Abstract
Patients with schizophrenia demonstrate marked impairments on most clinical neuropsychological tests. These findings suggest that patients suffer from a generalized form of cognitive impairment, with little evidence of spared performance documented in several large meta-analytic reviews of the clinical literature. In contrast, we review evidence for relative sparing of aspects of attention, procedural memory, and emotional processing observed in studies that have employed experimental approaches adapted from the cognitive and affective neuroscience literature. These islands of preserved performance suggest that the cognitive deficits in schizophrenia are not as general as they appear to be when assayed with clinical neuropsychological methods. The apparent contradiction in findings across methods may offer important clues about the nature of cognitive impairment in schizophrenia. The documentation of preserved cognitive function in schizophrenia may serve to sharpen hypotheses about the biological mechanisms that are implicated in the illness.
Keywords: Schizophrenia, neuropsychology, cognitive neuroscience, preserved function
The study of cognitive performance in patients with schizophrenia has become one of the central topics in the schizophrenia research literature. Indeed, a Pubmed search using the terms “cognition and schizophrenia” produces over 5700 abstracts. This enormous literature has largely focused on several issues. First, there is a long research tradition, perhaps best exemplified by the pioneering work of David Shakow (Shakow, 1972) where different measures of cognitive performance are used as probes to isolate the specific cognitive processes that are differentially impaired in the illness (Cautin 2008). When clinical neuropsychological assessment methods became commonplace in the schizophrenia literature in the late 1970s–1980s, interpretive attention turned towards the anatomic localization implied by the pattern of observed behavioral deficits, with many suggesting a locus of impairment in the frontal and medial temporal lobes (Goldberg, Weinberger, Berman, Pliskin, & Podd 1987; Saykin et al 1994). Supported by converging structural and functional neuroimaging evidence (Weinberger, Berman, Suddath, & Torrey 1992; Gur et al 2000A; Gur et al 2000B), these findings have played an important role in informing more recent efforts to develop animal models of impaired cognition in schizophrenia, whether by genetic, environmental, surgical, or neurochemical manipulation (Chen, Lipska, & Weinberger 2006; Lipska, Lerman, Khaing, Weinberger 2003; Li et al 2007).
In the past decade, cognitive measures have also been extensively studied as correlates/predictors of functional outcome, as targets for pharmacological and psychosocial intervention, and as potential intermediate phenotype markers of genetic effects that may run within families of a person with schizophrenia (Egan et al 2001; Gold 2004; Green, Kern, Braff, Mintz 2000; Hogarty et al 2004). Thus, cognitive measurement approaches have been used to do some serious “heavy lifting” about the nature of the brain systems implicated in the illness and in the evaluation of treatment effects. Given the centrality of these issues for schizophrenia research, progress depends on the validity of the inferences that can be drawn from available measurement approaches. The goal of this paper is to highlight some of the conceptual limitations of the clinical neuropsychological methods that have been the mainstay of the field, and to review some empirical findings that are very difficult to reconcile with the conclusions that seem to necessarily flow from the neuropsychological literature.
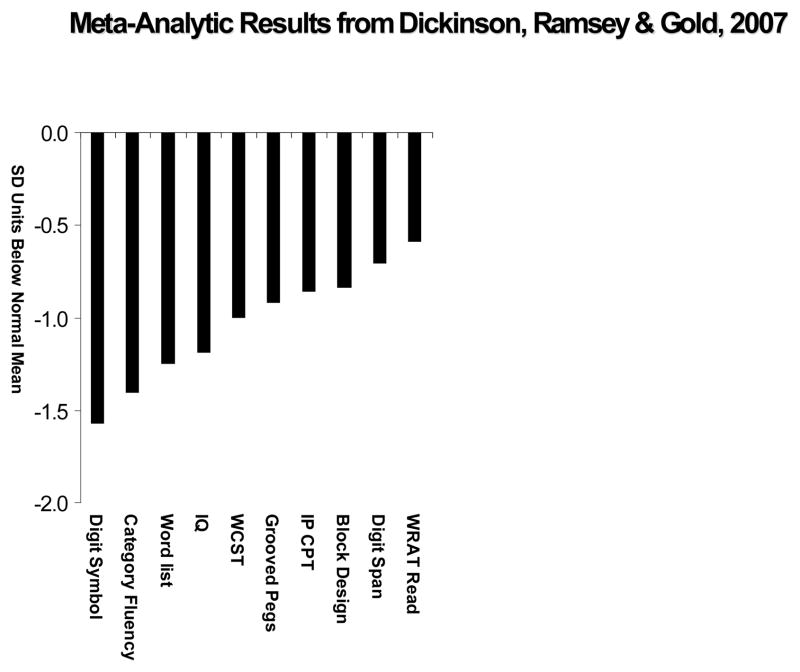
Findings from standard cognitive and neuropsychological methods have been the subject of several meta-analyses (Aleman, Hijman, de Haan, Kahn 1999; Dickinson, Ramsey, Gold 2007; Heinrichs & Zakzanis 1998; Henry & Crawford 2005; Lee & Park 2005; Reichenberg & Harvey 2007). While differing in study and measure inclusion details, these meta-analyses all yield consistent evidence that patients with schizophrenia demonstrate a substantial level of impairment (on the order one standard deviation) across many cognitive measures. An example is shown in Figure 1, adapted from Dickinson, Ramsey & Gold (2007). As seen in the Figure, there is some variability in the extent of impairment across measures, but all measures are a substantial distance from the mean healthy volunteer performance level, including measures of single word reading that are often considered as measures of premorbid cognitive ability.
Figure 1.
Meta-Analytic results adapted from Dickinson, Ramsey, & Gold 2007. The meta-analysis synthesized data from 37 studies involving 1961 patients with schizophrenia and 1444 healthy comparison subjects, and found that the largest effect size documented in the clinical neuropsychological literature was observed on digit symbol coding tasks.
To the degree that some of the measures have anatomical implications and are largely (or relatively) independent of one another, patients with schizophrenia appear to have multiple substantial impairments and no real areas of preserved performance. While differing to some degree in severity, the deficits shown in Figure 1 implicate a range of cognitive processes and anatomic regions, suggesting widespread impairment, with effect sizes varying from the medium to large range. This could result from multiple independent focal impairments or a single pathological process that impacts much of the brain.
Alternatively, one could hypothesize that the pattern of general impairment seen in Figure 1 is actually the consequence of impairments in a limited number of specific cognitive processes that have generalized consequences. That is, impairment could be limited to specific cognitive processes that impact the function of many other cognitive functions. This sharply limits cognitive “candidates” to mechanisms that are involved in multiple cognitive systems, and the two most likely candidates are attention and working memory. Attention serves a modulatory role across cognitive systems, facilitating task performance in the face of information overload (Luck & Vecera 2002). Attention serves to focus perceptual systems when they are taxed by multiple inputs, serves to guide working memory encoding in order to optimize the use of limited storage capacity, and serves to guide response selection when confronted by response incompatibility. Thus, impairments of attention might be expected to have broad consequences. Similarly, working memory — the system that forms, maintains, and manipulates short-term representations in the service of task performance — is a fundamental building block of more complex cognitive operations such as reasoning and language comprehension (Baddelely, 1986). Thus, impairment in either the attention or working memory systems could provide a plausible explanation of the general deficit pattern observed in the literature. Alternatively, impairment in affective and motivational systems could plausibly undermine the generation of goal directed behavior in general, including the effortful cognitive processes assayed by clinical neuropsychological tests.
One advantage that the specific-deficits-having-general-consequences view has above that of a more generalized form of cerebral dysfunction is that it is compatible with areas of normal, or nearly normal cognitive performance in schizophrenia that are occasionally found in the literature. That is, some aspects of cognitive performance may be “beyond the reach” of the specific impairments, resulting in areas of spared performance. However, it is difficult to reconcile the observation of areas of preserved performance with the view that patients suffer from a form of generalized deficit that impacts all aspects of cognition as suggested by Figure 1. Below, we will selectively review some of the evidence that such islands of preserved performance have been documented in the literature, including in some very surprising places including aspects of attention, reinforcement learning, and emotional processing. Such evidence may have important implications for the nature of cognitive dysfunction in schizophrenia.
Attention
Multiple aspects of attention would need to be impaired in order to produce the generalized form of cognitive impairment observed in schizophrenia. At first glance, such evidence appears to emerge from the clinical assessment literature. For example, patients routinely demonstrate substantial impairment on the Wisconsin Card Sorting and Trail Making B tests, which are discussed as requiring attentional set shifts (Mirsky, Ingraham, Kugelmass 1995). Similarly, the Wechsler Digit Symbol Substitution Test (Wechsler, 1997) is often considered a measure of attention and is among the most, if not the most, reliable impairment documented in the clinical neuropsychological literature (Dickinson et al. 2007). However, each of these tests clearly involves multiple cognitive operations, and it is difficult to establish which operation is responsible for the overall level of impairment documented in the summary performance score. Thus, the clinical assessment literature provides suggestive evidence of an impairment in attention but it is not possible to rule out the impact of deficits in other component processes that are involved in task performance.
In the experimental cognitive neuroscience literature, the role of attention is explicitly manipulated, and the pattern of performance is evaluated across different task conditions, not just overall task success or speed as is common in the clinical assessment literature. Thus, for example, subjects may be cued to respond to certain spatial locations and not others, and performance is compared at cued and uncued locations. Thus, the role of attention in this type of task is captured by the relationship between reaction times or accuracies at different locations, thereby isolating the specific impact of attentional selection on performance.
A paradigmatic example of this type of task is the attentional orienting task developed by Posner (1980) where the subject is instructed to respond as quickly as possible to the appearance of a target stimulus at one of two peripheral locations while maintaining central fixation. On a high proportion of trials, a precue indicates the likely target location along with smaller numbers of trials with misleading (invalid) and neutral cues. By comparing performance across cue types, the ability to perform preparatory shifts of attention and to filter out unlikely target locations is assayed. Comparison of peripheral versus central symbolic cues provides for measures of exogenous versus endogenous attentional shifts, i.e., triggered by the onset of a stimulus event or by abstract information and a cognitive decision process. In Posner-type paradigms, using both central and peripheral cues, the ability to orient spatial attention appears at least as robust in patients with schizophrenia as in healthy controls. In Figure 2, the mean performance of patients and controls is shown across seven (exogenous cues) and three (endogenous cues) published studies using comparable variants of the Posner task. Despite overall slower responding, the average reaction time benefit of valid cues and reaction time costs of invalid cues are comparable across patients and controls. In other words, patients are able to use the advance information conveyed by spatial cues to allocate attentional resources to the expected target location prior to target onset. To ensure that reaction time differences between conditions reflect specific differences in functions of attentional selection rather than in general alerting effects of cues, several studies employed a neutral cue instead of a no-cue condition (e.g., Carter et al. 1992, 1994, Bustillo et al. 1997, Sapir et al. 2001, Gouzoulis-Mayfrank et al. 2004, 2007). These studies report essentially the same findings on cue usage.
Figure 2.

Reaction time of patients with schizophrenia and normal control subjects in Posner-type visuospatial attention paradigms with target-predictive cues. The left graph presents averages across mean values reported by Bustillo et al (1997), Gold et al. (1992), Oie et al. (1998), Pardo et al. (2000), Posner et al. (1988), Wigal et al. (1997), and Strauss et al. (1991). These experiments include a total of 154 patients and 130 healthy controls. The right graph presents averages across mean values reported by Carter et al. (1992, 1994) and Bustillo et al. (1997) and is based on a total of 82 patients and 51 healthy controls.
