Abstract
Amphypterygium adstringens is a Mexican tree known as cuachalalate whose bark is habitually used for the treatment of fresh wounds, gastric ulcers, gastrointestinal cancer and various inflammatory conditions. The aim of this study was to evaluate the immunostimulant effect of the aqueous extract of A. adstringens on immune cellular response in immunosuppressed mice. An aqueous extract from the bark of cuachalalate was administered into BALB/c mice for 10 days. We assessed their immunostimmulant activity on cellular immune response by Delayed Type Hypersensitivity Response (DHT) to dinitrofluorobencene (DNFB) and by MTT assay. L5178Y lymphoma was used as immunossuppression model. An increase in DHT was observed after treatment with 10 and 100 mg/kg of the aqueous extract from A. adstringens oral treatment in lymphoma bearing mice. Splenocyte proliferation rate was significantly increased (2.5 time) in immunosuppresed mice treated with 10 mg/kg oral treatment compared with group that received vehicle only. The present study showed for the first time the aqueous extract from A. adstringens as a positive immunostimulant agent in lymphoma bearing mice and we demonstrated evidence to support the traditionally use of cuachalalate in conditions in which the immune system is depressed.
Keywords: A. Adstringens, Cuachalalate, immunostimulant, lymphoma, medicinal plants, Cancer
Introduction
Amphypterygium adstringens Schiede ex Schlecht (synonym Juliania adstringens) is a Mexican tree that belongs to Julianaceae family, whose bark is traditionally used for the treatment of cholelithiasis, fresh wounds, hypercholesterolemia, gastritis, as a cicatrizant agent, anti-inflammatory, to treat gastric ulcers and tract digestive cancer (Olivera-Ortega et al., 1999; Argueta,1994; Estrada, 1992). Active principles and extracts from bark have been shown to posses anti-ulcer (Navarrete et al., 1998, 2005), anti-inflammatory (Navarrete et al., 2005; Oviedo-Chávez et al., 2004) and antitumor properties. A methanol extract showed an anti-tumor effect in a murine mammary tumor model (González et al., 1962). Further, Makino et al. (2004) showed growth inhibitory activity against leukemia cells (L-1210) of five tirucallane-type triterpenes were isolated along with nine known triterpenes from the bark of J. adstringens. For these reason, the aim of this study was to evaluate the immunostimulant effect from aqueous extract of A. adstringens on immune cellular response in murine immunosuppression model.
Material and Methods
Plant materials and extraction
Bark of Amphypterygium adstringens Schiede ex Schlecht were collected in Cabo Corrientes, Jalisco (Mexico). A voucher specimen has been deposited at the Herbarium of Botany Department from University of Guadalajara under the number IBUG-169301. Bark from A. adstringens (80 g) was dried and powdered. The aqueous extract was prepared by the addition of 2 L of twice distilled water at boiling point for 5 minutes. The crude extract was then concentrated in a rotary evaporator (MOD. RE47, YAMATO Scientific CO., LTD. Tokyo, Japan) and lyophilized (Freeze Dry System/Freezone 4.5. LABCONCO Corporation. Kansas City Missouri 64132). The lyophilized was solubilised in distilled water before being fed to the mice.
Animals
Male BALB/c mice aged 6–8 weeks were maintained and bred under conventional laboratory conditions at the University of Guadalajara, Guadalajara, Mexico, according to the guidelines for the use and care of laboratory animals and World Medical Association Declaration of Helsinki (amended by the 52nd WMA General Assembly, Edinburgh, Scotland, October 2000). Animal protocols were approved by the Biomedicine Sciences Committee.
Lymphoma cell line
The murine lymphoma cell line L5178Y was derived from murine thymic lymphoma of DBA/2 mice (H-2d/d), and was maintained in ascitic form by weekly intraperitoneal (i.p.) passages of 1×106 cells in syngenic BALB/c mice (H-2d/d) (Puebla-Pérez et al., 1998).
