Abstract
Objective
Working memory (WM) capacity, typically measured with cognitively complex span tasks, is correlated with higher-order cognitive abilities in healthy adults. The goals of the present study were to determine: 1) if a more focused measure of visual WM storage capacity would show similar higher-order ability correlations in healthy adults and in people with schizophrenia (PSZ) thereby demonstrating the importance of simple storage capacity, 2) determine if the illness alters the pattern of correlations across cognitive domains, and, 3) evaluate whether between-group differences in WM capacity could account for the generalized cognitive impairment in PSZ.
Method
Ninety-nine PSZ and 77 healthy controls (HCs) completed a visual WM change localization task, the Wechsler Abbreviated Scale of Intelligence (WASI) and the MATRICS Consensus Cognitive Battery (MCCB).
Results
PSZ performed more poorly than HC on all cognitive measures. The between-group effect size for WM capacity was large (d = 1.11). WM robustly correlated with WASI and MCCB performance with no significant differences in the magnitude or pattern of correlations across groups. When the groups were pooled, WM capacity correlated at r=0.68 with MCCB composite score and at r=0.56 with WASI estimated Full Scale IQ. WM capacity accounted for approximately 40% of the between-group variance across the WASI and MCCB.
Conclusions
A simple measure of WM storage capacity is robustly associated with the higher-order cognitive abilities assessed by the WASI and MCCB in both HC and PSZ. WM capacity reduction may be a critical determinant of the general cognitive impairment in PSZ.
Keywords: working memory, schizophrenia, intelligence
Introduction
There is a large body of evidence that individual differences in working memory (WM) storage capacity are predictive of performance on many broad measures of complex cognition in healthy people, including scholastic aptitude, fluid intelligence, and executive function (Cowan et al., 2005; Fukuda, Vogel, Mayr, & Awh, 2010; Miyake, Friedman, Rettinger, Priti, & Hegarty, 2001). Much of this evidence comes from correlations between criterion cognitive measures and complex WM span measures that assess the ability of participants to maintain information in WM while performing distracting cognitive operations (Daneman & Carpenter, 1980; Kail, 2001; Kane, Hambrick, & Conway, 2005). However, complex span measures involve multiple cognitive processes beyond simple WM capacity, including task switching and suppression of proactive interference, and it is not clear which of the cognitive demands posed by complex span measures accounts for the relationships with higher-order cognitive performance.
Despite the importance of task switching and inhibitory processes, some evidence suggests that the WM storage capacity per se may account for some of the variance in criterion measures. For example, studies have shown associations between higher order abilities and simple span measures, which depend to a much greater degree on pure WM storage capacity (Colom, Shih, Flores-Mendoza & Quiroga, 2006; Miyake et al, 2001; Unsworth & Engle, 2007). These associations were of similar magnitude as with the more complex span tasks described above.
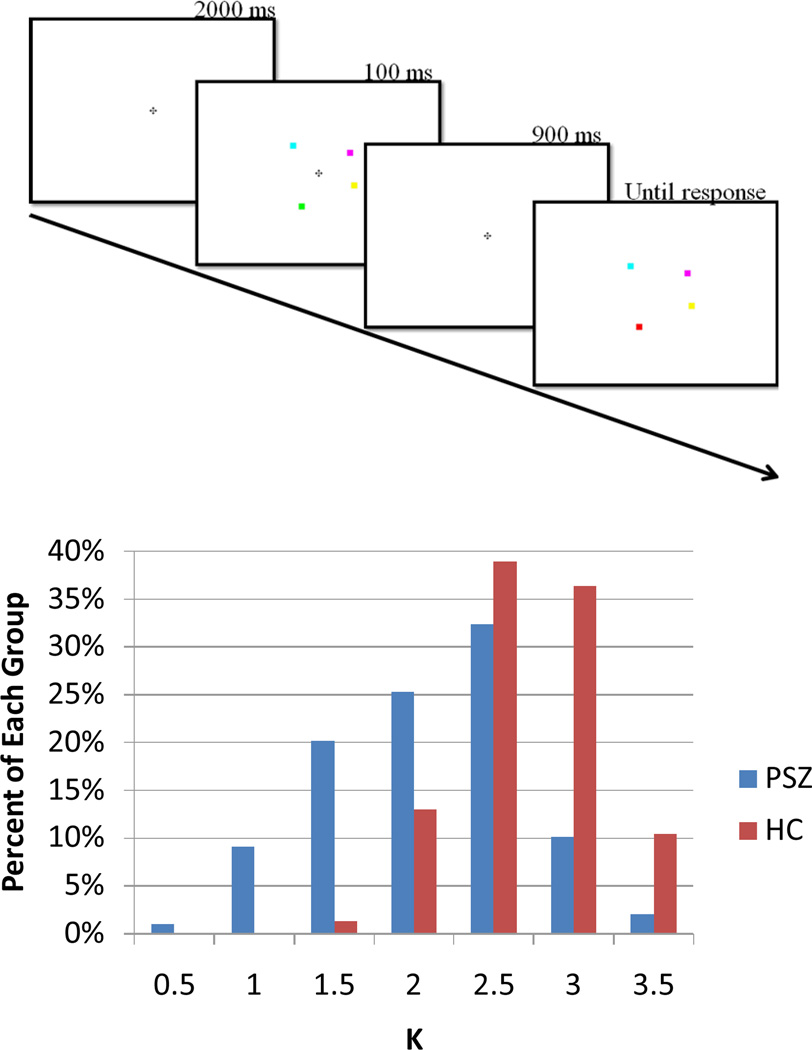
For decades, researchers have assumed that WM span is correlated with broader measures of cognition because complex cognitive tasks require the active representation of multiple pieces of information in a short-term storage buffer (Anderson, 1993; Baddeley 1986; Meyer & Kieras 1997). However, traditional WM span tasks do not appear to provide a pure measure of this ability because they are heavily influenced by executive control processes (Cowan, 2001 Saults, & Cowan, 2007). Visual change detection tasks appear to provide a purer measure of the amount of information present in a short-term storage system. In these tasks (see Figure 1), observers are shown a sample array containing a few simple stimuli (e.g., 4 colored squares) for a short period (e.g., 100 ms). After a brief delay (e.g., 1000 ms), a test array appears that is either identical to the sample array or differs in some way (e.g., one of the items might have changed colors). Participants then report whether or not a change was detected. A change-localization task can also be used, in which a change occurs on every trial and the participants report the location of the change (Gold et al., 2006, Kyllingsbaek, & Bundesen, 2009). A simple equation can be used to estimate a participant’s storage capacity from performance in these tasks (Cowan, et al., 2005; Pashler, 1988). Healthy college students typically have a capacity of 3–5 items, although substantial individual differences are observed even in this population (Vogel, & Awh, 2008).
Figure 1.
Top panel: Time-line of the change localization task. Bottom panel: the distribution of K scores in PSZ and HC. The value under each bar reflects the lower limit of the corresponding K bin, with a bin width of 0.5.
