Specifically, Brette et al (2000) (Brette et al., 2000) used membrane labeling with di-8-ANEPPS to test whether hypo-osmotic swelling affects t-tubules in rat ventricular myocytes. The data showed that t-tubules remained accessible to the extracellular dye even after 10 min exposure to hypo-osmotic solution of ~180 mOsm (i.e. during swelling phase) when myocytes displayed signs of truly severe stress (including membrane blebbing). The interpretation of the data is of importance, however. For example, if significant t-tubular constriction upon swelling did occur it still might not have been tight enough to prevent diffusion of relatively small di-8-ANEPPS dye through the constricted t-tubule lumen. In other words, membrane labeling technique is only useful in detecting really tight constriction (although no number can be easily put here). In contrast, electrophysiological markers (e.g. IK1 tail currents; (Clark et al., 2001; Cheng et al., 2011)) of t-tubular integrity are useful in detecting even partial constriction or dilation, although the quantitative interpretation of the data is quite difficult at this time (Cheng et al., 2011). Therefore, the extent and the direction of t-tubular remodeling (constriction or dilation) during hypo-osmotic swelling remained an open question although the results of the present study essentially rule out strong (or even measurable) sealing of t-tubules. Specifically, the data in Fig. 7 show that neither IK1 tail currents nor membrane capacitance (CM) are affected by swelling of the myocytes in any significant way.
With regard to the above, our recent findings show that ‘hidden’ t-tubular remodeling occurs even under conditions which are far from being as harsh as the exposure to 1.5 M formamide (Cheng et al., 2011). In particular, even simple dialysis of ventricular myocytes with commonly used intracellular solutions or poisoning with cyanide may lead to changes in various markers of t-tubular integrity or even to nearly complete detubulation. Therefore, in this study we reassessed some consequences of hypo-osmotic swelling addressed by Brette et al (2000) (Brette et al., 2000) and went further by looking at the post-shock state of t-tubules using different approaches.
The central, unexpected and equally important finding of our study is that the resolution of hypo-osmotic stress but not its induction leads to dramatic t-tubular remodeling (Fig. 2). In particular, wash-out of hypo-osmotic solution of ~200 mOsm is associated with a loss of membrane capacitance (Fig. 6D; top left) comparable to that of entire t-tubular system, trapping of extracellular dextran (Fig. 2) in sealed t-tubules and inaccessibility of a significant part of t-tubular system to di-8-ANEPPS dye applied after removal of hypo-osmotic solution (Fig. 4).
The effects of short (few min) exposure to hypo-osmotic solutions in several previous electrophysiological experiments (whole-cell patch-clamp) are usually fully reversible (Li et al., 2002; Missan et al., 2011). This is seemingly inconsistent with our central finding that washout of 0.6 Na hypo-osmotic solution leads to nearly complete and nearly permanent detubulation (the fluorescence of trapped 3K dextran declines less than 10% per hour; data not shown). Differences in species (mouse in this study vs guinea-pig, rat etc in others) may be one of the underlying reasons. Experimental details may be of importance as well. In particular, the speed of washout of osmotic solutions may potentially contribute to the differences between the studies, with slower speeds leading to less abrupt and thus milder stress. The relevant quantitative data, however, are not available. Another plausible explanation is that the whole-cell dialysis (in whole cell-patch clamp experiments) during cell swelling and shrinking may reduce the magnitude of stress with patch-pipette opening acting as a ‘relief valve’ against a buildup and resolution of intracellular pressure. Experiments with perforated patches (Missan et al., 2011) and the current experiments using conventional whole-cell patch method (Fig. 7), however, are against this hypothesis. However, it does not seem implausible that in many previous studies t-tubules might have been lost even before applying hypo-osmotic stress, either during isolation procedure or soon after establishing whole-cell configuration of the patch clamp. This hypothesis is strongly supported by our recent study showing that various types of relatively mild stress may lead to significant detubulation characterized, in particular, by disappearance of electrophysiological markers of intact t-tubules such as IK1 tail currents (Fig. 6) (Cheng et al., 2011). In further support for this, inspection of published whole-cell recordings of relevant K+ currents shows that in several previous studies IK1 tail currents are essentially missing (London et al., 1998; Xu et al., 1999; Bodi et al., 2003; Borg et al., 2004; Wang et al., 2005), while they can be clearly seen in others (Tomita et al., 1994; Wickenden et al., 1999; Clark et al., 2001; Cheng et al., 2011), suggesting significant variability in the integrity of t-tubular system due to different experimental conditions. The data in our study show that t-tubules are well preserved during myocytes isolation procedure and various other manipulations (e.g. centrifugation steps etc) but can be easily sealed by the removal of hypo-osmotic stress.