Lethal-dose 50 (LD50) and Acute Toxicity
Oral single doses of aqueous extract from A. adstringens at 1200, 1600 and 2000 mg/kg body weight were administered in mice from three independent groups (n=10 each). Clinical signs of acute toxicity (postration, grooming, piloerection and motor activity) and death were evaluated parameters by 72 hours (Loomis, 1992)
In vivo cellular immune response
Groups of 10 male BALB/c mice were used in a murine immunosuppression model (Orozco-Barocio, 1998), in which the animals were inoculated intraperitoneally (i.p) with 0.1 ml of suspension of fresh ascitic fluid containing 1 × 107 lymphoma murine L5178Y cells (day 0). Treatments with A. adstringens and vehicle (distilled water) were started the same day of lymphoma inoculation and, were administered orally during 10 days. Control groups (healthy and lymphoma bearing mice) received 0.1 ml distilled water and experimental groups (healthy and lymphoma bearing mice) were treated with 10 and 100 mg/kg body weight of aqueous extract of A. adstringens. Five days after lymphoma evolution, Delayed Type Hypersensitivity Response (DTH) to dinitrofluorobenzene (DNFB) (1-Fluoro-2,4-dinitrobenzene. Sigma-Aldrich Co. LLC. St. Louis, Missouri) in the four groups were tested. The tumor-bearing or normal mice were sensitized to DNFB by placing one drop of 0.5% DNFB in acetone-olive oil on their shaved abdomens on days 7 and 8. On day 11, one drop of 0.2% DNFB solution was placed on the right ear. Ear thickness was monitored using a precision micrometer (General 102, USA), before challenge and 48 h after challenge. (Streilein et al.1980). The results were expressed as the Percentage Increment (PI) and it was calculated using the following formula:
PI = (Final thickness — basal thickness / Basal thickness) × 100.
Cell Proliferation Assay
Cell cultures were used to evaluate cellular immune response in healthy and lymphoma bearing mice (immunosuppressed), treated or not with 10 mg/kg body weight with aqueous extract of A. adstringens (10 mice per group). Concanavalin A (Sigma Chemical, St. Louis, MO) was used as mitogenic stimuli. At eleven day of lymphoma evolution, the spleens were removed aseptically, splenocytes were isolated and cultured and measured by MTT assay (Sigma-Aldrich, Inc; St. Louis, MO.) described by Hansen et al. (1989). Cell proliferation was measured using 3,(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT). The method involves conversion of MTT to coloured formazan by the living cells. Then, 40 µl of MTT (5 mg/ml) was added to each well. After 2 h incubation at 37° C, 160 µl of extraction buffer pH 4.7 containing 20% w/v of SDS (Bio-Rad, Richmond, CA) in a solution of 50% N,N-dimethyl formamide (Sigma Chemical, St Louis, MO) in demineralized water were added. After overnight incubation at 37° C, optical densities at 570 nm were measured with an ELISA plate reader using the extraction buffer as blank. Cellular proliferation was determinated by Stimulation Index (SI) according to the following formula: SI = (Optical Density with mitogen/ Optical Density without mitogen).
Statistics
The results are expressed as means ± standard deviation. Statistical significance was calculated using a one-way analysis of variance (ANOVA). Significant differences between means were determined by Tukey test with respect to control group. Values of p < 0.05 were considered significant.
Results and Discussion
The aqueous extract of A. adstringens showed no lethal effects at high doses (1200–2000 mg/Kg body weight). Animals were observed until 72 hours after administration. However, these animals did not die or show signs of acute toxicity such as prostration, grooming, piloerection and altered motor activity. Starting from this evaluation, we decided to use a range of doses (10 and 100 mg/kg body weight) that are 20–200 times lower than doses administrated in the LD50 test (Kent, 1998).