Spared performance on the Posner orienting task may have limited implications for more challenging forms of selection. The target stimuli in the Posner task are luminance onsets that attract attention nearly automatically. Further, it is not possible to be certain that attention serves to enhance perceptual processing because reaction time speeding could arise on the basis of changes in response criterion alone given the uneven cue probabilities. That is, subjects may be more willing to initiate a response at a cued location (relative to an uncued location) on the basis of less perceptual evidence given that cues are almost always valid. Thus, the Posner task does not provide unambiguous evidence that attentional cues serve to improve target signal processing.
Clear evidence for intact attentional selection has been documented in the context of selection for working memory encoding. In brief, because working memory capacity is sharply limited, selective attention plays a critical role in ensuring that only relevant items are encoded. In a series of four visual change detection experiments, patients were presented with arrays of items to remember (colored rectangles, circles, and oriented bars) that were at or above working memory capacity (Gold et al. 2006). In each experiment, memory for only half the items was tested. A variety of selection cues were used, including central arrows or cue boxes, indicating the hemifield likely to be tested, or subjects were instructed to attend to items of a particular color or shape. The cues correctly predicted the test items on the majority of trials in order to motivate cue use; however, on a minority of trials, memory for the uncued items was tested. In this type of design, intact selective attention should result in nearly exclusive encoding of cued items whereas impaired selection would lead to a performance advantage for the uncued items. In each experiment, patients had lower overall working memory capacity than controls but showed clearly superior performance for cued items relative to uncued items. Further, patients showed minimal encoding of uncued items—the same performance pattern observed in healthy controls, suggesting that this form of attentional selection is surprisingly spared in the illness. One potential limitation of the evidence provided by these experiments is that cued items were either of the same or possibly greater visual salience as the distractors. Thus it remains for future work to determine if selective attention operates efficiently when challenged by the demand to exclude distractors that have a competitive salience advantage relative to target items. However, it appears that patients are surprisingly able to use a variety of salient cue types to control access to working memory.
Compatible results were obtained in visual search paradigms where participants identified a target in an array of distractor items that shared either the color or shape of the target. In one experiment (Gold et al. 2007), the target was always among three items of one color, while the number of distractors of the other color varied. The item subsets were highly discriminable, and there were only small increases in search time with increasing numbers of distractor items, i.e., attention during search was effectively restricted to items of the relevant color. Patients with schizophrenia, although slower overall, displayed the same shallow search slope, indicating that they, too, were able to focus on the relevant items and ignore the irrelevant ones. In another experiment (Elahipanah et al. 2008), the relative frequency of the distractors that shared either the color or the shape of the target was varied to be equally or unequally frequent. Search times were faster in the unequal conditions, indicating that subjects focused attention on whichever distractor subset was smaller. Patients with schizophrenia, although slowed overall, displayed the same improvements with smaller subsets, suggesting (a) intact segregation of the item types, (b) intact attentional selection, and (c) intact flexibility in adjusting their search according to the distractor frequencies. Again, the two types of categories were highly salient and discriminable, and the modulation of visual search was likely governed by bottom-up, stimulus-driven factors.
Another study employing event-related potentials investigated whether attentional selection may be implemented at a slower speed in schizophrenia (Luck et al. 2006). The N2pc component is a negative occipitotemporal deflection initiated by focusing visual attention to a target in the contralateral hemifield. Two arrays of target stimuli were presented, one in each hemifield. Participants responded to an item from either the left or right array based on its color, providing a salient cue. Despite slowed response times, patients with schizophrenia displayed virtually identical N2pc onset latencies. An additional behavioral experiment provided further evidence for a normal speed of attentional resource allocation: one item from an array was cued for a varying length of time before being masked, and recall accuracy increased with the length of the cueing period. This increase was barely shallower in patients, but this small difference was due to four outlier subjects in the patient group, suggesting overall comparable speed of attentional resource allocation between patients and controls. This result was particularly surprising given that impairments on Digit Symbol, which clearly requires rapid shifts of visual attention, are often thought to reflect slowing in processing speed. However, when speed of attentional resource allocation is assessed directly there is simply no evidence of slowing, highlighting the conflicting results between performance indicators derived from clinical neuropsychological tasks and experimental cognitive neuroscience.
The above studies suggest that patients with schizophrenia are unimpaired in controlling the allocation of attentional resources based on salient cues and in selecting relevant and filtering irrelevant material. This preservation of basic attentional selection mechanisms constitutes clear evidence against a model of generalized cognitive dysfunction in schizophrenia. What the above studies have in common, however, is a low dependence on top-down control processes. For these more effortful control processes, there is evidence for patient impairment in the context of attentional selection (Fuller et al. 2006, Gold et al. 2007, Luck & Gold 2008), consistent with findings of top-down executive control and working memory dysfunction (Kerns et al. 2008, Barch and Smith 2008). That is, when attentional selection fails in schizophrenia it appears to implicate higher order processes involved in control, not the actual implementation of selection. For example, Maruff, Dankert, Pantellis & Currie (1998) designed a version of the Posner task where the cue indicated that the target would appear at the opposite location. Thus, the task demands a shift of attention away from rather than towards the cue onset. Patients demonstrated marked impairments in this task, where the salience of a strong bottom-up stimulus needs to be overridden by a top-down rule. This finding is fully analogous to the well-documented finding that patients with schizophrenia have difficulty performing anti-saccade tasks that require a powerful top-down signal to override the nearly reflexive orienting to the cue (Radant et al. 2007). The fact that prosaccades are preserved in schizophrenia provides evidence that the abnormality is in attentional control processes rather than intrinsic to the eye-movement system (Hutton and Ettinger 2006). A similar argument can be made about the nature of the deficit observed in “expectancy” versions of the CPT studied by Cohen and colleagues where approximately 70% of trials are A-followed-by-X-targets (Servan-Schreiber, Cohen, Steingard 1996; Cohen, Barch, Carter, & Servan-Schreiber 1999). This leads to a powerful “respond to X” bias that leads to commission errors on B-followed-by-X lure trials. Again, the top-down response selection rule is at a competitive disadvantage relative to the bias that is established by the frequent target responses to the X stimulus. Thus, both perceptual input selection and response selection processes may fail where the role of the attentional system is to resolve competition between task rules. In contrast, when a task environment does not involve competition at the level of task rules, attentional processes that boost the processing of high priority items and suppress the processing of irrelevant distractors may function surprisingly normally in patients with schizophrenia.
Thus, the evidence suggests that the impairment of attention in schizophrenia is not generalized: basic selection mechanisms appear to be preserved but fail in a predictable fashion when tasks require a high degree of top down control. In accordance with the specific-deficits-general-consequences view, a deficit in such top down control processes may account for impairment in a wide range of tasks, while allowing for islands of preserved function described above.
Implicit and Feedback-Driven Learning
The idea that deficits in reinforcement learning are central to schizophrenic psychopathology is intuitively appealing, in that such deficits would implicate brain dopamine systems, widely considered to function abnormally in schizophrenia. The identification of specific deficits in reinforcement learning or specific areas of preserved function has the potential to tell us, for example, whether the processing of reinforcement is abnormal in schizophrenia, or whether the problem lies more with reward-based learning or value-based response-selection. However, addressing this question is not straightforward as there are multiple forms of reinforcement learning as well as multiple forms of implicit memory more generally, likely involving different neural substrates.
There are clearly some aspects of reinforcement learning that are severely impaired in schizophrenia. Patients with schizophrenia, for example, routinely do poorly on tests of rule learning that rely on hypothesis testing. A well-documented example of this is the reduction in number of categories achieved by patients on the Wisconsin Card Sort Task (Goldberg et al 1987). Patients with schizophrenia also show impaired performance on conditional associative learning tasks (Gold et al 2000; Kemali et al 1987; Rushe et al 1999), which require them to use feedback to learn one-to-one stimulus-response mappings. There is also relatively strong evidence that patients with schizophrenia have deficits in the ability to use feedback to make rapid adjustments to behavior in an adaptive way (as is required for tasks such as reversal learning). These results have been interpreted as pointing to a reduced ability of prefrontal cortical (PFC) processes to contribute to reinforcement learning, especially the ventral, or orbital, regions of PFC.
Beginning in the late-1980s and early-1990s, new evidence of multiple systems for learning and memory began to inspire attempts to identify learning capacities that might be unaffected by schizophrenia. Motivated by findings of relatively intact non-declarative, or implicit, learning abilities in amnesic individuals with extensive medial temporal lobe damage, researchers tested the hypothesis that implicit aspects of learning might also be relatively spared in schizophrenia, thereby evaluating the adequacy of a medial temporal lobe model of schizophrenia memory performance across declarative and implicit memory systems. In general, implicit learning processes are thought to occur without access to consciousness, operating largely independently of frontal-hippocampal systems supporting declarative memory (Seger 1994), and are thought to depend critically on the intact functioning of the basal ganglia.
It is clearly not the case, however, that patients with schizophrenia show consistently intact performance on all tasks considered to be probes of implicit learning. Further, this literature is marked by contradictory findings across laboratories from many individual paradigms. Thus, patient performance on supposed implicit learning paradigms is influenced by the details of implementation, the demands that are placed on other cognitive systems in order to perform the tasks, the characteristics of the patient and control samples, and numerous other factors. In this section, we discuss the possible roles of these factors, offer a principled explanation of why patients with schizophrenia may show evidence of intact implicit learning under some circumstances but not others, and identify specific aspects of reinforcement processing and learning that may be preserved in the illness.
One area where patients may have produced a relatively consistent pattern of results is in the domain of unsupervised learning tasks. These are implicit learning tasks that do not require the integration of trial-by-trial feedback at all, involving incidental learning through repeated stimulus exposures or repeated performance of specific motor sequences or routines. Such tasks include artificial grammar learning (Danion et al 2001; Horan et al 2008), mirror reading (Takano et al 2002), target tracking on the pursuit rotor task (Goldberg et al 1993; Kern et al 1997; Wexler et al 1997), and some tests of prototype-based classification (Keri et al 2001). These sorts of processes do not appear to rely on hippocampal-dependent explicit memory processes or dopaminergic/prefrontal cortical mechanisms of prediction-error signaling, thought to be disrupted in schizophrenia/acute psychosis (Corlett et al 2007; Heckers 2001; Murray et al 2007). Note that patients may not fully match normal performance levels on these tasks but often show the same learning slope as controls over increasing task exposure. It remains unclear if the performance gap between groups is due to limitations in perceptual and motor processes required to execute the task or a subtle compromise in the early phases of learning that may involve declarative systems.
Between reinforcement learning tasks dependent on explicit memory and unsupervised learning tasks are a range of tasks involving the use of feedback to guide the learning of procedures believed to rely largely on the basal ganglia. Examples of these include Serial Response Time (SRT) tasks, the Rutgers Acquired Equivalence Task (S-R learning), the Chaining Task, information-integration classification learning tasks, and probabilistic stimulus-selection, or probabilistic response-learning tasks. Tasks like these generally present difficulty for patients suffering from Parkinson’s disease (Ashby et al., 2003; Shohamy et al., 2005; Siegert et al., 2006) and Huntington’s disease (St. Cyr et al., 1988; Bylsma et al., 1990; Gabrieli et al., 1997), neurological disorders that involve basal ganglia dysfunction (Cepeda et al., 2007; Smith et al., 2009).
