Abstract
This study was specifically designed to identify anticancer constituents in methanol-water extract of Polygonum bistorta L. and evaluate its cytotoxicity. For this purpose methanol-water (40:60 v/v) extract was subjected to conventional preparative high pressure liquid chromatography and 13 fractions were obtained. Constituents of obtained fractions were separated and identified with the help of GC-MS and LC-DAD-ESI-MS. Anticancer phenolic compounds such as gallic acid, protocatechuic acid, p-hydroxybenzoic acid, chlorogenic acid, vanillic acid, syringic acid, catechol, 4-methyl catechol, syringol and pyrogallol and fatty acids such as linoleic acid, myristic acid and palmitic acid were separated from different fractions. Fractions were evaluated for their cytotoxic activity on a rarely studied human hepatocellular carcinoma cell line (HCCLM3). 11 fractions showed good to strong cytotoxicity in a range of 200 µg/mL-800 µg/mL, whereas 2 fractions did not show any activity even at 800 µg/mL and no anticancer constituent was detected from them. 50 percent growth inhibition (GI50) values for five most active fractions were calculated and results were in a range of 86.5 (±3) µg/mL–126.8 (±3) µg/mL. 3 out of these 5 most active fractions were found to contain phenolic content in them whereas all other fractions containing phenolic content did possess cytotoxic activity that may suggest the importance of phenolic constituents in anticancer activity. Moreover, the results also showed a definite dose dependent relationship between amount of fractions and cytotoxic activity.
Keywords: cytotoxicity, Polygonum bistorta, phenolic compounds, fatty acids, anticancer activity
Introduction
Polygonum bistorta L. (PB) belongs to the family Polygonaceae and is a well known traditional Chinese medicine. It is used for various curative purposes in different parts of the world especially Asian (China, India, Pakistan, Singapore, Japan), African, European and American countries. On the other hand, its roots and leaves are cooked and eaten as raw food in different regions of Europe and America (Moerman et al., 1998; Couplan and Duke, 1998). In India and Pakistan, it has traditionally been used in making heat clearing drinks and syrups. “Chinese Pharmacopoeia” (2005), and other online literature have also cited this medicine as a single cure for various diseases such as dysentery with bloody stools, diarrhoea in acute gastroenteritis, hematemesis, epitasis, leucorrhoea, cholera, scrofula, oral ulcer haemorrhoidal bleeding and acute respiratory infection with cough and even snakebites. Previous literature on PB cites that it has anti-inflammatory (Duwiejua et al., 1994, 1999), antibacterial (Liu et al., 2006; Khalid et al., 2011), antiviral, anti-mutative (Miki et al., 1995), antioxidant (Chang et al., 2009) whereas Manoharan et al., (2007) evaluated it for cytotoxic activity by using solvent extraction technique and found strong cytotoxic activity for its chloroform and hexane fractions and few of their sub-fractions.
PB has been widely studied for its chemical profiling and found to possess different classes of constituents such as phenolics (Liu et al., 2004; Intisar et al., 2012), flavonoids (Smolarz, 2002), steroids (Sun et al., 2007), triterpenoids (Manoharan et al., 2005; Sun et al., 2007) and tannins (Liu et al., 2006). High pressure preparative liquid chromatography is a technique that has been used for decades for the separation, isolation and purification of bioactive plant constituents leading to their identification by the use of mass spectroscopic techniques (Shang et al., 2010). In our research work, 40 % methanol-water extract of PB was studied for the cytotoxic activity as it contains phenolic constituents (especially anticancer phenolic acids) that are well-known compounds that possess anticancer activity (Cai et al., 2004; Gomes et al., 2003) as well as anticancer fatty acids (Sagar and Das, 1995; Salerno and Smith, 1991). For this purpose, the extract was subjected to conventional preparative high pressure liquid chromatography to obtain different fractions followed by the identification of their phenolic and fatty acid content and evaluation of each fraction for cytotoxic activity against cancer line. The main aims of this study were the separation and identification of anticancer constituents and evaluation of methanol-water extract of this plant for cytotoxic activity.