Several pieces of evidence indicate that the measure of storage capacity obtained from this task provides a relatively pure index of the amount of information that can be actively maintained in a short-term buffer. First, both fMRI and ERP studies have shown that the maintenance of information in memory in this task is accompanied by sustained neural activity during the delay interval, indicating that the information is actively maintained; as the number of items to be stored increases, the sustained neural activity increases, reaching an asymptote at the individual observer’s behaviorally-measured capacity level (Todd, & Marois, 2004; Vogel & Machizawa, 2004). Second, performance is strongly related to the visual properties of the stimuli (Alvarez & Cavanagh 2004; Zhang & Luck, 2004) but is not strongly influenced by links to long-term memory (Olson & Jiang, 2004; Pashler, 1988). Third, whereas other span measures are strongly influenced by proactive interference from previous trials, no evidence of proactive interference is observed in the change-detection task when typical parameters are used (Lin, & Luck, 2012). Fourth, the memory representations that underlie change detection can be created in as little as 50 ms (Vogel, Woodman, & Luck, 2006) which is presumably too fast for the creation of long-term memory representations. Fifth, the creation of these representations immediately impacts other ongoing cognitive processes (Carlisle, Arita, Pardo, & Woodman, 2011; Hollingworth, Richard, & Luck, 2008) indicating that they constitute a working memory.
Like the measures of capacity provided by other span tasks, the relatively pure measure of WM storage capacity that can be obtained in change-detection tasks is strongly correlated with broader measures of cognitive ability (Cowan, et al., 2005; Fukuda, et al., 2010; Gold, et al., 2010). Demonstrating such effects with simple measures of visual change detection is particularly attractive from an interpretive point of view because individual differences in WM capacity derived from this type of task have been linked to a relatively circumscribed neural system (Edin, et al., 2009; McNab & Klingberg, 2008; Todd, & Marois, 2005) thereby implicating the functional integrity of this system to much broader aspects of cognition.
In addition to varying across healthy individuals, WM capacity is also impaired in individuals with psychiatric disorders. In particular, dozens of studies have shown that people with schizophrenia (PSZ) reliably show impairments in WM performance (see meta-analyses by Lee & Park, 2005; Piskulic, Olver, Norman & Maruff, 2007). Many other studies have shown substantial impairments in broader measures of cognitive function in PSZ, and these impairments are among the best predictors of functional outcome in the disease (Green, 1996; Heinrichs & Zakzanis, 1998). However, relatively little research has attempted to link the WM impairment to the broader general cognitive deficit of schizophrenia. Some research on PSZ has demonstrated that complex span measures such as letter-number span and listening span are related to complex problem solving measures (such as the Wisconsin Card Sorting Task, Tower of Hanoi), as well as verbal free recall (Chan, Wang, Cao, & Chen 2010; Gold, Carpenter, Randolph, Goldberg, & Weinberger 1997; Perry et al., 2001, Stone, Gabrieli, Stebbins, & Sullivan, 1998), but this research did not examine broad composite measures of cognitive ability. Moreover, it is not known whether the observed correlations reflect the storage capacity component or the executive component of the complex span tasks. Thus, despite overwhelming evidence that WM capacity and broad cognitive abilities are both impaired in PSZ, there has been little research asking whether and how the WM deficit can explain the broad cognitive impairment.
Our previous research (Gold et al., 2010; Leonard et al., 2012) has provided some preliminary evidence that visual WM storage capacity is correlated with IQ and overall performance on the MATRICS Consensus Cognitive Battery (MCCB; Nuechterlein & Green, 2006), a battery of cognitive tasks across a wide range of domains, including processing speed, attention, learning, and problem solving. Large reductions in WM storage capacity were found in PSZ, and strong correlations were observed between WM storage capacity and the MCCB, but this research was limited to relatively small sample sizes.
In the present study, we examined reductions in visual WM capacity and their correlations with MCCB and WASI IQ in a more comprehensive fashion in a much larger group of participants. We sought to address four key issues. First, we asked how severely visual WM capacity is reduced in PSZ. The specific issue of capacity reduction was not addressed in the previous meta-analyses (Lee & Park, 2005; Piskulic, Olver, Norman & Maruff, 2007) which integrated results from studies using many different procedures and performance metrics that may be measuring different underlying abilities and that have varying levels of reliability, whereas we used a single task that is known to isolate visual WM storage capacity and has high reliability. Second, we asked whether the highly focused measure of storage capacity that is obtained from the change detection/localization task is correlated with measures of broad cognitive function. Previous research in healthy populations has shown that complex WM span is moderately correlated with broad measures of cognitive function (Unsworth & Engle, 2007), but much of this may reflect the executive components of the complex span tasks (Lustig, May, & Hasher, 2001). The relatively pure measure of storage capacity provided by the change detection/localization task is correlated with narrow measures of global fluid intelligence in college students (Fukuda, Vogel, Mayr, & Awh, 2010), but no studies have examined correlations with broader measures of cognitive function in more diverse samples of adults.
A third key question is whether the relationship between visual WM capacity and broader measures of cognition are altered by schizophrenia. On the one hand, this relationship might be quite different in PSZ because the mechanisms that produce reduced capacity in PSZ appear to be different from those that cause variation in capacity among healthy individuals. Specifically, individual differences among healthy young adults mainly reflect differences in the ability to use selective attention to control the contents of WM (Vogel, McCollough & Machizawa, 2005; McNab & Klingberg, 2008), but the pattern of behavioral performance and brain activity in PSZ is not like the pattern observed in low-capacity healthy individuals (Gold et al., 2006; Leonard et al., 2012). Consequently, correlations between WM capacity and broader measures of cognitive ability could be either amplified or attenuated by in PSZ. The few studies that have examined this issue—using very different methods—have produced contradictory findings (Gold et al., 1997; Perry et al., 2001; Silver, Feldman, Bilker & Gur, 2003) However, if WM capacity is associated with the ability to perform a broad array of cognitive tasks, then reduced capacity should be correlated with impaired cognitive performance no matter what originally caused the reduced capacity. This is a key question for the development of treatments for impaired cognition in schizophrenia: If reduced WM capacity in PSZ is strongly predictive of impaired cognitive performance, then it is reasonable to develop treatments aimed at increasing WM capacity.
A fourth and related question addressed by the present study is whether deficits in WM capacity among PSZ can account for a substantial proportion of their impairment in overall cognitive ability. If so, this provides additional incentive to develop treatments designed to increase WM storage capacity. Of course, our correlational methods cannot readily determine whether deficits in WM capacity actually cause the impairments in broader cognitive ability. Nonetheless, if a substantial proportion of the broad cognitive impairment is statistically associated with the degree of WM reduction, then this will be an important first step toward understanding the causes of impaired cognition in schizophrenia, especially given that our measure of WM capacity is closely linked to a circumscribed and well-studied set of neural mechanisms.
Method
Participants
Ninety-nine people who met DSM-IV (American-Psychiatric-Association, 2000) criteria for schizophrenia (42 paranoid, 27 undifferentiated, 3 disorganized, 9 residual, 1 catatonic) or schizoaffective disorder (n=17) and 77 matched healthy controls (HCs) participated in this study. Diagnosis was established using a consensus best estimate approach combining the results of a Structured Clinical Interview for DSM-IV (First, Spitzer, Gibbon, & Williams, 1997) with medical records and collateral informants when available. Demographic information is summarized in the Table 1. There was a significant difference in years of education between groups (t(174) = 5.85, p<.001), which presumably reflects the effects of SZ on educational attainment rather than a premorbid demographic difference. Consistent with this, the groups did not differ in parental education (t(168) = .371, p = .711). They also did not differ in gender distribution (χ2(1, n=176 )=. 002, p =. 969), race (χ2 (4, n=176) = 5.39, p =. 25), ethnicity (χ2 (2, n=176) = 1.56, p = .45) or age (t(174) = .08, p = .94).