Stem bark from A. adstringens has shown anti-inflammatory (Oviedo-Chávez, 2004) and anti-tumor activities (Gonzalez et al., 1962; Makino et al., 2004). However, the aqueous extract from this plant (folk use) has not been tested before in an in vivo immunosuppression model. We showed that the aqueous extract from A. adstringens had no lethal effects at high doses in mice. These findings are consistent with its anti-cytotoxic effect from anacardic acid isolated from bark of A. adstringens evaluated in murine lymphocytes and which did not produce chromosome damage (Acevedo et al., 2006). Furthermore, extracts of the powdered bark, did not exert toxic effects (LD50 < 5 g), (Déciga-Campos et al., 2007; Navarrete et al., 2006).
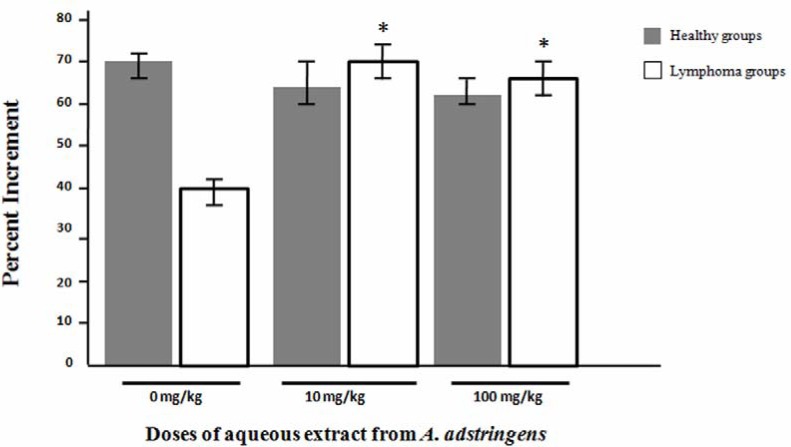
In order to evaluate in vivo effect from aqueous extract from A. adstringens in cellular immune response, we used DTH test (Figure 1). Healthy and lymphoma groups that received vehicles showed a Percentage Increment (PI) of 69.79 ± 3.36 and 40.95 ± 1.85 respectively. The treatment of aqueous the extract from A. adstringens in lymphoma bearing mice at doses of 10 and 100 mg/kg body weight , significantly increased the magnitude of ear swelling on DHT to DNFB test respect to lymphoma group without treatment (69.35 ± 3.56 and 66.88 ± 3.71 vs 40.95 ± 1.85 respectively). Furthermore, there was no statistical significant difference between healthy group and immunosuppressed mice treated with A. adstringens at doses of 10 and 100 mg/kg body weight.
Figure 1.
Effect of aqueous extract from A. adstringens on DHT to DNFB.
The figure shows the effect of aqueous extract from A. adstringens on the cellular immune response in vivo of healthy and immunosuppressed mice with L5178Y lymphoma cells, by measuring the Delayed Type Hypersensitivity Response (DTH) to DNFB. The data are mean ± standard deviation of the percentages of increment of the ear thickness. * p < 0.05 respect to lymphoma group that received vehicle only (0 mg/kg).