Subcellular and molecular mechanisms of stress-induced sealing of t-tubules remain unclear. However, the strong regulatory effect of NaCl suggests potential involvement of Na/K ATPase and/or Cl− channels. In this regard, the contribution of other major ions (e.g. K+) and membrane transport systems (e.g. Na/Ca exchanger) is also highly likely. However, addressing relevant underlying mechanisms is clearly beyond the scope of this study and will be investigated separately.
Physiological relevance
Swelling of cardiac myocytes is a common complication in various pathological conditions including ischemia (Vandenberg et al., 1996) or during application of cardioplegic solutions (Handy et al., 1996; Shaffer et al., 1998). Since in most cases osmotic stress is resolved (or at least needs to be resolved) significant t-tubular remodeling may accompany the recovery from insult leading to secondary complications. The described phenomenon formally resembles, and likely is linked to, a well-known effect of ischemia-reperfusion injury (Kloner & Jennings, 2001), where the most severe complications occur not during the insult itself but during its resolution. The exact underlying mechanisms are likely to be different in every case but it is also highly likely that t-tubular remodeling during reperfusion may be a significant contributor to an overall injury of the myocardium.
Conclusion
This study discloses a novel phenomenon of stress-induced t-tubular remodeling in ventricular myocytes. The data show that resolution but not induction of hypo-osmotic shock leads to a loss of membrane capacitance, changes in electrophysiological markers of t-tubular integrity and trapping low molecular weight dextran inside the cells – all being consistent with sealing of t-tubules.
New findings.
What is the central question of this study?
T-tubules of ventricular myocytes are critical elements in the excitation-contraction coupling. They become disorganized or even lost in various cardiac pathologies. However, the mechanisms leading to disruption of t-tubules are essentially not known. Therefore, this study was aimed to identify physiologically relevant processes which underlie remodeling of t-tubules.
What is the main finding and its importance?
We show that the resolution of physiologically relevant hypo-osmotic swelling, not the application of osmotic shock itself, leads to dramatic t-tubular remodeling including sealing of individual t-tubules. The results point to an important and likely general mechanism of acute and fast stress-induced t-tubular remodeling that may underlie various relevant pathologies of the heart.
Acknowledgments
This work was supported by R01-HL-069052 (ANL) grant from the National Institutes of Health.
