Abstract
Rheumatoid arthritis (RA) is a chronic inflammatory disease of the synovium. It is involved in up-regulation of pro-inflammatory cytokines and matrix metalloproteinases (MMPs), resulting in joint inflammation and erosion. Zingiber cassumunar Roxb. has long been used to reduce joint pain and inflammation. This study aimed to investigate the inhibitory activities of an active compound of Z. cassumunar, (E)-4-(3′,4′-dimethoxyphenyl)but-3-en-1-ol (compound D), against cytokine-induced up-regulation of catabolic genes involved in cartilage degradation in RA. Synovial fibroblast cell line, SW982, was cultured in media containing interleukin-1β (IL-1β), in the presence or absence of compound D at the concentration range of 1 to 100 µM. After 24 hours, the cells were analyzed for the expressions of MMPs, IL-1β and interleukin-1β-converting enzyme (ICE) by RT-PCR. MMPs activities in the culture media were analyzed by zymographic techniques. Dexamethasone was used as the positive control. It was found that compound D at the concentration of 10 – 100 µM significantly decreased the mRNA expressions of MMP-1, -2, -3, and -13 which was induced by IL-1β (P<0.05) concomitantly with a decrease in activities of these MMPs in the culture media. An increase in the mRNA expression of IL-1β and ICE was also suppressed by compound D. The results suggest that the potent activities of this compound may be involved in the reduction of IL-1β protein synthesis in both pro-form and active form which played an important role in up-regulation of MMPs. This study first revealed the chondroprotective activity of Z. cassumunar in the transcriptional level by suppressing cytokine-induced catabolic genes which caused cartilage erosion in RA.
Keywords: (E)-4-(3′,4′-dimethoxyphenyl)but-3-en-1-ol; compound D; matrix metalloproteinases; interleukin-1β; interleukin-1β-converting enzyme; Zingiber cassumunar
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease which causes chronic inflammation of the joints and the surrounding tissues, leading to joint pain and deformity (Scher and Abramson, 2011). The inflammatory process of the synovial membrane causes the progressive destruction of cartilage and bone (Feldmann et al., 1996). The mechanism of action is involved in an elevation of the pro-inflammatory cytokines, especially interleukin-1beta (IL-1β) and thereby results in the up-regulation of matrix metalloproteinases (MMPs) which are responsible for joint erosion (Mohammed et al., 2003).
The current treatment of RA includes three categories; non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids such as dexamethasone, and disease-modifying anti-rheumatic drugs (DMARDs) (Peng-Thim and Keng-Hong, 2007). However, long-term use of these pharmacological agents causes deleterious side effects (Weisman, 2005). Thus, alternatively some medicinal plants have been used for pain relief, which is a regular symptom of this disease.
Zingiber cassumunar Roxb. is one of the most widely used medicinal plants of Asian folk remedies for joint and muscular pain. (E)-4-(3′,4′-dimethoxyphenyl)but-3-en-1-ol, compound D, is an active ingredient of the essential oil isolated from the hexane extract of Z. cassumunar (Amatayakul, 1979). There have been reports that compound D exhibited a strong anti-inflammatory response in vivo (Masuda and Jitoe, 1994) and protected cartilage degradation in the cartilage explant model (Chaiwongsa, 2012). We therefore attempted to verify the chondroprotective activities of compound D at the molecular level against the IL-1β-induced expression of catabolic genes which caused joint destruction. Results of this study may provide alternative medical treatment for RA.
Materials and Methods
Compound D was provided by Professor Vichai Reutrakul, Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Mahidol University, Bangkok 10400, Thailand. It was isolated from a hexane fraction of Z. cassumunar as previously reported (Tuntiwachwuttikul et al., 1981).
Human synovial fibroblast cell line culture and treatments
SW982, a human synovial sarcoma cell line, was obtained from ATCC® Number HTB-93. The cells were cultured in a 25 cm2 flask at a density of 1X106 cells/5 ml in Leibovitz's L-15 medium (L-15 medium) supplemented with ten percent fetal calf serum, penicillin G sodium (100 U/ml), and streptomycin (100 µg/ml). The culture medium was changed every day. In the study of gene expressions, SW982 cells were cultured in L-15 medium as high density upto 80% confluence. These cells were maintained in culture at 37°C, 95% air and without 5% CO2. The cells were co-treated with 1.0 ng/ml IL-1β in the absence or presence of compound D (1–100 µM), this active compound was dissolved in 0.1% dimethyl sulfoxide. Dexamethasone, a commercially available steroidal anti-inflammatory drug, was performed in parallel as a positive control. Following 24 hours of incubation, the culture medium was collected to analyze the MMP-2 activity by gelatin zymography and groups of MMP-1, -3, -13 activities by casein zymography. The total RNA was extracted to analyze mRNA expressions of MMP-1, -2, -3, and -13 including IL-1β and ICE by reverse-transcriptase polymerase chain reaction (RT-PCR).