Results from studies of schizophrenia patients, using most of these tasks, are mixed. One particularly dramatic example comes from the numerous studies done in schizophrenia patients using SRT tasks (Nissen and Bullemer 1987). These tasks typically involve having subjects observe a screen with four spatial locations, one of which is highlighted on a given trial. Subjects are prompted to respond as quickly and accurately to the highlighted location by pressing a compatible button on a response box. On some sets of trials, location selection occurs in a particular sequence (unbeknownst to the subject); during other sets of trials, locations are highlighted in a pseudo-random order. A subject’s ability to accelerate his/her response times when location selection is guided by an underlying sequence is a measure of motor/procedural learning. From studies of SRT-task performance in schizophrenia patients, examples exist of both apparently disrupted learning (Kumari et al 2002; Pedersen et al 2008; Schwartz et al 2003) and seemingly intact learning (Foerde et al 2008; Perry et al 2000; Stevens et al 2002). In fact, Manoach et al. (2004) observed that patients and controls showed similar initial acquisition performance on a finger tapping motor sequence task, but that patients failed to show the same overnight (sleep-dependent) improvement on the task that controls did.
Another task that has been a source of mixed results in schizophrenia patients is the Weather, or Probabilistic Classification Task (PCT; Knowlton et al 1994). In this task, subjects are shown 1–4 cue cards that predict an outcome (sun or rain) with some probability. Subjects are prompted to guess the outcome, and are given feedback as to whether they guessed correctly or incorrectly. Because the task is probabilistic, people are thought to improve their performance on this task in an implicit, procedural way, without explicit knowledge of the individual contingencies. Successful learning on this task has also been demonstrated in patients with medial temporal lobe damage, while basal ganglia dysfunction, resulting from Parkinson’s Disease, has been found to disrupt learning (Knowlton et al 1996).
Multiple studies have included this task as a probe of implicit learning abilities in schizophrenia. Some studies (Keri et al 2000; Weickert et al 2002) have found evidence of normal learning rates in SZ patients, while others (Foerde et al 2008; Horan et al 2008) have not. Differences among the findings of these studies could be due to details of implementation and analysis. Importantly, Foerde and colleagues (2008) note that, although patients and controls show similar learning rates on the PCT in previous studies (Keri et al 2000; Weickert et al 2002), patients never achieved the same level of performance as controls. Foerde et al. (2008) contrast the impaired performance of SZ patients on their version of the PCT, with their finding of intact performance on the SRT, concluding that impaired performance on this task in schizophrenia may reflect relatively greater dysfunction of the more cognitive, as opposed to more motor, components of the neostriatum (see Middleton and Strick 2000, e.g., for a review).
One possible explanation for the apparent range of performance in SZ patients is that it is likely the case that all such tasks involve both the implicit and explicit systems to varying degrees and at various stages of performance (Atallah et al 2004). In fact, the PCT has been subjected to detailed analyses (Gluck et al 2002), revealing that declarative memory and conscious awareness make greater contributions to successful performance on these tasks than originally believed. Thus, patients with schizophrenia may be most successful in performing implicit learning tasks that place the least demands on explicit memory, such as unsupervised tasks, or motor learning tasks. Consistent with this formulation, Heerey et al. (2008) observed that patients with schizophrenia acquired a normal reward-seeking response bias on a difficult perceptual discrimination task where explicit knowledge was systematically assessed after the task was completed. Patients were fully unaware of task-reward contingencies yet showed a clear preference for the more frequently rewarded stimulus.
Aside from the characteristics of the tasks themselves, it is evident that the characteristics of subject samples influence the conclusions that are drawn about the overall performance of a patient group on these tasks. First, there is suggestive evidence that different antipsychotic medications differentially affect performance on procedural learning tasks in patients with schizophrenia. Indirect evidence of an influence of D2-receptor-blocking antipsychotic drugs on procedural learning comes from a variety sources. There is some evidence that procedural learning impairments may be relatively mild in neuroleptic-naïve patients (Scherer et al 2003). Furthermore, at least one study has observed a correlation between chlorpromazine-equivalent doses of antipsychotics in patients and procedural learning performance, as assessed using the Rutgers Acquired Equivalence Test (Face-Fish Task; Keri et al 2005), while another (Paquet et al 2004) has found evidence of a correlation between procedural learning and D2-receptor occupancy, using SPECT.
Several studies have contrasted performances of patients on first-generation antipsychotics (FGAs) with those on second-generation antipsychotics (SGAs), although patients in these studies were never randomly assigned to drug. Both Scherer et al. (2004; using a mirror-drawing task) and Stevens et al. (2002; using an SRT task) found that patients on FGAs performed significantly worse than patients on SGAs. These findings fit with the observation of Foerde et al. (2008) that deficits in performance on SRT tasks have been observed largely in samples predominantly treated with FGAs (Schwartz et al 2003). In their own study (Foerde et al 2008), as well as another in which patients were predominantly treated with SGAs (Perry et al 2000), patients and controls showed similar learning rates across the session.
In a study comparing the effects of first- and second-generation antipsychotics on reinforcement learning performance, Beninger and colleagues (2003) found that patients on FGAs showed considerably worse performance on the PCT than those on SGAs. By contrast, Beninger et al. (2003) found that SGAs seem to adversely affect performance on a supposed probe of ventral PFC function, the Iowa Gambling Task (Bechara et al 1994). These authors concluded that the comparatively-low affinity of SGAs for striatal D2 receptors may have led to a sparing of procedural learning abilities, whereas the comparatively-high affinity of SGAs for serotonin receptors (possibly in the PFC) may have affected learning mechanisms dependent on ventral PFC.
Thus, multiple examples exist of relatively worse procedural learning in patients medicated with more potent D2-antagonists. These results are consistent with findings from the experimental psychology literature, in which the administration of potent D2-antagonists to healthy volunteers leads to reductions in learning performance (Pessiglione et al 2006). Are procedural learning mechanisms largely unaffected by SGAs? This appears unlikely, though SGAs may also vary in their impact on reinforcement learning, as they vary in their affinities for dopamine receptors. Sweeney and colleagues (Harris et al 2006), for example, have found that oculomotor response learning is not impaired in neuroleptic-naïve schizophrenia patients, although a deficit emerges after stable treatment with risperidone. In contrast, Exner and colleagues (2006) found that patients who showed a deficit on an SRT task while acutely psychotic actually showed normal performance at a 20-month follow-up, at which point they were stably-medicated. Thus, easy generalization across studies is difficult as treatments have not been randomly assigned and it is difficult to disentangle state-related clinical factors from the impact of different compounds. That said, it appears likely that some of the studies reporting impaired implicit learning in patients may be explained on the basis of very high levels of D2 blockade.
Second, clinical subtype also seems to play a role in determining how preserved implicit learning capacities are in schizophrenia patients. Such a relationship fits well with the idea that negative symptoms in schizophrenia stem primarily from motivational deficits (Foussias and Remington 2008). An important relationship between motivational deficits in schizophrenia patients and their abilities to use reinforcement to learn and guide future behavior makes a great deal of intuitive sense, and the critical implication would be that procedural learning abilities would be most impaired in patients with more severe negative symptoms (and more preserved in patients with predominantly positive symptoms).
There is some correlational evidence that this may be the case. Our group (Waltz et al 2007), for example, observed a significant correlation between acquisition performance on the PSS and patients’ total scores from the SANS. Furthermore, Keri and colleagues (Farkas et al 2008; Polgar et al 2008) found that deficit syndrome patients showed greater impairment than non-deficit patients on two separate measures of procedural learning. Such correlations, while suggestive from a theoretical point of view, are not consistently found (across laboratories and experimental paradigms), however, and are often moderate when they are observed (see Gold et al 2008 for discussion).
Evidence of normal procedural learning does not necessarily imply normal basal ganglia function is schizophrenia. In fact, there is some direct evidence from neuroimaging studies (Kodama et al 2001; Reiss et al 2006; Weickert et al 2009) that normal learning in patients with schizophrenia is sometimes accompanied by abnormal brain activity, including in the basal ganglia. Evidence of striatal abnormalities also comes from PET studies of never-medicated patients (Buchsbaum et al 1992; Rubin et al 1991), suggesting that striatal abnormalities are not simply a consequence of medication. Thus, patients with schizophrenia may use a variety of mechanisms to achieve performance improvement on putative implicit memory tasks, and it is possible that patients rely on neural substrates outside of the neostriatum in accomplishing supposedly BG-dependent learning tasks, or alternatively, rely on different striatal mechanisms than do controls to reach the same level of learning.
Our group (Waltz et al 2007), for example, has found that patients may be able to use negative feedback to accomplish procedural learning, even when the ability to use positive feedback to drive learning seems disrupted. Specifically, using Frank’s Probabilistic Stimulus Selection task (Frank et al 2004), we observed that patients did not differ from controls in their tendency to avoid a frequently punished stimulus in novel pairings with less-frequently-punished stimuli, although patients exhibited a reduced tendency to choose a frequently rewarded stimulus in novel pairings with less-frequently-rewarded stimuli (Waltz et al 2007). These findings contribute to evidence of a dissociation between processes underlying reward-driven “Go” learning and those punishment-driven “NoGo” learning.
Recent physiological evidence from our group (Morris et al 2008; Waltz et al 2008) further suggests that patients’ sensitivity to negative prediction errors (unexpectedly aversive outcomes) may be, at most, mildly disturbed, whereas their sensitivity to positive prediction errors (unexpectedly pleasant outcomes) may be severely attenuated. Our findings are also consistent with reports that patients show relatively normal post-error slowing on learning tasks (Laurens et al 2003; Mathalon et al 2002; Polli et al 2006), as well as relatively normal error correction when demands on explicit memory are low (Kopp and Rist 1994; Kopp and Rist 1999; Polli et al 2006). By contrast, patients show reduced facilitation under conditions of reduced response conflict (Kopp et al 1994; Maier et al 1994), consistent with abnormal response potentiation or “Go” learning.
Behavioral-genetic (Frank et al 2007; Klein et al 2007) and pharmacological studies (Frank and O’Reilly 2006; Pessiglione et al 2006) indicate that reward-driven (Go) learning and punishment-driven (NoGo) learning may be linked to transmission at different dopamine receptors types (D1 vs. D2). In the case of schizophrenia, intact punishment-driven “NoGo” learning in SZ would be suggestive of intact (or restored) D2-receptor function. Impaired reward-driven “Go” learning in SZ would be point to still-dysfunctional D1-receptor function.
Even while acknowledging that findings in the procedural memory domain are far from uniform, it remains the case that the frequent demonstrations of performance improvement using a variety of implicit memory tasks stand in sharp contrast to the seemingly consistent picture of profound deficits on tasks of verbal and working memory, which are dependent on fronto-hippocampal neural systems. This dissociation is recapitulated by the often-intact ability of patients to gradually acquire contingencies, despite a clear deficit in the ability to do rapid reinforcement learning over the course of one or two trials in the context of tasks such as the WCST, Conditional Associative Learning, and Probabilistic Reversal Learning, etc. Within the procedural task catalog, there is suggestive evidence for maximal sparing of tasks that require the development of a motor or perceptual skill, in the absence of explicit knowledge, or the explicit representation of feedback from single trials. Impaired performance is more likely to be observed on tasks that benefit from the ability to generalize from positive reinforcement, or tasks where explicit memory for reinforcement contingencies can boost early learning, such as on the PCT. Thus, the overall pattern of findings in this literature argues against the presence of a large, undifferentiated generalized deficit.