Table 1.
Demographic and Psychiatric Characteristics of Sample
| Schizophrenia | Healthy Control | |
|---|---|---|
| Age | 39.4 (10.5) | 39.6 (10.4) |
| Age Range | 18–58 | 18–54 |
| Participant Education, years | 12.79(2.3) | 14.71 (2.0) |
| Parental Education years | 13.5 (3.0) | 13.6 (2.5) |
| Gender | 61% male | 61% male |
| Race | ||
| Native-American | 2 | 0 |
| Asian | 3 | 0 |
| African-American | 36 | 32 |
| Mixed | 4 | 1 |
| Caucasian | 54 | 44 |
| Mean Total Symptom Scores | ||
| BPRS Anxiety/Depression | 7.72(2.65) | |
| BPRS Anergia | 6.88(2.46) | |
| BPRS Negative Symptoms | 5.8(2.39) | |
| BPRS Psychosis | 9.26(4.12) | |
| BPRS Activation | 4.14(1.42) | |
| BPRS Hostile/Suspiciousness | 5.36(2.26) | |
| BPRS Total | 32.57(6.97) | |
| SANS Affective Flattening/Blunting | 7.75(5.71) | |
| SANS Alogia | 1.88(1.83) | |
| SANS Avolition/Apathy | 7.95(4.53) | |
| SANS Anhedonia/Asociality | 8.02(3.97) | |
| SANS Total | 25.58(11.51) | |
Note. BPRS=Brief Psychiatric Rating Scale, SANS=Schedule for the Assessment of Negative Symptoms
The PSZ were clinically stable outpatients. At the time of testing, PSZ were mildly/moderately symptomatic as measured by the Scale for the Assessment of Negative Symptoms and the Brief Psychiatric Rating Scale (Table 1) (Andreasen, 1983; Overall & Gorham, 1962). All PSZ were receiving antipsychotic medication; 17 were treated with a first-generation antipsychotic; 4 with a combination of a first generation and a second generation antipsychotic; 29 with a single, second-generation antipsychotic other than clozapine; 33 received clozapine; 11 received clozapine with an additional antipsychotic, and 5 received two different second generation antipsychotics other than clozapine. Additional psychotropic medication use was as follows: 17 received a mood stabilizer, 17 received an anticholinergic, 47 received antidepressants, and 29 anti-anxiety medication. PSZ were taking stable medications for a minimum of 4 weeks prior to testing. Control participants were recruited from the community, primarily through random digit dialing in local zip codes and by word of mouth among the friends of participants recruited in this fashion, and had no current Axis I or II diagnoses as established by the Structured Clinical Interview for DSM-IV Axis I Disorders (First, Spitzer, Gibbon, & Williams, 1997), had no family history of psychosis, and were not taking any psychotropic medication. All participants provided informed consent for a protocol approved by the University of Maryland School of Medicine institutional review board.
Neuropsychological Measures
All participants completed the Wechsler Abbreviated Scale of Intelligence (WASI, Wechsler, 1999) and the MATRICS Consensus Cognitive Battery (MCCB, Nuechterlein & Green, 2006). The MCCB yields measures of seven cognitive domains: processing speed, working memory, verbal learning, visual learning, attention/vigilance, reasoning/problem solving, and social cognition which are combined in the calculation of an overall composite T score.
WM Change Localization Stimuli and Task
All participants completed 60 trials of a change localization task, an experimental paradigm that measures visual WM capacity. The task was programmed in E-Prime, and stimuli were presented on a cathode ray tube monitor at a nominal viewing distance of 70 cm. As illustrated in Figure 1, participants were presented with a sample array of four colored squares, each measuring 0.7 × 0.7° of visual angle, for 100 ms. After a 900-ms delay during which only the fixation cross was shown, participants were presented with a test array that was identical to the sample array except that one color had changed to a value that had not been present in the sample array. The colors were selected at random without replacement from a set of six colors with the following red, green, blue values: red (255, 0,0), green (0, 255, 0), yellow (255, 255, 0), magenta (255, 0, 255), cyan (0, 255, 255), and bright blue (80, 60, 255).
From trial to trial, the colored squares were randomly placed around an imaginary circle with a radius of 3°, with the constraints that one item appeared in each of the 4 screen quadrants, and each item was separated by a minimum of 30° on the circle from the next item. Participants used a mouse to click on the location of the item in the test array that they believed had changed color from the sample array. They were encouraged to respond accurately, with no speed pressure, and a response was required on every trial. The test array was visible until the response was made, and trials were separated by a 2000-ms inter-trial interval. A total of 60 trials was tested in each subject, which typically required approximately 8–10 minutes of testing time. The E-Prime script is available at http://mindbrain.ucdavis.edu/labs/luck-lab/change-localization.
It should be noted that this is an extremely simple task: Participants view two arrays of highly discriminable items, separated by a brief delay, and make an unspeeded response to indicate the location of the one item that differed between arrays. There is no time pressure or task switching. Moreover, it is very easy for subjects to understand the instructions, and no background knowledge (e.g., vocabulary or math facts) is needed to perform the task. This makes it a purer measure of WM storage capacity than complex span tasks. In addition, the fact that chance is 25% rather than 50% for this task minimizes guessing effects and increases measurement reliability (Kyllingsbaek, & Bundesen, 2009).
This task yields highly reliable results. We have retested a total of 45 participants (14 HC and 31 PSZ) twice with a mean retest interval 1.5 years (SD = 1.1 years). The mean K was 2.68 (SD = 0.56) at time 1 and was 2.63 (SD = .60) at time 2. The Pearson correlation between testing occasions was 0.77, P<.001, while the Intraclass Correlation Coefficient was 0.76.
Statistical Analysis
WM storage capacity was quantified using a variant of the Pashler/Cowan K equation, where K represented how many items worth of information have been stored in WM (Cowan, et al., 2005; Pashler, 1988). Since each trial contained a change, there was no potential for false alarms. As a result, K was calculated by multiplying each participant’s proportion correct by 4 (the number of items in the memory array; see Gold et al., 2006; Kyllingsbaek & Bundesen, 2009).
