Abstract
BACKGROUND
It is well known that individuals with schizophrenia have dopaminergic abnormalities as well as working memory difficulties, both of which are associated with impulsive decision making. We used a delay discounting measure to test the degree to which patients make future-oriented decisions.
METHODS
Forty-two patients with schizophrenia and 29 healthy comparison participants completed a delay discounting measure, along with tests of cognitive function and, in patients, current symptoms.
RESULTS
Patients discounted more steeply than did comparison participants. Discounting among patients related to measures of memory and tended to relate inversely to negative symptoms.
CONCLUSIONS
The impulsive decision-making evidenced by patients suggests that they may be prone to choosing immediate over long-term rewards, even when their interests are better served by choosing the latter. Improving working memory may enhance their ability to make future-oriented decisions.
Keywords: Schizophrenia, Dopamine, Working Memory, Impulsive Choice
Each of us is regularly confronted with choices involving outcomes that differ in magnitude or that are nearer or farther away in time. For example, one may choose to forgo purchasing a second car now, in favor of saving for future retirement (or vice versa). It is no surprise that when faced with such choices, the temporal remoteness of an outcome is related to the likelihood that it will be preferred. Indeed, numerous studies have shown that the more a reward is delayed the less subjective value it holds (e.g., Holt, Green, & Myerson, 2003; Kirby & Santiesteban, 2003; Petry, Kirby, & Kranzler, 2002). Thus, remote outcomes must be of relatively greater value to be preferred over temporally proximal ones.
The discounting of future outcomes among humans is most frequently characterized by a delay discounting (DD) function, which can be estimated from the degree to which an individual prefers smaller rewards sooner to larger rewards later. As DD increases, individuals become more susceptible to proximal rewards and begin to make decisions that are described as “temporally myopic” or “impulsive” (Kirby, Petry, & Bickel, 1999).
Several lines of evidence provide clues to the neurobiology of DD. Individuals who are dependent on heroin (Bornovalova, Daughters, Hernandez, Richards, & Lejuez, 2005; Kirby, Petry, & Bickel, 1999), cocaine (Kirby & Petry, 2004), alcohol (Mitchell, Fields, D’Esposito, & Boettiger, 2005), and possibly nicotine (Ohmura, Takahashi, & Kitamura, 2005) are more temporally myopic, as are individuals with damage to ventromedial cortex (Winstanley, Theobald, Cardinal, & Robbins, 2004). In addition, dopamine in the orbital frontal cortex affects DD (Winstanley, Theobald, Dalley, Cardinal, & Robbins, 2006; Winstanley, Theobald, Dalley, & Robbins, 2005), suggesting that propensity to discount future outcomes may relate to the reward system. Other research points to a critical role of working memory in DD. For example, increases in working memory load during DD tasks cause future rewards to be more steeply discounted (Hinson, Jameson, & Whitney, 2003). Accordingly, functional imaging results show greater activity in dorsolateral prefrontal cortex (DLPFC) than ventromedial regions when individuals choose a delayed over an immediate reward and decreased DLPFC activity when they choose impulsively (McClure, Laibson, Loewenstein, & Cohen, 2004). Thus, there is converging evidence that the extent of preference for smaller immediate rewards over larger delayed rewards represents an important individual difference dimension that captures the relative contributions of different neural systems to decision making.
Given their compromised working memory, impairment in DLPFC function and abnormalities in dopamine function one might expect patients with schizophrenia to demonstrate greater discounting of future rewards than healthy comparison individuals. The present study was designed to test this prediction.
Methods
Participants
Participants included 42 outpatients with diagnoses of schizophrenia or schizoaffective disorder (SC) and 29 healthy comparison (HC) participants. Patient diagnoses were assessed with the Structured Clinical Interview for DSM-IV (SCID, First, Spitzer, Miriam, & Williams, 2002). All patients were receiving stable doses of antipsychotic medications with no medication changes for at least 4 weeks prior to participation (see Table 1 for sample characteristics). Patients were deemed clinically stable by their clinicians and were capable of providing informed consent, as assessed by a set of standard probes. Symptom assessments included the Brief Psychiatric Rating Scale (BPRS, Overall & Gorham, 1962) and the Scale for the Assessment of Negative Symptoms (SANS, Andreasen, 1989). HC participants were free of psychiatric diagnoses as indicated by the SCID, receiving no psychiatric medications, and had no family history of psychosis. Potential participants were excluded if there was evidence of neurological injury/disorder, substance abuse/dependence, or other disorder capable of affecting symptoms and task performance. After study procedures were described, participants gave written informed consent. The University of Maryland’s institutional review board approved the study.
Table 1.