Cell viability by MTT assay
The cytotoxic effect of compound D on cell viability was performed by the well established MTT assay (Mosmann, 1983). This colorimetric method allows assessing the viability of the treated cells by measuring the activity of mitochondrial succinate dehydrogenase activity of the viable cells that reduce MTT dye to a blue formazan crystal. SW982 cells were treated with different concentrations of compound D (0–800 µM) or dexamethasone (0–1200 nM). After 24 hours of incubation, the cell viability was determined with the MTT assay method. The results were presented as a percentage relative to the control.
Zymographic assays for enzyme activities
The assay for MMP-2 activity in the culture media was performed by gelatin zymography as described by Ongchai et al. (Ong-chai et al., 2008). The mixed activities of MMP-1, -3 and -13 were analyzed by casein zymographic technique modified from Boonsing et al. (Boonsing et al., 2010). Briefly, samples of the culture media (15 µl) were loaded into 10% polyacrylamide gel containing 0.2% (w/v) bovine casein (Sigma-Aldrich). The gel electrophoresis was performed for 200 minutes at 90 volt. Then, the gels were incubated at 37°C for 16 hours in activating buffer. Subsequently, they were stained with 0.2% Coomassie® brilliant blue G250 for five hours at room temperature, destained with 50% methanol and 10% acetic acid to reveal zone of the lysis within the casein matrix. The caseinolytic activity was detected as the clear bands against a blue background corresponding to the hydrolysis of milk casein.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
The harvested cells were analyzed for gene expression by semiquantitative PCR methods as previously described by Boonsing et al. (Boonsing et al., 2010). Briefly, the cells were extracted for total RNA by RNAspin Mini (GE Healthcare UK Ltd, Buckinghamshire, UK). Reverse transcription reaction was performed by using 1.0 µg of total RNA and the RNA was reverse-transcribed into cDNA using RevertAid™ First Strand cDNA synthesis kit (Fermentus Inc, Ontario, Canada). The cDNA was amplified for 35 cycles by using two oligonucleotide primers derived from previously published sequences of MMP -1, -2, -3, -13, IL-1β, ICE, and GAPDH genes (Pothacharoen et al., 2006). The PCR products were subjected to 1.5% agarose gel electrophoresis. Quantitative data normalized to GAPDH were obtained using Scion Image software for PC (Scion Corporation, Maryland, USA), working in the Gel Plot 2 mode.
Statistical analysis
All statistical analyses were performed using Microsoft Excel 2000 or the SPSS 11.5 for windows software package. Data were expressed as mean±S.D. of triplicate independent experiments. Statistically significant values were compared by using of one-way analysis of variance (ANOVA) at the significant level of P<0.05.
Results
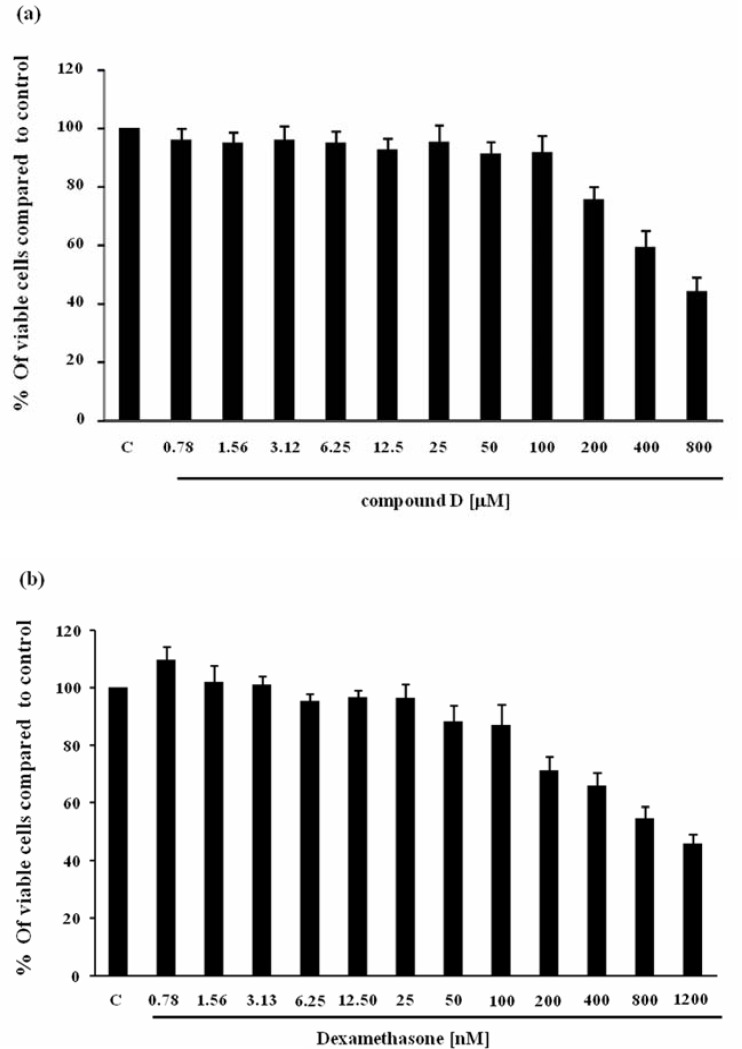
Cytotoxicity effect of compound D and dexamethasone on SW982 cell line were investigated by MTT cell viability assay.Figure 1 illustrates the effects of compound D and dexamethasone on SW982 cell viability. Nevertheless, these effects of compound D in the concentration ranging from 1 to 100 µM and dexamethasone from 1 to 100 nM, used throughout the experiments, were not significantly different from the untreated control. Higher concentration of both test compounds suppressed cell viability with the IC50 of 640 µM for Compound D and IC50 of 980 nM for dexamethasone, respectively.