Effect sizes (Cohen’s d) for group differences on K, the WASI and MCCB were calculated by d = (difference of group means)/pooled s.d., where the pooled s.d. was calculated as sdp =[ ( (n1−1)sd12 + (n2−1) sd22 )/(n1 + n2 − 2) ]1/2. Partial Spearman correlations between WM capacity (K) and the MCCB and WASI domain scores were computed, adjusted for age, separately for each group. Spearman correlations were used due to non-normality of distributions and to reduce the impact of outlier values. Age was included due to its well-documented relationship to WM (Jost, Bryck, Vogel, & Mayr, 2011; Myerson, Emery, White, & Hale, 2003). Fisher’s Z- transformation—Z(R) = 0.5ln((1+R)/(1-R))—was used to compare the magnitude of the differences in age-adjusted Spearman correlations between PSZ and HC (Kleinbaum, Kupper, & Muller, 1988). As described below, none of these differences was statistically significant, so we: 1) re-computed the correlations after pooling the data from HC and PSZ; 2) applied the method of SMeng, Rosenthal, & Rubin (1992) to test for all pairwise differences in magnitude of correlations of MCCB individual cognitive domain T scores (i.e., working memory, processing speed, etc.) with K; and 3) used Benjamini and Hochberg’s (1996) false discovery rate (FDR) method of multiple testing to assess the significance of the p-values for these 41 pairwise correlations. In addition, we fitted linear regression models separately in HC and PSZ to predict WASI and MCCB scores from K, using the models of the general form domain score = intercept + K. Separate pairs of models (for HC and PSZ) were fitted for each WASI and MCCB domain score, as well as the MCCB composite score. For each domain or composite score, we then tested the significance of the difference of the estimated slopes for K (βhc and βpsz ) in the two groups, using the formula Z = (βhc - βpsz)/[s.e.(βhc)2 + s.e.(βpsz)2 ]1/2, rejecting equality of the two slopes if |Z|>1.96 (Kleinbaum, Kupper, & Muller, 1988). No HC - PSZ differences of slopes were statistically significant. We chose to focus our report on the Spearman results as these are more easily understood.
To assess the extent to which the magnitude of HC- PSZ differences in broader cognition can be explained by differences in K, we compared the magnitude of the group differences for each MCCB cognitive domain, the WASI vocabulary and matrix reasoning subtests, as well as the MCCB overall composite score and WASI estimated IQ score after adjustment for age alone, and then after adjustment for both age and K, using analysis of covariance (ANCOVA) models: 1) cognitive T-score = age + group, and 2) cognitive T-score = age + K + group. Inclusion of age in these models is motivated by the observation that, while MCCB and WASI T-scores are normed for age, change localization K-scores decrease with age.
Results
Table 2 shows mean scores for K and each of the domain-specific and composite measures of cognitive ability. PSZ performed significantly lower than HC on the MATRICS Battery Composite Score and all seven Domain scores as well as on the WASI Estimated IQ and composite scores (all ps < 0.001).
Table 2.
Intellectual and Neuropsychological Test Scores of Sample
| Schizophrenia Mean, SD |
Healthy Control Mean, SD |
Cohen d Effect Size | |
|---|---|---|---|
| Change Localization K Score | 2.34 (0.6) | 2.93 (0.43) | 1.11 |
| WASI Estimated IQ | 97.08 (14.39) | 113.86 (10.77) | 1.30 |
| WASI Matrix Reasoning T-Score | 49.32 (9.94) | 58.64 (6.47) | 1.08 |
| WASI Vocabulary T-Score | 46.28 (11.18) | 56.52 (8.41) | 1.02 |
| MCCB Composite T-score | 30.71 (13.51) | 52.55 (11.72) | 1.71 |
| MCCB Processing Speed | 34.85 (11.75) | 54.38 (10.84) | 1.72 |
| MCCB Attention Vigilance | 39.43 (10.84) | 52.25 (9.37) | 1.25 |
| MCCB Working Memory | 38.67 (10.68) | 53.14 (10.70) | 1.35 |
| MCCB Verbal Learning | 38.81 (10.42) | 51.42 (11.23) | 1.17 |
| MCCB Visual Learning | 34.1 (13.53) | 46.52 (11.24) | 0.99 |
| MCCB Reasoning/Problem Solving | 42.46 (10.02) | 52.95 (10.29) | 1.03 |
| MCCB Social Cognition | 38.76 (13.23) | 51.68 (10.77) | 1.06 |
Note. WASI=Wechsler Abbreviated Scale of Intelligence, MCCB=MATRICS Consensus Cognitive Battery. The WASI Estimated IQ score is reported as a standard score with a mean of 100 and SD of 15 while the individual subtests are reported as T scores with a mean of 50 and SD of 10. All of the MCCB scores are reported as T scores.
As seen in Figure 1, the overall distribution of K scores was substantially shifted toward lower values in PSZ relative to HC, even though it appears that the use of 4-item arrays created an artificial performance ceiling for HC. Nearly half of the HC (47%) had a K score of 3 or higher whereas only 12% of PSZ achieved scores of 3 or greater. In contrast, nearly a third of PSZ (31.3%) had K scores of 2 or lower whereas only 2.6% of the HC group had K scores in that range. The difference between groups on K was robust: t = 7.61, p<.001; Cohen’s d = 1.11.
The Spearman correlations, adjusting for age, between K and the MATRICS and WASI scores are shown in Table 3, along with the results of statistical tests of whether the correlations differed across groups. Although the correlations were consistently higher among PSZ than HC (which could reflect the greater range of values among PSZ), none of these correlations differed significantly between the two groups. Accordingly, the two samples were pooled to compute age-adjusted partial Spearman correlations in the total sample.
Table 3.
Age-Adjusted Spearman Correlations of Change-Location K with MCCB and WASI Domain Scores, by Diagnosis
| MCCB and WASI Domains |
RHC N=77 | RPSZ N=99 | RPooled N=176 | Test for HC vs PSZ difference in correlation |
|
|---|---|---|---|---|---|
| T | P-value | ||||
| WASI Estimated IQ | 0.26* | 0.44*** | 0.56*** | −1.36 | 0.172 |
| WASI Matrix Reason. T-score | 0.28* | 0.40*** | 0.51*** | −0.92 | 0.359 |
| WASI Vocabulary T-Score | 0.24* | 0.35*** | 0.48*** | −0.81 | 0.415 |
| MCCB Composite | 0.45*** | 0.57*** | 0.68*** | −1.01 | 0.313 |
| MCCB Attention/Vigilance | 0.31*** | 0.51*** | 0.60*** | −1.60 | 0.110 |
| MCCB Processing Speed | 0.46*** | 0.48*** | 0.67*** | −0.16 | 0.876 |
| MCCB Reason/Problem solving | 0.43*** | 0.41*** | 0.57*** | 0.21 | 0.835 |
| MCCB Social Cognition | 0.17 | 0.23* | 0.40*** | −0.39 | 0.696 |
| MCCB Verbal Learning | 0.25* | 0.41*** | 0.55*** | −1.12 | 0.261 |
| MCCB Visual Learning | 0.25* | 0.37*** | 0.47*** | −0.84 | 0.402 |
| MCCB Working Memory | 0.28* | 0.49*** | 0.58*** | −1.63 | 0.103 |
RHC, RPSZ and Rpooled are the Spearman coefficients between the change location K score and MCCB or WASI domain T-scores in healthy control subjects (HC), people with schizophrenia (PSZ) and all participants combined (pooled), respectively. WASI=Wechsler Abbreviated Scale of Intelligence, MCCB= MATRICS Consensus Cognitive Battery. T= test statistic for difference in correlation coefficients based on normal approximation to the Fisher’s Z-transformation of RHC and RPSZ.
=p<0.05
= p<0.01
=p<0.001
The correlations calculated on the combined sample were higher than in either group alone, likely because the range of values was greater in the pooled group than in either diagnostic group considered separately (see the within group correlation matrix in Supplementary Table 1 and the combined group matrix in Supplementary Table 2). Correlations between K and the individual MCCB and WASI domains in the pooled sample ranged from a low of r = 0.40 for social cognition to a high of r = 0.67 for processing speed, with 8 of 11 correlations exceeding Cohen’s r≥ 0.50 criterion for a large effect size. Interestingly, although K is a measure of WM, its correlation with the MCCB working memory domain was no higher than the correlations observed with some of the other domains. This likely reflects the coarseness of the MCCB domain measures, which are highly intercorrelated (August, Kiwanuka, McMahon, & Gold, 2012).