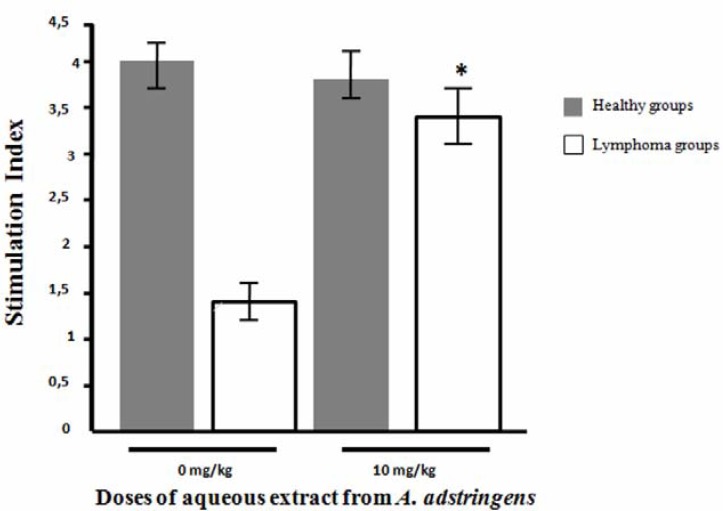
We also evaluated lymphoproliferative response in healthy mice and lymphoma bearing mice by MTT assay (Figure 2). We used the dose of 10 mg/kg due to increase in vivo cellular immune response in immunosuppressed mice with DTH test. According to the in vivo results, there was a statistical significant increase in cellular immune response in immunossuppresed mice that received 10 mg/kg of A. adstringens compared with immunosupressed mice without treatment (Stimulation Index [SI]: 3.35 ± 0.40 vs 1.36 ± 0.12 respectively, p<0.05). However, the healthy mice groups with and without treatment of aqueous extract of A. adstringens did not have any difference in the splenocyte proliferation (SI: 3.89 ± 0.22 vs 4.01 ± 0.26 respectively).
Figure 2.
Effect of treatment of aqueous extract from A. adstringen on splenocytes proliferation rate. The figure shows the effect of aqueous extract from A. adstringens on the splenocytes proliferation of healthy and immunosuppressed mice with L5178Y lymphoma cells. The data are mean ± standard deviation of the Stimulation Index. * p < 0.05 respect to lymphoma group that received vehicle only (0 mg/kg).
In this work, we found that A. adstringens stimulated in vivo some aspects of immune response, such as delayed type hypersensitivity response and cell proliferation assay in BALB/c mice bearing L5178Y, a model characterized by defective immune response. However, in healthy mice neither delayed type hypersensitivity response to DNFB nor cell proliferation assay were increased by cuachalalate. This indicates that the A. adstringens could restore the cellular immunity of immunosuppressed mice to levels of healthy mice.
To conciliate our findings, we need to consider that delayed type hypersensitivity response is a complex process where many cells are involved; namely, T-lymphocytes, dendritic cells, keratinocytes, endothelial cells, macrophages, and polymorphonuclear cells (Akiba, et al 2002). For these reasons, A. adstringens may modulate delayed type hypersensitivity response at sensitization phase (dendritic cells, macrophages, keratinocytes, and Tcells) or efferent phase (T-cells, mastocytes, macrophages, and polymorphonuclear cells). In any case, in the present work we found that deficient T-cell proliferative response was restored by the addition of A. adstringens.
This work demonstrates for the first time that A. adstringens improves immunological parameters in a tumor-bearing host. The next step would be to answer questions related with A. adstringens mechanisms, such as cellular pathways implicated in macrophage activation, cell subpopulations as CD4+, CD8+ and CD4+/CD25+ cells and the study of different components of innate and specific immunity.
Acknoledgement
This study was supported by the University of Guadalajara.