Materials and Methods
Chemicals and materials.
Methanol, ethanol and PBS were purchased from Sinopharm (Shanghai, China). DMEM (Dulbecco's modified eagle medium), FCS (foetal calf serum), penicillin and streptomycin were purchased from Sigma-Aldrich (Shanghai, China). Water used in all experiments was purified by a Sartorius Arium 611 system (Goettingen, Germany). Healthy rhizomes of PB were purchased from the local market of Lahore, Pakistan in January, 2011. Samples were identified by the “School of Pharmacy, East China University of Science and Technology” as “Polygonum bistorta L.” All chemicals used in the experiments were of analytical grade.
Instruments
Preparative HPLC system, consisted of an Elite HPLC system with a P270 constant pressure pump and a UV230+ detector (equipped with a preparative flow cell), was provided by Dalian Elite Analytical Instruments Co., Ltd, (Dalian, China). GC instrumentation from Agilent technologies (Shanghai, China), consisted of an Agilent 7890A GC system, an Agilent 7683B auto-sampler and a split/splitless injector, was used during the chromatographic run coupled to 6975C mass spectrometer, operating in electron ionization (EI) mode. HPLC system was a Waters ACQUITY UPLC™ and attached with a Quattro Micro MS equipped with a DAD detector by Waters Technologies (Shanghai, China) was used. System operations and data acquisition were performed by MassLynx NT software (v 4.1).
Extraction
PB rhizomes were dried, cleaned and ground to make a fine powder and passed through a 0.45 mm mesh. The sample was extracted according to the previously reported method with minor modification (Intisar et al., 2012 ) as followed: 300 grams of this fine powder was dissolved in 20 litres of 40% methanol-water solvent and heated at 50°C for 4 hours. The sample was filtered through a 0.45 µm filter and concentrated by vacuum distillation.
Preparative chromatography conditions
The preparative separation was performed on an ODS glass column (26 mm × 460 mm) with a particle size of 30 µm. Pure water (A) and methanol (B) were used as the mobile phase. The optimized gradient elution program was 0∼38 min: B 5%∼18%, 38∼76min: B 18%∼28%, 76∼95min: B 28∼50%, 95∼118min: B 50∼85%, 118∼123 min: B isocratic 85%. The flow rate for the experiment was 30 mL/min and injection volume of 25 mL was used in each run. The detection wavelength was 280 nm and the whole experiment was carried out at room temperature (25°C). 13 fractions were obtained.
GC-MS conditions for obtained fractions
All fractions obtained from preparative LC were dissolved in pure methanol and run through GC-MS. The separation was performed by using an HP-5 fused silica capillary column (30 m × 0.25 mm ID × 0.25 µm film thickness) manufactured by Agilent. The operating conditions used were a temperature gradient as followed: 60°C for 3 minutes and then increased at a rate of 20°C/min to 280°C and then held at 280°C for 15 minutes. An injection volume of 5µl was used in split mode (split ratio 1:30). Helium was used as carrier gas at a flowrate of 1 mL/min. The mass detector conditions were: transfer line temperature of 250°C and a solvent delay of 3 min were selected, the mass range m/z was 30∼500 and a multi-channel plate voltage of 2200V was used. The detector temperature was 280°C. The ion source temperature of 230°C and ionizing voltage of 70eV was used.
HPLC-DAD-ESI-MS conditions
HPLC separation was performed by using a Waters XTerra MS column (2.1 mm × 150 mm) with a particle size of 5 µm. Solvent A was 0.1% formic acid in water and solvent B was 0.1% formic acid in Acetronitrile (ACN). The gradient elution program of 0∼20 min: B 10%∼30%, 20∼30 min: B 30%∼50% was used for the separation of fractions 3–12. The flow rate for the experiment was 0.2 mL/min and injection volume was 2 µl. The detection wavelengths were calculated by DAD detector in a range of 214 nm-360 nm. All experiments were carried out at room temperature (25°C). The ESI-MS was operated in negative ion mode. ESI-MS conditions were as followed: capillary voltage and cone voltage were 3 kV and 15 V respectively, nitrogen was used as a the desolvation gas at 600 L/h, source temperature and desolvation temperature were 120°C and 380°C, respectively. The scan range was selected between 100∼600 m/z.