After adjusting for multiple comparisons, the Spearman correlation between K and processing speed ( r= 0.67) was significantly higher than the correlation of any other MCCB domain with K, and higher than the WASI Estimated IQ, matrix reasoning and vocabulary correlations. The correlation between K and social cognition was significantly lower than the correlations between K and processing speed, reasoning/problem solving, and working memory. The MCCB overall composite score had a significantly higher correlation (r = 0.68) with K than any individual MCCB domain except processing speed (which had a correlation of r = 0.90 with the MCCB composite score), and higher than the correlations between K and the WASI estimated IQ, matrix reasoning and vocabulary scores. Note that the interpretation of these differences among correlations may be impacted by differences in reliability among the domain and composite scores and therefore should be regarded with caution.
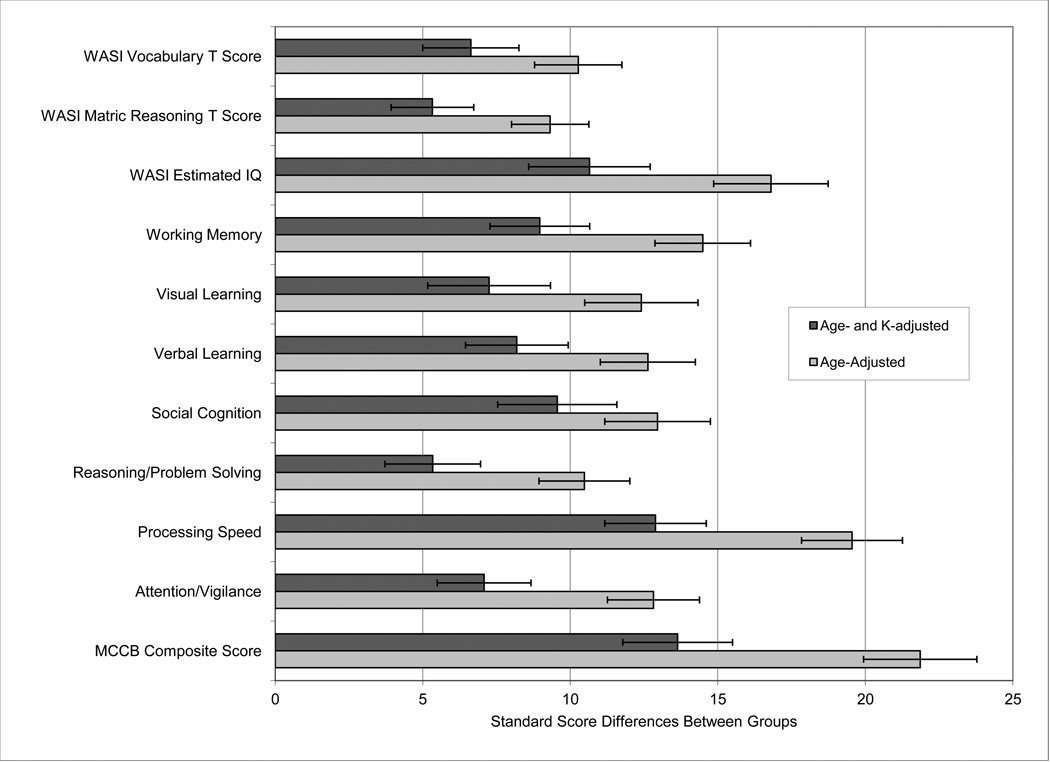
Given that WM capacity (assessed by change localization K score) was correlated with measures of multiple cognitive domains, we addressed the question of whether impaired patient performance in these domains could be explained by reduced K. As described in the statistical methods, we ran separate ANCOVA models for each cognitive domain with domain score as the dependent variable, group as the independent variable, and age as a covariate. We next added the K score as a covariate to determine the amount of between group variance that could be accounted for by K. K was a significant covariate at p<.001 in all models except when Social Cognition was the independent variable, where p = 0.18. Diagnosis was significant at the p = 0.001 level in all models, with and without adding K; however, including K in the model resulted in substantial reductions in between-group differences across all domains.
Figure 2 illustrates these results, with the age-adjusted group difference shown in light gray and the age- and K-adjusted difference score shown in dark gray. On average, the between-group difference across all scores was reduced by approximately 40% when K was added as a covariate. That is, the difference in K between groups accounted for approximately 40% of the between-group differences across the MATRICS battery and the WASI. This is a large proportion of the variance, but K did not account for the entirety of the reduced MATRICS and WASI performance in PSZ. A similar conclusion can be reached by examining the regression lines in Figure 3: The slopes of the regression lines are virtually identical for PSZ and HCs, but the line indicating the expected value of MCCB scores for PSZ at each value of K is lower than the line indicating the expected values for HC, indicating that PSZ have reduced MCCB performance compared to HC beyond what would be predicted from their lower K scores. For example, at K = 2.6, the mean value of K in the pooled sample, the predicted between group difference ± s.e. for the MCCB composite score is 13.6 ± 1.9, t = 7.10,df = 172, p<0.001. Thus, although we cannot be certain of the direction of causation, the decreased WM capacity in PSZ can potentially account for 40% of the broad cognitive deficit measured by the MCCB.
Figure 2.
Age-adjusted and age plus K-adjusted MCCB and WASI marginal mean group differences. All differences are calculated from T-scores except for WASI Estimated IQ Score, which is the difference of standard scores, rescaled to have an SD of 10. Adjusted differences are estimated with analysis of covariance models. All p-values for adjusted between group differences were p <0.001.
Figure 3.