References
- 1.Acevedo HR, Rojas MD, Arceo SD, Soto-Hernández M, Martínez-Vázquez M, Terrazas T, del Toro GV. Effect of 6-nonadecyl salicilic acid and its metil ester on the induction of micronuclei in polychromatic erythrocytes in Mouse peripheral blood. Mutation Research. 2006;609(1):43–46. doi: 10.1016/j.mrgentox.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Akiba H, Kehren J, Ducluzeau MT, Krasteva M, Horand F, Kaiserlian D. Skin inflammation during contact hypersensitivity is mediated by early recruitment of CD8+T cytotoxic 1 cells inducing keratinocyte apoptosis. J Immunol. 2002;168(6):3079–3087. doi: 10.4049/jimmunol.168.6.3079. [DOI] [PubMed] [Google Scholar]
- 3.Argueta A. Atlas de las plantas de Medicina Tradicional Mexicana. first ed. III. México: Instituto Nacional Indigenista; 1994. [Google Scholar]
- 4.Déciga-Campos M, Rivero-Cruz I, Arriaga-Alba M, Castañeda-Corral G, Angeles-López G E, Navarrete A, Mata R. Acute toxicity and mutagenic activity of Mexican plants used in traditional medicine. Journal of Ethnopharmacol. 2007;110(2):334–342. doi: 10.1016/j.jep.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Estrada E. Plantas Medicinales de México Introducción a su estudio. Unidad de Estudios Etnobotánicos, Universidad Autónoma de Chapingo; 1992. [Google Scholar]
- 6.González E E, Mckenna G P, Delgado J N. Anticancer activity of Amphypteryghum adstringens. Journal of Pharmaceutical Sciences. 1962;51:901–905. [Google Scholar]
- 7.Hansen MB, Nielsel SE, Berg K. Re-examination and further develop of precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989;119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- 8.Kent C. Basics of Toxicology. Willey & Sons; 1998. [Google Scholar]
- 9.Loomis TD. Fundamentos de Toxicología. España: Acribia; 1982. [Google Scholar]
- 10.Makino M, Motegi T, Fujimoto Y. Tyrucallane-type triterpenes from Juliania adstringens. Phytochemistry. 2004;65:891–896. doi: 10.1016/j.phytochem.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Navarrete A, Martínez-Uribe L S, Reyes B. Gastroprotective activity of the stem bark of Amphypterygium adstringens in rats. Phytother Res. 1998;12(1):1–4. [Google Scholar]
- 12.Navarrete A, Oliva I, Sánchez-Mendoza M, Arrieta J, Cruz-Antonio L, Castañeda-Hernández G. Gastroprotection and effect of the simultaneous administration of cuachalalate (Amphypterygium adstringens) on the pharmacokinetics and anti-inflamatory activity of diclofenac in rats. Journal of Pharmacy and Pharmacology. 2005;57(12):1629–1636. doi: 10.1211/jpp.57.12.0013. [DOI] [PubMed] [Google Scholar]
- 13.Navarrete A, Arrieta J, Hersh P, Déciga M, Rivero I, Mata R. Farmacología preclínica, seguridad, autenticidad y metodología analítica de la corteza de cuachalalate (Amphypterygium adstringens) Fitoterapia. 2006;6(S1):85. [Google Scholar]
- 14.Olivera-Ortega AG, Soto-Hernández M, Martínez-Vázquez M, Terrazas-Salgado T, Solares-Arenas F. Phytochemical study of cuachalate. J Ethnopharmacol. 1999;68(1–3):109–113. doi: 10.1016/s0378-8741(99)00047-1. [DOI] [PubMed] [Google Scholar]
- 15.Orozco-Barocio A. Modulación de la respuesta inmune en ratones BALB/c con linfoma murino L5178Y tratados con acíbar de Aloe vera. 1998. Doctoral Thesis from Universidad de Guadalajara. [Google Scholar]
- 16.Oviedo-Chávez I, Ramírez-Apan T, Soto-Hernández M, Martínez-Vázquez M. Principles of the bark of Amphypterygium adstringens (Julianaceae) with anti-inflammatory activity. Phytomedicine. 2004;11(5):436–445. doi: 10.1016/j.phymed.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Puebla-Pérez AM, Huacuja-Ruiz L, Rodríguez-Orozco G, Villaseñor-García MM, Miranda-Beltran ML, Celis A, Sandoval-Rairez L. Cytotoxic and antitumor activity from Bursera fagaroides ethanol extract in mice with L5178Y lymphoma. Phytother Res. 1998;12:545–548. [Google Scholar]
- 18.Streilein JW, Toews GT, Gilliam JN, Bergstresser PR. Tolerance or hypersensitivity to 2,4dinitro-1-fluorobenzene: the role of Langerhans cell density within epidermis. J Invest Dermatol. 1980;74(5):319–322. doi: 10.1111/1523-1747.ep12543557. [DOI] [PubMed] [Google Scholar]