Cell culture
Human hepatocellular carcinoma cell line (HCCLM3) with high metastatic capacity was obtained from cell bank of Zhongshan Hospital (Shanghai, China) for performing cytotoxicity experiments. Cells were grown in Dulbecco's modified eagle's medium (DMEM) with high glucose, containing 10% foetal calf serum (FCS) in a 5% CO2 humidified atmosphere at 37°C. The medium was supplied with penicillin (50 IU/mL) and streptomycin (50 µg/mL).
Cytotoxicity assay
Exponentially growing cells were seeded in 96-well microplates at a density of 3.5 × 103 cells per well in 100 µl of culture medium and were allowed to adhere for 18 h before treatment. PBS was used as a negative control. Fractions were initially dissolved in pure water and their final volumes were adjusted with PBS. Then increasing concentrations of these fractions were evaluated against the selected cancer cell line. Three replicates were carried out for each dosage. Cells were incubated for 24 h in the presence or absence of different amounts of fractions. Cytotoxicity was calculated using the Cell Counting Kit-8 (CCK-8) and the absorbance was measured at 450 nm using a microplate reader. Percent cell viabilities were calculated against selected concentrations. Cell survival curves were then calculated for the fractions showing strongest cytotoxic activities and 50 percent growth inhibition (GI50) values were calculated from the curve for each fraction. GI50 was defined as the concentration at which each cell line growth was inhibited by 50 percent.
Results and discussion
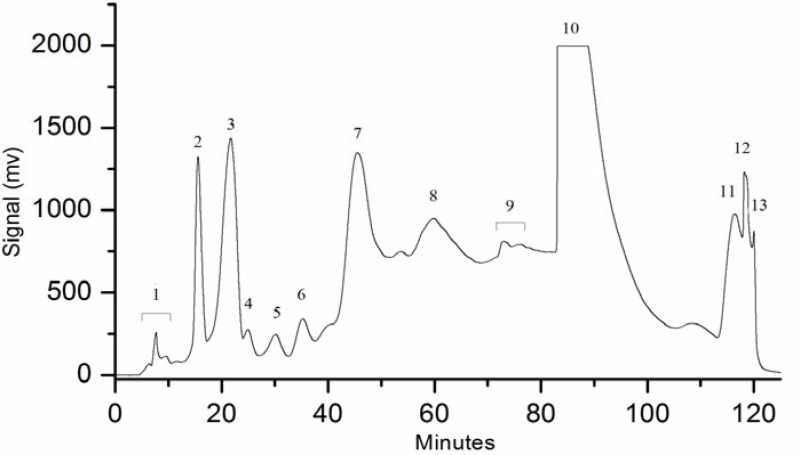
A simple water/methanol mobile phase was used to avoid any acidity or buffer content in the obtained fractions. Different gradients and percentage compositions of mobile phase were tried and other conditions were optimized to finally obtain 13 fractions as shown in Figure 2.
Figure 2.
Preparative high pressure liquid chromatogram showing 13 obtained fractions.