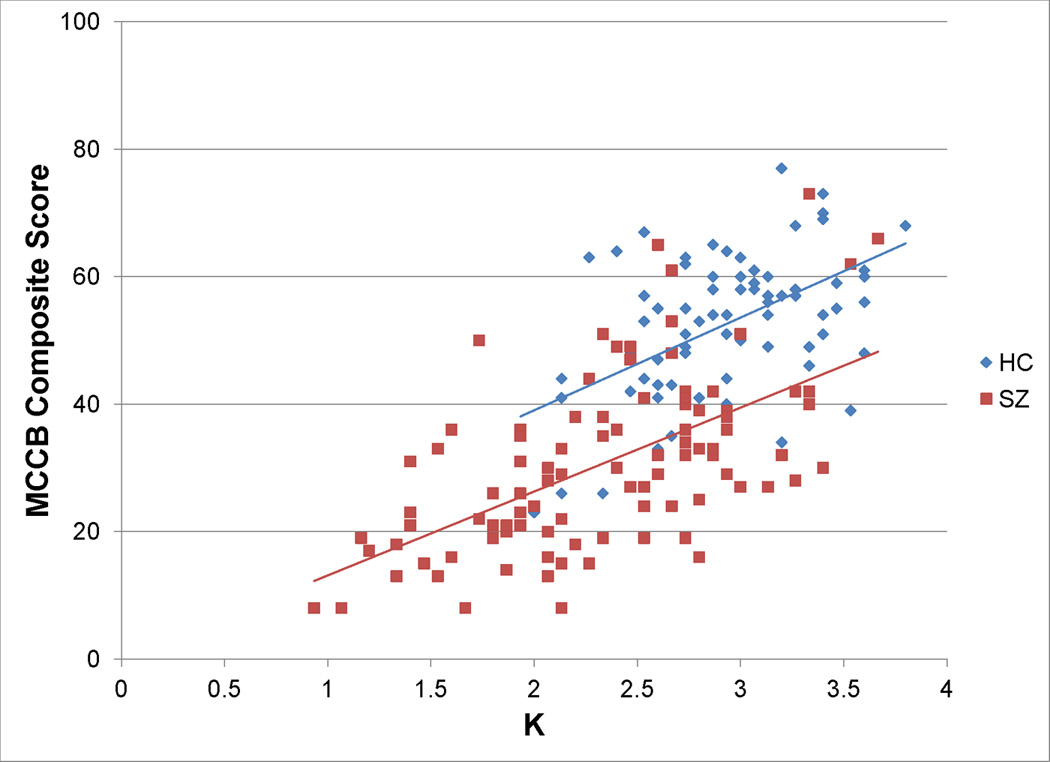
MATRICS Consensus Cognitive Battery (MCCB) composite score versus K by group, with plotted linear regressions (not adjusted for age) for HCs (composite score = 9.93 + 14.55 × K) and PSZ (composite score = −0.05 + 13.16 × K). Differences between the slopes of the two regression lines are not statistically significant (t=0.42, df=172, p=0.68).
To examine possible effects of antipsychotic medications, we used the method of Andreasen et al. (2010) to convert all antipsychotics onto a common scale of haloperidol equivalents and correlated this dose burden with K scores: This correlation was not significant, r = 0.051, p = 0.62. (One patient who used a medication not included in the conversion tables of Andreasen et al. (2010) was excluded from this analysis).
Discussion
These results provide several important insights into the relationship between WM capacity and broad measures of cognitive ability in the healthy population and in people with schizophrenia. First, PSZ demonstrated a substantial reduction in visual WM capacity (Cohen’s d = 1.11) using a simple visual change localization task that is believed to provide a relatively pure measure of the amount of information that can be actively maintained in a short-term buffer. Thus, it is not necessary for a WM task to involve memory manipulation, task switching, or distractor suppression to yield a very robust deficit in SZ. These more complex processes may also be an area of deficit in PSZ, but the current results clearly demonstrate a robust impairment in WM storage capacity.
The extent of impairment in our sample was substantially larger than documented in a prior meta-analysis of WM in schizophrenia that examined a wide range of tasks but did not include change detection measures (Lee & Park 2005). However, our results are consistent with a meta-analysis of spatial WM performance that reported an overall effect size of 1.00 (Piskulic, Olver, Norman & Maruff, 2007). Further, the size of the K reduction was comparable to the effect size for the MCCB working memory domain score in the present study, which was in turn comparable to the effect size observed for the MCCB working memory domain score in a previous large study (Keefe et al., 2011). Effect sizes are limited by the reliability of the measure, and one possible reason for the discrepancy between these large effect sizes and the smaller average effect size in the Lee and Park meta-analysis is that their meta-analysis included studies using a wide variety of tasks, many of which may have had lower reliability than the highly reliable K and MCCB scores (which were designed to maximize reliability). An additional possibility is that K is a purer measure of WM capacity than the measures used in some of the studies included in the meta-analysis, which may reduce variance related to factors that do not differ as much between PSZ and HC.
A second key finding was that WM capacity estimates derived from this simple task were strongly correlated with broader measures of cognitive ability, which provides evidence that robust correlations with higher cognitive abilities can be found for a relatively pure measure of WM storage capacity and does not require the inclusion of more complex executive processes such as the manipulation of representations, task switching, or distractor suppression. The relationship between K and higher cognitive abilities was very broad and robust, with correlations ranging from r = 0.47 to 0.68 in the pooled sample, suggesting that WM capacity is a critical cognitive capacity that is powerfully associated with performance across the types of cognitive functions demanded in the MCCB and the WASI. This is consistent with prior findings in healthy populations (Fukuda, Vogel, Mayr, & Awh, 2010) but this issue has not been addressed in the schizophrenia literature beyond two studies from our group with relatively modest sample sizes ( Gold et al., 2010; Leonard et al., 2012).
The correlations between K and the MCCB and WASI did not vary significantly as a function of group, although they were consistently higher in the PSZ, likely reflecting the greater range of scores in this group compared to the control group. This may be somewhat surprising because one typically thinks of WM deficits in schizophrenia as a consequence of the disease process, which might be expected to alter the relationship between WM and other aspects of the cognitive architecture. Some, but not all prior studies that examined the relationship of complex WM measures and other aspects of cognitive performance in PSZ suggest an amplification of these relationships (Gold et al., 1997; Perry et al., 2001; Silver et al., 2003). Indeed, recent electrophysiological evidence from our group suggests that WM capacity reduction in PSZ is mediated by different processes than those that produce variations in K among healthy individuals (Leonard et al., 2012), raising the possibility that the relationship between WM and other aspects of cognitive performance might be altered in PSZ. However, the present findings, based on large samples of PSZ and HC, suggest that the normative patterns of covariation between abilities are preserved in PSZ. PSZ may have arrived at their low K scores as the result of a pathological process, but the effect on broader cognitive function remains the same. By analogy, automobile tires normally lose air pressure over time due to simple diffusion, but air pressure can also be decreased by a nail puncture; in either case, the reduced air pressure has the same effect on the automobile’s performance. This suggests that new treatments designed to “reinflate” WM capacity in PSZ could potentially have a substantial impact on broader cognitive abilities.
Interestingly, K was correlated approximately equally with most of the individual cognitive domains sampled by the WASI and MCCB, with no compelling evidence of a stronger correlation with the MCCB working memory domain. There were two exceptions: a larger correlation with processing speed and a smaller correlation with social cognition. Note, however, that even the smallest correlation in the pooled-groups analysis exceeded Cohen’s r≥.30 criteria for a medium effect size. Thus, although some of the correlations differed statistically across domains, the theoretical or practical implications of these differences appear to be minimal. WM storage capacity may play a particularly large role in measures of processing speed, because greater storage capacity may allow people to process multiple items in parallel, avoiding the need for slower serial processing. The lower correlation with the MCCB social cognition domain may indicate that “cold” cognitive factors such as WM capacity play a smaller role in social cognition than in traditional cognitive tasks.
The most striking aspect of the data is that a relatively pure measure of working memory storage correlated with all of the abilities sampled by the WASI and the MCCB. There are at least two different means of accounting for this observation. First, one could argue that the MATRICS and WASI subscales do a poor job of isolating specific cognitive abilities, evidenced by the fact that the different domain and composite scores are robustly intercorrelated (see Supplementary Tables 1 and 2 and August, Kiwanuka, McMahon & Gold, 2012). From this perspective, the WM storage correlations might be interpreted as evidence for a relationship with general cognitive ability, not broad correlation across discrete abilities. Alternatively, WM capacity may play a similar role across these various domains, perhaps explaining the correlations among the domain scores as well as with the WM capacity score. Given that this broad pattern of correlations was observed in both HC and PSZ, it is likely to be true in other clinical populations, although this remains to be documented.