References
- Argiro V. Excitation-contraction uncoupling of striated muscle fibres by formamide treatment: evidence of detubulation. J Muscle Res Cell Motil. 1981;2:283–294. doi: 10.1007/BF00713267. [DOI] [PubMed] [Google Scholar]
- Bodi I, Muth JN, Hahn HS, Petrashevskaya NN, Rubio M, Koch SE, Varadi G, Schwartz A. Electrical remodeling in hearts from a calcium-dependent mouse model of hypertrophy and failure: complex nature of K+ current changes and action potential duration. J Am Coll Cardiol. 2003;41:1611–1622. doi: 10.1016/s0735-1097(03)00244-4. [DOI] [PubMed] [Google Scholar]
- Borg JJ, Hancox JC, Hogg DS, James AF, Kozlowski RZ. Actions of the anti-oestrogen agent clomiphene on outward K+ currents in rat ventricular myocytes. Clin Exp Pharmacol Physiol. 2004;31:86–95. doi: 10.1111/j.1440-1681.2004.03956.x. [DOI] [PubMed] [Google Scholar]
- Boyett MR, Frampton JE, Kirby MS. The length, width and volume of isolated rat and ferret ventricular myocytes during twitch contractions and changes in osmotic strength. Exp Physiol. 1991;76:259–270. doi: 10.1113/expphysiol.1991.sp003492. [DOI] [PubMed] [Google Scholar]
- Brette F, Calaghan SC, Lappin S, White E, Colyer J, Le Guennec JY. Biphasic effects of hyposmotic challenge on excitation-contraction coupling in rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2000;279:H1963–H1971. doi: 10.1152/ajpheart.2000.279.4.H1963. [DOI] [PubMed] [Google Scholar]
- Brette F, Komukai K, Orchard CH. Validation of formamide as a detubulation agent in isolated rat cardiac cells. Am J Physiol Heart Circ Physiol. 2002;283:H1720–H1728. doi: 10.1152/ajpheart.00347.2002. [DOI] [PubMed] [Google Scholar]
- Brette F, Orchard C. Resurgence of cardiac t-tubule research. Physiology (Bethesda) 2007;22:167–173. doi: 10.1152/physiol.00005.2007. [DOI] [PubMed] [Google Scholar]
- Brette F, Salle L, Orchard CH. Differential modulation of L-type Ca2+ current by SR Ca2+ release at the T-tubules and surface membrane of rat ventricular myocytes. Circ Res. 2004;95:e1–e7. doi: 10.1161/01.RES.0000135547.53927.F6. [DOI] [PubMed] [Google Scholar]
- Cazorla O, Pascarel C, Brette F, Le Guennec JY. Modulation of ions channels and membrane receptors activities by mechanical interventions in cardiomyocytes: possible mechanisms for mechanosensitivity. Prog Biophys Mol Biol. 1999;71:29–58. doi: 10.1016/s0079-6107(98)00036-4. [DOI] [PubMed] [Google Scholar]
- Chase A, Orchard CH. Ca efflux via the sarcolemmal Ca ATPase occurs only in the t-tubules of rat ventricular myocytes. J Mol Cell Cardiol. 2011;50:187–193. doi: 10.1016/j.yjmcc.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Cheng L, Wang F, Lopatin AN. Metabolic stress in isolated mouse ventricular myocytes leads to remodeling of t-tubules. Am J Physiol Heart Circ Physiol. 2011;301:H1984–H1995. doi: 10.1152/ajpheart.00304.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RB, Tremblay A, Melnyk P, Allen BG, Giles WR, Fiset C. T-tubule localization of the inward-rectifier K(+) channel in mouse ventricular myocytes: a role in K(+) accumulation. J Physiol. 2001;537:979–992. doi: 10.1111/j.1469-7793.2001.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg B, Eisenberg RS. Selective disruption of the sarcotubular system in frog sartorius muscle. A quantitative study with exogenous peroxidase as a marker. J Cell Biol. 1968;39:451–467. doi: 10.1083/jcb.39.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh WJ, Gibson KJ, Maylie JG. Hypotonic-induced stretch counteracts the efficacy of the class III antiarrhythmic agent E-4031 in guinea pig myocytes. Cardiovasc Res. 1996;31:237–245. [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Handy JR, Jr, Dorman BH, Cavallo MJ, Hinton RB, Roy RC, Crawford FA, Spinale FG. Direct effects of oxygenated crystalloid or blood cardioplegia on isolated myocyte contractile function. J Thorac Cardiovasc Surg. 1996;112:1064–1072. doi: 10.1016/S0022-5223(96)70108-3. [DOI] [PubMed] [Google Scholar]