GC-MS and LC-DAD-ESI-MS analyses were applied and identified anticancer compounds from these fractions were shown in the Table 1. Compounds were identified by matching their mass spectral data with NIST-08/NBS-75K library and the retention index matching (http://webbook.nist.gov/chemistry/) for GC-MS analysis, while online massbank source (http://www.massbank.jp/) and previous PB literature was used for LC-DAD-ESI-MS analysis. These data showed that PB is rich in phenolic content. Considerable amounts (as assessed by chromatographic peak areas, but not quantified) of chlorogenic acid, gallic acid, and relatively lower amounts of p-hydroxybenzoic acid, hydroquinone, vanillic acid, syringic acid, 4-methyl catechol, syringol, catechol and pyrogallol were detected in fractions 1,2,3,5,6,7,8, whereas, anticancer fatty acids such as myristic acid, palmitic acid and linoleic acid were identified from fractions 10, 11, 12. To the best of our knowledge, syringol and 4-methyl catechol were identified for the first time from this plant.
Table 1.
Anticancer constituents identified from fractions (1–13).
| Fraction No. | Phenolic compounds identified by GC-MS/HPLC-DAD-MS |
| 1 | gallic acid, protocatechuic acid |
| 2 | p-hydroxy benzoic acid |
| 3 | chlorogenic acid |
| 4 | ND |
| 5 | pyrogallol |
| 6 | hydroquinone, vanillic acid |
| 7 | syringic acid, catechol |
| 8 | syringol, 4-methyl catechol |
| 9 | ND |
| 10 | myristic acid |
| 11 | palmitic acid |
| 12 | linoleic acid |
| 13 | ND |
ND = No anticancer compound detected
In recent years, plant-derived bioactive substances especially anticancer ones have gained considerable attention (Oueslati et al., 2012). Moreover, many plants containing phenolic compounds have been found to possess good anticancer activity (Cai et al., 2004; Owen et al., 2000) and based on this fact plants rich in phenolic content have been considered as interesting source of anticancer potential (Huang et al., 2009; Vuorela et al., 2005). This unidirectional literature and the difference in the mode of previous cytotoxic evaluation of PB (on hexane and chloroform fractions) by Manoharan et al., (2007), directed us towards the investigation of its methanol-water extract for identification of anticancer compounds especially phenolic ones and anticancer activity.
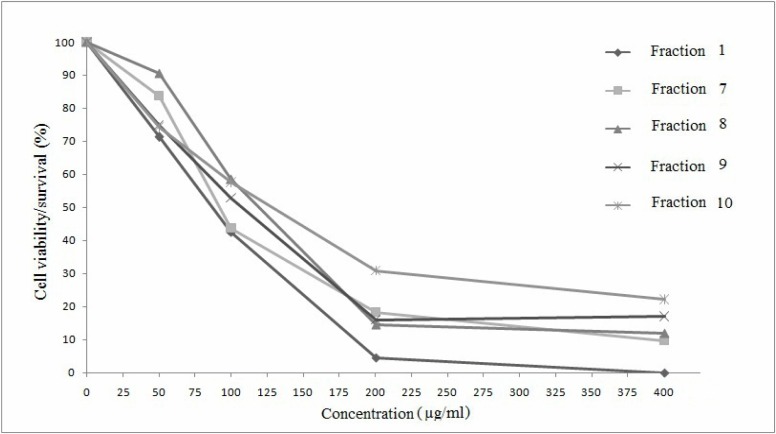
The cytotoxicity of obtained fractions evaluated against human hepatocellular carcinoma cell line (HCCLM3) showed strong cytotoxic activities for fractions 1, 7, 8, 9, 10 (50 µg/mL–400 µg/mL), while good activities for fractions 2, 3, 5, 6, 11, 12 (200 µg/mL–800 µg/mL), whereas almost no activity was found for fractions 4 and 13 even at a concentration of 800 µg/mL as shown in Table 2. A curve was plotted between percent cell viability/survival and concentration for the fractions showing strong activities, and 50 percent growth inhibition (GI50) values were calculated from this curve, according to the similar method described in literature (Sylvestre et al., 2006). The curve is shown in Figure 3. The lowest GI50 values were found to be 86.5 (±3), 92.3 (±3), 