Figure 1.
Effect of compound D (a) and dexamethasone (b) on cell viability. SW982 cells were incubated with various concentrations of compound D or dexamethasone for 24 hours, and then analyzed by MTT assay. The data were presented as percentage relative to the control.
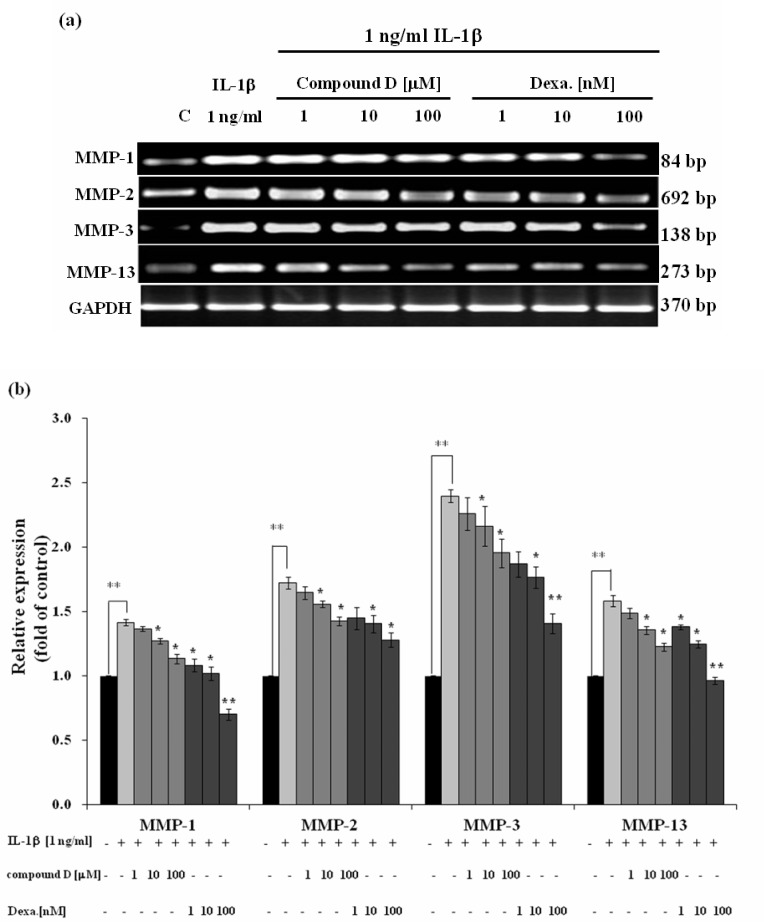
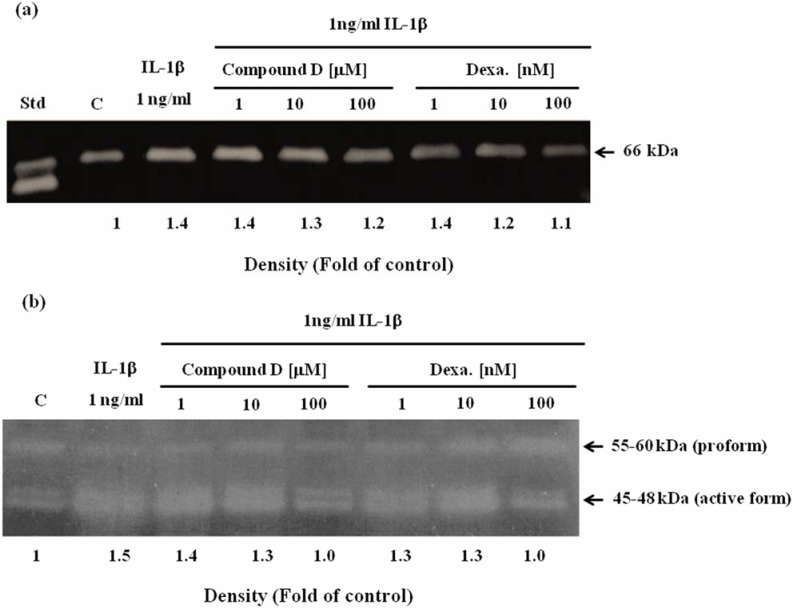
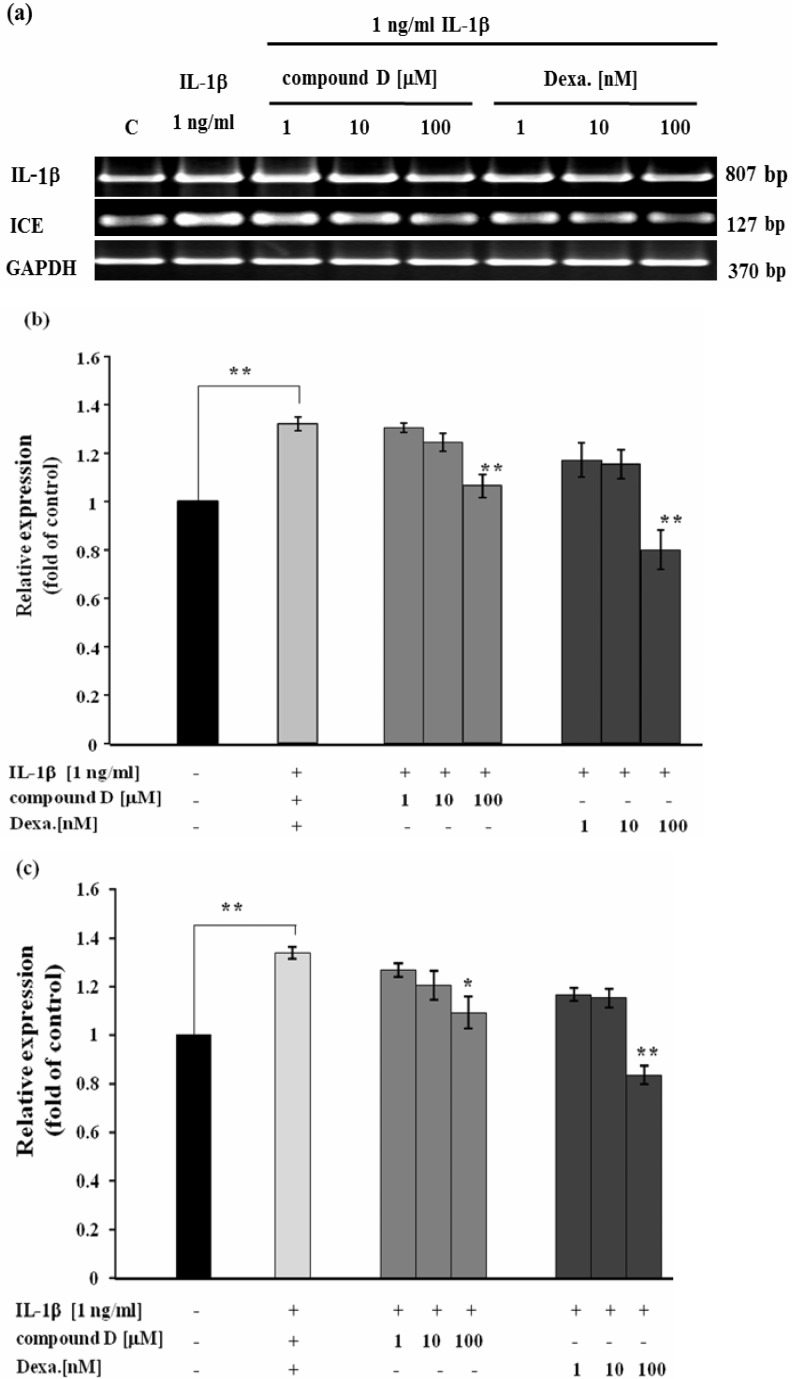
Effect of compound D on RA-involved gene expression was investigated in SW982 cell line. In conditioned medium containing only human recombinant IL-1β caused the dramatic increase in mRNA levels of MMP-1, -2, -3, -13 (Figure 2). This was consistent to the increase in activities of MMP-2 (Figure 3a) and the mixed activities of MMP-1, 3, 13 (Figure 3b) measured from the cultured media. In the co-treatment with compound D, the expressions of those genes were gradually decreased in a dose response manner, consistently with the reduction of activities of those MMPs in the cultured medium (Figure 2 and Figure 3). Figure 4 clearly demonstrates significant induction of mRNA expression of IL-1β and ICE in SW982 cell line in the present of external IL-1β. This mRNA expression was suppressed when the cells were co-treated with compound D. These effects were found to be comparable to those of the positive control, dexamethasone. It was found that compound D at the concentration of 100 µM was able to significantly down-regulate all those of studied genes induced by the external IL-1β.