Participant Characteristics
| Healthy Comparison Participants (n=29) | Schizophrenia Patients (n=42) | p-value | |
|---|---|---|---|
| Age | 44.45 (10.57) | 44.05 (9.13) | .865 |
| Age at illness onset | — | 22.75 (7.26) | — |
|
|
|||
| Education | 14.76 (2.50) | 12.86 (2.28) | .001 |
|
|
|||
| Paternal Education | 13.28 (4.58) | 13.51 (4.24) | .835 |
|
|
|||
| Gender (M:F)* | 12:17 | 26:16 | .098 |
|
|
|||
| Race* | .348 | ||
| African American | 8 | 16 | |
| Caucasian | 21 | 24 | |
| Other | 0 | 2 | |
|
|
|||
| Cognition | |||
| WTAR | 109.18 (13.26) | 96.92 (17.01) | .002 |
| LNS | 15.62 (3.25) | 11.95 (3.41) | <.001 |
| SS | 11.41 (2.23) | 7.89 (2.98) | <.001 |
| HVLT | 29.31 (4.28) | 21.55 (5.51) | <.001 |
|
|
|||
| Antipsychotic Medication | |||
| Atypical Antipsychotics | — | 33 | — |
| Typical Antipsychotics | — | 9 | — |
| Atypical + Typical | — | 1 | — |
|
|
|||
| Clinical Ratings | |||
| BPRS Total | — | 36.20 (8.56) | — |
| SANS Total | — | 32.88 (15.58) | — |
Note: Table includes means and standard deviations. T-tests were conducted to determine group differences except where noted.
Group differences tested with chi-square
Procedure
As part of a larger study of decision-making, participants completed a computerized version of the monetary choice questionnaire for hypothetical monetary rewards (Kirby, Petry, & Bickel, 1999). The measure includes 27 items in which participants choose between a smaller, immediate reward (SIR) and a larger, delayed reward (LDR). Participants pressed either the left or right button on a response pad to indicate their choice. LDRs included rewards of 3 sizes, small ($25–35), medium ($50–60) and large ($75–85). The DD parameter (denoted as k) was estimated according to methods reported by Kirby (2000). Briefly, the geometric mean of a window bounded by the largest k-value at which a participant chose the LDR and the smallest k-value at which a participant chose the SIR was calculated. For example, if a question’s k-value was .0060 and a participant chose the LDR, a choice consistent with k smaller than .0060, and at the next smallest k-value (.0025) chose the SIR, then that participant’s k-value was estimated by taking the geometric mean of k-values .0060 and .0025. k-values at small, medium and large LDRs were estimated independently. Three SC participants were excluded at 1 or more LDR-sizes due to inconsistent responding (fewer than 7 items consistent with the estimated value of k in one or more LDR-sizes).
In addition to the task above, participants completed Chapman Physical and Social Anhedonia Scales (Chapman, Chapman, & Raulin, 1976), Spatial Span (SS) and Letter-Number Sequencing (LNS, Wechsler, 1997), Hopkins Verbal Learning Test (HVLT, Shapiro, Benedict, Schretlen, & Brandt, 1999), and Wechsler Test of Adult Reading (WTAR, Wechsler, 2001).
Results
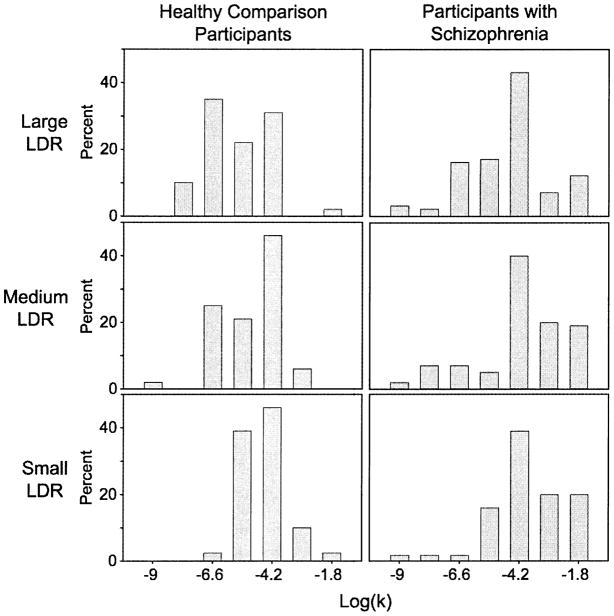
Both groups responded to items with similar degrees of consistency (HC: Mean=97.3% consistent, sd=.03; SC: Mean=95.6% consistent, sd=.06; F(1,66)=2.20, p=.14). Using the natural log transformation to approximately normalize the data (see Figure 1), SAS® PROC MIXED was used to fit a mixed model for incomplete repeated measures of the form: log(k) = group + LDR size + group × LDR-size. As shown in Figure 2, estimated discount rates depended on group (SC > HC; F(1,69)=4.94, p=.03) and LDR-size (F(2,65)=20.30, p<.001). Larger delayed rewards held their value better than smaller ones (small, medium and large discount rates differed from each other; min p=.002). The group × LDR-size interaction was not significant (F(2,65)=.92, p=.41).