118.9 (±3), 107.2 (±3), 126.5 (±3) for fractions 1, 7, 8, 9, 10, respectively. These values show strong activities of these fractions against cancer cell line that may be due to the presence of phenolic compounds especially well-known anticancer phenolic acids such as gallic acid and protocatechuic acid in fraction 1 (You et al., 2010; Yip et al., 2006), while syringic acid and catechol in fraction 7 (Babich et al., 2003), and syringol and 4-methyl catechol in fraction 8 (Morita et al., 2003). On the other hand, fractions 2,3 possessed p-hydroxybenzoic acid and chlorogenic acid respectively, where chlorogenic acid is a well known anticancer phenolic acid (Kulisic-Bilusic et al., 2012; Marques et al., 2009) but fraction 2 showed sudden strong cytotoxicity at a concentration of 800 µg/mL whereas fraction 3 showed cytoxicity between 400 µg/mL–800 µg/mL. Fraction 5 contained pyrogallol, whereas 6 contained hydroquinone and vanillic acid and all three of them, especially pyrogallol, have been cited in literature as anticancer agents (Yang, et al., 2009; Terasaka et al., 2005; Arisawa et al., 1984; Babich et al., 2003) and these fractions showed good cytotoxicity at 800 µg/mL. Since, all fractions containing phenolic constituents showed good to strong cytotoxic activity especially low GI50 values of fractions 1, 7, 8 may suggest the presence of a relationship of phenolic content and cytotoxicity. The cytotoxic activities of fractions 10, 11, 12 indicate the presence of other active compounds in Polygonum bistorta L. such as fatty acids: myristic acid, especially linoleic acid and palmitic acid (Sagar and Das, 1995; Salerno and Smith 1991) whereas low GI50 value of fraction 9 indicates the presence of other unknown active compounds present in it. Anticancer activity of these 11 out of total 13 fractions and its anticancer constituents strongly showed the remarkable importance of this plant in anticancer activity.
Table 2.
Cytotoxic activity of obtained fractions (1–13).
| Samples | Cell Viability (%) at 50 µg/mL |
Cell Viability (%) at 100 µg/mL |
Cell Viability (%) at 200 µg/mL |
Cell Viability (%) at 400 µg/mL |
Cell Viability (%) at 800 µg/mL |
| Control (PBS) | 100 | 100 | 100 | 100 | 100 |
| Fraction 1 | 71.29 | 42.56 | 4.48 | 0 | NT |
| Fraction 2 | NT | NT | NT | 101.22 | 14.00 |
| Fraction 3 | NT | NT | NT | 86.70 | 25.90 |
| Fraction 4 | NT | 117.94 | 117.10 | 102.16 | 98.11 |
| Fraction 5 | NT | NT | NT | 78.03 | 33.03 |
| Fraction 6 | NT | NT | NT | 85.52 | 54.82 |
| Fraction 7 | 83.67 | 43.68 | 18.29 | 9.69 | NT |
| Fraction 8 | 90.52 | 58.57 | 14.56 | 11.88 | NT |
| Fraction 9 | 74.91 | 52.75 | 15.99 | 16.97 | NT |
| Fraction 10 | 74.25 | 57.54 | 30.72 | 22.10 | NT |
| Fraction 11 | NT | NT | NT | 52.69 | 34.99 |
| Fraction 12 | NT | NT | 71.14 | 50.07 | NT |
| Fraction 13 | NT | NT | 91.12 | 98.66 | 93.58 |
NT = not tested
Figure 3.
Curves showing the dose-dependent relationship between percent cell viability/survival and concentration of most active fractions 1, 7, 8, 9, 10. GI50 values were calculated from the curve for each of these specified fractions.