Figure 2.
Effect of compound D on IL-1-induced expression of MMP genes analyzed by semiquantitative RT-PCR; (a) representative images of agarose gel electrophoresis, (b) bar graphs indicating relative expression as fold change compared to the control (C). SW982 cells were treated with 1 ng/ml IL-1β in with or without the various concentrations of compound D or dexamethasone (Dexa). After 24 hours, mRNA levels of MMP genes were analyzed by semiquantitative RT-PCR. Bar graphs are expressed as mean ± SD of three independent experiments.
Figure 3.
Effect of compound D on reduction of MMPs activities in culture media analyzed by gelatin zymography (a) and casein zymography (b). SW982 cells were treated with 1 ng/ml IL-1β in with or without the various concentrations of compound D or dexamethasone (Dexa). After 24 hours, cultured media were analyzed for the mixed activities of MMP-2 activity by gelatin zymography and MMP-1, -3, -13 by casein zymography, respectively. The activities were quantified by band densitometry as showed in the representative MMPs zymograms. All values are fold change compared to the control (C). The fold change of the mixed MMP-1, -3 and -13 activities are calculated from the total activities of pro- and active forms. Std = standard MMP-2
Figure 4.
Effect of compound D on IL-1β -induced expression of IL-1β and ICE genes analyzed by semiquantitative RTPCR; (a) representative images of agarose gel electrophoresis, bar graphs indicating relative expression of IL-1β (b) and ICE (c) as fold change compared to the control (C). SW982 cells were treated with 1 ng/ml IL-1β in with or without the various concentrations of compound D or dexamethasone (Dexa). After 24 hours, mRNA levels of IL-1β and ICE genes were analyzed by semiquantitative RT-PCR. The bar graph is presented as mean + SD of three independent experiments. The significant differences were evaluated by one-way ANOVA. Asterisks denote values significantly different from IL-1β -treated group; P<0.05 (*) and P< 0.01 (**), respectively.
Discussion
Rheumatoid arthritis (RA) is an autoimmune disease that causes inflammation and progressive destruction of joints. There is growing evidence that activated synovial fibroblasts play an exceptional role in the genesis and progression of RA (Aryeh et al., 2006). RA-synovial fibroblasts over proliferate in the synovium, consistently with up-regulation of pro-inflammatory cytokines and matrix-degrading enzymes such as MMP-1, -2, -3, and -13, which results in the erosion of joints (Muller-Ladner and Gay, 2002; Walsh, 1999). Numerous studies have indicated that the activated synovial fibroblasts play an integral role in the tissue damage observed in the rheumatoid arthritis patients (Huber et al., 2006; Pap et al., 2002).
Although RA-derived synovial fibroblasts are potential targets for therapeutic intervention, the attempted use of a primary cell culture of synovial fibroblasts as a tool for studying the cellular mechanisms of chronic inflammatory joint diseases is limited by its finite life span. The SW982 cell line, a human synovial fibroblast which is derived from the human synovial sarcoma cell line, was used throughout the present study. This cell line responds to IL-1β-induced expression of IL-1β and MMPs, including MMP-1, -2, and -13, but fails to express TNF-α and MMP-9 (Zhang et al., 1997). Our study was able to demonstrate the similar response in expression of IL-1β and MMPs as previously reported. In addition, MMP-3 and interleukin-1β-converting enzyme (ICE) were found to be up-regulated in response to IL-1β induction. It was found that expression of these genes was suppressed by dexamethasone similar to the previous report (Yamazaki et al., 2003). The expression of these genes performed in the SW982 cell line was comparable with those of human primary fibroblast-like synoviocytes (data not shown). These suggest that the SW982 cell line is great facilitating tool for studying the gene expressions which are involved in RA pathology.