- Howell JN. A lesion of the transverse tubules of skeletal muscle. J Physiol. 1969;201:515–533. doi: 10.1113/jphysiol.1969.sp008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Ma J, Zhang P, Zheng J. Extracellular hypotonicity induces disturbance of sodium currents in rat ventricular myocytes. Physiol Res. 2009;58:807–815. doi: 10.33549/physiolres.931692. [DOI] [PubMed] [Google Scholar]
- Kawai M, Hussain M, Orchard CH. Excitation-contraction coupling in rat ventricular myocytes after formamide-induced detubulation. Am J Physiol. 1999;277:H603–H609. doi: 10.1152/ajpheart.1999.277.2.H603. [DOI] [PubMed] [Google Scholar]
- Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 1. Circulation. 2001;104:2981–2989. doi: 10.1161/hc4801.100038. [DOI] [PubMed] [Google Scholar]
- Li GR, Zhang M, Satin LS, Baumgarten CM. Biphasic effects of cell volume on excitation-contraction coupling in rabbit ventricular myocytes. American Journal of Physiology-Heart and Circulatory Physiology. 2002;282:H1270–H1277. doi: 10.1152/ajpheart.00946.2001. [DOI] [PubMed] [Google Scholar]
- London B, Jeron A, Zhou J, Buckett P, Han X, Mitchell GF, Koren G. Long QT and ventricular arrhythmias in transgenic mice expressing the N terminus and first transmembrane segment of a voltage-gated potassium channel. Proc Natl Acad Sci U S A. 1998;95:2926–2931. doi: 10.1073/pnas.95.6.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo A-t, Luo H-y, Hu X-w, Gao L-l, Liang H-m, Tang M, Hescheler J. Hyposmotic challenge modulates function of L-type calcium channel in rat ventricular myocytes through protein kinase C. Acta Pharmacologica Sinica. 2010;31:1438–1446. doi: 10.1038/aps.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda N, Hagiwara N, Shoda M, Kasanuki H, Hosoda S. Enhancement of the L-type Ca2+ current by mechanical stimulation in single rabbit cardiac myocytes. Circ Res. 1996;78:650–659. doi: 10.1161/01.res.78.4.650. [DOI] [PubMed] [Google Scholar]
- McLerie M, Lopatin AN. Dominant-negative suppression of I(K1) in the mouse heart leads to altered cardiac excitability. J Mol Cell Cardiol. 2003;35:367–378. doi: 10.1016/s0022-2828(03)00014-2. [DOI] [PubMed] [Google Scholar]
- Missan S, Linsdell P, McDonald TF. Role of kinases and G-proteins in the hyposmotic stimulation of cardiac IKs. Biochim Biophys Acta. 2006;1758:1641–1652. doi: 10.1016/j.bbamem.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Missan S, Linsdell P, McDonald TF. Involvement of tyrosine kinase in the hyposmotic stimulation of I (Ks) in guinea-pig ventricular myocytes. Pflugers Archiv-European Journal of Physiology. 2008;456:489–500. doi: 10.1007/s00424-007-0424-y. [DOI] [PubMed] [Google Scholar]
- Missan S, Shuba LM, Zhabyeyev P, McDonald TF. Osmotic modulation of slowly activating IKs in guinea-pig ventricular myocytes. Cardiovasc Res. 2011;91:429–436. doi: 10.1093/cvr/cvr074. [DOI] [PubMed] [Google Scholar]
- Priebe L, Beuckelmann DJ. Cell swelling causes the action potential duration to shorten in guinea-pig ventricular myocytes by activating IKATP. Pflugers Arch. 1998;436:894–898. doi: 10.1007/pl00008087. [DOI] [PubMed] [Google Scholar]