Conclusion
Anticancer phenolic compounds and fatty acids were identified from different fractions of methanol-water extract of Polygonum bistorta L. 11 fractions out of 13 possessed good to strong cytotoxicity against HCCLM3 cancer cell line that demonstrated good bioactivity of this herbal plant. Additionally, all of the fractions containing phenolic content showed good to strong cytotoxic activity and 3 out of the 5 most active fractions also exhibited phenolic content that signifies the idea that phenolic compounds containing plants can be interesting source of anticancer potential. However, further studies can be conducted to identify the unknown active constituents to fully evaluate the anticancer importance and medicinal value of this plant. Moreover, compounds like catechol may be dangerous at high concentration levels, hence it should be quantified to ensure the safe use of this plant.
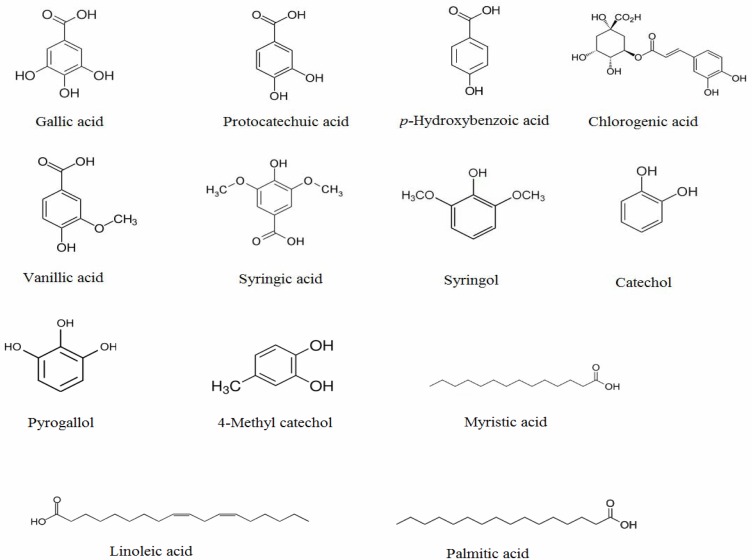
Figure 1.
Structures of the phenolic compounds and fatty acids identified from 40 % methanol-water extract of Polygonum bistorta L.
Acknowledgement
This work was kindly supported by “The 973 Project from the Ministry of Science and Technology of People's Republic of China (No. 2011CB910404)”, “The Fundamental Research Funds for the Central Universities of China (WK1014042) and “The Science and Technology Commission of Shanghai Municipality (10dz2220500)”
References
- 1.Arisawa M, Funayama S, Pezzuto JM, Kinghorn AD, Cordell GA, Farnsworth NR. Potential anticancer agents XXXII. Hydroquinone from Ipomopsis aggregata. J Nat Prod. 1984;47(2):393–394. doi: 10.1021/np50032a037. [DOI] [PubMed] [Google Scholar]
- 2.Babich H, Visioli F. In vitro cytotoxicity to human cells in culture of some phenolics from olive oil. Farmaco. 2003;58:403–407. doi: 10.1016/S0014-827X(03)00048-X. [DOI] [PubMed] [Google Scholar]
- 3.Cai YZ, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang X, Liu YX, Kang WY. Antioxidant activity of extracts from Polygonum bistorta L. Fine Chem Interm. 2009;39(2) [Google Scholar]
- 5.Couplan F, Duke JA. The encyclopedia of edible plants of North America. New Canaan, Connecticut: Keats Publishing Inc.; 1998. p. 128. [Google Scholar]
- 6.Duwiejua M, Zeitlin IL, Gray AI, Waterman PG. Anti-inflammatory activity of Polygonum bistorta, Quaiacum officinale and Hamamelis virginiana in rats. J Pharm Pharmacol. 1994;46:286–290. doi: 10.1111/j.2042-7158.1994.tb03795.x. [DOI] [PubMed] [Google Scholar]
- 7.Duwiejua M, Zeitlin IL, Gray AI, Waterman PG. The anti-inflammatory compounds of Polygonum bistorta: isolation and characterization. Planta Med. 1999;65:371–374. doi: 10.1055/s-2006-960791. [DOI] [PubMed] [Google Scholar]
- 8.Gomes CA, Girão T, Andrade JL, Milhazes N, Borges F, Marques MPM. anticancer activity of phenolic acids of natural or synthetic origin: A structure-activity study. J Med Chem. 2003;46:5395–5401. doi: 10.1021/jm030956v. [DOI] [PubMed] [Google Scholar]