Figure 1.
Natural log transformed histograms of discounting rate estimates
Figure 2.
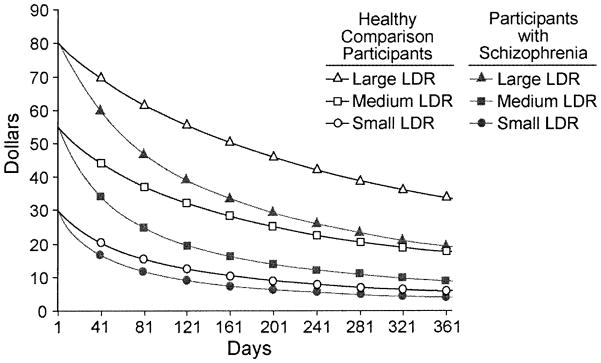
Delay discounting by LDR-size in participants with and without schizophrenia
To test the relationship between DD and cognition, an overall discounting factor was estimated from the geometric mean of k across all LDR-sizes (excluding participants who had responded inconsistently). This measure was correlated using spearman’s rho with the Chapman measures of anhedonia, cognitive function and symptoms in each group. Among SC participants, the DD estimate did not relate to physical or social anhedonia, or WTAR (see Table 2). However, DD was inversely related to total HVLT score (rs=−0.33, p=.04) and tended to be inversely correlated with SS as well (rs=−0.29, p=.08). Thus, SC participants with better episodic and working memory function demonstrated less severe discounting. Among HC participants, none of these correlations was significant.
Table 2.
Relationship between delay discounting, cognitive measures and symptoms
| 1* | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1 Delay Discounting* | — | −24 (.22) | −17 (.37) | .20 (.30) | .13 (.51) | −.15 (.45) | −.16 (.42) | — | — |
| 2 Physical Anhedonia | .04 (.80) | — | .07 (.72) | −.57 (<.01) | −.70 (<.01) | −.16 (.41) | −.26 (.19) | — | — |
| 3 Social Anhedonia | −.15 (.37) | .28 (.09) | — | .30 (.12) | −.09 (.64) | −15 (.45) | .12 (.54) | — | — |
| 4 Spatial Span | −.29 (.08) | −.02 (.93) | −.04 (.83) | — | .49 (<.01) | .36 (.05) | .17 (39) | — | — |
| 5 HVLT | −.33 (.04) | −.27 (.11) | .15 (.36) | .55 (<.01) | — | .33 (.09) | .31 (.11) | — | — |
| 6 LNS | −.13 (.32) | −.21 (.22) | −.01 (.94) | .49 (<.01) | .53 (<.01) | — | .77 (<.01) | — | — |
| 7 WTAR | −.12 (.47) | −.10 (.56) | −.03 (.84) | .19 (.26) | .36 (.03) | .40 (.01) | — | — | — |
| 8 SANS Total | −.31 (.06) | .07 (.67) | .42 (.01) | −.20 (.23) | −.02 (.89) | −.06 (.72) | −.21 (.21) | — | — |
| 9 BPRS Anergia | −.30 (.07) | .14 (.43) | .31 (.07) | −.16 (.34) | −.08 (.62) | −.18 (.27) | −.06 (.71) | .83 (<.01) | — |
Note: cells show correlation coefficient (p-values in parentheses). HC group correlations are displayed above the diagonal (shaded cells); SC group below the diagonal (un-shaded cells). Except where noted, correlation is the Pearson correlation coefficient.
Spearman’s rho
Although DD was not significantly related to positive symptoms (rs.=.09; p=.58), it did tend to relate inversely to negative symptoms such that smaller k-values (i.e., more normal discounting) were associated with more negative symptoms (SANS total: rs=−0.31, p=.07; BPRS anergia: rs=−0.30, p=.06). Thus, negative symptoms were related to a decreased tendency to prefer smaller immediate rewards.
Discussion
Individuals with schizophrenia discount the value of future rewards at a significantly greater rate than do healthy individuals. Moreover, in the present sample, SC participants with better cognitive function and those with higher levels of negative symptoms showed more normal delay discounting. Delay discounting did not relate to self-reported anhedonia, or to positive symptoms.