Emotional Processing
While emotional processing is clearly a different domain than cognitive performance as assessed on most clinical measures, it is plausibly relevant to the concept of the generalized cognitive deficit for several reasons. First, impairments in motivation are often discussed as potentially underlying global cognitive deficits. In essence, the suspicion is that patients fail to exert an adequate level of effort while performing taxing cognitive tasks due to a lack of engagement with the testing situation or lack of concern over the adequacy of their performance. However, little empirical work has examined the effects of motivational processes on cognitive performance in schizophrenia to establish the claim that cognitive impairment can be attributed in part to poor effort. A recent study by Barch et al. (2008) examined the relationship between cognitive impairment and self-reported intrinsic motivation and found no support for the notion that cognitive deficits are simply byproducts of decreased motivation to perform well. Second, there is an increasing appreciation of the interactive nature of cognitive and affective processes, and that deficits in affective processes could result in impaired cognitive performance. For example, anhedonia is considered to be a central clinical feature of the illness, particularly in patients with negative symptoms, and has also been studied as a potential marker of schizophrenia liability (Chapman, Chapman & Raulin 1976). The importance of anhedonia for motivation is straightforward: if achieving behavioral goals does not result in the experience of pleasure, then there is little reason for vigorous pursuit of such goals. Insofar as cognitive tasks offer the opportunity for a sense of mastery and self-efficacy that is normatively experienced as being pleasurable, then a reduction in the capacity to experience pleasure would be expected to undermine performance. In order for this type of affective impairment to be responsible for the generalized cognitive deficit observed in schizophrenia, one would think that the impairments would need to be of substantial magnitude and impact basic hedonic processing.
Although affective impairment (e.g., anhedonia) has long been considered a core clinical feature of schizophrenia, there is surprisingly little empirical evidence that individuals with the illness experience pleasant stimuli as being less pleasant than healthy controls. A variety of laboratory-based paradigms and stimulus types have been used to elicit emotional experience, including facial expressions, evocative photographs, film clips, valenced words, odors, food, drinks, and social interactions (for review see Kring & Moran, 2008). These studies typically record valence (i.e., pleasantness) and arousal (i.e., intensity) levels evoked during stimulus exposure, or require participants to complete a comprehensive mood questionnaire before and after a block of stimulus presentations. Remarkably, in the clear majority of these studies, across these varied stimulus types and paradigms, individuals with schizophrenia report experiencing levels of valence and arousal that are remarkably and surprisingly similar to those of healthy controls when responding to positive stimuli. Only a small minority of studies have reported that patients rate pleasant stimuli as being less pleasant than controls. Furthermore, patients also generally do not differ from controls in rating the valence and arousal of negative stimuli. Thus, patients do not appear to display a deficit in the ability to appropriately experience hedonic and aversive emotions when presented with pleasant and unpleasant stimuli.
Several factors may call the validity of these laboratory-based findings into question. First, the majority of laboratory studies have been relatively underpowered and included smaller sample sizes that would make all but large effects undetectable. Second, there is significant variability in patient characteristics, such as gender composition, whether patients are medicated vs. unmedicated, and inpatient vs. outpatient status. Third, task instructions, stimulus type, and rating scale methods have been inconsistent across studies. Finally, it is simply hard to believe that patients do not have a phenomenologically attenuated experience of pleasure given the large number of studies documenting diminished experience of positive emotion using clinical interviewing procedures, trait questionnaires, and naturalistic experience sampling methods. However, a recent meta-analysis of the laboratory-based emotional experience literature, which looked at 26 published studies, systematically examined the influence of such factors (Cohen & Minor, 2008). Results of the meta-analysis indicated that: 1) patients did not evidence a hedonic deficit relative to controls (i.e., patients reported experiencing similar levels of pleasure in response to pleasant stimuli), 2) patient self-report did not differ as a function of clinical characteristics, gender, or antipsychotics medication use, and 3) stimulus type, task instructions, and rating scale procedures did not differentially influence patient performance. Thus, even though it stands in sharp contrast to years of clinical lore, the rapidly growing body of laboratory-based studies examining evoked emotional response shows no consistent support for a decrement in the momentary experience of emotion in schizophrenia.
Psychophysiological measurement provides another means of assessing the integrity of affective response in relation to positive and negative stimuli, as well as additional support for the notion that momentary affective response is preserved in schizophrenia. It is now widely documented that the startle reflex is modulated by stimulus valence. Specifically, when shown a series of emotionally evocative pictures, healthy individuals consistently show a pattern of startle response that varies by stimulus valence, whereby unpleasant stimuli yield a larger blink response than neutral stimuli, which in turn elicit a greater response than pleasant stimuli (Bradley, Lang, Cuthbert, 2003; Bradley et al., 2001; Bradley, Codispoti, Lang, 2006; Lang, Bradley, Cuthbert, 1990). It has been proposed that startle potentiation evoked by unpleasant stimuli reflects an activation of the defensive motivational system, whereas the blink response is inhibited during the presentation of pleasant stimuli when the appetitive motivational system is presumed to be active (Bradley et al., 2001). In the three studies published to date examining affect modulation of startle in schizophrenia, all report that patients display normal startle modulation in response to pleasant and unpleasant photographs when stimuli are presented at relatively longer viewing periods (Curtis et al., 1999; Schlenker et al., 1995; Volz et al., 2003). Volz et al. (2003) added a novel manipulation to the startle paradigm, measuring blink reflex in both early (e.g., 150–300ms) and later (e.g., 3800ms) viewing periods, and found that probes presented relatively early in picture viewing potentiated startle response for unpleasant stimuli in healthy controls but not patients. These findings suggest that schizophrenia patients may display a delayed autonomic response to unpleasant stimuli, potentially resulting from deficient information transfer within amygdala circuitry and subordinate structures, which regulate the defensive motivational system. It therefore appears that there is normal affective modulation of the startle response in individuals with schizophrenia for both pleasant and unpleasant stimuli, but that the startle reflex may be delayed in response to unpleasant stimuli. Thus, studies examining affect-modulated startle are generally consistent with laboratory-based self-report studies indicating that patients display a normal response in relation to affective stimuli.
Although there is consistent evidence that individuals with schizophrenia have normal evoked response to emotional stimuli, it has become apparent that they may have difficulty using that experience to motivate an appropriate behavioral response (Gold et al., 2008). Our group explored this issue by examining the association between wanting (i.e., the extent to which patients put forth effort to seek or avoid stimulus exposure) and liking (i.e., the degree to which patients rated stimuli as pleasant or unpleasant) using a key-press procedure that allowed participants to press a button to indicate the extent to which they wanted to seek or avoid future stimulus exposure. The procedure was performed both during (evoked responding) and after (representational responding) stimulus exposure to determine whether behavior differed when patients were required to form a working memory representation of stimulus value. Results indicated that although patients did not differ from controls in their subjective rating of affective stimuli, they showed weaker correspondence between their volitional motor behavior and affective ratings than controls. Additionally, the effect was magnified during the representational relative to the evoked condition. These findings suggest that even when affective values are assigned in a normative fashion, these assignments have reduced impact on the modulation of motivated behavior in patients with schizophrenia, potentially due in part to difficulty generating, accessing, or maintaining internal representations of affective value (Heerey & Gold, 2007). Thus, even though patients may adequately experience emotion in the moment, cognitive factors mediate the extent to which patients are able to couple affect and behavior (Barch, 2005).
In addition to studies investigating emotional experience, there is also a significant literature indicating that patients demonstrate both preserved performance and impairment on tasks that examine the interaction between cognitive and affective processing. Evidence for impairment exists on tasks that require processing facial expressions of emotion (Edwards, Jackson, & Pattison, 2002; Hooker & Park, 2002; Kohler, Bilker, Hagendoorn, Gur, & Gur, 2000; Martin, Baudouin, Tiberghien, & Franck, 2005; Mueser, Penn, Blanchard, & Bellack, 1997; Salem, Kring, & Kerr, 1996), interpreting the valence of affective speech productions (Billenber & Johnson, 1965; Bozikas, Kosmidis, Anezoulaki, Giannakou, Karavatos, 2004; Edwards et al., 2001; Fricchione, Sedler, & Shukla, 1986; Jonsson & Sjostedt, 1973; Murphy & Cutting, 1990), integrating audio-visual emotion productions (de Gelder et al., 2005; de Jong et al., in press), and automatically processing emotional stimuli (Epstein et al., 1999; Fear et al., 1996; Strauss et al., 2008; Suslow, et al., 2003a, b, 2005).
Studies on emotional memory have shown mixed results, with some evidence for preserved function when memory is assessed at shorter delay intervals (e.g., 4 hrs.) and some evidence for impairment at intervals of a much longer delay (e.g., 24 hrs.) (for a review see Herbener, 2008). Matthews and Barch (2004) examined immediate verbal recall and recognition for emotional and neutral words after an incidental encoding task (word ratings), and found that patients displayed generally poorer recall ability, but no evidence for a different pattern of performance across valence conditions. Horan, Green, Kring, and Nuechterlein (2006), presented patients and controls with a series of pleasant and neutral stimuli and asked them to rate their level of evoked emotional experience. After a four-hour delay period, participants were given an unannounced recall task where they were asked to indicate how they felt while previously being exposed to each stimulus. Results indicated that, although substantially anhedonic as determined through clinical rating, patients did not report experiencing less positive emotion in the moment, nor did they recall their experience of the stimuli as being any less pleasant than they did at initial presentation. Contrary findings have been reported by Herbener, Rosen, Khine, and Sweeney (2007) who presented patients and controls a series of positive, negative, and neutral images, required participants to indicate how emotionally intense each image made them feel, and then complete an unannounced recognition session after a 24-hour delay. Results indicated that although patients did not show an attenuated experience of positive emotion during initial stimulus exposure, they failed to show the same enhancement for positive stimuli that was found for controls after a 24-hour delay, ostensibly indicating a failure to integrate positive emotional experiences into the memory consolidation process. Herbener (in press) has followed-up on these findings with an implicit preference conditioning task, which associates different abstract stimulus patterns with different reward frequencies, and tested preference for the patterns both after an immediate and 24-hour delay. Immediately after training, both patients and controls showed a preference for selecting more frequently rewarded stimuli over less frequently rewarded stimuli; however, after the 24-hour delay, controls continued to prefer the more often rewarded patterns over the less rewarded ones, while patients did not. Thus, although findings in this area are mixed, there is evidence that patients are capable of initially learning and experiencing emotional stimuli appropriately, but that they are unable to retain this information in long-term memory after a 24-hour delay.
In sum, the literature on affective disturbance in schizophrenia provides striking evidence for areas of normality, as well as areas of impairment, thereby resembling the attention and reinforcement learning literatures discussed above. Given the consistent evidence of normal evoked emotional experience in schizophrenia, we speculate that it is unlikely that affective disturbances could be the mechanism that is responsible for the gross cognitive deficits documented in the neuropsychological literature. Further, as with the attention and reinforcement learning literatures, the evidence of areas of spared performance is inconsistent with the hypothesis that cognitive and affective processes in schizophrenia are simply impaired in an undifferentiated and generalized fashion.
Discussion
The demonstration of islands of preserved performance in schizophrenia is important for several reasons. First, this type of evidence should serve to constrain the understanding of the general cognitive deficit that has been amply documented in the neuropsychological literature: not every form of cognition is equally impaired in schizophrenia and some cognitive processes may function fairly normally. Further this type of evidence undermines the claim that patients generally perform poorly on tests due to inadequate effort: they don’t always perform poorly. It may be tempting to dismiss some of this evidence as simply being the result of small underpowered studies, quirky paradigms, or plain bad luck in sampling. While possible, such explanations seem very unlikely. For example, the evidence for largely intact emotional experience comes from a large body of studies using a variety of converging methods. Our evidence for intact attentional selection for working memory storage came from four converging experiments, while evidence for intact speed of attention shifting has come from converging behavioral and electrophysiological experiments. The evidence suggesting relative sparing of aspects of gradual learning comes from multiple studies using very different methodologies. Thus, the evidence base for selective sparing is actually surprisingly large in certain areas, and given the “file-drawer” problem, we suspect it is much larger than it appears in the published literature.