- 9.Huang W, Cai YZ, Zhang Y. Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutr Cancer. 2009;62(1):1–20. doi: 10.1080/01635580903191585. http://webbook.nist.gov/chemistry/ [DOI] [PubMed] [Google Scholar]
- 10. http://www.massbank.jp/
- 11.Intisar A, Kiazolu JB, Wang Y, Zhang L, Zhang W. Effect of mobile phase composition and pH on the separation of rhizome of Polygonum bistorta. J Liq Chromatogr Relat Technol. 2012;35(7):977–987. [Google Scholar]
- 12.Khalid A, Waseem A, Saadullah M, Rehman U-U-, Khiljee S, Sethi A, Asad MHHB, Rasool F, Waqas MK, Murtaza G. Antibacterial activity analysis of extracts of various plants against gram-positive and -negative bacteria. Afr J Pharm Pharmacol. 2011;5(7):887–893. [Google Scholar]
- 13.Kulisic-Bilusic T, Schmöller I, Schnäbele K, Siracusa L, Ruberto G. The anticarcinogenic potential of essential oil and aqueous infusion from caper (Capparis spinosa L.) Food Chem. 2012;132:261–267. doi: 10.1016/j.foodchem.2011.10.074. [DOI] [PubMed] [Google Scholar]
- 14.Liu CQ, Wang XL, Zeng J. Preliminary study on antimicrobial activity of Polygonum bistorta L. J Gannan Med Univ. 2006;26(4):489–490. [Google Scholar]
- 15.Liu XQ, Chen FK, Wu LJ, Wang ST, Li WW. Studies on the chemical constituents of Polygonum bistorta L. J Shenyang Pharm Univ. 2004;3:187–189. [Google Scholar]
- 16.Liu XQ, Hua HM, Liu J, Chen FK, Wu LJ. A new tannin-related compound from the rhizome of Polygonum bistorta L. J Asian Nat Prod Res. 2006;8(4):299–302. doi: 10.1080/10286020500034956. [DOI] [PubMed] [Google Scholar]
- 17.Manoharan KP, Benny TKH, Yang D. Cycloartane type triterpenoids from the rhizomes of Polygonum bistorta. Phytochem. 2005;66(19):2304–2308. doi: 10.1016/j.phytochem.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Manoharan KP, Yang D, Hsu A, Huat BTK. Evaluation of Polygonum bistorta for anticancer potential using selected cancer cell lines. J Med Chem. 2007;3(2):121–126. doi: 10.2174/157340607780059495. [DOI] [PubMed] [Google Scholar]
- 19.Marques V, Farah A. Chlorogenic acids and related compounds in medicinal plants and infusions. Food Chem. 2009;113:1370–1376. [Google Scholar]
- 20.Miki N, A-Fu W, Takahiko S, Hisamitsu N, Hideaki K. Effects of Chinese medicinal plant extracts on mutagenecity of Trp-P-1. J Nat Med. 1995;49:329–331. [Google Scholar]
- 21.Moerman DE. Native American food plants: an ethnobotanical dictionary. Portland, Oregon: Timber Press Inc.; 1998. p. 189. [Google Scholar]
- 22.Morita K, Arimochi H, Ohnishi Y. In vitro cytotoxicity of 4-methylcatechol in murine tumor cells: induction of apoptotic cell death by extracellular pro-oxidant action. J Pharmacol Exp Therap. 2003;306:317–323. doi: 10.1124/jpet.103.050351. [DOI] [PubMed] [Google Scholar]
- 23.Owen RW, Giacosa A, Hull WE, Haubner AR, Spiegelhalder B, Bartsch H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. EurJ Cancer. 2000;36:1235–1247. doi: 10.1016/s0959-8049(00)00103-9. [DOI] [PubMed] [Google Scholar]