The ANCOVA results shown in Figure 2 are consistent with the correlational data. Co-varying K substantially reduced the magnitude of between-group differences across all WASI and MCCB measures and accounted for nearly 40% of the between-group difference. Thus, the between-group difference in WM capacity does not fully account for the generalized cognitive deficit observed in schizophrenia, but it accounts for an impressively large proportion. It is possible that similar ANCOVA findings would be observed using measures of processing speed (or others) as potential covariates. However, one important advantage of the use of WM in these types of analyses is that the neural system involved with variation in WM capacity (especially visual WM storage capacity) is much better defined than that involved with processing speed. Progress in understanding the neural basis and cognitive mechanisms implicated in WM impairment in schizophrenia may offer critical targets for psychological and pharmacological treatment development as any improvement in this area will likely have a positive impact on broad aspects of cognitive performance.
One possible concern about the interpretation of these data is the role of other cognitive processes. Specifically, might reductions in processing speed or degradation of perceptual processing be implicated in reduced working memory capacity? In a prior study, we directly compared performance with 100-ms and 500-ms sample durations and did not find better patient performance with the longer arrays, arguing against a role for perceptual encoding speed deficits (Gold, Wilk, McMahon, Buchanan & Luck 2003). Further, we have shown that the rate of decay of iconic memory is not different between PSZ and HC (Hahn et al., 2011). In another study, we documented reductions in WM capacity in the absence of any evidence that the perceptual precision of WM representations was altered in PSZ (Gold et al., 2006). Thus, it does not appear likely that impairments in processing speed or perceptual processing can account for the WM capacity deficit in PSZ.
This study has two potential limitations. Most importantly, correlations are not evidence of causation nor can they establish the directionality of the effects. For example, one could argue that people who are more cognitively able may be able to manage their use of WM capacity more efficiently than those who are less cognitively able. This explanation, while a logical possibility, fails to address why they are more cognitively able. Thus, the idea that variation in working memory capacity is predictive of broader cognitive performance appears to be more parsimonious but cannot be proven by the correlational approach taken here. Further, we cannot rule out the possibility of an unknown third variable that is driving variation in both WM capacity and broad cognitive performance. The fact that our results are consistent with prior studies in healthy adults may serve to reduce the concern that an obscure third variable, specific to PSZ, is driving the findings.
Our healthy controls, despite being recruited by random digit dialing, scored well above the population mean on the WASI, and it is possible that different relationships would be observed in a lower ability sample. It is also possible that the magnitude of our effect size for WM capacity may be amplified by this IQ difference. Note, however, that our groups were matched on parental education years, suggesting they were drawn from similar backgrounds. Moreover, our controls scored very close to the standardization sample mean of the MCCB. Thus, it is unlikely that our effect size was substantially overestimated because of a “super normal” control group. Indeed, the effect size may have been somewhat underestimated because we only had a single, relatively brief measure of WM capacity. Moreover, the fact that our measure of WM capacity accounted for approximately 40% of the patient deficit on the MCCB cannot be explained by the unexpectedly high IQ scores of the HC (given that their MCCB scores were typical), nor can the strong correlations between WM capacity and both MCCB and WASI IQ. Thus, the higher-than-expected IQ scores of the HC are not a major cause for concern.
Supplementary Material
Acknowledgements
This work was supported by NIMH MH065034. We would also like to thank Samuel Kaiser, Jacqueline Kiwanuka, Leeka Hubzin, Sharon August, and Brad Gray for their work in the coordination and conduct of this study.
References
- Alvarez GA, Cavanagh P. The capacity of visual short-term memory is set both by information load and by number of objects. Psychological Science. 2004;15:106–111. doi: 10.1111/j.0963-7214.2004.01502006.x. [DOI] [PubMed] [Google Scholar]
- American-Psychiatric-Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, D.C: American Psychiatric Association; 2000. Fourth, Text Revision ed. [Google Scholar]
- Anderson JR. Rules of the Mind. Hillsdale, NJ: Erlbaum; 1993. [Google Scholar]
- Andreasen NC. The scale for the assessment of negative symptoms (SANS) Technical Report. University of Iowa; 1983. [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biological. Psychiatry. 2010;67:255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- August SM, Kiwanuka JN, McMahon RP, Gold JM. The MATRICS Consensus Cognitive Battery (MCCB): Clinical and Cognitive Correlates. Schizophrenia Research. 2012;134:76–82. doi: 10.1016/j.schres.2011.10.015. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley AD. Working Memory. Oxford: Clarendon; 1986. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A new and powerful approach to multiple hypothesis testing. Journal of the Royal Statistical Society, Series B. 1995;57:1289–1300. [Google Scholar]
- Carlisle NB, Arita JT, Pardo D, Woodman GF. Attentional templates in visual working memory. Journal of Neuroscience. 2011;31:9315–9322. doi: 10.1523/JNEUROSCI.1097-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RCK, Wang YN, Cao XY, Chen EYH. Contributions of working memory components to the performance of the Tower of Hanoi in schizophrenia. East Asian Archives of Psychiatry. 2010;20:69–75. [PubMed] [Google Scholar]
- Cohen J. A Power Primer. Quantitative Methods in Psychology. 1992;112:1155–1159. [Google Scholar]
- Colom R, Shih PC, Flores-Mendoza C, Quiroga MA. The real relationship between short-term memory and working memory. Memory. 2006;14(7):804–813. doi: 10.1080/09658210600680020. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences. 2001;24:87–185. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Cowan N, Elliot EM, Saults JS, Morey CC, Mattox S, Hismjatullina A, et al. On the capacity of attention: Its estimation and its role in working memory and cognitive aptitudes. Cognitive Psychology. 2005;51:42–100. doi: 10.1016/j.cogpsych.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman M, Carpenter PA. Individual differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior. 1980;19:450–466. [Google Scholar]
- Edin F, Klingberg T, Johansson P, McNab F, Tegner J, Compte A. Mechanism for top-down control of working memory capacity. Proceedings of the National Acadamy of Sciences. 2009;106:6802–6807. doi: 10.1073/pnas.0901894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV- Axis I Disorders (SCID-I) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Fukuda K, Vogel E, Mayr U, Awh E. Quantity, not quality: The relationship between fluid intelligence and working memory capacity. Psychonomic Bulletin & Review. 2010;175:673–679. doi: 10.3758/17.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR. Auditory working memory and the Wisconsin Card Sorting Test performance in schizophrenia. Archives of General Psychiatry. 1997;54:159–165. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- Gold JM, Wilk CM, McMahon RP, Buchanan RW, Luck SJ. Working memory for visual features and conjunctions in schizophrenia. Journal of Abnormal Psychology. 2003;112:61–71. [PubMed] [Google Scholar]