Several factors are suspected to trigger inflammation in RA, consequently with persistence and vast production of the potent pro-inflammatory cytokines, TNF-α and IL-1β (Fionula et al., 2008). ICE, also called Caspase-1, has biological function to degrade the newly synthesized pro-IL-1β to yield an active cytokine (Boileau et al., 2002). It has been reported to be up-regulated in RA. Our study revealed that the exogenous IL-1β induced expression of IL-1β and ICE mRNA in the SW982 cell line. These indicated the amplifying effects of IL-1β on expressions of its gene including the ICE gene, resulted in an elevation of IL-1β protein synthesis and a release of the IL-1β active form. These evidences supported the self-amplifying fashion of IL-1β which has been suggested to play an important role in neurodegenerative diseases (Griffin, 2006). Therefore, the further study of these effects will be verified in the primary fibroblast-like synoviocytes, cell of the synovial membrane. This cell type has been claimed to play a key role in the pathological mechanisms of rheumatoid arthritis (Bartok, B. and Firestein, G.S., 2011). In the present study, compound D at concentration of 100 µM exerted the inhibitory effects on the expression of IL-1β-induced IL-1β and ICE genes. These may hypothesize the effects of this compound on other cytokines production which are able to be induced by IL-1β especially IL-8 (Porat et al., 1992; Ryll et al., 2011). This cytokine has been reported to play role in elevation of MMPs synthesis in degenerative joint diseases (Merz et al., 2003). It has been reported that the inhibition of IL-1β by IL1receptor antagonist protein clearly demonstrated the reduction of IL-8 synthesis (Porat et al., 1992). This will be interesting to explore the effects of compound D on IL-8 including the cytokine cascade which is triggered by IL-1β. In the present study, compound D was found to diminish the gene expressions of MMPs which are responsible for joint erosion. These were accompanied by the decrease in enzyme activities as analyzed in the cultured media. These results suggested that the efficacy of compound D may be involved in the molecular level of those catabolic gene regulations. Although the casein zymographic analysis could not distinguish the mixed activities of MMP-1, 3 and -13, it appeared that compound D was likely to have an effect on the low molecular weight forms of the mixed MMPs (45–48 kDa) rather than the pro-forms of these enzymes (55–60 kDa). The results obtained may suggest the influence of compound D upon the synthesis and activation of other MMPs which are able to produce a stepwise activation of MMP-1, 3 and -13. It has been reported that plasmin activates proMMP-1 and proMMP-3 (Saito et al., 1998), while MT1-MMP can activate proMMP-13 (Knäuper et al., 1996). In order to confirm the results of this study, there will be the research of interest for further investigation in the effect of compound D on synthesis and activation of these MMPs network.
Compound D is a major lipophilic compound of hexane extract of Z. cassumuna which has been claimed to contain potent anti-inflammatory activity (Jeenapongsa et al., 2003). Interestingly, the present study demonstrated the comparable efficacy of compound D and dexamethasone, the steroidal anti-inflammatory drug, against the IL-1β-induced catabolic genes of RA. Nevertheless, the inside mechanisms of these two compounds may not be similar. Dexamethasone has been reported to strongly suppress prostaglandin E2 production, (Newton et al., 1998) while compound D has no inhibitory effect on lipopolysaccharide-induced prostaglandin E2 synthesis (Han et al., 2005). This indicates that the anti-inflammatory effect of compound D is not involved in the cyclooxygenase pathway. In addition, compound D has been recently reported to protect cartilage explant degradation induced by IL-1β (Chaiwongsa et al., 2012). On the contrary, dexamethasone has been found to suppress chondrocyte proliferation and extracellular matrix formation, leading to cartilage degeneration (Chrysis et al., 2005). Diacerein, another anti-inflammatory agent, has been claimed to protect against cytokine-induced cartilage degradation. It is currently used as an anti-arthritic drug for the treatment of osteoarthritis. However, this drug has been proved to be effective on chondrocytes rather than synovial fibroblasts (Álvarez-Soria et al., 2008). NSAIDs, non-steroidal anti-inflammatory drugs, also inhibit the onset of inflammation and prostaglandins synthesis. However, few of them have chondrobeneficial effect (Kenneth, 2011). Besides, several types of NSAIDs exhibit the reverse effects on cartilage matrix components (Changhai, 2002). Taken together, the anti-inflammatory and chondroprotective activities of compound D provided the beneficial information for therapeutic purpose in osteoarthritis and synovitis. Further studies are required to elucidate the intracellular signaling cascades that involve in the inhibitory effect of compound D on the expression of those catabolic genes.
The significant differences were evaluated by one-way ANOVA. Asterisks denote values significantly different from IL-1β -treated group; P<0.05 (*) and P< 0.01 (**), respectively.
Conclusion
The present study clearly demonstrated the inhibitory activities of compound D, an anti-inflammatory compound of Z. cassumunar against the IL-1β-induced catabolic gene expression that involves in cartilage degeneration. The results indicated that compound D can serve as an upstream inhibitor of the catabolic cascade in chronic inflammatory joint erosion. These data provided the additional scientific-based information that Z. cassumunar may not only be effective on the reduction of joint pain and inflammation, but also delay joint destruction. Our study supports complementary and alternative medicines' utilization of Z. cassumunar for the treatment of chronic inflammatory joint diseases.
Acknowledgments
This study was supported by a grant from Center of Excellence for Innovation in Chemistry (PERCH-CIC) and Thailand Excellence Center for Tissue Engineering and Stem Cells, Faculty of Medicine, Chiang Mai University, Thailand. Commission on Higher Education and The Graduate School Chiang Mai University are gratefully acknowledged.