Alternatively, one might dismiss the evidence as being simply comprised of oddball, splinter-skills and abilities that are not terribly important for everyday cognitive function or irrelevant for current thinking about the nature of impairment in schizophrenia. In our view, it is difficult to justify such a blanket dismissal. Some of the abilities that appear to be spared in schizophrenia are in areas where many have argued for impairments. For example, we fully expected that patients would be unable to selectively encode for working memory storage hypothesizing that this type of goal-driven selection would be impaired as part of the broader executive control impairment thought to be characteristic of the illness. Indeed, we were motivated to do multiple experiments because the results from our initial experiment were so unexpected. However, the value of such unexpected findings is that it forces a greater degree of precision in conceptualizing the nature of cognitive impairment in schizophrenia, and opens up new areas of experimental work. Similarly, the fact that some forms of gradual learning guided by reinforcement may be spared, as is evoked emotional responding, should serve to modify any straightforward linkage of amotivation and a disruption of reward sensitivity and ability to learn from outcomes. Minimally, the findings in these latter two areas suggest that impairments, if observed, are likely limited to distinct subpopulations of patients and are not characteristic of the illness per se. This evidence should also serve to constrain some of the biological explanations offered for the general deficit: it must be able to explain areas of sparing in a principled fashion.
Second, the appearance of the general deficit may be a function of assessment approach. That is, the general deficit appears to be a highly reliable and robust result when measurement approaches that have predominated in the clinical neuropsychological literature are used to evaluate cognitive function. In contrast, methods inspired by cognitive neuroscience appear more likely to yield evidence of selective impairments as well as areas of preserved function. Our focus here is on the possible implications of the observations of preserved function.
There may be an important signal about the nature of cognitive impairment in schizophrenia in these contrasting findings as a function of measurement approach. In many clinical neuropsychological tests, multiple cognitive processes are queried simultaneously. For example, in Digit Symbol type tasks, performance requires working memory of the symbol linked to the target response, shifts of visual attention through the series of cues, and programming and execution of the grapho-motor response. In addition, task performance requires substantial interference control to manage previously primed motor responses. These processes must be rapidly sequenced and coordinated. Impairment or slowing in any one of these processes, or the coordination of these processes could serve to degrade performance. Thus, in one task, requiring only a couple of minutes to administer, multiple processes, subserved by multiple, widely distributed neural systems are interrogated. This type of cognitive complexity characterizes many clinical neuropsychological tests. This likely serves to increase the sensitivity of these tests to detect many different types of impairments while simultaneously serving to reduce interpretive specificity, as it is not possible to isolate the role of any of the specific processes.
In contrast to common off the shelf clinical neuropsychological assessment methods that have been the mainstays of schizophrenia research, most cognitive neuroscience approaches strive to isolate specific functions, building upon the findings established in the experimental neuropsychological research tradition. This is achieved in a variety of ways. For example, in the Posner visual orienting paradigm the perceptual and response selection demands of the task are minimal, and the target luminance increment is salient and serves to attract attention in a largely automatic fashion, reducing the role of strategy differences between individuals that often emerge in less structured task environments. When queried this way, patients with schizophrenia appear to shift attention in a largely normative fashion, in marked contrast to results observed on the Trailmaking Test, often considered a measure of processing speed and attentional shifting. Trailmaking, however, assesses attentional shifting while also demanding working memory and graphomotor speed. This would suggest that the patient impairment on Trailmaking is likely attributable to impairments in these later two processes, or the need to integrate the multiple processes that are involved in task performance.
A similar argument can be made on the nature of the processing speed impairment observed on Digit Symbol type tasks. Perhaps the simplest interpretation of this observed deficit is that it implies that multiple processes are slowed in schizophrenia. However, we have observed that the speed of attentional shifting, one of the key demands of the task, appears to be normal using both behavioral and electrophysiological methods (Luck et al., 2006). Thus, it is simply not viable to assert that all processes involved in Digit Symbol are slowed. However, it is clearly the case that when many such processes need to be integrated behavioral slowing is marked.
While speculative, it appears that the need to integrate multiple distributed processing streams, many of which are being challenged by task demands, is a feature of many neuropsychological tasks. Without doubt, many cognitive neuroscience methods also assess multiple processing streams. However, most tasks are designed to maximally stress specific processes while minimizing the challenge to other systems. Thus, the integrative challenge posed by the two methods appears to be different. When an impaired process is required, and specifically challenged, to meet a cognitive neuroscience task demand, impaired performance is to be expected. However, when an unimpaired process is isolated (such as speed of attention shifting, certain forms of reinforcement learning, or evoked emotional responsivity), and the role of other systems has been minimized, islands of preserved function, such as we documented above are likely to emerge. We acknowledge that the sparing of function in patients may not always be evident in fully normal performance levels. However, for patients to perform at even approximate normal levels is noteworthy and stands in sharp contrast to the magnitude of deficits encountered in the clinical neuropsychology literature.
If the cognitive impairments in schizophrenia are limited to focal processes, then there is every reason to be optimistic that the widespread adaptation of cognitive neuroscience methods will yield a new, more molecular understanding of the disorder. Indeed, the recent NIMH initiative to evaluate the translation of basic cognitive neuroscience paradigms for use as endpoints in clinical trials suggests that this perspective is at the leading edge of the field (Barch et al 2009). Implicitly, the field has adopted the specific deficits have general consequences viewpont. However, if the illness more critically involves the ability to mobilize and coordinate widely distributed networks, then cognitive neuroscience methods are likely to miss the signal that has been amply documented using clinical neuropsychological methods, unless they specifically target such coordination demands. That is, many available clinical neuropsychological assessment approaches unintentionally stress coordinated processing, whereas cognitive neuroscience methods typically seek to minimize this demand. Interestingly, there may be one important exception to this formulation. The cognitive control functions of the prefrontal cortex are necessarily implemented through the modulation of other cognitive systems. That is, cognitive control necessarily requires the coordination of widely distributed networks. Thus, it is possible that the appearance of a specific, focal deficit in prefrontal control systems, a deficit that is the focus of much contemporary research, could actually be the result of a far more general problem. To use a metaphor, cognitive control deficits could simply be the visible tip of a much larger cognitive coordination iceberg (Phillips & Silverstein, 2003). In essence, a deficit in broader cognitive coordination could easily be most manifest as a failure in prefrontal control processing, or be the manifest evidence of subtle impairments in multiple cognitive processes (Goldberg & Bilder, 1987). Alternatively, prefrontal control deficits might be truly a specific deficit with general consequences, impacting the ability to coordinate distributed processing streams, and it will take further research to decide between these accounts (Yoon et al 2008).
After the last decades of research on cognitive dysfunction in schizophrenia, the catalog of impairments documented in the literature is voluminous and the significance and specificity of additional entries to the catalog are increasingly difficult to evaluate. We do not mean to suggest that the pursuit of evidence of specific and differential impairments using methods that could support such claims is unimportant or may not provide new leverage on understanding the disorder. However, it is our view that documenting areas of preserved cognitive function in schizophrenia may be more theoretically informative than simply enlarging the catalog of deficits. Areas of normal performance present a difficult interpretive challenge in light of the number and magnitude of impairments documented in the literature, and may serve to lead to more precise hypotheses about the nature of cognitive impairment in schizophrenia. Expanding the catalog of preserved function in schizophrenia can serve a clear useful role in establishing the inconvenient findings that are in need of explanation, and serve as powerful constraints on the translation of the clinical implications of biological and genetic findings. Further, accounting for such findings will require a greater degree of theoretical precision than is common in the current literature. Any theory that can generate testable and falsifiable hypotheses about areas of spared and impaired function could help push the field in a principled fashion. While difficult to test with available methods, we hypothesize that the apparent contradiction between evidence of generalized dysfunction that emerges from the clinical neuropsychological tradition and the emerging evidence for a mix of spared and impaired functions that is coming from cognitive neuroscience inspired methods may reflect the different demands these two assessment approaches put on the need to coordinate the function of different processing streams—a demand maximized in the former and minimized in the latter approach. It appears that the illness-related deficit emerges most reliably when the demand for coordination is maximized. To test this idea, it will be necessary to develop novel experimental methods that can operationalize and parametrically vary this construct in an explicit fashion. Thus, it appears likely that an inference that emerges from the clinical neuropsychological tradition will require the use of novel methods from cognitive neuroscience to be empirically validated.
Acknowledgments
Supported, in part, by USPHS Grants MH080066, MH065034, MH72647, P30 MH068580 from the National Institute of Mental Health, USA (Dr. Gold).