- Gold JM, Fuller RL, Robinson BM, McMahon RP, Braun EL, Luck SJ. Intact attentional control of working memory encoding in schizophrenia. Journal of Abnormal Psychology. 2006;115(4):658–673. doi: 10.1037/0021-843X.115.4.658. [DOI] [PubMed] [Google Scholar]
- Gold JM, Hahn B, Zhang WW, Robinson BM, Kappenman ES, Beck VM, et al. Reduced capacity but spared precision and maintenance of working memory representations in schizophrenia. Archives of General Psychiatry. 2010;67:570–577. doi: 10.1001/archgenpsychiatry.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? American Journal of Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Hahn B, Kappenman ES, Robinson BM, Fuller RL, Luck SJ, Gold JM. Iconic decay in schizophrenia. Schizophrenia Bulletin. 2011;37:950–957. doi: 10.1093/schbul/sbp164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Hollingworth A, Richard AM, Luck SJ. Understanding the function of visual short-term memory: Transsaccadic memory, object correspondence, and gaze correction. Journal of Experimental Psychology: General. 2008;137:163–181. doi: 10.1037/0096-3445.137.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost K, Bryck RL, Vogel EK, Mayr U. Are old adults just like low working memory young adults? Filtering efficiency and age differences in visual working memory. Cerebral Cortex. 2011;21:1147–1154. doi: 10.1093/cercor/bhq185. [DOI] [PubMed] [Google Scholar]
- Kail R, Hall LK. Distinguishing short-term memory from working memory. Memory & Cognition. 2001;29:1–9. doi: 10.3758/bf03195735. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Hambrick DZ, Conway ARA. Working memory capacity and fluid intelligence are strongly related constructs: Comment on Ackerman, Beier, and Boyle. Psychological Bulletin. 2005;131:66–71. doi: 10.1037/0033-2909.131.1.66. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Fox KH, Harvey PD, Cucchiaro J, Siu C, Loebe A. Characteristics of the MATRICS Consensus Cognitive Battery in a 29-site antipsychotic schizophrenia clinical trial. Schizophrenia Research. 2011;125:161–168. doi: 10.1016/j.schres.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Kleinbaum DG, Kupper LL, Muller KE. Applied Regression Analysis and Other Multivariable Methods. 2nd Ed. Boston: PWS-Kent Publishing; 1988. [Google Scholar]
- Kyllingsbaek S, Bundesen C. Changing change detection: improving the reliability of measures of visual short-term memory capacity. Psychonomic Bulletin & Review. 2009;16:1000–1010. doi: 10.3758/PBR.16.6.1000. [DOI] [PubMed] [Google Scholar]
- Lee J, Park S. Working Memory Impairments in Schizophrenia: A Meta-Analysis. Journal of Abnormal Psychology. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Leonard CJ, Kaiser ST, Robinson BM, Kappenman ES, Hahn B, Gold JM, et al. Toward the Neural Mechanisms of Reduced Working Memory Capacity in Schizophrenia. Cerebral Cortex. Cerebral Cortex, June1. 2012 doi: 10.1093/cercor/bhs148. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Lin PH, Luck SJ. Proactive interference does not meaningfully distort visual working memory capacity estimates in the canonical change detection task. Frontiers in Psychology. 2012;3:1–9. doi: 10.3389/fpsyg.2012.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, May CP, Hasher L. Working memory span and the role of proactive interference. Journal of Experimental Psychology. General. 2009;130:199–207. doi: 10.1037//0096-3445.130.2.199. [DOI] [PubMed] [Google Scholar]
- McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nature Neuroscience. 2008;11:103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- Meng XL, Rosenthal R, Rubin DB. Comparing correlated correlation coefficients. Psychological Bulletin. 1992;111:172–175. [Google Scholar]
- Meyer DE, Kieras DE. A computational theory of executive cognitive processes and multiple-task performance: Part 1. Basic mechanisms. Psychological Review. 1997;104:3–65. doi: 10.1037/0033-295x.104.1.3. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Rettinger D, Priti S, Hegarty M. How are visuospatial working memory, executive functioning and spatial abilities related? A latent variable analysis. Journal of Experimental Psychology: General. 2001;130:621–640. doi: 10.1037//0096-3445.130.4.621. [DOI] [PubMed] [Google Scholar]
- Myerson J, Emery L, White DA, Hale S. Effects of Age, Domain, and Processing Demands on Memory Span: Evidence for Differential Decline. Aging, Neuropsychology, and Cognition. 2003;10:20–27. [Google Scholar]
- Nuechterlein KH, Green MF. MATRICS Consensus Cognitive Battery, Manual. Los Angeles, CA: MATRICS Assessment Inc; 2006. [Google Scholar]
- Overall J, Gorham D. The brief psychiatric rating scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Pashler H. Familiarity and visual change detection. Perception and Psychophysics. 1988;44:369–378. doi: 10.3758/bf03210419. [DOI] [PubMed] [Google Scholar]
- Perry W, Heaton RK, Potterat E, Roebuck T, Minassian A, Braff DL. Working memory in schizophrenia:transient “online” storage versus executive functioning. Schizophrenia Bulletin. 2001;27:157–176. doi: 10.1093/oxfordjournals.schbul.a006854. [DOI] [PubMed] [Google Scholar]
- Piskulic D, Olver JS, Norman TR, Maruff P. Behavioral studies of spatial working memory dysfunction in schizophrenia: a quantitative review. Psychiatry Research. 2007;150:111–121. doi: 10.1016/j.psychres.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Rouder JN, Morey RD, Morey CC, Cowan N. How to measure working memory capacity in the change detection paradigm. Psychonomic Bulletin & Review. 2011;18:324–330. doi: 10.3758/s13423-011-0055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saults JS, Cowan N. A central capacity limit to the simultaneous storage of visual and auditory arrays in working memory. Journal of Experimental Psychology: General. 2007;136:663–684. doi: 10.1037/0096-3445.136.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver H, Feldman P, Bilker W, & Gur RC. Working memory dysfunction as a core neuropsychological dysfunction in schizophrenia. American Journal of Psychiatry. 2003;160:1809–1816. doi: 10.1176/appi.ajp.160.10.1809. [DOI] [PubMed] [Google Scholar]
- Stone M, Gabrieli JDE, Stebbins GT, Sullivan EV. Working and strategic memory deficits in schizophrenia. Neuropsychology. 1998;12:278–288. doi: 10.1037//0894-4105.12.2.278. [DOI] [PubMed] [Google Scholar]
- Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428:751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Engle R. On the Division of Short-Term and Working Memory: An Examination of Simple and Complex Span and Their Relation to Higher Order Abilities. Psychological Bulletin. 2007;133:1038–1066. doi: 10.1037/0033-2909.133.6.1038. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Redick TS, Heitz RP, Broadway JM, Engle RW. Complex working memory span tasks and higher-order cognition: A latent-variable analysis of the relationship between processing and storage. Memory. 2009;17:635–654. doi: 10.1080/09658210902998047. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428:784–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- Vogel EK, McCollough AW, Machizawa MG. Neural measure reveal individual differences in controlling accesss to working memory. Nature. 2005;438:500–503. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Woodman GF, Luck SJ. The time course of consolidation in visual working memory. Journal of Experimental Psychology: Human Perception and Performance. 2006;32:1436–1451. doi: 10.1037/0096-1523.32.6.1436. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Awh E. How to exploit diversity for scientific gain: Using individual differences to constrain cognitive theory. Current Directions in Psychological Science. 2008;17:171–176. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. (WASI): Psychological Corporation; 1999. [Google Scholar]
- Zhang W, Luck SJ. Discrete fixed-resolution representations in visual working memory. Nature. 2004;453(7192):233–235. doi: 10.1038/nature06860. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.