- Rees SA, Vandenberg JI, Wright AR, Yoshida A, Powell T. Cell swelling has differential effects on the rapid and slow components of delayed rectifier potassium current in guinea pig cardiac myocytes. J Gen Physiol. 1995;106:1151–1170. doi: 10.1085/jgp.106.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos KP. Length, width, and volume changes in osmotically stressed myocytes. Am J Physiol. 1986;251:H1373–1378. doi: 10.1152/ajpheart.1986.251.6.H1373. [DOI] [PubMed] [Google Scholar]
- Shaffer RF, Baumgarten CM, Damiano RJ., Jr Prevention of cellular edema directly caused by hypothermic cardioplegia: studies in isolated human and rabbit atrial myocytes. J Thorac Cardiovasc Surg. 1998;115:1189–1195. doi: 10.1016/S0022-5223(98)70420-9. [DOI] [PubMed] [Google Scholar]
- Soeller C, Cannell MB. Examination of the transverse tubular system in living cardiac rat myocytes by 2-photon microscopy and digital image-processing techniques. Circ Res. 1999;84:266–275. doi: 10.1161/01.res.84.3.266. [DOI] [PubMed] [Google Scholar]
- Sorota S. Swelling-induced chloride-sensitive current in canine atrial cells revealed by whole-cell patch-clamp method. Circ Res. 1992;70:679–687. doi: 10.1161/01.res.70.4.679. [DOI] [PubMed] [Google Scholar]
- Stewart JM, Page E. Improved stereological techniques for studying myocardial cell growth: application to external sarcolemma, T system, and intercalated disks of rabbit and rat hearts. J Ultrastruct Res. 1978;65:119–134. doi: 10.1016/s0022-5320(78)90050-3. [DOI] [PubMed] [Google Scholar]
- Tomita F, Bassett AL, Myerburg RJ, Kimura S. Diminished transient outward currents in rat hypertrophied ventricular myocytes. Circ Res. 1994;75:296–303. doi: 10.1161/01.res.75.2.296. [DOI] [PubMed] [Google Scholar]
- Vandenberg JI, Rees SA, Wright AR, Powell T. Cell swelling and ion transport pathways in cardiac myocytes. Cardiovasc Res. 1996;32:85–97. [PubMed] [Google Scholar]
- Vandenberg JI, Yoshida A, Kirk K, Powell T. Swelling-activated and isoprenaline-activated chloride currents in guinea pig cardiac myocytes have distinct electrophysiology and pharmacology. J Gen Physiol. 1994;104:997–1017. doi: 10.1085/jgp.104.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Wang GX, Yamamoto S, Ye L, Baxter H, Hume JR, Duan D. Molecular mechanisms of regulation of fast-inactivating voltage-dependent transient outward K+ current in mouse heart by cell volume changes. J Physiol. 2005;568:423–443. doi: 10.1113/jphysiol.2005.091264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley DW, Hemsworth PD, Rasmussen HH. Sodium-hydrogen exchange in guinea-pig ventricular muscle during exposure to hyperosmolar solutions. J Physiol. 1991;444:193–212. doi: 10.1113/jphysiol.1991.sp018873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley DW, Hool LC, Ten Eick RE, Rasmussen HH. Effect of osmotic swelling and shrinkage on Na(+)-K+ pump activity in mammalian cardiac myocytes. Am J Physiol. 1993;265:C1201–C1210. doi: 10.1152/ajpcell.1993.265.5.C1201. [DOI] [PubMed] [Google Scholar]
- Wickenden AD, Jegla TJ, Kaprielian R, Backx PH. Regional contributions of Kv1.4, Kv4.2, and Kv4.3 to transient outward K+ current in rat ventricle. Am J Physiol. 1999;276:H1599–H1607. doi: 10.1152/ajpheart.1999.276.5.H1599. [DOI] [PubMed] [Google Scholar]
- Xu H, Guo W, Nerbonne JM. Four kinetically distinct depolarization-activated K+ currents in adult mouse ventricular myocytes. J Gen Physiol. 1999;113:661–678. doi: 10.1085/jgp.113.5.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui K, Anno T, Kamiya K, Boyett MR, Kodama I, Toyama J. Contribution of potassium accumulation in narrow extracellular spaces to the genesis of nicorandil-induced large inward tail current in guinea-pig ventricular cells. Pflugers Arch. 1993;422:371–379. doi: 10.1007/BF00374293. [DOI] [PubMed] [Google Scholar]