References
- Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. American Journal of Psychiatry. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Noble S, Filoteo JV, Waldron EM, Ell SW. Category learning deficits in Parkinson’s disease. Neuropsychology. 2003;17:115–124. [PubMed] [Google Scholar]
- Atallah HE, Frank MJ, O’Reilly RC. Hippocampus, cortex, and basal ganglia: insights from computational models of complementary learning systems. Neurobiology of Learning and Memory. 2004;82:253–267. doi: 10.1016/j.nlm.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working memory. Oxford, UK: 1986. [Google Scholar]
- Barch DM, Carter CS, Arnsten A, Buchanan RW, Cohen JD, Geyer M, Green MF, Krystal JH, Nuechterlein K, Robbins T, Silverstein S, Smith EE, Strauss M, Wykes T, Heinssen R. Selecting Paradigms From Cognitive Neuroscience for Translation into Use in Clinical Trials: Proceedings of the Third CNTRICS Meeting. Schizophrenia Bulletin. 2009;35:109–114. doi: 10.1093/schbul/sbn163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, Noll DC, Cohen JD. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Archives of General Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- Barch DM, Yodkovik N, Sypher-Locke H, Hanewinkel M. Intrinsic motivation in schizophrenia: relationships to cognitive function, depression, anxiety, and personality. Journal of Abnormal Psychology. 2008;117:776–87. doi: 10.1037/a0013944. [DOI] [PubMed] [Google Scholar]
- Barch DM, Smith E. The cognitive neuroscience of working memory: relevance to CNTRICS and schizophrenia. Biological Psychiatry. 2008;64:11–17. doi: 10.1016/j.biopsych.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM. The relationships among cognition, motivation, and emotion in schizophrenia: How much and how little we know. Schizophrenia Bulletin. 2005;31:875–881. doi: 10.1093/schbul/sbi040. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Beninger RJ, Wasserman J, Zanibbi K, Charbonneau D, Mangels J, Beninger BV. Typical and atypical antipsychotic medications differentially affect two nondeclarative memory tasks in schizophrenic patients: a double dissociation. Schizophrenia Research. 2003;61:281–292. doi: 10.1016/s0920-9964(02)00315-8. [DOI] [PubMed] [Google Scholar]
- Bozikas VP, Kosmidis MH, Anezoulaki D, Giannakou M, Karavatos A. Relationship of affect recognition with psychopathology and cognitive performance in schizophrenia. Journal of the International Neuropsychological Society. 2004;10(4):549–558. doi: 10.1017/S1355617704104074. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–298. [PubMed] [Google Scholar]
- Bradley MM, Lang PJ, Cuthbert BN. Emotion, novelty, and the startle reflex: habituation in humans. Behavioral Neuroscience. 1993;107:970–80. doi: 10.1037//0735-7044.107.6.970. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Haier RJ, Potkin SG, Nuechterlein K, Bracha HS, Katz M, et al. Frontostriatal disorder of cerebral metabolism in never-medicated schizophrenics. Archives of General Psychiatry. 1992;49:935–942. doi: 10.1001/archpsyc.1992.01820120023005. [DOI] [PubMed] [Google Scholar]
- Bustillo JR, Thaker G, Buchanan RW, Moran M, Kirkpatrick B, Carpenter WT., Jr Visual information-processing impairments in deficit and nondeficit schizophrenia. American Journal of Psychiatry. 1997;154:647–654. doi: 10.1176/ajp.154.5.647. [DOI] [PubMed] [Google Scholar]
- Bylsma FW, Brandt J, Strauss ME. Aspects of procedural memory are differentially impaired in Huntington’s disease. Archives of Clinical Neuropsychology. 1990;5:287–297. [PubMed] [Google Scholar]
- Carter CS, Robertson LC, Chaderjian MR, Celaya LJ, Nordahl TE. Attentional asymmetry in schizophrenia: controlled and automatic processes. Biological Psychiatry. 1992;31:909–918. doi: 10.1016/0006-3223(92)90117-i. [DOI] [PubMed] [Google Scholar]
- Carter CS, Robertson LC, Chaderjian MR, O’Shora-Celaya L, Nordahl TE. Attentional asymmetry in schizophrenia: the role of illness subtype and symptomatology. Progress in Neuropsychopharmacology and Biological Psychiatry. 1994;18:661–683. doi: 10.1016/0278-5846(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Cautin RL. David Shakow and schizophrenia research at Worcester State Hospital: the roots of the scientist-practitioner model. Journal of the History of the Behavioral Sciences. 2008;44:219–237. doi: 10.1002/jhbs.20312. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Wu N, André VM, Cummings DM, Levine MS. The corticostriatal pathway in Huntington’s disease. Progress in Neurobiology. 2007;81:253–271. doi: 10.1016/j.pneurobio.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. Journal of Abnormal Psychology. 1976;85:374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Weinberger DR. Genetic mouse models of schizophrenia: from hypothesis-based to susceptibility gene-based models. Biological Psychiatry. 2006;59:1180–1188. doi: 10.1016/j.biopsych.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Barch DM, Carter C, Servan-Schreiber D. Context-processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks. Journal of Abnormal Psychology. 1999;108:120–133. doi: 10.1037//0021-843x.108.1.120. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Minor KS. Emotional Experience in Schizophrenia patients revisited: Meta-analysis of laboratory studies. Schizophrenia Bulletin. doi: 10.1093/schbul/sbn061. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlett PR, Murray GK, Honey GD, Aitken MR, Shanks DR, Robbins TW, et al. Disrupted prediction-error signal in psychosis: evidence for an associative account of delusions. Brain. 2007;130:2387–2400. doi: 10.1093/brain/awm173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CE, Lebow B, Lake DS, Katsanis J, Iacono WG. Acoustic startle reflex in schizophrenia patients and their first-degree relatives: evidence of normal emotional modulation. Psychophysiology. 1999;36:469–475. doi: 10.1017/s0048577299980757. [DOI] [PubMed] [Google Scholar]
- Danion JM, Meulemans T, Kauffmann-Muller F, Vermaat H. Intact implicit learning in schizophrenia. American Journal of Psychiatry. 2001;158:944–948. doi: 10.1176/appi.ajp.158.6.944. [DOI] [PubMed] [Google Scholar]
- de Gelder B, Vroomen J, de Jong SJ, Masthoff ED, Trompenaars FJ, Hodiamont P. Multisensory integration of emotional faces and voices in schizophrenics. Schizophrenia Research. 2005;72:195–203. doi: 10.1016/j.schres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Archives of General Psychiatry. 2007;64:532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- Edwards J, Jackson HJ, Pattison PE. Emotion recognition via facial expression and affective prosody in schizophrenia: A methodological review. Clinical Psychology Reviews. 2002;22:789–832. doi: 10.1016/s0272-7358(02)00130-7. [DOI] [PubMed] [Google Scholar]
- Edwards J, Pattison PE, Jackson HJ, Wales RJ. Facial affect and affective prosody recognition in first-episode schizophrenia. Schizophrenia Research. 2001;48(2):235–253. doi: 10.1016/s0920-9964(00)00099-2. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Gscheidle T, Weirich M, Rawlings R, Hyde TM, Bigelow L, Weinberger DR. Relative risk for cognitive impairments in siblings of patients with schizophrenia. Biological Psychiatry. 2001;50:98–107. doi: 10.1016/s0006-3223(01)01133-7. [DOI] [PubMed] [Google Scholar]
- Elahipanah A, Christensen BK, Reingold EM. Visual selective attention among persons with schizophrenia: The distractor ratio effect. Schizophrenia Research. 2008;105:61–67. doi: 10.1016/j.schres.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Epstein J, Stern E, Silbersweig D. Mesolimbic activity associated with psychosis in schizophrenia: Symptom-specific PET studies. In: McGinty JF, editor. Advancing from the ventral striatum to the extended amygdala: Implications for neuropsychiatry and drug use: In honor of Lennart Heimer. New York, NY: New York Academy of Sciences; 1999. pp. 562–574. [DOI] [PubMed] [Google Scholar]
- Exner C, Boucsein K, Degner D, Irle E. State-dependent implicit learning deficit in schizophrenia: evidence from 20-month follow-up. Psychiatry Research. 2006;142:39–52. doi: 10.1016/j.psychres.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Farkas M, Polgar P, Kelemen O, Rethelyi J, Bitter I, Myers CE, et al. Associative learning in deficit and nondeficit schizophrenia. Neuroreport. 2008;19:55–58. doi: 10.1097/WNR.0b013e3282f2dff6. [DOI] [PubMed] [Google Scholar]
- Fear C, Sharp H, Healy D. Cognitive processes in delusional disorders. British Journal of Psychiatry. 1996;168:61–67. doi: 10.1192/bjp.168.1.61. [DOI] [PubMed] [Google Scholar]
- Foerde K, Poldrack RA, Khan BJ, Sabb FW, Bookheimer SY, Bilder RM, et al. Selective corticostriatal dysfunction in schizophrenia: examination of motor and cognitive skill learning. Neuropsychology. 2008;22:100–109. doi: 10.1037/0894-4105.22.1.100. [DOI] [PubMed] [Google Scholar]
- Foussias G, Remington G. Negative Symptoms in Schizophrenia: Avolition and Occam’s Razor. Schizophrenia Bulletin. 2008 doi: 10.1093/schbul/sbn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Moustafa AA, Haughey HM, Curran T, Hutchison KE. Genetic triple dissociation reveals multiple roles for dopamine in reinforcement learning. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:16311–16316. doi: 10.1073/pnas.0706111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, O’Reilly RC. A mechanistic account of striatal dopamine function in cognition: Psychopharmacological studies with cabergoline and haloperidol. Behavioral Neuroscience. 2006;120:497–517. doi: 10.1037/0735-7044.120.3.497. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, O’Reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- Fuller RL, Luck SJ, Braun EL, Robinson BM, McMahon RP, Gold JM. Impaired control of visual attention in schizophrenia. Journal of Abnormal Psychology. 2006;115:266–275. doi: 10.1037/0021-843X.115.2.266. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Stebbins GT, Singh J, Willingham DB, Goetz CG. Intact mirror-tracing and impaired rotary-pursuit skill learning in patients with Huntington’s disease: evidence for dissociable memory systems in skill learning. Neuropsychology. 1997;11:272–281. doi: 10.1037//0894-4105.11.2.272. [DOI] [PubMed] [Google Scholar]
- Gluck MA, Shohamy D, Myers C. How do people solve the “weather prediction” task?: individual variability in strategies for probabilistic category learning. Learning & Memory. 2002;9:408–418. doi: 10.1101/lm.45202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Bish JA, Iannone VN, Hobart MP, Queern CA, Buchanan RW. Effects of contextual processing on visual conditional associative learning in schizophrenia. Biological Psychiatry. 2000;48:406–414. doi: 10.1016/s0006-3223(00)00930-6. [DOI] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward Processing in Schizophrenia: A Deficit in the Representation of Value. Schizophrenia Bulletin. 2008;34:835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM. Cognitive deficits as treatment targets in schizophrenia. Schizophrenia Research. 2004;72:21–28. doi: 10.1016/j.schres.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Gold JM, Fuller RL, Robinson BM, Braun EL, Luck SJ. Impaired top-down control of visual search in schizophrenia. Schizophrenia Research. 2007;94:148–155. doi: 10.1016/j.schres.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Fuller RL, Robinson BM, McMahon RP, Braun EL, Luck SJ. Intact attentional control of working memory encoding in schizophrenia. Journal of Abnormal Psychology. 2006;115:658–673. doi: 10.1037/0021-843X.115.4.658. [DOI] [PubMed] [Google Scholar]
- Gold JM, Randolph C, Coppola R, Carpenter CJ, Goldberg TE, Weinberger DR. Visual orienting in schizophrenia. Schizophrenia Research. 1992;7:203–209. doi: 10.1016/0920-9964(92)90013-u. [DOI] [PubMed] [Google Scholar]
- Goldberg E, Bilder RM. The frontal lobes and hierarchic organization of cognitive control. In: Perecman E, editor. The Frontal Lobes Revisited. New York: IRBN Press; 1987. pp. 159–187. [Google Scholar]