References
- 1.Álvarez-Soria MA, Herrero-Beaumont G, Sánchez-Pernaute O, Bellido M, Largo R. Diacerein has a weak effect on the catabolic pathway of human osteoarthritis synovial fibroblast comparison to its effects on osteoarthritic chondrocytes. Rheumatology. 2008;47:627–633. doi: 10.1093/rheumatology/ken116. [DOI] [PubMed] [Google Scholar]
- 2.Amatayakul T, Cannon JR, Dampawan P, Dechatiwong T, Giles RG, Huntrakul C, Kusamran K, Mokkhasamit M, Raston CL, Reutrakul V, White AH. Chemistry and crystal structures of some constituents of Zingiber cassumuar. Aust J Chem. 1979;32:71–88. [Google Scholar]
- 3.Aryeh MA, Michael HP. The role of the synovial fibroblast in rheumatoid arthritis. cartilage destruction and the regulation of matrix metalloproteinases. Bull NYU Hosp Jt Dis. 2006;64(1–2):20–24. [PubMed] [Google Scholar]
- 4.Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2011;233(1):233–255. doi: 10.1111/j.0105-2896.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boileau C, Martel-Pelletier J, Moldovan F, Jouzeau JY, Netter P, Manning PT, Pelletier JP. The in situ up-regulation of chondrocyte interleukin-1-converting enzyme and interleukin-18 levels in experimental osteoarthritis is mediated by nitric oxide. Arthritis Rheum. 2002;46(10):2637–2647. doi: 10.1002/art.10518. [DOI] [PubMed] [Google Scholar]
- 6.Boonsing P. Effect of Plai (Zingiber cassumunar Roxb) extract on hyaluronan and proteoglycan degradation in cartilage explant. Chiang Mai, Thailand: Master of Science (Biochemistry), Chiang Mai University; 2010. [Google Scholar]
- 7.Chaiwongsa R, Ongchai S, Tangyuenyong S, Kongtawelert P, Panthong A, Reutrakul R. Chondroprotective potential of bioactive compounds of Zingiber cassumunar Roxb. against cytokine-induced cartilage degradation in explant culture. J Med Plants Res. 2012 [Epub ahead of print] [Google Scholar]
- 8.Changhai D. Do NSAIDs affect the progression of osteoarthritis? Inflammation. 2002;26(3):139–142. doi: 10.1023/a:1015504632021. [DOI] [PubMed] [Google Scholar]
- 9.Chrysis D, Zaman F, Chagin AS, Takigawa M, Sävendahl L. Dexamethasone induces apoptosis in proliferative chondrocytes through activation of caspases and suppression of the Akt-Phosphatidylinositol 3-kinase signaling pathway. Endocrinology. 2005;146(3):1391–1397. doi: 10.1210/en.2004-1152. [DOI] [PubMed] [Google Scholar]
- 10.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 11.Fionula MB, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118(11):3537–3545. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin WST. Inflammation and neurodegenerative diseases. Am J Clin Nutr. 2006;83(2):470S–474S. doi: 10.1093/ajcn/83.2.470S. [DOI] [PubMed] [Google Scholar]
- 13.Han AR, Kim MS, Jeong YH, Lee SK, Seo EK. Cyclooxygenase-2 inhibitory phenylbutenoids from the rhizomes of Zingiber cassumunar. Chem Pharm Bull (Tokyo) 2005;53:1466–1468. doi: 10.1248/cpb.53.1466. [DOI] [PubMed] [Google Scholar]
- 14.Huber LC, Distler O, Tarner I, Gay RE, Gay S, Pap T. Synovial fibroblasts: key players in rheumatoid arthritis. Rheumatology. 2006;45:669–675. doi: 10.1093/rheumatology/kel065. [DOI] [PubMed] [Google Scholar]
- 15.Jeenapongsa R, Yoovathaworn K, Sriwatanakul KM, Pongprayoon U, Sriwatanakul K. Anti-inflammatory activity of (E)-1-(3,4-dimethoxyphenyl) butadiene from Zingiber cassumunar Roxb. J Ethnopharmacol. 2003;87:143–148. doi: 10.1016/s0378-8741(03)00098-9. [DOI] [PubMed] [Google Scholar]
- 16.Kenneth DB. Issues in pharmacological management of osteoarthritis. J Muscoskel Med. 2011;28(2):45–55. [Google Scholar]
- 17.Knäuper V, Will H, López-Otin C, Smith B, Atkinson SJ, Stanton H, Hembry RM, Murphy G. Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to generate active enzyme. J Biol Chem. 1996;19(29):17124–17131. doi: 10.1074/jbc.271.29.17124. 271. [DOI] [PubMed] [Google Scholar]
- 18.Masuda T, Jitoe A. Antioxidative and antiinflammatory compounds from tropical gingers: Isolation, structure determination, and activities of cassumunins A, B, and C, new complex curcuminoids from Zingiber cassumunar. J Agr Food Chem. 1994;42:1850–1856. [Google Scholar]