- 24.Oueslati S, Ksouri R, Falleh H, Pichette A, Abdelly C, Legault J. Phenolic content, antioxidant, anti-inflammatory and anticancer activities of the edible halophyte Suaeda fruticosa Forssk. Food Chem. 2012;132:943–947. [Google Scholar]
- 25.Sagar SP, Das UN. Cytotoxic action of cis-unsaturated fatty acids on human cervical carcinoma (HeLa) cells in vitro. Prostaglandins, Leukotrienes and Essential Fatty Acids. 1995;53(4):287–299. doi: 10.1016/0952-3278(95)90129-9. [DOI] [PubMed] [Google Scholar]
- 26.Salerno JW, Smith DE. The use of sesame oil and other vegetable oils in the inhibition of human colon cancer growth in vitro. Anticancer Res. 1991;11(1):209–215. [PubMed] [Google Scholar]
- 27.Shang YF, Kim SM, Song DG, Pan CH, Lee WJ, Um BH. Isolation and identification of antioxidant compounds from Ligularia fischeri. J Food Sci. 2010;75(6):C530–C535. doi: 10.1111/j.1750-3841.2010.01714.x. [DOI] [PubMed] [Google Scholar]
- 28.Smolarz HD. Comparative study on the free flavonoid aglycones in herbs of different species of Polygonum L. Acta Pol Pharm Drug Res. 2002;59(2):145–148. [PubMed] [Google Scholar]
- 29.Sun XB, Zhao PH, Xu YJ, Sun LM, Cao MA, Yuan CS. Chemical constituents from the roots of Polygonum bistorta. Chem Nat Compd. 2007;43(5):563–566. [Google Scholar]
- 30.Sylvestre M, Pichette A, Longtin A, Nagau F, Legault J. Essential oil analysis and anticancer activity of leaf essential oil of Croton flavens L. from Guadeloupe. J Ethnopharmacol. 2006;103:99–102. doi: 10.1016/j.jep.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Terasaka H, Morshed SR, Hashimoto K, Sakagami H, Fujisawa S. Hydroquinone-induced Apoptosis in HL-60 Cells. Anticancer Res. 2005;25:161–170. [PubMed] [Google Scholar]
- 32.The State Pharmacopoeia Commission of People's Republic of China, author. “Pharmacopoeia of the People's Republic of China”. I. Beijing, China: Chemical Industry Press; 2005. p. 202. [Google Scholar]
- 33.Vuorela S, Kreander K, Karonen M, Nieminen R, Hämäläinen M, Galkin A. Preclinical evaluation of rapeseed, raspberry, and pine bark phenolics for health related effects. J Agric Food Chem. 2005;53:5922–5931. doi: 10.1021/jf050554r. [DOI] [PubMed] [Google Scholar]
- 34.Yang CJ, Wang CS, Hung JU, Huang HW, Chia YC, Wang PH, Weng CF, Huang MS. Pyrogallol induces G2-M arrest in human lung cancer cells and inhibits tumor growth in an animal model. Lung Cancer. 2009;66(2):162–168. doi: 10.1016/j.lungcan.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 35.Yip ECH, Chan ASL, Pang H, Tam YK, Wong YH. Protocatechuic acid induces cell death in HepG2 hepatocellular carcinoma cells through a c-Jun N-terminal kinase-dependent mechanism. Cell Biol Toxicol. 2006;22(4):293–302. doi: 10.1007/s10565-006-0082-4. [DOI] [PubMed] [Google Scholar]
- 36.You BR, Moon HJ, Han YH, Park WH. Gallic acid inhibits the growth of HeLa cervical cancer cells via apoptosis and/or necrosis. Food Chem Toxicol. 2010;48(5):1334–1340. doi: 10.1016/j.fct.2010.02.034. [DOI] [PubMed] [Google Scholar]