- Goldberg TE, Torrey EF, Gold JM, Ragland JD, Bigelow LB, Weinberger DR. Learning and memory in monozygotic twins discordant for schizophrenia. Psychological Medicine. 1993;23:71–85. doi: 10.1017/s0033291700038861. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Weinberger DR, Berman KF, Pliskin NH, Podd MH. Further evidence for dementia of the prefrontal type in schizophrenia? A controlled study of teaching the Wisconsin Card Sorting Test. Archives of General Psychiatry. 1987;44:1008–1014. doi: 10.1001/archpsyc.1987.01800230088014. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Balke M, Hajsamou S, Ruhrmann S, Schultze-Lutter F, Daumann J, et al. Orienting of attention in unmedicated patients with schizophrenia, prodromal subjects and healthy relatives. Schizophrenia Research. 2007;97:35–42. doi: 10.1016/j.schres.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Heekeren K, Voss T, Moerth D, Thelen B, Meincke U. Blunted inhibition of return in schizophrenia-evidence from a longitudinal study. Progress in Neuropsychopharmacology and Biological Psychiatry. 2004;28:389–396. doi: 10.1016/j.pnpbp.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophrenia Bulletin. 2000;26:119–36. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Gur RE, Cowell PE, Latshaw A, Turetsky BI, Grossman RI, Arnold SE, Bilker WB, Gur RC. Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Archives of General Psychiatry. 2000;57:761–768. doi: 10.1001/archpsyc.57.8.761. [DOI] [PubMed] [Google Scholar]
- Gur RE, Turetsky BI, Cowell PE, Finkelman C, Maany V, Grossman RI, Arnold SE, Bilker WB, Gur RC. Temporolimbic volume reductions in schizophrenia. Archives of General Psychiatry. 2000;57:769–775. doi: 10.1001/archpsyc.57.8.769. [DOI] [PubMed] [Google Scholar]
- Harris MS, Reilly JL, Keshavan MS, Sweeney JA. Longitudinal studies of antisaccades in antipsychotic-naive first-episode schizophrenia. Psychological Medicine. 2006;36:485–494. doi: 10.1017/S0033291705006756. [DOI] [PubMed] [Google Scholar]
- Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 2001;11:520–528. doi: 10.1002/hipo.1068. [DOI] [PubMed] [Google Scholar]
- Heerey EA, Bell-Warren KR, Gold JM. Decision-Making Impairments in the Context of Intact Reward Sensitivity in Schizophrenia. Biological Psychiatry. 2008;64:62–69. doi: 10.1016/j.biopsych.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerey EA, Gold JM. Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. Journal of Abnormal Psychology. 2007;116:268–278. doi: 10.1037/0021-843X.116.2.268. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Henry JD, Crawford JR. A meta-analytic review of verbal fluency deficits in schizophrenia relative to other neurocognitive deficits. Cognitive Neuropsychiatry. 2005;10:1–33. doi: 10.1080/13546800344000309. [DOI] [PubMed] [Google Scholar]
- Herbener ES, Harrow M, Hill SK. Change in the relationship between anhedonia and functional deficits over a 20-year period in individuals with schizophrenia. Schizophrenia Research. 2005;75:97–105. doi: 10.1016/j.schres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Herbener ES. Emotional memory in schizophrenia. Schizophrenia Bulletin. 2008;34:875–887. doi: 10.1093/schbul/sbn081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbener ES. Impairment in Long-Term Retention of Preference Conditioning in Schizophrenia. Biological Psychiatry. doi: 10.1016/j.biopsych.2009.01.020. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbener ES, Rosen C, Khine T. Failure of Positive but Not Negative Emotional Valence to Enhance Memory in Schizophrenia. Journal of Abnormal Psychology. 2007;116:43–55. doi: 10.1037/0021-843X.116.1.43. [DOI] [PubMed] [Google Scholar]
- Hogarty GE, Flesher S, Ulrich R, Carter M, Greenwald D, Pogue-Geile M, Keshavan M, Cooley S, DiBarry AL, Garrett A, Parepally H, Zoretich R. Cognitive enhancement therapy for schizophrenia: effects of a 2-year randomized trial on cognition and behavior. Archives of General Psychiatry. 2004;61:866–876. doi: 10.1001/archpsyc.61.9.866. [DOI] [PubMed] [Google Scholar]
- Horan WP, Green MF, Knowlton BJ, Wynn JK, Mintz J, Nuechterlein KH. Impaired implicit learning in schizophrenia. Neuropsychology. 2008;22:606–617. doi: 10.1037/a0012602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Green MF, Kring AM, Nuechterlein KH. Does Anhedonia in Schizophrenia Reflect Faulty Memory for Subjectively Experienced Emotions? Journal of Abnormal Psychology. 2006;115:496–508. doi: 10.1037/0021-843X.115.3.496. [DOI] [PubMed] [Google Scholar]
- Hutton SB, Ettinger U. The antisaccade task as a research tool in psychopathology: a critical review. Psychophysiology. 2006;43:302–313. doi: 10.1111/j.1469-8986.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- Kemali D, Maj M, Galderisi S, Monteleone P, Mucci A. Conditional associative learning in drug-free schizophrenic patients. Neuropsychobiology. 1987;17:30–34. doi: 10.1159/000118337. [DOI] [PubMed] [Google Scholar]
- Keri S, Kelemen O, Benedek G, Janka Z. Intact prototype learning in schizophrenia. Schizophrenia Research. 2001;52:261–264. doi: 10.1016/s0920-9964(00)00092-x. [DOI] [PubMed] [Google Scholar]
- Keri S, Kelemen O, Szekeres G, Bagoczky N, Erdelyi R, Antal A, et al. Schizophrenics know more than they can tell: probabilistic classification learning in schizophrenia. Psychological Medicine. 2000;30:149–155. doi: 10.1017/s0033291799001403. [DOI] [PubMed] [Google Scholar]
- Keri S, Nagy O, Kelemen O, Myers CE, Gluck MA. Dissociation between medial temporal lobe and basal ganglia memory systems in schizophrenia. Schizophrenia Research. 2005;77:321–328. doi: 10.1016/j.schres.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Kern RS, Green MF, Wallace CJ. Declarative and procedural learning in schizophrenia: A test of the integrity of divergent memory systems. Cognitive Neuropsychiatry. 1997;2:39–50. doi: 10.1080/135468097396405. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Nuechterlein KH, Braver TS, Barch DM. Executive functioning component mechanisms and schizophrenia. Biological Psychiatry. 2008;64:26–33. doi: 10.1016/j.biopsych.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Buchanan RW. Anhedonia and the deficit syndrome of schizophrenia. Psychiatry Research. 1990;31:25–30. doi: 10.1016/0165-1781(90)90105-e. [DOI] [PubMed] [Google Scholar]
- Klein TA, Neumann J, Reuter M, Hennig J, von Cramon DY, Ullsperger M. Genetically determined differences in learning from errors. Science. 2007;318:1642–1645. doi: 10.1126/science.1145044. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR, Gluck MA. Probabilistic classification learning in amnesia. Learning & Memory. 1994;1:106–120. [PubMed] [Google Scholar]
- Kodama S, Fukuzako H, Fukuzako T, Kiura T, Nozoe S, Hashiguchi T, et al. Aberrant brain activation following motor skill learning in schizophrenic patients as shown by functional magnetic resonance imaging. Psychological Medicine. 2001;31:1079–1088. doi: 10.1017/s0033291701004196. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Brennan AR. Recognition of facial emotions in schizophrenia. Current Opinion in Psychiatry. 2004;17:81–86. [Google Scholar]
- Kopp B, Mattler U, Rist F. Selective attention and response competition in schizophrenic patients. Psychiatry Research. 1994;53:129–139. doi: 10.1016/0165-1781(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Kopp B, Rist F. Error-correcting behavior in schizophrenic patients. Schizophrenia Research. 1994;13:11–22. doi: 10.1016/0920-9964(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Kopp B, Rist F. An event-related brain potential substrate of disturbed response monitoring in paranoid schizophrenic patients. Journal of Abnormal Psychology. 1999;108:337–346. doi: 10.1037//0021-843x.108.2.337. [DOI] [PubMed] [Google Scholar]
- Kring AM, Moran EK. Emotional response deficits in schizophrenia: insights from affective science. Schizophrenia Bulletin. 2008;34:819–34. doi: 10.1093/schbul/sbn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Gray JA, Honey GD, Soni W, Bullmore ET, Williams SC, et al. Procedural learning in schizophrenia: a functional magnetic resonance imaging investigation. Schizophrenia Research. 2002;57:97–107. doi: 10.1016/s0920-9964(01)00270-5. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97:377–395. [PubMed] [Google Scholar]
- Laurens KR, Ngan ET, Bates AT, Kiehl KA, Liddle PF. Rostral anterior cingulate cortex dysfunction during error processing in schizophrenia. Brain. 2003;126:610–622. doi: 10.1093/brain/awg056. [DOI] [PubMed] [Google Scholar]
- Lee E, Kim JJ, Namkoong K, et al. Aberrantly flattened responsivity to emotional pictures in paranoid schizophrenia. Psychiatry Research. 2006;143:135–145. doi: 10.1016/j.psychres.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. Journal of Abnormal Psychology. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Li W, Zhou Y, Jentsch JD, Brown RA, Tian X, Ehninger D, Hennah W, Peltonen L, Lönnqvist J, Huttunen MO, Kaprio J, Trachtenberg JT, Silva AJ, Cannon TD. Specific developmental disruption of disrupted-in-schizophrenia-1 function results in schizophrenia-related phenotypes in mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18280–18285. doi: 10.1073/pnas.0706900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipska BK, Lerman DN, Khaing ZZ, Weinberger DR. The neonatal ventral hippocampal lesion model of schizophrenia: effects on dopamine and GABA mRNA markers in the rat midbrain. European Journal of Neuroscience. 2003;18:3097–104. doi: 10.1111/j.1460-9568.2003.03047.x. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Gold JM. The construct of attention in schizophrenia. Biological Psychiatry. 2008;64:34–39. doi: 10.1016/j.biopsych.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Vecera SP. Attention. In: Pashler H, Yantis S, editors. Stevens’ Handbook of Experimental Psychology: Vol. 1. Sensation and Perception. 3. New York: Wiley; 2002. pp. 235–286. [Google Scholar]
- Luck SJ, Fuller RL, Braun EL, Robinson B, Summerfelt A, Gold JM. The speed of visual attention in schizophrenia: electrophysiological and behavioral evidence. Schizophrenia Research. 2006;85:174–195. doi: 10.1016/j.schres.2006.03.040. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Carter CS, Kerns JG, Ursu S, Barch DM, Holmes AJ, Stenger VA, Cohen JD. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. American Journal of Psychiatry. 2005;162:475–84. doi: 10.1176/appi.ajp.162.3.475. [DOI] [PubMed] [Google Scholar]
- Maier W, Franke P, Kopp B, Hardt J, Hain C, Rist F. Reaction time paradigms in subjects at risk for schizophrenia. Schizophrenia Research. 1994;13:35–43. doi: 10.1016/0920-9964(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Cain MS, Vangel MG, Khurana A, Goff DC, Stickgold R. A failure of sleep-dependent procedural learning in chronic, medicated schizophrenia. Biological Psychiatry. 2004;56:951–956. doi: 10.1016/j.biopsych.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Maruff P, Danckert J, Pantelis C, Currie J. Saccadic and attentional abnormalities in patients with schizophrenia. Psychological Medicine. 1998;28:1091–100. doi: 10.1017/s0033291798007132. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Fedor M, Faustman WO, Gray M, Askari N, Ford JM. Response-monitoring dysfunction in schizophrenia: an event-related brain potential study. Journal of Abnormal Psychology. 2002;111:22–41. [PubMed] [Google Scholar]
- Mathews JR, Barch DM. Episodic memory for emotional and nonemotional words in schizophrenia. Cognition & Emotion. 2004;18(6):721–740. [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Research Reviews. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Mirsky AF, Ingraham LJ, Kugelmass S. Neuropsychological assessment of attention and its pathology in the Israeli cohort. Schizophrenia Bulletin. 1995;21:193–204. doi: 10.1093/schbul/21.2.193. [DOI] [PubMed] [Google Scholar]
- Morris SE, Heerey EA, Gold JM, Holroyd CB. Learning-related changes in brain activity following errors and performance feedback in schizophrenia. Schizophrenia Research. 2008;99:274–285. doi: 10.1016/j.schres.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D, Cutting J. Prosodic comprehension and expression in schizophrenia. Journal of Neurology, Neurosurgery & Psychiatry. 1990;53:727–730. doi: 10.1136/jnnp.53.9.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray GK, Corlett PR, Clark L, Pessiglione M, Blackwell AD, Honey G, et al. Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Molecular Psychiatry. 2007;13:267–276. doi: 10.1038/sj.mp.4002058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer P. Attentional requirements of learning: evidence from performance measures. Cognitive Psychology. 1987;19:1–32. [Google Scholar]