- 19.Merz D, Liu R, Johnson K, Terkeltaub R. IL-8/CXCL8 and Growth-Related Oncogene α/CXCL1 Induce Chondrocyte Hypertrophic Differentiation. J Immunol. 2003;171(8):4406–4415. doi: 10.4049/jimmunol.171.8.4406. [DOI] [PubMed] [Google Scholar]
- 20.Mohammed FF, Smookler DS, Khokha R. Metalloproteinases, inflammation, and rheumatoid arthritis. Ann Rheum Dis. 2003;62:43–47. doi: 10.1136/ard.62.suppl_2.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 22.Muller-Ladner U, Gay S. MMPs and rheumatoid synovial fibroblasts: Siamese twins in joint destruction? Ann Rheum Dis. 2002;61(11):957–959. doi: 10.1136/ard.61.11.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newton R, Seybold J, Kuitert LME, Bergmanni M, Barnes PJ. Repression of cyclooxygenase-2 and prostaglandin E2 release by dexamethasone occurs by transcriptional and post-transcriptional mechanisms involving loss of polyadenylated mRNA. J Biol Chem. 1998;273(48):32312–32321. doi: 10.1074/jbc.273.48.32312. [DOI] [PubMed] [Google Scholar]
- 24.Ong-Chai S, Chaiwongsa R, Viriyakhasem N, Pompimon W, Tangyuenyong S. Effect of active compounds from Andrographis paniculata (Nees) on protection of equine articular cartilage degradation In Vitro. KKU Vet J. 2008;18(2):81–96. [Google Scholar]
- 25.Pap T, Müller-Ladner U, Gay RE, Gay S. Fibroblast biology: Role of synovial fibroblasts in the pathogenesis of rheumatoid arthritis. Arthritis Res. 2002;2(5):361–367. doi: 10.1186/ar113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng-Thim F, Keng-Hong L. The use of biological agents in the treatment of rheumatoid arthritis. Ann Acad Med Singapore. 2007;36:128–134. [PubMed] [Google Scholar]
- 27.Porat R, Poutsiaka D D, Miller L C, Granowitz E V, Dinarello C A. Interleukin-1 (IL-1) receptor blockade reduces endotoxin and Borrelia burgdorferi-stimulated IL-8 synthesis in human mononuclear cells. FASEB J. 1992;6(7):2482–2486. doi: 10.1096/fasebj.6.7.1532945. [DOI] [PubMed] [Google Scholar]
- 28.Pothacharoen P, Choocheep K, Pitak T, Pompimon W, Premanode B, Hardingham TE, Kongtawelert P. Effect of Alpinia galanga extract on cartilage degradation and on gene expression in human chondrocyte and synovial fibroblast metabolism. Cent Eur J Biol. 2006;1(3):430–450. [Google Scholar]
- 29.Ryll A, Samaga R, Schaper F, Alexopoulos LG, Klamt S. Large-scale network models of IL1 and IL-6 signalling and their hepatocellular specification. Mol BioSyst. 2011;7:3253–3270. doi: 10.1039/c1mb05261f. [DOI] [PubMed] [Google Scholar]
- 30.Saito S, Katoh M, Masumoto Mari, Matsumoto S, Masuho Y. Involvement of MMP-1 and MMP-3 in collagen degradation induced by IL-1 in rabbit cartilage explant culture. Life Sci. 1998;62(22,24):PL359–PL365. doi: 10.1016/s0024-3205(98)00181-7. [DOI] [PubMed] [Google Scholar]
- 31.Scher JU, Abramson SB. The microbiome and rheumatoid arthritis. Nat Rev Rheumatol. 2011;7:569–578. doi: 10.1038/nrrheum.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuntiwachwuttikul P, Pancharoen O, Jaipetch T, Reutrakul V. Phenylbutanoids from Zingiber cassumunar. Phytochemistry. 1981;20:1164–1165. [Google Scholar]
- 33.Walsh DA. Angiogenesis and arthritis. Rheumatology. 1999;38:103–112. doi: 10.1093/rheumatology/38.2.103. [DOI] [PubMed] [Google Scholar]
- 34.Weisman MH. Progress toward the cure of rheumatoid arthritis? The Best study. Arthritis Rheum. 2005;52:3326–3332. doi: 10.1002/art.21503. [DOI] [PubMed] [Google Scholar]
- 35.Yamazaki T, Yokoyama T, Akatsu H, Tukiyama T, Tokiwa T. Phenotypic characterization of a human synovial sarcoma cell line, SW982, and its response to dexamethasone. In Vitro Cell Dev Biol Anim. 2003;39(8–9):337–339. doi: 10.1290/1543-706X(2003)039<0337:PCOAHS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 36.Zhang HG, Blackburn WD, Jr, Minghetti PP. Characterization of a SV40-transformed rheumatoid synovial fibroblast cell line which retains genotypic expression patterns: a model for evaluation of anti-arthritic agents. In Vitro Cell Dev Biol Anim. 1997;33(1):37–41. doi: 10.1007/s11626-997-0020-7. [DOI] [PubMed] [Google Scholar]