Insofar as delay discounting constitutes a measure of impulsive decision-making, it has implications for understanding the clinical phenomenology of schizophrenia. In essence, patients have difficulty representing the value of future outcomes, preferring more immediate, albeit smaller rewards. This may explain, in part, the difficulty that patients experience in developing and following through with longer-range educational and vocational programs. Metaphorically, the pot of gold at the end of the rainbow simply does not shine brightly enough to justify the sacrifice of more proximal rewards. The imaging data of McClure et al. (2004) suggest that this behavioral tendency results from a reduced role of the DLPFC and greater reliance on limbic areas in decision making among individuals with schizophrenia.
Surprisingly, elevations in negative symptoms tended to relate to more future-oriented decision-making. Although it seems paradoxical that symptoms as debilitating as anergia might be protective, it is nonetheless possible that these reflect a disorder in sensitivity to reward (Shurman, Horan, & Nuechterlein, 2005). Negative symptoms then, may serve to dampen the pleasure associated with immediate rewards (Juckel et al., 2006), making impulsive choices easier to resist.
Individuals with schizophrenia discount future rewards to a greater degree than do healthy individuals. Although replication of the present findings will be necessary, it appears that learning and working memory, along with negative symptoms, affect the degree to which individuals with schizophrenia act in a temporally myopic fashion. Unfortunately, the present results do not address whether these findings relate to schizophrenia, its treatment, or both. Nonetheless, it seems clear that individuals with the illness are less able to resist the pull of an immediate reward than are healthy individuals. Improving cognition may remediate some of this discrepancy.
Acknowledgments
Funding support was provided by the National Institutes of Mental Health, Grant # MH72647, to J.G. We wish to thank Sharon August, Pablo Diego, Mary Beth Ramsey, Kimberly Warren and Christopher Wilk for their assistance with data collection.
References
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl. 1989;(7):49–58. [PubMed] [Google Scholar]
- Bornovalova MA, Daughters SB, Hernandez GD, Richards JB, Lejuez CW. Differences in impulsivity and risk-taking propensity between primary users of crack cocaine and primary users of heroin in a residential substance-use program. Exp Clin Psychopharmacol. 2005;13(4):311–318. doi: 10.1037/1064-1297.13.4.311. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. J Abnorm Psychol. 1976;85(4):374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen. New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Hinson JM, Jameson TL, Whitney P. Impulsive decision making and working memory. J Exp Psychol Learn Mem Cogn. 2003;29(2):298–306. doi: 10.1037/0278-7393.29.2.298. [DOI] [PubMed] [Google Scholar]
- Holt DD, Green L, Myerson J. Is discounting impulsive?. Evidence from temporal and probability discounting in gambling and non-gambling college students. Behav Processes. 2003;64(3):355–367. doi: 10.1016/s0376-6357(03)00141-4. [DOI] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Wustenberg T, Villringer A, Knutson B, et al. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006;29(2):409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Kirby KN. Instructions for inferring discount rates from choices between immediate and delayed rewards. Williams College; 2000. p. 7. [Google Scholar]
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99(4):461–471. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128(1):78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Santiesteban M. Concave utility, transaction costs, and risk in measuring discounting of delayed rewards. J Exp Psychol Learn Mem Cogn. 2003;29(1):66–79. [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306(5695):503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Fields HL, D’Esposito M, Boettiger CA. Impulsive responding in alcoholics. Alcohol Clin Exp Res. 2005;29(12):2158–2169. doi: 10.1097/01.alc.0000191755.63639.4a. [DOI] [PubMed] [Google Scholar]
- Ohmura Y, Takahashi T, Kitamura N. Discounting delayed and probabilistic monetary gains and losses by smokers of cigarettes. Psychopharmacology (Berl) 2005;182(4):508–515. doi: 10.1007/s00213-005-0110-8. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:790–812. [Google Scholar]
- Petry NM, Kirby KN, Kranzler HR. Effects of gender and family history of alcohol dependence on a behavioral task of impulsivity in healthy subjects. J Stud Alcohol. 2002;63(1):83–90. [PubMed] [Google Scholar]
- Shapiro AM, Benedict RH, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. Clin Neuropsychol. 1999;13(3):348–358. doi: 10.1076/clin.13.3.348.1749. [DOI] [PubMed] [Google Scholar]
- Shurman B, Horan WP, Nuechterlein KH. Schizophrenia patients demonstrate a distinctive pattern of decision-making impairment on the Iowa Gambling Task. Schizophr Res. 2005;72(2–3):215–224. doi: 10.1016/j.schres.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale. 3. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Winstanley CA, Theobald DE, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci. 2004;24(20):4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Cardinal RN, Robbins TW. Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cereb Cortex. 2006;16(1):106–114. doi: 10.1093/cercor/bhi088. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Robbins TW. Interactions between serotonin and dopamine in the control of impulsive choice in rats: therapeutic implications for impulse control disorders. Neuropsychopharmacology. 2005;30(4):669–682. doi: 10.1038/sj.npp.1300610. [DOI] [PubMed] [Google Scholar]