- Oie M, Rund BR, Sundet K. Covert visual attention in patients with early-onset schizophrenia. Schizophrenia Research. 1998;34:195–205. doi: 10.1016/s0920-9964(98)00092-9. [DOI] [PubMed] [Google Scholar]
- Paquet F, Soucy JP, Stip E, Levesque M, Elie A, Bedard MA. Comparison between olanzapine and haloperidol on procedural learning and the relationship with striatal D2 receptor occupancy in schizophrenia. Journal of Neuropsychiatry & Clinical Neurosciences. 2004;16:47–56. doi: 10.1176/jnp.16.1.47. [DOI] [PubMed] [Google Scholar]
- Pardo PJ, Knesevich MA, Vogler GP, Pardo JV, Towne B, Cloninger CR, et al. Genetic and state variables of neurocognitive dysfunction in schizophrenia: a twin study. Schizophrenia Bulletin. 2000;26:459–477. doi: 10.1093/oxfordjournals.schbul.a033466. [DOI] [PubMed] [Google Scholar]
- Pedersen A, Siegmund A, Ohrmann P, Rist F, Rothermundt M, Suslow T, Arolt V. Reduced implicit and explicit sequence learning in first-episode schizophrenia. Neuropsychologia. 2008;46:186–195. doi: 10.1016/j.neuropsychologia.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Perry W, Light GA, Davis H, Braff DL. Schizophrenia patients demonstrate a dissociation on declarative and non-declarative memory tests. Schizophrenia Research. 2000;46:167–174. doi: 10.1016/s0920-9964(99)00229-7. [DOI] [PubMed] [Google Scholar]
- Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442:1042–1045. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips WA, Silverstein SM. Convergence of biological and psychological perspectives on cognitive coordination in schizophrenia. Behavioral and Brain Sciences. 2003;26:65–82. doi: 10.1017/s0140525x03000025. [DOI] [PubMed] [Google Scholar]
- Polgar P, Farkas M, Nagy O, Kelemen O, Rethelyi J, Bitter I, et al. How to find the way out from four rooms? The learning of “chaining” associations may shed light on the neuropsychology of the deficit syndrome of schizophrenia. Schizophrenia Research. 2008;99:200–207. doi: 10.1016/j.schres.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Polli FE, Barton JJ, Vangel M, Goff DC, Iguchi L, Manoach DS. Schizophrenia patients show intact immediate error-related performance adjustments on an antisaccade task. Schizophrenia Research. 2006;82:191–201. doi: 10.1016/j.schres.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Early TS, Reiman E, Pardo PJ, Dhawan M. Asymmetries in hemispheric control of attention in schizophrenia. Archives of General Psychiatry. 1988;45:814–821. doi: 10.1001/archpsyc.1988.01800330038004. [DOI] [PubMed] [Google Scholar]
- Quirk SW, Strauss ME, Sloan DM. Emotional response as a function of symptoms in schizophrenia. Schizophrenia Research. 1998;32:31–39. doi: 10.1016/s0920-9964(98)00039-5. [DOI] [PubMed] [Google Scholar]
- Radant AD, Dobie DJ, Calkins ME, Olincy A, Braff DL, Cadenhead KS, et al. Successful multi-site measurement of antisaccade performance deficits in schizophrenia. Schizophrenia Research. 2007;89:320–329. doi: 10.1016/j.schres.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Harvey PD. Neuropsychological, impairments in schizophrenia: Integration of performance-based and brain imaging findings. Psychological Bulletin. 2007;133:833–858. doi: 10.1037/0033-2909.133.5.833. [DOI] [PubMed] [Google Scholar]
- Reiss JP, Campbell DW, Leslie WD, Paulus MP, Ryner LN, Polimeni JO, et al. Deficit in schizophrenia to recruit the striatum in implicit learning: a functional magnetic resonance imaging investigation. Schizophrenia Research. 2006;87:127–137. doi: 10.1016/j.schres.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Rubin P, Holm S, Friberg L, Videbech P, Andersen HS, Bendsen BB, et al. Altered modulation of prefrontal and subcortical brain activity in newly diagnosed schizophrenia and schizophreniform disorder: A regional cerebral blood flow study. Archives of General Psychiatry. 1991;48:987–995. doi: 10.1001/archpsyc.1991.01810350027004. [DOI] [PubMed] [Google Scholar]
- Rushe TM, Woodruff PW, Murray RM, Morris RG. Episodic memory and learning in patients with chronic schizophrenia. Schizophrenia Research. 1999;35:85–96. doi: 10.1016/s0920-9964(98)00117-0. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr JA, Taylor AE, Lang AE. Procedural learning and neostriatal dysfunction in man. Brain. 1988;111:941–959. doi: 10.1093/brain/111.4.941. [DOI] [PubMed] [Google Scholar]
- Sapir A, Henik A, Dobrusin M, Hochman EY. Attentional asymmetry in schizophrenia: disengagement and inhibition of return deficits. Neuropsychology. 2001;15:361–370. doi: 10.1037//0894-4105.15.3.361. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Archives of General Psychiatry. 1994;51:124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- Scherer H, Bedard MA, Stip E, Paquet F, Richer F, Beriault M, et al. Procedural learning in schizophrenia can reflect the pharmacologic properties of the antipsychotic treatments. Cognitive & Behavioral Neurology. 2004;17:32–40. doi: 10.1097/00146965-200403000-00004. [DOI] [PubMed] [Google Scholar]
- Scherer H, Stip E, Paquet F, Bedard MA. Mild procedural learning disturbances in neuroleptic-naive patients with schizophrenia. Journal of Neuropsychiatry & Clinical Neurosciences. 2003;15:58–63. doi: 10.1176/jnp.15.1.58. [DOI] [PubMed] [Google Scholar]
- Schlenker R, Cohen R, Hopmann G. Affective modulation of the startle reflex in schizophrenic patients. European Archives of Psychiatry and Clinical Neurosciences. 1995;245:309–318. doi: 10.1007/BF02191873. [DOI] [PubMed] [Google Scholar]
- Schneider F, Gur RC, Gur RE, Shtasel DL. Emotional processing in schizophrenia: neurobehavioral probes in relation to psychopathology. Schizophrenia Research. 1995;17:67–75. doi: 10.1016/0920-9964(95)00031-g. [DOI] [PubMed] [Google Scholar]
- Schwartz BL, Howard DV, Howard JH, Jr, Hovaguimian A, Deutsch SI. Implicit learning of visuospatial sequences in schizophrenia. Neuropsychology. 2003;17:517–533. doi: 10.1037/0894-4105.17.3.517. [DOI] [PubMed] [Google Scholar]
- Seger CA. Implicit learning. Psychological Bulletin. 1994;115:163–196. doi: 10.1037/0033-2909.115.2.163. [DOI] [PubMed] [Google Scholar]
- Servan-Schreiber D, Cohen JD, Steingard S. Schizophrenic deficits in the processing of context: A test of a theoretical model. Archives of General Psychiatry. 1996;53:1105–1112. doi: 10.1001/archpsyc.1996.01830120037008. [DOI] [PubMed] [Google Scholar]
- Shakow D. The Worcester State Hospital Research on schizophrenia(1927–1946) Journal of Abnormal Psychology. 1972;80:67–110. doi: 10.1037/h0033412. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Grossman S, Sage J, Gluck MA. The role of dopamine in cognitive sequence learning: evidence from Parkinson’s disease. Behavioural Brain Research. 2005;156:191–199. doi: 10.1016/j.bbr.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Siegert RJ, Taylor KD, Weatherall M, Abernethy DA. Is implicit sequence learning impaired in Parkinson’s disease? A meta-analysis. Neuropsychology. 2006;20:490–495. doi: 10.1037/0894-4105.20.4.490. [DOI] [PubMed] [Google Scholar]
- Smith Y, Raju D, Nanda B, Pare JF, Galvan A, Wichmann T. The thalamostriatal systems: anatomical and functional organization in normal and parkinsonian states. Brain Research Bulletin. 2009;78:60–68. doi: 10.1016/j.brainresbull.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A, Schwarz J, Schwarz B, Ruf I, Kolter T, Czekalla J. Implicit and explicit learning in schizophrenics treated with olanzapine and with classic neuroleptics. Psychopharmacology (Berl) 2002;160:299–306. doi: 10.1007/s00213-001-0974-1. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Allen DN, Duke LA, Ross SA, Schwartz J. Automatic affective processing impairments in patients with deficit syndrome schizophrenia. Schizophrenia Research. 2008;102:76–87. doi: 10.1016/j.schres.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Allen DN, Ross SA, Duke LA, Schwartz J. Olfactory hedonic judgment in patients with deficit syndrome schizophrenia. Schizophrenia Bulletin. doi: 10.1093/schbul/sbn178. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss ME, Novakovic T, Tien AY, Bylsma F, Pearlson GD. Disengagement of attention in schizophrenia. Psychiatry Research. 1991;37:139–146. doi: 10.1016/0165-1781(91)90071-v. [DOI] [PubMed] [Google Scholar]
- Suslow T, Droste T, Roestel C. Automatic processing of facial emotion in schizophrenia with and without affective negative symptoms. Cognitive Neuropsychiatry. 2005;10:35–56. doi: 10.1080/13546800344000318. [DOI] [PubMed] [Google Scholar]
- Suslow TS, Roestel C, Arolt V. Affective priming in schizophrenia and without affective negative symptoms. European Archives of Psychiatry & Clinical Neuroscience. 2003a;253:292–300. doi: 10.1007/s00406-003-0443-4. [DOI] [PubMed] [Google Scholar]
- Suslow TS, Roestel C, Droste T, Arolt V. Automatic processing of verbal emotion stimuli in schizophrenia. Psychiatry Research. 2003b;120:131–144. doi: 10.1016/s0165-1781(03)00173-2. [DOI] [PubMed] [Google Scholar]
- Takano K, Ito M, Kobayashi K, Sonobe N, Kurosu S, Mori Y, et al. Procedural memory in schizophrenia assessed using a mirror reading task. Psychiatry Research. 2002;109:303–307. doi: 10.1016/s0165-1781(02)00021-5. [DOI] [PubMed] [Google Scholar]
- Tan HY, Callicott JH, Weinberger DR. Dysfunctional and compensatory prefrontal cortical systems, genes and the pathogenesis of schizophrenia. Cerebral Cortex. 2007;17(Suppl 1i):171–81. doi: 10.1093/cercor/bhm069. [DOI] [PubMed] [Google Scholar]
- Volz M, Hamm AO, Kirsch P, Rey ER. Temporal course of emotional startle modulation in schizophrenia patients. International Journal of Psychophysiology. 2003;49:123–137. doi: 10.1016/s0167-8760(03)00100-4. [DOI] [PubMed] [Google Scholar]
- Waltz JA, Frank MJ, Robinson BM, Gold JM. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biological Psychiatry. 2007;62:756–764. doi: 10.1016/j.biopsych.2006.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Schweitzer JB, Gold JM, Kurup PK, Ross TJ, Jo Salmeron B, et al. Patients with Schizophrenia have a Reduced Neural Response to Both Unpredictable and Predictable Primary Reinforcers. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Third Edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Weickert TW, Goldberg TE, Callicott JH, Chen Q, Apud JA, Das S, et al. Neural correlates of probabilistic category learning in patients with schizophrenia. Journal of Neuroscience. 2009;29:1244–1254. doi: 10.1523/JNEUROSCI.4341-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert TW, Terrazas A, Bigelow LB, Malley JD, Hyde T, Egan MF, et al. Habit and skill learning in schizophrenia: evidence of normal striatal processing with abnormal cortical input. Learning & Memory. 2002;9:430–442. doi: 10.1101/lm.49102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Suddath R, Torrey EF. Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. American Journal of Psychiatry. 1992;149:890–897. doi: 10.1176/ajp.149.7.890. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Hawkins KA, Rounsaville B, Anderson M, Sernyak MJ, Green MF. Normal neurocognitive performance after extended practice in patients with schizophrenia. Schizophrenia Research. 1997;26:173–180. doi: 10.1016/s0920-9964(97)00053-4. [DOI] [PubMed] [Google Scholar]
- Wigal SB, Swanson JM, Potkin SG. Lateralized attentional deficits in drug-free and medicated schizophrenic patients. Neuropsychologia. 1997;35:1519–1525. doi: 10.1016/s0028-3932(97)00087-0. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Minzenberg MJ, Ursu S, Ryan Walter BS, Wendelken C, Ragland JD, Carter CS. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: relationship with impaired cognition, behavioral disorganization, and global function. American Journal of Psychiatry. 2008;165:1006–1014. doi: 10.1176/appi.ajp.2008.07060945. [DOI] [PMC free article] [PubMed] [Google Scholar]